Abstract
Purpose
Low-dose radiation therapy (LD-RT) has been shown to have an anti-inflammatory effect, and preliminary results suggest it is feasible to treat patients with coronavirus disease 2019 (COVID-19) pneumonia.
Materials and Methods
We conducted a prospective, single-arm, phase 1/2 clinical trial enrolling patients aged ≥50 years, who were coronavirus disease 2019 (COVID-19) positive, at phase 2 or 3 with lung involvement at imaging study and oxygen requirement. Patients received 100 cGy to total lungs in a single fraction. Primary outcome was radiologic response using severity and extension score on baseline computed tomography (CT), at days 3 and 7 after LD-RT. Secondary outcomes were toxicity using Common Terminology Criteria for Adverse Events v.5.0, duration of hospitalization, blood work evolution, and oxygen requirements using SatO2/FiO2 index (SAFI), at days 3 and 7 after LD-RT.
Results
Nine patients were included. Median age was 66 (interquartile range, 57-77). Severity score was stable or decreased in the third CT but was not statistically significant (P = .28); however, there were statistically significant changes in the extension score (P = .03). SAFI index significantly improved 72 hours and 1 week after LD-RT (P = .01). Inflammatory blood parameters decreased 1 week after RT compared with baseline; only lactate dehydrogenase decreased significantly (P = .04). Two patients presented grade 2 lymphopenia after RT and another (with baseline grade 3) worsened to grade 4. Overall, the median number of days of hospitalization was 59 (range, 26-151). After RT the median number of days in the hospital was 13 (range, 4-77). With a median follow-up after RT of 112 days (range, 105-150), 7 patients were discharged and 2 patients died, 1 due to sepsis and the other with severe baseline chronic obstructive pulmonary disease from COVID-19 pneumonia.
Conclusions
Our preliminary results show that LD-RT was a feasible and well-tolerated treatment, with potential clinical improvement. Randomized trials are needed to establish whether LD-RT improves severe pneumonia.
Postmortem analysis in a series of coronavirus disease 2019 (COVID-19)–infected patients has shown diffuse alveolar damage with inflammatory infiltrate present, which compromises gas exchange.1 Radiation therapy administered at low doses (LD-RT) has anti-inflammatory properties such as lowering levels of pro-inflammatory cytokines (eg, IL-1a) or inhibiting leukocyte recruitment.2, 3, 4, 5 In addition to its anti-inflammatory effect, LD-RT was used for pneumonia in the first half of the 20th century with several reports suggesting potential efficacy.6 , 7 Preliminary results have shown that 0.5 Gy LD-RT is feasible in patients with COVID-19 pneumonia.8
Taking into account the low risk of toxicity and the potential benefit of LD-RT, we conducted a prospective phase 1-2 study to evaluate the radiologic and clinical efficacy of LD-RT in patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Herein, we report preliminary results.
Materials and Methods
Study design and patient selection
Patients aged ≥50 years with confirmed COVID-19 by polymerase chain reaction (PCR) with lung involvement at imaging study (chest radiograph, chest computed tomography [CT], or positron emission tomography [PET]-CT) and oxygen requirement and previous provision of written informed consent were enrolled into a prospective single-arm phase 1-2 clinical trial at a single institution (NCT-04420390). This study was approved by our institutional research ethics board.
Patients in phase 2 (lung phase, patients develop a viral pneumonia, with cough, fever, and hypoxia defined as PaO2/FiO2 < 300 mm Hg) or phase 3 (hyperinflammatory phase; the disease manifests as an extrapulmonary systemic hyperinflammation syndrome; C-reactive protein, ferritin, and D-dimer are significantly elevated)9 were eligible. Exclusion criteria included severe comorbidities that could hamper the radiation treatment, such as an impossibility of holding a supine position.
Treatment details
Patients were immobilized in the supine position with a wedge-shaped mattress to perform a CT scan (Toshiba Aquilion LB 1800-mm CT device, Toshiba Corp, Tokyo, Japan) for simulation purposes. Clinical target volume included both lungs. Planning target volume (PTV) was generated adding 1 cm cranial, antero-posterior, and lateral, and 2 cm caudal. Heart and esophagus were contoured as organs at risk retrospectively. Participants received 100 cGy in a single fraction prescribed to the PTV. Dose planning goals were 80% of the dose received by >95% of the PTV volume and maximal dose (Dmax) <115%.
Treatment planning was carried out using the 3-dimensional conformed radiation therapy technique (Eclipse v.15.6, Varian Medical Systems, Palo Alto, CA), with 2 opposite antero-posterior beams and 6MV photons.
Outcomes evaluation
The primary outcome was radiologic response. Patients underwent 3 thoracic CTs: simulation CT and days 3 and 7 after RT. Image analysis was performed by 2 experienced thoracic radiologist (>10 years’ experience) and a second-year radiology resident using the institutional digital database system (IMPAX 6.5.33, Agfa-Gevaert, Mortsel, Belgium). To rate the COVID-19 lung involvement 2 scores described by Chung et al were used.10 A severity score was assigned to each lobe based on the lung abnormalities detected being:
-
-
0: no lung abnormalities
-
-
1: ground-glass opacities (GGO)
-
-
2: GGO and consolidations, with GGO predominance
-
-
3: GGO and consolidations, with no predominance
-
-
4: GGO and consolidations, with consolidation predominance
According to the extension of the lung involvement (extension score) each of the 5 lung lobes was assessed for the percentage of the lobar involvement and classified as none (0% = score 0), minimal (1%-25% = score 1), mild (26%-50% = score 2), moderate (51%-75% = score 3), or severe (76%-100% = score 4). The total severity and extension score was reached by summing the 5 lobe scores in each patient (range from 0-20).
Oxygen requirement was measured using SatO2/FiO2 index (SAFI)11 , 12 previous to RT, 72 hours, and a week after (normal SAFI index >315, mild respiratory failure <300, and severe <200). Blood tests including ferritin, blood cell count, C-reactive protein (CRP), D-dimer, and lactate dehydrogenase (LDH) were performed before RT; 24, 48, and 72 hours after RT; and then every 2 days until normal levels.
Toxicity data were collected prospectively using the Common Terminology Criteria for Adverse Events version 5.0 (CTCAE v.5.0), at 15 days and 30 days after treatment.13
Discharge criteria included: resolution of fever for at least 48 hours without use of antipyretic medication, maintaining O2 saturation >95% with low flow rate oxygen therapy with nasal glasses at 3 liters per minute, improvement of signs and symptoms requiring minimal supportive care (oral medication), ability to adhere to home isolation recommendations, and sufficient support at home.
Statistical analyses
Descriptive analyses were summarized as means with standard deviation (SD) and medians with interquartile ranges (IQRs) for normally and non-normally distributed continuous characteristics, respectively, frequencies, and proportions for categorical characteristics. A Wilcoxon sign rank test for paired data was used to assess the statistical significance of the CT scores, and it was established as statistically significant at P < .05.
Results
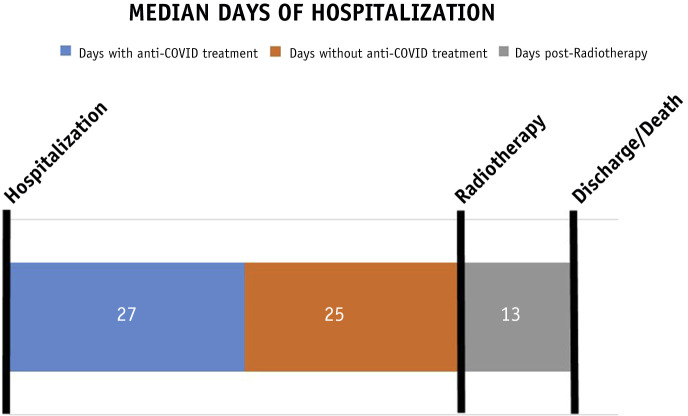
From April to June 2020, 9 patients consented and were treated with 100 cGy to both lungs on the same day as enrollment. Patient characteristics are summarized in Table 1 . Median age was 66 (IQR, 57-77). Details about RT are included in Supplementary Table E1. All patients were on tapering steroid dose (prednisone or methylprednisolone, median dose 40 mg/24 h) while on RT. Median time to receive RT from the date of admission was 52 days (range, 17-85) and from the last anti-COVID treatment (hydroxychloroquine, lopinavir/ritonavir, tocilizumab, or remdesivir) was 25 days (range, 10-75). Median time of hospitalization after RT was 13 days (range, 4-77) and overall was 59 days (range, 26-151) (Fig. 1 and Fig. E1).
Table 1.
Patient characteristics
| Age | Sex | Comorbidities | Domiciliary O2 | Baseline Sat O2% | Previous COVID treatment |
DC | Death | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HCQ∗ | L/R† | TZM‡ | RDM§ | ATB | GC | AT‖ | ||||||||
| 1 | 76 | F | DM | N | 95 | Y | Y | Y | N | Y | Y | Y | - | Y |
| 2 | 86 | M | HBP, parotid cancer | N | - | Y | N | N | N | Y | Y | N | Y | N |
| 3 | 68 | M | DM, HBP, COPD | Y | 95 | Y | N | N | N | Y | Y | N | Y | Y |
| 4 | 90 | M | DM, HBP, ischemic heart disease | N | 90 | Y | N | N | N | Y | Y | Y | Y | N |
| 5 | 53 | M | Obesity, DL | N | 90 | Y | N | Y | Y | Y | Y | Y | Y | N |
| 6 | 58 | F | Obesity, DL | N | 88 | Y | N | N | N | Y | Y | Y | Y | N |
| 7 | 55 | M | DL, ischemic heart disease, chronic hepatitis B, mixed connective tissue disease | N | 86 | Y | Y | N | N | Y | Y | Y | Y | N |
| 8 | 64 | M | HBP, obesity, hepatitis B, OSA | N | 86 | Y | Y | N | N | Y | Y | Y | Y | N |
| 9 | 56 | M | - | N | 96 | Y | Y | Y | N | Y | Y | Y | Y | N |
Abbreviations: AT = antithrombotic; ATB = antibiotic; COPD = chronic obstructive pulmonary disease; DC = discharged; DL = dyslipidemia; DM = diabetes; GC = glucocorticoids; HBP = high blood pressure; HCQ = hydroxychloroquine; L/R = lopinavir/ritonavir; OSA = obstructive sleep apnea; RDM = remdesivir; TZM = tocilizumab.
HCQ was administered at a dose of 400 mg/12 h the first day and then 200 mg/12 h for 5 d.
Lopinavir 200 mg/ritonavir 100 mg, 2 tablets/12 h for 7 d.
Tocilizumab 400 mg with a maximum of 3 doses.
Remdesivir 100 mg/24 h during 9 d.
Antithrombotic 40 mg/24 h.
Fig. 1.
Hospitalization evolution.
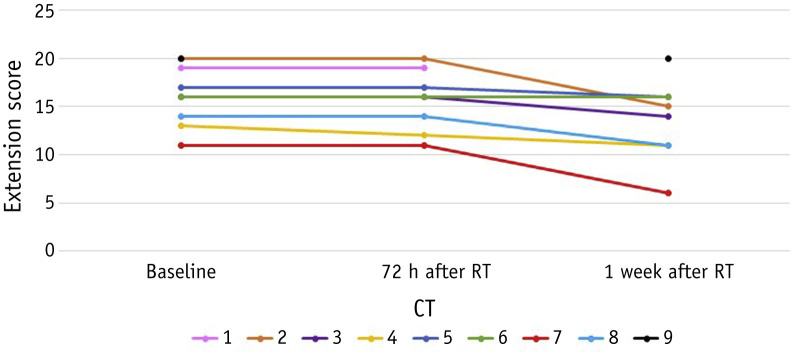
One patient could not undergo the second CT due to hemodynamic instability and another died before the third CT was performed. Severity scores are shown in Table 2 . In the first CT, 26 lobes (57.8%) had only GGO and 8 lobes (17.8%) had clear predominance of consolidation; in the third CT, 25 lobes (62.5%) had only GGO and 4 lobes (10%) had clear predominance of consolidation (Fig. 2 ). Severity score was stable or decreased in the third CT, but not significantly (P = .28). Extension scores are shown in Figure 3 and Table E2. No significant differences were found between the first and the second CT (P = .32), but there was a significant improvement between the first and the third CT (P = .03).
Table 2.
Severity score of lung abnormalities
| Patients |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| First CT | 8 | 7 | 13 | 16 | 8 | 8 | 7 | 5 | 12 |
| Second CT | 8 | 7 | 13 | 6 | 8 | 8 | 7 | 5 | |
| Third CT | 7 | 10 | 5 | 8 | 8 | 7 | 5 | 14 | |
Abbreviation: CT = computed tomography.
Fig. 2.
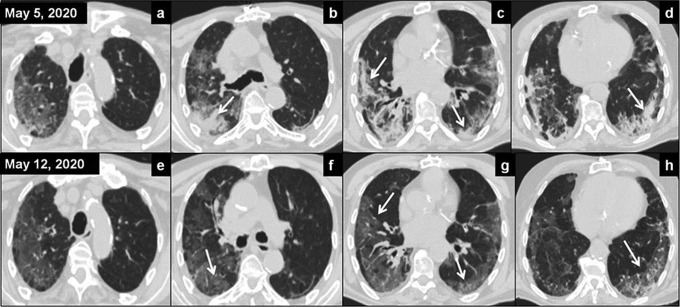
Axial computed tomography (CT) images before radiation therapy (A-D) showed extensive areas of increased attenuation in ground-glass opacities (GGO) and pulmonary consolidations, predominantly basal and peripheral (arrows); extension score was 13 and severity score was 16. Surveillance CT on day 7 (E-H images) showed radiologic improvement with disappearance of the lung consolidations with persistence of the GGO (arrows); extension score was 11 and severity score was 5.
Fig. 3.
Extension score of lung abnormalities. Abbreviations: CT = computed tomography; RT = radiation therapy.
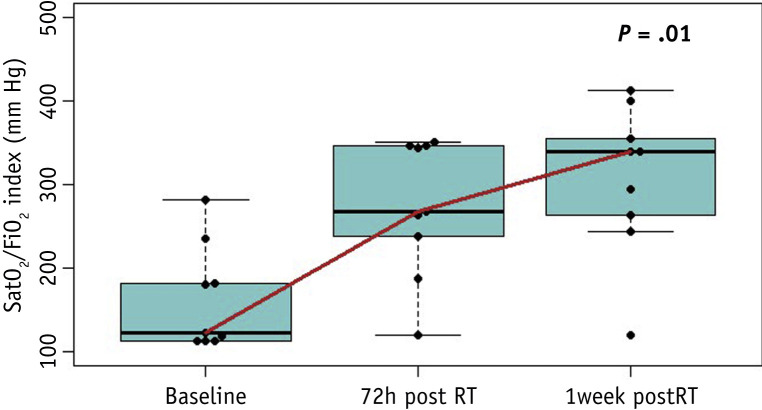
Clinically, 7 patients presented with baseline severe respiratory failure (SRF) and 2 with mild respiratory failure (MRF). Seventy-two hours after RT there was a significant improvement (P = .01): 2 patients continued with SRF, 3 patients with MRF, and 4 patients recovered normal SAFI index. A week later the significant improvement persisted (P = .01), 1 patient continued with SRF, 3 with MRF, and 5 recovered normal SAFI (Fig. 4 and Table E3).
Fig. 4.
Median SatO2/FiO2 (SAFI) index evolution. Abbreviation: RT = radiation therapy.
All patients but 1 (inpatient at an intensive care unit [ICU]) fulfilled the protocol of blood work. Inflammatory blood parameters among the other 8 patients (ferritin, D-dimer, LDH, and CRP) decreased 1 week after RT compared with baseline; only LDH (comparing baseline to 1 week after RT) declined significantly (P = .04) (Fig. E2).
Two patients developed grade 2 lymphopenia 72 hours after RT, and 1 patient with baseline grade 3 worsened to grade 4 one week after RT.
With a median follow-up after RT of 112 days (range, 105-150), 2 patients died, one 13 days after RT due to bacterial sepsis, and the other, with severe baseline chronic obstructive pulmonary disease, 34 days after RT from COVID pneumonia. The other 7 patients were discharged and maintained supplemental O2 (maximum 3 L, 5 of them 8 hours per day and 2 more than 16 hours).
Discussion
According to the expected LD-RT anti-inflammatory effect, our results showed a decrease in the acute phase reactants (CRP, ferritin, LDH, and D-dimer) 1 week after LD-RT similar to Amari and RESCUE 1-19 trial results.8 , 14
Radiologically, there was no significant difference in severity score; however, there was a statistically significant improvement on the extension score by 1 week (P = .03). Although radiologic changes were modest, clinically there was a significant improvement in SAFI 72 hours after RT (P = .01). One week after, only 1 patient continued with SRF, 3 with MRF, and 5 recovered normal SAFI index (P = .01). This rapid clinical improvement allowed patients to be discharged with a median of 13 days.
Patients were treated after a median of 52 days from admission, mainly because the referral criteria to consider LD-RT were after another anti-COVID treatment failed. According to Calabrese, LD-RT earlier than day 14 may be more effective6; however, our results suggest that LD-RT could be an option even later and after failure of currently known anti-COVID treatment.
Two patients developed grade 2 lymphopenia 72 hours after RT and 1 patient with baseline grade 3 worsened to grade 4 one week after RT. Nakamura et al15 studied lymphocyte radiosensitivity and concluded that 2 Gy is the lethal dose required to reduce the surviving fraction of lymphocytes by 50%. In our study we administered 1 Gy, and although lymphopenia can be produced by RT, COVID-19 infection itself or the use of some drugs such as steroids can also contribute to it.
A risk of radiation-induced heart disease has been described at a dose of 1 Gy, with an increased risk of major coronary events of 7.4% for each Gy of mean heart dose.16 The mean dose to the heart in our study was 0.91 Gy (0.85-0.92). The risk of radiation-induced tumors after RT is low (<5%) at moderate doses (20 Gy on average),17, 18, 19 with a long latency time (approximately 20 years) being the most important factors for carcinogenesis the young age at the time of exposure and the time elapsed after RT. Our patients received a much lower dose (1 Gy), but because this low dose of radiation can cause cancer,20 , 21 we did not include patients younger than age 50 years.
All patients included were treated uniformly with a prospective assessment. However, the small number of patients and the short follow-up are important limitations. Furthermore, the heterogeneity of the medical treatments previously received by patients and the absence of randomization with a control group make it difficult to really know the extent of improvement due to LD-RT.
Our preliminary results show that LD-RT was a feasible and well-tolerated treatment, with potential clinical improvement. A randomized trial with longer follow-up is needed to determine whether LD-RT provides benefit in this setting.
Footnotes
Disclosures: none.
All data generated and analyzed during this study are included in this published article (and its supplementary information files).
Supplementary material for this article can be found at https://doi.org/10.1016/j.ijrobp.2020.11.049.
Supplementary Materials
References
- 1.Carsana L., Sonzogni A., Nasr A., et al. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: A two-centre descriptive study. Lancet Infect Dis. 2020;20:1135–1140. doi: 10.1016/S1473-3099(20)30434-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodel F., Keilholz L., Herrmann M., et al. Radiobiological mechanisms in inflammatory diseases of low-dose radiation therapy. Int J Radiat Biol. 2007;83:357–366. doi: 10.1080/09553000701317358. [DOI] [PubMed] [Google Scholar]
- 3.Torres Royo L., Antelo Redondo G., Arquez Pianetta M., et al. Low-dose radiation therapy for benign pathologies. Rep Pract Oncol Radiother. 2020;25:250–254. doi: 10.1016/j.rpor.2020.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schaue D., Jahns J., Hildebrandt G., et al. Radiation treatment of acute inflammation in mice. Int J Radiat Biol. 2005;81:657–667. doi: 10.1080/09553000500385556. [DOI] [PubMed] [Google Scholar]
- 5.Arenas M., Gil F., Gironella M., et al. Time course of anti-inflammatory effect of low-dose radiotherapy: Correlation with TGF-beta(1) expression. Radiother Oncol. 2008;86:399–406. doi: 10.1016/j.radonc.2007.10.032. [DOI] [PubMed] [Google Scholar]
- 6.Calabrese E.J., Dhawan G. How radiotherapy was historically used to treat pneumonia: Could it be useful today? Yale J Biol Med. 2013;86:555–570. [PMC free article] [PubMed] [Google Scholar]
- 7.Oppenheimer A. Roentgen therapy of interstitial pneumonia. J Pediatr. 1943;41:404–414. [Google Scholar]
- 8.Ameri A., Rahnama N., Bozorgmehr R., et al. Low-dose whole-lung irradiation for COVID-19 pneumonia: Short course results. Int J Radiat Oncol Biol Phys. 2020;108:1134–1139. doi: 10.1016/j.ijrobp.2020.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siddiqi H.K., Mehra M.R. COVID-19 illness in native and immunosuppressed states: A clinical-therapeutic staging proposal. J Heart Lung Transplant. 2020;39:405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung M., Bernheim A., Mei X., et al. CT imaging features of 2019 novel coronavirus (2019-nCoV) Radiology. 2020;295:202–207. doi: 10.1148/radiol.2020200230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bilan N., Dastranji A., Ghalehgolab Behbahani A. Comparison of the SpO2/FiO2 ratio and the PaO2/FiO2 ratio in patients with acute lung injury or acute respiratory distress syndrome. J Cardiovasc Thorac Res. 2015;7:28–31. doi: 10.15171/jcvtr.2014.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pandharipande P.P., Shintani A.K., Hagerman H.E., et al. Derivation and validation of SpO2/FiO2 ratio to impute for PaO2/FiO2 ratio in the respiratory component of the Sequential Organ Failure Assessment score. Crit Care Med. 2009;37:1317–1321. doi: 10.1097/CCM.0b013e31819cefa9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Cancer Institute at the National Institutes of Health Common terminology criteria for adverse events: CTCAE v5.0. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_50 Available at:
- 14.Hess C.B., Buchwald Z.S., Stokes W., et al. Low-dose whole-lung radiation for COVID-19 pneumonia: Planned day 7 interim analysis of a registered clinical trial. Cancer. 2020;126:5109–5113. doi: 10.1002/cncr.33130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakamura N., Kusunoki Y., Akiyama M. Radiosensitivity of CD4 or CD8 positive human T-lymphocytes by an in vitro colony formation assay. Radiat Res. 1990;123:224–227. [PubMed] [Google Scholar]
- 16.Darby S.C., Ewertz M., McGale P., et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 17.Trott K.R., McKeown S.R., Hatfield P., et al. Radiotherapy for benign disease; assessing the risk of radiation-induced cancer following exposure to intermediate dose radiation. Br J Radiol. 2015;88 doi: 10.1259/bjr.20150405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trott R., Kamprad F. Estimation of cancer risks from radiotherapy of benign diseases. Strahlenther Onkol. 2006;182:431–436. doi: 10.1007/s00066-006-1542-8. [DOI] [PubMed] [Google Scholar]
- 19.Mazonakis M., Damilakis J. Cancer risk after radiotherapy for benign diseases. Phys Med. 2017;42:285–291. doi: 10.1016/j.ejmp.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 20.Salomaa S., Cardis E., Bouffler S.D., et al. Low dose radiation therapy for COVID-19 pneumonia: Is there any supportive evidence? Int J Radiat Biol. 2020;96:1224–1227. doi: 10.1080/09553002.2020.1762020. [DOI] [PubMed] [Google Scholar]
- 21.Kirsch D.G., Diehn M., Cucinotta F.A., et al. Lack of supporting data make the risks of a clinical trial of radiation therapy as a treatment for COVID-19 pneumonia unacceptable. Radiother Oncol. 2020;147:217–220. doi: 10.1016/j.radonc.2020.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.