Abstract
We describe 4 children (11–17 years in age) at our institution with acute appendicitis in the setting of SARS-CoV-2 infection, suggesting a possible association. Providers should consider testing for this infection in patients with severe gastrointestinal symptoms, in order to take appropriate transmission based precautions, until more is understood.
Keywords: COVID-19, SARS-CoV-2, Appendicitis
Abbreviations: COVID-19, novel coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; ED, emergency department; CT, computed tomography; PCR, polymerase-chain-reaction; NP, nasopharyngeal; IV, intravenous; MIS-C, multisystem inflammatory syndrome in children; ACE2, angiotensin-converting enzyme 2; HEPA, high-efficiency particulate air
1. Introduction
The clinical spectrum in children with the novel coronavirus disease 2019 (COVID-19) has continued to evolve since the rapid emergence and spread of the causative severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) at the end of 2019. To date, COVID-19 appears to cause milder manifestations in the majority of children compared to adults. Fever and respiratory symptoms are among the most common symptoms reported in children, though gastrointestinal and cutaneous findings are also reported. Severe disease can occur in children less frequently, but the full scope is yet to be elucidated. In this report we describe four patients admitted to our institution who were diagnosed with acute appendicitis in the setting of SARS-CoV-2 infection.
2. Case report
2.1. Patient 1
An obese but otherwise healthy 11-year-old Hispanic girl presented with two-days of abdominal pain, recurrent non-bloody, non-bilious emesis and fever. She was transferred to our emergency department (ED) for surgical consultation after her second presentation to a community hospital with these symptoms, now with concern for appendicitis based upon contrast enhanced computed tomography (CT) abdomen/pelvis showing a dilated fluid-filled appendix with peri-appendiceal inflammation (Fig. 1 a). Abdominal exam was remarkable for diffuse tenderness to palpation, most pronounced over the right lower quadrant, with guarding and rebound. (Initial vital signs and laboratory results for all patients are described in Table 1 .) SARS-CoV-2 was detected by polymerase-chain-reaction (PCR) of her nasopharyngeal (NP) swab. Empiric intravenous (IV) antimicrobial treatment with ceftriaxone and metronidazole was started and she was taken to the operating room for laparoscopic appendectomy. The resected appendix was perforated and necrotic. She developed isolated fever on post-operative day two, but no other signs of infection. She was discharged on post-operative day six to complete a ten-day course of antibiotic therapy with oral amoxicillin-clavulanate.
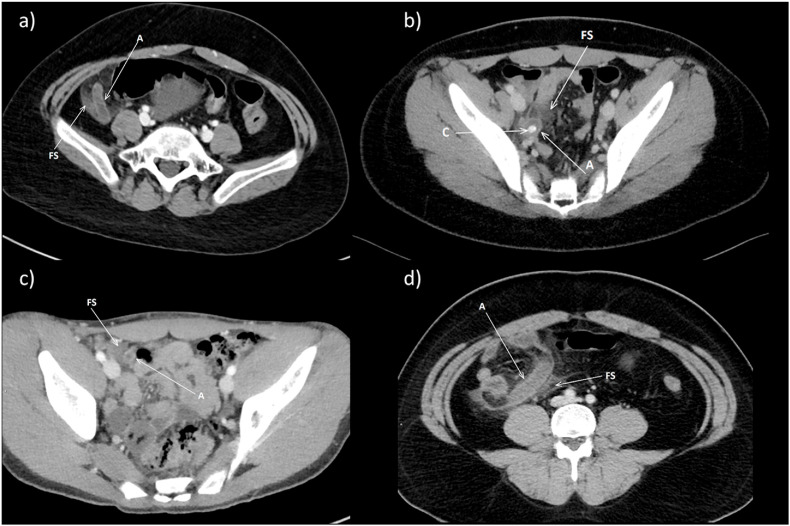
Fig. 1.
a) Patient 1. Axial contrast enhanced CT of the pelvis demonstrates a dilated fluid filled appendix (A); surrounding fat stranding (FS) is seen consistent with edema and acute inflammation. b) Patient 2. Axial contrast enhanced CT of the pelvis demonstrates a dilated fluid filled appendix (A) with a calcified (C) appendicolith inside; surrounding fat stranding (FS) is seen consistent with edema and acute inflammation. c) Patient 3. Axial contrast enhanced CT of the pelvis demonstrates a dilated fluid filled appendix (A). Surrounding fat stranding (FS) is seen consistent with edema and acute inflammation. d) Patient 4. Axial contrast enhanced CT of the pelvis demonstrates a dilated fluid filled appendix (A). Surrounding fat stranding (FS) is seen consistent with edema and acute inflammation.
Table 1.
Patient demographics and clinical characteristics.
| Characteristic | Patient 1 | Patient 2 | Patient 3 | Patient 4 |
|---|---|---|---|---|
| Age (years) | 11 | 13 | 17 | 14 |
| Gender | Female | Male | Male | Male |
| Ethnicity/Race | Hispanic/White | Hispanic/White | Hispanic/White | Non-Hispanic/White |
| Relevant medical history | Obesity (BMI 30, 98% for age) | Severe aplastic anemia on immunosuppresive therapy | SARS-CoV-2 PCR positive 7 weeks prior to presentation | Obesity (BMI 32; 99% for age) |
| Presenting symptoms | 2 days of generalized abdominal pain, vomiting, fever | 1 day of generalized abdominal pain, nausea and vomiting | 1 day of generalized abdominal pain, nausea and vomiting | 4 days of abdominal pain, diarrhea, vomiting, congestion, anosmia, ageusia |
| Initial vital signs | T 37.3, HR 196, RR 22, BP 103/67, SpO2 99% RA | T 37.4, HR 98, RR 20, BP 117/63, SpO2 97% RA | T 37, HR 70, RR 22, BP 100/59, SpO2 97% RA | T 37.7, HR 97, RR 20, BP 132/87, SpO2 97% RA |
| Initial labs | ||||
| Hemoglobin (g/dL) | 11.2 | 7.7a | 13.0 | 16.9 |
| White blood count (103/μL) | 15.4 | 4.4a | 13.4 | 15.4 |
| Neutrophils (103/μL) | 12.5 | 3.7a | 11.6 | 11.5 |
| Lymphocytes (103/μL) | 1.27 | 0.18a | 0.92 | 2.3 |
| Platelets (103/μL) | 273 | 12a | 224 | 345 |
| Blood urea nitrogen (mg/dL) | 8 | 16 | 13 | 7 |
| Creatinine (mg/dL) | 0.5 | 0.8 | 0.9 | 0.8 |
| Aspartate transaminase (U/L) | 22 | 55 | 16 | 10 |
| Alanine transaminase (U/L) | 18 | 97 | 8 | 25 |
| Albumin (g/dL) | 4.6 | 4.4 | 4.0 | 4.5 |
| Blood culture | NA | +EC, +PA | NA | NA |
| SARS-CoV2 PCR | +NP swab (POD 0) | +NP swab (POD 0) +stool (POD7) |
+NP swab (POD -48) +NP swab (POD 0) |
+NP swab (POD 0) |
| Imaging findings | Dilated, fluid filled appendix with surrounding inflammation consistent with acute appendicitis without perforation or abscess | Dilated, fluid appendix with appendicolith and surrounding inflammation consistent with acute appendicits without perforation or abscess | Early acute appendicits without perforation or abscess | Dilated appendix with surrounding inflammation consistent with acute appendicits without perforation or abscess |
| Operative findings | Perforated appendictis with peritonitis | Necrotic appendicitis with peritonitis | Acutely inflamed appendix without perforation or surrounding inflammatory changes | Gangrenous appendix with associated peritonitis without rupture |
| Pathology findings | Acute appendicitis | Acute gangrenous appendicitis with perforation | Acute appendicitis and periappendicitis | Gangrenous appendicitis with periappendicitis |
| Day of discharge | POD 6 | POD 14 | POD 0 | POD 0 |
BMI, body mass index, kg/m2; T, temperature, degrees celcius; HR, heart rate, beats per minute; RR, respiratory rate, breaths per minute; BP, blood pressure, mm Hg; SpO2, oxygen saturation; RA, room air; POD, post-operative day; NA, not assessed; EC, Escherichia coli; PA, Pseudomonas aeruginosa; NP, nasopharyngeal; PICU, pediatric intensive care unit.
Baseline range over prior month: hemoglobin 4.9–8.5 g/dL; white blood count 1.56–2.64 103/μL; absolute neutrophil count 0.92–1.80 103/μL; absolute lymphocyte count 0.32–0.44 103/μL; platelets 5–13 103/μL (received platelet transfusions two days prior).
2.2. Patient 2
A 13-year-old Hispanic boy with a history of severe, acquired aplastic anemia treated with intensive immunosuppressive therapy with anti-thymocyte globulin and cyclosporine plus eltrombopag and frequent transfusions via an indwelling central line presented with one-day of diffuse, crampy abdominal pain, nausea and multiple episodes of non-bloody, non-bilious emesis. On presentation to the ED, diffuse abdominal tenderness with guarding was noted by examination. The white blood cell count was higher than expected for him, with relative neutrophilia and lymphopenia (Table 1). He was also anemic and severely thrombocytopenic as expected for his underlying condition. SARS-CoV-2 was detected by PCR of his NP swab. Shortly after admission to the pediatric ward, he became febrile to 39.5 °C. Empiric IV antibiotic therapy with cefepime was begun after his blood was cultured. His abdominal pain became localized to the right lower quadrant. Abdominal ultrasound and contrast enhanced CT of the abdomen/pelvis showed findings consistent with acute appendicitis (Fig. 1b). Gram-negative bacillary bacteremia was detected. Empiric antimicrobial therapy was broadened and he was taken to the operating room for laparoscopic appendectomy which was converted to an open procedure for removal of the necrotic appearing appendix. He received multiple platelet and red blood cell transfusions throughout his hospitalization. Post-operatively, the patient was briefly administered oxygen via high flow nasal cannula for hypoxemia and a left lobe opacity involving the left hemi-thorax was noted on chest radiograph. Respiratory support was quickly weaned and he was back to breathing room air the day after surgery, despite persistence of infrahilar opacities on repeat chest radiographs. SARS-CoV-2 was detected by PCR of his stool when tested on his 7th day of hospitalization. Escherichia coli and Pseudomonas aeruginosa were grown from his initial blood culture. Subsequent blood culture was sterile and he completed treatment of polymicrobial gram-negative bacteremia with 5 days of IV metronidazole and 14 days of IV cefepime prior to discharge home. At surgery, the appendix was not felt to be perforated although pathology indicated the appendix to be necrotic with perforation based on a defect appreciated on the appendix.
2.3. Patient 3
An otherwise healthy Hispanic 17-year-old male presented with one-day of generalized abdominal pain, nausea and vomiting. He was transferred to our center from a community ED for surgical consultation for acute non-perforated appendicitis seen on contrast enhanced CT of the abdomen/pelvis (Fig. 1c). Abdominal exam demonstrated tenderness in the right and left lower quadrants without rebound tenderness. Notably, SARS-CoV-2 was detected by PCR after developing nasal congestion and cough seven weeks prior to presentation. His respiratory symptoms had resolved, however, SARS-CoV-2 was again detected by PCR of his NP swab on admission. Upon diagnosis of appendicitis, patient was started on IV piperacillin-tazobactam. Laparoscopic appendectomy was performed demonstrating an inflamed, non-ruptured appendix without surrounding inflammatory changes. Post-operative course was unremarkable, and he was discharged home later the same day without additional antibiotics.
2.4. Patient 4
An obese, white 14-year-old male presented with four-days of generalized abdominal pain, associated with one episode of emesis and several days of diarrhea preceding the onset of abdominal pain. He initially presented to a community ED, where a contrast enhanced CT abdomen/pelvis demonstrated findings of acute, non-ruptured appendicitis (Fig. 1d). He was transferred to our institution for surgical consultation. Initial exam demonstrated pain to palpation of his right lower quadrant, without guarding or rebound tenderness. SARS-CoV-2 was detected by PCR of his NP swab. On further review of his history, he did note several weeks of nasal congestion and four days of anosmia and ageusia. He was given empiric IV piperacillin-tazobactam and brought to the OR for a laparoscopic appendectomy. Surgery revealed a gangrenous appendix without perforation and associated injection of the peritoneum and small bowel. The patient recovered well from surgery and was discharged home later the same day without additional antibiotics.
3. Discussion
We report four children presenting to our center with typical symptoms of appendicitis and also found to be infected with SARS-CoV-2 virus. During the time period of presentation of these patients, 4 out of 13 patients (31%) with acute appendicitis tested positive for COVID-19. Interestingly, this rate of COVID-19 in children with appendicitis was much higher than the 8% positivity rate for all children tested at our institution. In all four cases, the diagnosis of acute appendicitis was confirmed intra-operatively and by pathological examination, and all had good outcomes. The course for our second case was complicated by the development of polymicrobial Gram-negative bacteremia, and it is unclear whether his underlying immunocompromised state contributed to a more severe presentation.
Appendicitis is the most common indication for emergency abdominal surgery in children, with a peak incidence in the adolescent age range, so this may be a coincidental association [1]. Reports of patients with appendicitis and COVID-19 have focused on the safe management of surgical conditions during the COVID-19 pandemic or described the adverse outcomes arising from delays in seeking care during this time [2]. Universally, healthcare systems have had to adjust the delivery of services in response to the pandemic. Under the guidance of health authorities, many have adopted a tiered approach to surgical operations by delaying elective procedures and prioritizing urgent ones during times of increased community-based transmission, so that utilization of health care resources is diverted to COVID-related care [3]. The approach to abdominal surgical emergencies, such as acute appendicitis, has varied under these conditions. Some centers have used more conservative approaches for uncomplicated cases of acute appendicitis in patients with COVID-19, including home care and non-operative management with antibiotics [[4], [5], [6]]. However, non-operative management many increase overall hospital stay and complications such as peritonitis at surgery [7].
Our health system initially suspended elective operations and only permitted emergent surgical procedures following the first surge of COVID-19 cases in our state in March 2020. For pediatric surgeries, we opted for surgical management of acute appendicitis in patients infected with SARS-CoV-2, with the plan to discharge uncomplicated cases after recovery from anesthesia, followed by close home monitoring using telehealth. Compared to non-operative management, this approach may shorten overall hospital length of stay and expose fewer healthcare workers. This approach worked well for both of our cases (case 3 and 4) with unperforated appendicitis and each child was safely discharged home on the same day of surgery. Our other two cases had more complicated presentations and required longer post-operative stays. One child had a delay in care due to initially unrecognized appendicitis at another facility (case 1) progressing to perforated appendicitis with peritonitis, followed by concern for post-operative fevers, but was discharged by post-operative day 6 and was able to complete her antibiotic course at home. Our patient with severe underlying immunocompromise (case 2) presented with Gram-negative sepsis and a necrotic appendix to the base necessitating conversion to open abdominal surgery to achieve source control. He was discharged on post-operative day 14 based on ongoing medical and social considerations. Our intra-operative protocols for COVID-19 patients includes venting with a “plume away” into a Neptune device equipped with high-efficiency particulate air (HEPA) filtration for all laparoscopic procedures. In addition, recommended infection prevention and control practices when caring for patients with COVID-19 are carefully observed [8]. Personal protection equipment includes the use of fit tested respirators and operations are performed in airborne infection isolation rooms. To date, good patient outcomes without known transmission to healthcare providers has been achieved with this approach.
In addition to these surgical and infection control considerations, we believe appendicitis may be a plausible association of COVID-19 in these children for several reasons. Firstly, gastrointestinal symptoms appear to be common in children with COVID-19 and for some children, symptoms such as nausea, emesis, abdominal pain and diarrhea may be their only complaint [9]. Gastrointestinal symptoms are also recognized to be a prominent presentation of multisystem inflammatory syndrome in children (MIS-C) [10]. Gastrointestinal symptoms were a presenting symptom in 84% of 44 MIS-C cases from New York, for example, including 3 children with bowel wall thickening noted on imaging [10]. Additionally, eight children with confirmed or suspected SARS-CoV-2 infection presenting with an appendicitis-like syndrome of fever, abdominal pain, diarrhea and emesis with elevated inflammatory markers were reported from a pediatric center in the United Kingdom, some requiring intensive care [11]. All children had abdominal imaging that confirmed terminal ileitis and none required surgical intervention [11]. Recent reports also describe infants with intussusception and SARS-CoV-2 infection [12,13]. Hence, gastrointestinal involvement in patients with COVID-19 is increasingly recognized.
Secondly, prolonged viral presence is observed in the gastrointestinal tract of COVID-19 patients, as was demonstrated in our second case (the other three cases were discharged prior to the ability to collect a stool sample for PCR testing) [14]. Infectious virus has been isolated from the stool and detection of SARS-CoV-2 in fecal samples by PCR and can be prolonged. More than 20% of patients with SARS-CoV-2 infection continued to have detectable viral RNA in fecal samples, for example, even after negative conversion of viral RNA in their respiratory tract [15]. While in a large animal model, viral RNA was found at higher levels within the gastrointestinal tract tissues than respiratory tract tissues at necropsy [16]. Cumulatively, data to date suggests that SARS-CoV-2 virus could be secreted from the infected enterocytes.
Thirdly, angiotensin-converting enzyme 2 (ACE2), the host receptor for viral entry into cells, is highly expressed by enterocytes [17]. Human small intestinal organoids are also readily infected by SARS-CoV-2, demonstrating that intestinal cells can support viral infection and replication [17]. As ACE2 is present on glandular cells in the appendix, the appendix is also a viral target of SARS-CoV-2 [18]. SARS-CoV-2 RNA was not detected by in situ hybridization of formalin fixed, paraffin-embedded tissue when tested in our first two cases. Further study may help to identify viral presence using other methodologies. Alternatively, SARS-CoV-2 could induce lymphoid follicular hyperplasia of the colonic epithelium lining the appendix leading to lumen obstruction, inflammation and ischemia [19].
In summary, in this first case series of children with COVID-19 and appendicitis, we draw attention to this possible association. We encourage consideration of testing for SARS-CoV-2 in pediatric patients presenting with severe gastrointestinal symptoms to inform mitigation strategies for transmission and monitor for respiratory decompensation. Reporting of these observations will help further our understanding of disease manifestations in children.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Consent
Consent to publish the case report was not obtained. This report does not contain any personal information that could lead to the identification of the patient.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to acknowledge Dr. Reza J. Daugherty, Chief of Pediatric Imaging, University of Virginia Health Department of Radiology and Medical Imaging for assistance with radiologic images.
References
- 1.Anderson J.E., Bickler S.W., Chang D.C. Examining a common disease with unknown etiology: trends in epidemiology and surgical management of appendicitis in California, 1995-2009. World J Surg. 2012;36(12):2787–2794. doi: 10.1007/s00268-012-1749-z. [published Online First: 2012/09/06] [DOI] [PubMed] [Google Scholar]
- 2.Snapiri O., Rosenberg Danziger C., Krause I. Delayed diagnosis of paediatric appendicitis during the COVID-19 pandemic. Acta Paediatr. 2020 doi: 10.1111/apa.15376. [published Online First: 2020/05/28] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.CfDCa Prevention. Healthcare facilities: managing operations during the COVID-19 pandemic 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-hcf.html [Available from: accessed.
- 4.Tankel J., Keinan A., Blich O. The decreasing incidence of acute appendicitis during COVID-19: a retrospective multi-centre study. World J Surg. 2020;44(8):2458–2463. doi: 10.1007/s00268-020-05599-8. [published Online First: 2020/05/28] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones B.A., Slater B.J. Non-operative management of acute appendicitis in a pediatric patient with concomitant COVID-19 infection. J Pediatr Surg Case Rep. 2020;59:101512. doi: 10.1016/j.epsc.2020.101512. [published Online First: 2020/06/17] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kvasnovsky C.L., Shi Y., Rich B.S. Limiting hospital resources for acute appendicitis in children: lessons learned from the U.S. epicenter of the COVID-19 pandemic. J Pediatr Surg. 2020 doi: 10.1016/j.jpedsurg.2020.06.024. [published Online First: 2020/07/06] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Podda M., Cillara N., Di Saverio S. Antibiotics-first strategy for uncomplicated acute appendicitis in adults is associated with increased rates of peritonitis at surgery. A systematic review with meta-analysis of randomized controlled trials comparing appendectomy and non-operative management with antibiotics. Surgeon. 2017;15(5):303–314. doi: 10.1016/j.surge.2017.02.001. [published Online First: 2017/03/13] [DOI] [PubMed] [Google Scholar]
- 8.CfDCa Prevention. Interim infection prevention and control recommendations for healthcare personnel during the coronavirus disease 2019 (COVID-19) pandemic 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html [November 15, 2020]. Available from:
- 9.Tian Y., Rong L., Nian W. Review article: gastrointestinal features in COVID-19 and the possibility of faecal transmission. Aliment Pharmacol Ther. 2020;51(9):843–851. doi: 10.1111/apt.15731. [published Online First: 2020/03/31] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller J., Cantor A., Zachariah P. Gastrointestinal symptoms as a major presentation component of a novel multisystem inflammatory syndrome in children (MIS-C) that is related to COVID-19: a single center experience of 44 cases. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.05.079. [published Online First: 2020/06/09] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tullie L., Ford K., Bisharat M. Gastrointestinal features in children with COVID-19: an observation of varied presentation in eight children. Lancet Child Adolesc Health. 2020 doi: 10.1016/s2352-4642(20)30165-6. [published Online First: 2020/05/23] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Makrinioti H., Mac Donald A., Lu X. Intussusception in two children with SARS-CoV-2 infection in children. J Pediatric Infect Dis Soc. 2020 doi: 10.1093/jpids/piaa096. [published Online First: 2020/08/10] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martínez-Castaño I., Calabuig-Barbero E., Gonzálvez-Piñera J. COVID-19 infection is a diagnostic challenge in infants with ileocecal intussusception. Pediatr Emerg Care. 2020;36(6):e368. doi: 10.1097/pec.0000000000002155. [published Online First: 2020/06/03] [DOI] [PubMed] [Google Scholar]
- 14.Xu Y., Li X., Zhu B. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020;26(4):502–505. doi: 10.1038/s41591-020-0817-4. [published Online First: 2020/04/15] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiao F., Tang M., Zheng X. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158(6):1831–1833. doi: 10.1053/j.gastro.2020.02.055. [published Online First: 2020/03/07] e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hartman A.L., Nambulli S., McMillen C.M. SARS-CoV-2 infection of African green monkeys results in mild respiratory disease discernible by PET/CT imaging and shedding of infectious virus from both respiratory and gastrointestinal tracts. PLoS Pathog. 2020;16(9) doi: 10.1371/journal.ppat.1008903. [published Online First: 2020/09/19] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamers M.M., Beumer J., van der Vaart J. SARS-CoV-2 productively infects human gut enterocytes. Science. 2020 doi: 10.1126/science.abc1669. [published Online First: 2020/05/03] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uhlen Tissue-based map of the human proteome. Science. 2015 doi: 10.1126/science.1260419. Image available at: the following URL: v19.proteinatlas.org/ENSG00000130234-ACE2/tissue/appendix [ [DOI] [PubMed] [Google Scholar]
- 19.Rabah R. Pathology of the appendix in children: an institutional experience and review of the literature. Pediatr Radiol. 2007;37(1):15–20. doi: 10.1007/s00247-006-0288-x. [published Online First: 2006/10/13] [DOI] [PubMed] [Google Scholar]