Abstract
Background
The COVID-19 pandemic has provided an opportune time to evaluate the efficacy of traditional medicine. Many clinical studies involving AYUSH systems are being initiated and registered with Clinical Trials Registry - India (CTRI) since last few months.
Objective
The present work is an analysis of different characteristics of these studies on the basis of available datasets.
Material and Methods
COVID-19 related clinical studies involving the healthcare systems of AYUSH, registered on CTRI between 1st February 2020 and 24th August 2020, were searched. They were analysed as per different characteristics such as registration month, study sites, aim, sample size, population, setting, sponsorship, intervention and comparators, duration & outcome measures.
Results
A total of 197 AYUSH studies were registered on CTRI of which majority (n = 113) were from Ayurveda, with another nine of them with an intra-AYUSH collaboration. The highest number of studies were registered in month of June (n = 57). Maximum study sites were in Maharashtra (n = 65). From the 197 total studies, only six were observational studies, with 191 being interventional studies. As an outcome, majority of the studies aimed at recovery (n = 112). Majority of studies (n = 105) were Government of India sponsored and proposed in AYUSH setting (n = 107). The proportion of comparative studies was more than single arm studies. Guduchi (Tinospora cordifolia) was the most frequently mentioned drug.
Conclusion
Our analysis revealed some interesting characteristics of the registered studies such as use of platform trial design, system specific criteria for assessment and personalized interventions. Though it was not possible to evaluate the quality of these studies in view of the limited dataset used for trial registration, we could notice variations in important characteristics like sample size, treatment arms, comparator used and study duration according to the primary aim of the studies. Overall, the present review underlines the formidable efforts of AYUSH sector in combating COVID-19 outbreak.
Keywords: Ayurveda, Preventive studies, Recovery studies, SARS-CoV-2
1. Introduction
The unexpected emergence of Corona Virus Disease-19 (COVID-19) pandemic has been a serious threat to entire world. In India, the first case of COVID-19 was documented on 31st January 2020 [1]. Although conventional medicine has contributed to a great extent in controlling the symptoms and reducing mortality rate, there is limited evidence for the efficacy of these medicines, in the present situation [2]. World Health Organisation (WHO) has also accepted that there is no specific medicine recommended to prevent or treat SARS-CoV-2 [3]. It extended an opportunity for traditional medicines around the world to explore their potential for treatment of COVID-19, backed with scientific evidence [4].
China, source of this pandemic, has successfully integrated the Traditional Chinese Medicine (TCM) with the mainstream conventional system of medicine in the treatment of COVID-19 [5]. In India, the Ministry of AYUSH (Ayurveda, Yoga and Naturopathy, Unani, Siddha & Homeopathy) has taken proactive steps in this crisis, right from the beginning and released an advisory that incorporated simple, household measures to boost immunity [6]. An ‘Interdisciplinary AYUSH Research and Development Task Force’ was also established under the Ministry of AYUSH which initiated systematic and well-planned clinical activities in this regard [7]. Apart from these trials, research proposals were invited by the Ministry through extra-mural research scheme. Considering the magnitude of the pandemic and need for the search of effective interventions, other stakeholders of AYUSH viz. teaching institutions and pharmaceutical industry have also undertaken clinical studies for prevention or management of COVID-19. These efforts certainly led to an increase in number of AYUSH clinical studies pertaining to COVID-19.
These studies were registered in Clinical Trials Registry- India (CTRI) as per the directives of the Drugs Controller General of India (DCGI) [8]. The present review was conducted to assess the characteristics of AYUSH studies, undertaken in view of COVID-19 and registered in CTRI, so as to understand the types and methodological aspects of these studies. It is expected that such an extensive analysis can provide ideas for future studies.
2. Methods
All AYUSH clinical studies for COVID-19 registered with CTRI during the period between 1st February 2020 and 24th August 2020 were retrieved using keywords like COVID/COVID-19/Coronavirus and AYUSH/Ayurveda/Yoga/Naturopathy/Siddha/Unani/Homeopathy/Nutraceuticals in various permutations and combinations. We considered inclusion of nutraceuticals with the justification that herbal drug under purview of AYUSH systems are frequently incorporated in nutraceuticals’ compositions, which were listed in ‘Others’ category.
The data was retrieved by the first two authors independently and cross-verified to check for any discrepancies. The collected data was inserted in spreadsheets as per various items of the CTRI registration dataset. Initially the studies were analysed according to general characteristics like month of registration, aim, study settings, sites and sponsorship. They were divided as per study design (observational/interventional). The interventional studies were sub-divided as per their aim such as prevention, recovery, mitigation of psychological impact of COVID-19 and both prevention and recovery studies. All studies were analysed with respect to sample size, study population, interventions and comparators, treatment duration, overall study duration & outcome measures. These data characteristics are presented as actual numbers.
3. Results
A total of 197 studies from the constituent units of AYUSH systems related to COVID-19 were registered with CTRI during the review period.
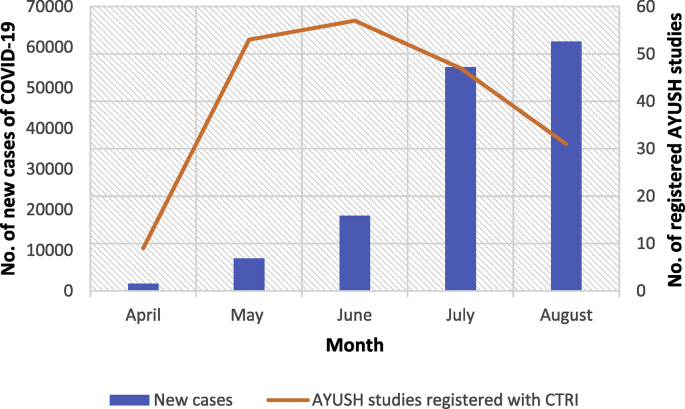
The month wise distribution of these studies has revealed that the highest number of studies were registered in the months of May (n = 53) and June (n = 57) following which there was a decline in these numbers after June 20. This was contradictory to steady increase in number of COVID-19 cases tested positive (Fig. 1).
Fig. 1.
Month-wise registered studies vis-à-vis Incidence of COVID-19.
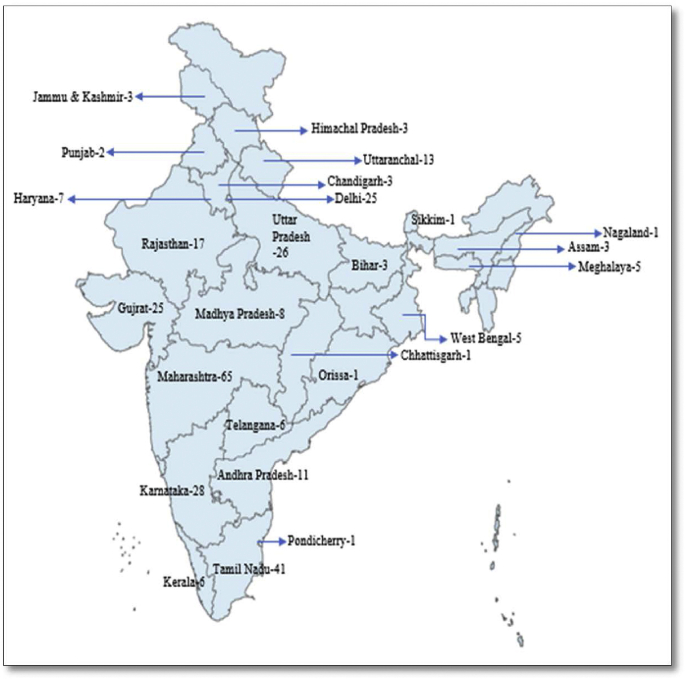
As per the registry records, these studies were conducted at 310 study sites. The geographical mapping of the sites revealed that maximum number of sites were located in Maharashtra (n = 65) followed by Tamil Nadu (n = 41). The states of Karnataka (n = 28), Uttar Pradesh (n = 26), Gujarat (n = 25) and Delhi (n = 25) were found to have almost equal number of study sites. Few study sites were also from states of Orissa, Nagaland, Sikkim, with lesser incidence of COVID-19 (Fig. 2).
Fig. 2.
State-wise distribution of study sites.
3.1. General characteristics of the registered studies
It was seen from the system wise analysis that studies from Ayurveda were much higher (n = 113) than the other AYUSH systems. There were 24 studies from Siddha, 23 studies from Homeopathy, 12 from Yoga & Naturopathy, seven from Unani system and nine from Others (Nutraceuticals) segment. The intra-AYUSH collaborative efforts were seen in nine studies. There were six observational studies and 191 interventional studies. The interventional studies could be further categorized as per their aims; prevention (n = 66) and recovery (n = 112). Eleven studies focused on mitigation of psychological impacts related to COVID-19, whereas two studies had both prevention and recovery as their aim. Of the 197 studies, maximum studies (n = 107) were conducted by either AYUSH Institutions or by personnel trained in AYUSH systems; 83 studies were planned by non-AYUSH institutions/personnel for conduct. Seven studies were collaborative in nature, between AYUSH and non-AYUSH institutions/personnel. A major number of studies were sponsored by the Government of India (n = 105) as compared to investigator initiated/academic studies (n = 52). From the 197 studies collected from CTRI, pharmaceutical industry funded studies were 40. There are 168 registered studies which were executed from one study site while 29 were multi-centric with two to three study sites. Few studies had more than three sites with a maximum of 20 sites. There were 56 studies with study population aged between 18 and 60 years while 110 studies included individuals above 60 years of age. A total of five studies mentioned inclusion criteria as below 18 years while one study reported inclusion of even new-borns. Studies that included both sets of population i.e. below 18 years and above 60 years were seen to be 26 (Table 1).
Table 1.
General characteristics of COVID-19 related AYUSH studies.
| Characteristics of study | Ayurveda (n = 113) | Yoga & Naturopathy (n = 12) | Unani (n = 7) | Siddha (n = 24) | Homeopathy (n = 23) | Others (n = 9) | Collaborative (n = 9) |
|---|---|---|---|---|---|---|---|
| Primary Aim | |||||||
| Observationsa (n = 6) | 2 | 0 | 0 | 4 | 0 | 0 | 0 |
| Prevention (n = 66) | 43 | 1 | 4 | 2 | 9 | 3 | 4 |
| Recovery (n = 112) | 66 | 1 | 3 | 18 | 14 | 6 | 4 |
| Mitigation of COVID-19 related stress (n = 11) | 1 | 10 | 0 | 0 | 0 | 0 | 0 |
| Both prevention & recovery (n = 2) | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Study setting (Organization & Personnel) | |||||||
| AYUSH | 61 | 3 | 4 | 16 | 20 | 1 | 2 |
| Non-AYUSH | 49 | 8 | 2 | 6 | 3 | 8 | 7 |
| Both | 3 | 1 | 1 | 2 | 0 | 0 | 0 |
| Sponsorship | |||||||
| Government agencies & Institutions | 63 | 7 | 6 | 17 | 9 | 1 | 2 |
| Private clinics & Colleges | 23 | 5 | 0 | 4 | 14 | 2 | 4 |
| Pharmaceutical industry | 27 | 0 | 1 | 3 | 0 | 6 | 3 |
| Study sites | |||||||
| Single | 95 | 10 | 6 | 23 | 20 | 7 | 7 |
| Multi-centric | 18 | 2 | 1 | 1 | 3 | 2 | 2 |
| Inclusion criteria for age | |||||||
| <18 years | 3 | 0 | 1 | 0 | 1 | 0 | 0 |
| 18–60 Years | 36 | 5 | 0 | 8 | 2 | 3 | 2 |
| >60 years | 62 | 7 | 6 | 13 | 10 | 6 | 6 |
| Both<18 years to > 60 years | 12 | 0 | 0 | 3 | 10 | 0 | 1 |
Observations of behavioural patterns, health status of population following AYUSH measures and awareness about AYUSH measures for prevention and control.
We further divided these studies as per their design as observational and interventional. Interventional studies were sub-divided according to their aim as prevention, recovery, stress mitigation and both prevention & recovery. All these studies were analysed with respect to the different characteristics viz. sample size, study population, type of interventions, comparator used (placebo, conventional care etc), duration of treatment, duration of study and outcome measures.
3.2. Observational studies (n = 6)
As shown in Table 1, of the 197 total studies, six studies were observational in nature; two from Ayurveda and four from Siddha system. These studies were planned to observe behavioural patterns, health status of population following implementation of AYUSH measures and awareness about the same for prevention and control of COVID-19.
3.3. Preventive studies (n = 66)
A total of 66 studies were planned for prevention of COVID-19, of which, 22 were single arm (i.e. without any comparator). Maximum number of studies (n = 40) were double arm while four studies were multi-arm. Though different kind of comparisons were proposed through these studies, the preference was seen to be for comparison between AYUSH treatment and Conventional Care (n = 23). One study from Ayurveda was a platform trial, wherein seven study arms were proposed. In case of sample size, there were maximum number of studies in the population range of 100–1000 (n = 27). A similar number of studies (n = 25) with sample size more than 1000 were also found to be registered. The maximum sample size employed in these studies was 2,00,000 (two lacs). At the same time, it should be noted that there were five studies with sample size less than 30. A trend to conduct preventive studies in high risk population (n = 57) rather than healthy individuals was observed. There was an almost equal distribution of studies with single drug formulations (n = 21), multi-drug formulations (n = 18) and multi-formulation regimen (n = 25). The treatment duration was observed to be ranging from less than seven days up to more than one month. Study duration was not mentioned in 20 studies. The overall study duration was seen to be less than six months in maximum studies. Since these studies were planned for prevention of COVID-19, the obvious outcome measure was incidence of infection which was seen in 61 studies. Interestingly, in four studies from Ayurveda, bala (Immuno-stimulatory potential) assessment was included as an outcome measure. These characteristics are summarized in Table 2.
Table 2.
Characteristics of preventive studies (n = 66).
| Characteristics of study | Ayurveda (n = 43) | Yoga & Naturopathy (n = 1) | Unani (n = 4) | Siddha (n = 2) | Homeopathy (n = 9) | Others (n = 3) | Collaborative (n = 4) |
|---|---|---|---|---|---|---|---|
| Study arms | |||||||
| Single arm | 16 | 0 | 0 | 0 | 3 | 1 | 2 |
| Double arm | 26 | 1 | 2 | 2 | 5 | 2 | 2 |
| Multi arm | 1 | 0 | 2 | 0 | 1 | 0 | 0 |
| Sample size | |||||||
| ≤30 | 3 | 0 | 0 | 0 | 1 | 1 | 0 |
| 31–100 | 6 | 1 | 1 | 0 | 0 | 0 | 1 |
| 101–1000 | 17 | 0 | 1 | 0 | 4 | 2 | 3 |
| >1001 | 17 | 0 | 2 | 2 | 4 | 0 | 0 |
| Study Population | |||||||
| Healthy volunteers | 7 | 0 | 0 | 0 | 1 | 0 | 1 |
| High Risk Individuals | 36 | 1 | 4 | 2 | 8 | 3 | 3 |
| Type of Intervention (where applicable) | |||||||
| Single drug formulation | 16 | 0 | 0 | 0 | 4 | 1 | 0 |
| Multi drug formulation | 13 | 0 | 1 | 1 | 2 | 1 | 0 |
| Multi-formulation regime | 14 | 0 | 3 | 1 | 3 | 1 | 3 |
| Non-Pharmacological | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| Use of comparators | |||||||
| AYUSH vs. CCa | 16 | 0 | 2 | 2 | 0 | 1 | 2 |
| AYUSH vs. Placebo/No Treatment | 7 | 1 | 2 | 0 | 5 | 1 | 0 |
| AYUSH vs. nutritional/ | 2 | 0 | 0 | 0 | 1 | 0 | 0 |
| dietary items | |||||||
| AYUSH + CC vs.CC or Placebo + CC | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Treatment duration | |||||||
| ≤7 days | 1 | 0 | 0 | 0 | 3 | 0 | 0 |
| >7 days to ≤15 days | 1 | 0 | 2 | 1 | 1 | 2 | 1 |
| >15 days to ≤30 days | 17 | 0 | 1 | 0 | 0 | 1 | 0 |
| >30 days | 12 | 0 | 0 | 0 | 1 | 0 | 2 |
| Not Specified | 12 | 1 | 1 | 1 | 4 | 0 | 1 |
| Primary Outcomes | |||||||
| Incidence of infection | 38 | 1 | 4 | 2 | 9 | 3 | 4 |
| Incidence of infection & AYUSH specific parameters | 4 | 0 | 0 | 0 | 0 | 0 | 0 |
| Acceptability of Intervention | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Study Duration | |||||||
| ≤3 months | 20 | 1 | 2 | 0 | 4 | 0 | 1 |
| >3 moths & ≤ 6 months | 15 | 0 | 2 | 2 | 5 | 3 | 1 |
| >6 months & ≤ 1 year | 6 | 0 | 0 | 0 | 0 | 0 | 2 |
| >1 year | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
CC: Conventional Care.
3.4. Recovery studies (n = 112)
Of the total 112 recovery studies, 84 studies were double arm, 20 with single arm and eight studies with triple arm. Different kinds of comparisons were proposed in recovery studies similar to preventive studies. Though the preference in recovery studies was also seen to be, to compare between AYUSH treatment and Conventional Care (n = 38), in almost equal number of studies (n = 35) Conventional Care was provided to both the groups. There were 14 studies with sample size less than 30, whereas maximum studies (n = 62) reported sample size between 31 and 100. The sample size within range of 100–1000 was seen in 36 studies with maximum number as 658. A major number of studies (n = 74) included individuals suffering from mild to moderate symptoms while seven studies had only asymptomatic individuals. There were 31 studies in which both these categories (asymptomatic + mild to moderate symptoms) were considered. A single study from Homeopathy included patients with symptoms of severe infection along with those with mild to moderate symptoms. The studies using multi-drug formulations (n = 51) were more compared to single drug formulations (n = 15) and multi-formulation regimen (n = 42). Intervention was not specified in three studies. The treatment duration ranged from less than seven days up to more than a month, with maximum studies (n = 54) with duration between seven and 15 days, even among recovery studies. The study duration was not mentioned in 29 studies. The overall study duration was found to be less than 6 months in majority of studies (n = 101). While 74 studies had documented clearance from infection as an outcome, 38 studies mentioned progression of severity as an outcome. These findings are presented in Table 3.
Table 3.
Characteristics of recovery studies (n = 112).
| Characteristics of study | Ayurveda (n = 66) | Yoga & Naturopathy (n = 1) | Unani (n = 3) | Siddha (n = 18) | Homeopathy (n = 14) | Others (n = 6) | Collaborative (n = 4) |
|---|---|---|---|---|---|---|---|
| Treatment arms | |||||||
| Single arm | 12 | 0 | 0 | 4 | 4 | 0 | 0 |
| Double arm | 49 | 1 | 2 | 13 | 9 | 6 | 4 |
| Multi arm | 5 | 0 | 1 | 1 | 1 | 0 | 0 |
| Sample size | |||||||
| ≤30 | 10 | 0 | 0 | 1 | 0 | 1 | 2 |
| 31–100 | 35 | 0 | 2 | 11 | 9 | 3 | 2 |
| 101–1000 | 21 | 1 | 1 | 6 | 5 | 2 | 0 |
| Study Population | |||||||
| Only Asymptomatic Positive | 2 | 0 | 0 | 2 | 3 | 0 | 0 |
| Asymptomatic Positive & Mild to moderate infection | 15 | 1 | 1 | 7 | 3 | 2 | 2 |
| Mild to moderate | 49 | 0 | 2 | 9 | 8 | 4 | 2 |
| Type of Intervention (where applicable) | |||||||
| Single drug formulation | 5 | 0 | 1 | 1 | 7 | 1 | 0 |
| Multi drug formulation | 34 | 0 | 2 | 10 | 3 | 2 | 0 |
| Multi-formulation regimen | 27 | 0 | 0 | 7 | 1 | 3 | 4 |
| Not Specified | 0 | 0 | 0 | 0 | 3 | 0 | 0 |
| Non-Pharmacological | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Type of Comparators (where applicable) | |||||||
| AYUSH vs. CC | 24 | 1 | 0 | 8 | 1 | 1 | 3 |
| AYUSH vs. Placebo/No Treatment | 7 | 0 | 3 | 1 | 2 | 0 | 0 |
| AYUSH vs. nutritional/dietary items | 3 | 0 | 0 | 3 | 0 | 0 | 0 |
| AYUSH + CC vs.CC or Placebo + CC | 20 | 0 | 0 | 2 | 7 | 5 | 1 |
| Treatment duration | |||||||
| ≤7 days | 4 | 0 | 0 | 4 | 2 | 0 | 0 |
| >7 days to ≤15 days | 29 | 1 | 2 | 10 | 2 | 6 | 4 |
| >15 days to ≤30 days | 12 | 0 | 0 | 0 | 2 | 0 | 0 |
| >30 days | 3 | 0 | 0 | 2 | 0 | 0 | 0 |
| Not Specified | 18 | 0 | 1 | 2 | 8 | 0 | 0 |
| Primary Outcomes | |||||||
| Clearance from infection (Symptomatic, Clinical, serological) | 47 | 0 | 1 | 11 | 11 | 2 | 2 |
| Progression of severity of infection | 19 | 1 | 2 | 7 | 3 | 4 | 2 |
| Study Duration | |||||||
| ≤3 months | 26 | 0 | 2 | 11 | 9 | 2 | 3 |
| >3 moths & ≤ 6 months | 32 | 1 | 1 | 7 | 4 | 3 | 0 |
| >6 months & ≤ 1 year | 8 | 0 | 0 | 0 | 1 | 1 | 1 |
| >1 year | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
3.5. Studies related to mitigation of psychological impact of COVID-19 (n = 11)
There were 11 studies aimed at relieving the psychological stress related to COVID-19 and associated symptoms like depression, anxiety and insomnia, of which 10 studies were from Yoga & Naturopathy while one was from Ayurveda.
The analysis of 10 studies from Yoga & Naturopathy revealed only one study to be single arm and rest nine to be double armed. Four controlled studies mentioned intervention along with Conventional Care (CC) in treatment arm and only CC in control arm. Rest of the five studies mentioned comparators such as placebo/no treatment/music or counselling. Six studies involved high risk population and only one study; healthy volunteers. Of the remaining three studies, one involved asymptomatic individuals and patients with mild to moderate infection and two included patients with mild to moderate infection. Duration of treatment was not specified in six studies. Duration of seven days was mentioned in one study, while another one documented 15 days duration, and yet another two of them mentioned duration of more than 30 days. There were two studies with a total study duration of three months while for five studies the same was between three and six months and for another three, it was one year. Primary outcomes of all the studies were concerned with assessment of depression, anxiety, stress levels, insomnia etc. A particular study also mentioned serum cortisol level along with stress scale, as primary outcome.
The only stress mitigation study from Ayurveda was a double-arm study. The intervention arm consisted of Brahmi (Bacopa monnieri) and no treatment was mentioned in comparator arm. A sample of 200 healthy volunteers was considered in this study with total study duration of two months. The duration of treatment was not specified.
3.6. Both prevention and recovery studies (n = 2)
There were two studies aiming at both prevention and recovery as their primary outcomes. Both studies included high risk individuals and patients with mild to moderate infection. The Ayurveda study consisted of sample size of 120 patients with duration of five months. Another study, a collaboration between Ayurveda, Yoga & Naturopathy and Siddha mentioned a sample size of 61,000 and duration of two years. The outcomes of both studies were noted as clinical cure and viral load estimation. The Ayurveda study included CC as comparator while collaborative study had dietary comparator in form of water & fruit juice.
3.7. Interventions used
As specified in Table 2, Table 3, a variety of interventions such as single drugs/single drug formulations, multi-drug formulations as well as multi-formulation regimen were used in these studies.
In case of Ayurveda studies, Guduchi (Tinospora cordifolia) has been the most frequently used drug in both preventive (n = 29) as well as recovery (n = 16) studies followed by Yashtimadhu (Glycyrrhiza glabra) which was seen in five preventive and four recovery studies. For preventive purposes, Ashwagandha (Withania somnifera) (n = 10) and Chyawanprasha (n = 8) were also seen to be preferred. Nasya using Anu Taila (n = 7) was explored as a preventive intervention. There was mention of personalized medication in a preventive study. AYUSH kwatha [9], a proprietary formulation promoted by Ministry of AYUSH for COVID-19, was utilized in both preventive (n = 7) and curative (n = 3) studies. AYUSH-64, a drug developed by CCRAS, was mentioned in one preventive and ten recovery studies. Though various patent & proprietary drugs have also been included as interventions, the details of their ingredients could not be retrieved. The details of Ayurvedic interventions are captured in Table 4.
Table 4.
Details of Ayurvedic interventions.
| Details of Intervention | Frequency of intervention |
|---|---|
| Prevention studies | |
| Guduchi (Tinospora cordifolia) churna, ghana, Sanshamani vati, Amritadi Guggulu | 29 |
| Ashwagandha (Withania somnifera) | 10 |
| Chyawanprasha | 8 |
| Anu Taila; AYUSH kwatha | Each 7 |
| Yashtimadhu (Glycyrrhiza glabra) | 5 |
| Haridra (Curcuma longa) churna and khanda; Shunthi (Zingiber officinale); Pippali and Pippalimoola (Piper longum) | Each 3 |
| Kiratatikta (Swertia chirayita) churna & kwatha | 2 |
| Haritaki (Terminalia chebula); Bhumyamalaki (Phyllanthus amarus); Marich (Piper nigrum); Tulsi (Ocimum sanctum);Twak (Cinnamonum zeylanicum); Nishamalaki churna; Kalmegh (Andrographis paniculata); Suvarna Bhasma; Tab. AYUSH-64, Personalized intervention | Each 1 |
| Patent & Proprietary formulations | 6 |
| Recovery studies | |
| Guduchi (Tinospora cordifolia)churna, ghana, Sanshamani vati | 16 |
| Tab. AYUSH 64 | 10 |
| Yashtimadhu (Glycyrrhiza glabra) ghanavati;Haridra (Curcuma longa) | Each 4 |
| Mahasudarshan ghanavati; Sudarshana ghanavati; Kamdhenuasava; Vyaghryadi Kashaya, AYUSH kwatha | Each 3 |
| Dashamula kwatha; Rasona (Allium sativum) kalka; Sitopaladi churna; Shunthi (Zingiber officinale) | Each 2 |
| Parijata(Nyctanthus arbortristis) swarasa; Pippali(Piper longum); Sahadevi(Vernonia cinerea); Tulsi (Ocimum sanctum);Bilvadi kwatha; Dasamoola Kadutrayadi Kashayam tablets; Elankanadi kwatha; Gojihvadi kwatha; Indukantham kashayam; Kanakasavam; Karpuradi kwatha; Kirattiktadi kwatha; Lavangadi vati; Malla Chandrodaya; Nagaradi kwatha; Pathyadi kwatha; Sanjeevani vati, Shirashadi kashaya; Surasadi Kadha capsules; Sutshekhara; Swasari rasa; Tribhuvan Kirti; Trikatu churna; Vasarishta, Panchagavya therapy, sesame oil, Tab. Guduchi (Tinospora cordifolia) + Pippali(Piper longum); Personalized intervention | Each 1 |
The Yoga & Naturopathy preventive studies utilised alternative nostril breathing (n = 2) and guided meditation (n = 2). For recovery studies, meditation (mindful happiness) (n = 1) and Pranayama Module (n = 1) were exercised.
Unani Joshanda was utilised in preventive (n = 4) as well as recovery (n = 2) studies. Similarly, Khameera Marwareed was mentioned in three preventive and one recovery study. Tiryaq-e-Arbawas was evaluated in two preventive studies. Aloe vera gel, Mur Makki and Senna leaves (for each, n = 1) were among the interventions in recovery studies.
Siddha formulation, Kabasura Kudineer was mentioned in two preventive and 14 recovery studies, while Nilavembu Kudineer in one preventive and four recovery studies. The formulations used in Siddha recovery studies included Adathoda manapagu (n = 7), Amukara chooranam; Brammhanandha Bairavam (each = 5), Thippili Rasayanam (n = 4), Adathodai Kudineer; Notchi Kudineer; Thaleesadhi Chooranam Mathirai (each n = 3), Maldevi Chendooram; Nelikai legiyum; Thoothuvalai nei; Curcuma longa (each n = 2), Alpinia officinarum; Anethum sowa; Cuminum cyminum; Glycyrrhiza glabra; Piper nigrum; Zingiber officinale; Amirtham Muthuparpam; Athimathuram; Mahasudarsan chooranam; Melia dubia; Mollugo serviana; Omatheeneeer; Pavalaparpam; Seenilnthil chooranam; Silasathuparpam; Swasakudorimaathirai; Thripala, Alum; Thulasi chooranam; Vasanthakusumakaram Mathirai and Vipro (each n = 1).
In the studies from Homeopathy system, Arsenic album was mentioned in eight preventive and six recovery studies. Bryonia alba was utilised in one preventive and five recovery studies. Another drug Camphora was used in two preventive and four recovery studies. The drugs such as Allium cepa, Calc Phos 6x, Chininum arsenicosum, CVN01 nosode, Echinacia eupatorium, Gelsemium, Influenzum, Sarcolactic Acid 30, Thuja, Tuberculinum 1M and Zincum metallicum featured in one study each in preventive category. Antimonium tartaricum, Cadamba, Eupatorium perfoliatum 30 C, Helleborus niger, Justica adhatoda and Zincum muriaticum 200C featured in one study each in category of recovery studies. Personalized intervention was used in three recovery studies similar to Ayurveda concepts of individualized approach.
We have also mapped the nutraceuticals used. Cap. Reimmugen, Cap. Suved and Neem capsule 50 mg were mentioned once each in preventive studies. SSV formulation tablets was mentioned in one preventive and one recovery study. ACT 13 dry syrup and ACT 13 tablets (each n = 2) were mentioned in recovery studies. Picovrid syrup, Tab. Virulina and ViraCide Soft gels (each n = 1) were also used in recovery studies.
The preventive studies involving intra-AYUSH collaboration mentioned Samshamani vati, Aresenic album, Khadiradi vati, Murchita Tila taila and Sudarshan ghana vati. Proprietary formulations such as, Energy Z Capsule, Immunofree tablet, Reginmune capsule and Virowin capsules were mentioned in the collaborative studies for recovery purpose. The collaborative study with both prevention and recovery as aim used proprietary formulations viz. Bio-immune powder and Covalix Vaccoil liquid.
4. Discussion
The present review was carried out to assess characteristic of AYUSH clinical studies registered with CTRI for COVID-19 during the period of 6 months. There are similar reviews available on TCM clinical trials for COVID-19 registered in global trial registries [10,11]. Recently, few reviews on CTRI registered clinical trials have been published, which have considered AYUSH studies. Charan et al. have compiled data pertaining to AYUSH as well as allopathic clinical trials registered till 11th July 2020 [12]. A short communication by Londhe et al. has reviewed only Ayurveda studies (observational & interventional) for COVID-19 registered with CTRI from 1st March 2020 to 25th June 2020 [13]. Rao et al. have published review of COVID-19 related allopathic as well as traditional medicine (AYUSH) studies registered with CTRI till 5th June, 2020 [14]. Our review has considered AYUSH studies, both observational and interventional, registered till 24th August 2020.
4.1. Contribution of different AYUSH systems
Our analysis revealed that a total of 197 studies were registered from AYUSH systems, in which the share of studies from Ayurveda was much larger (n = 113/197) than the other AYUSH systems. The reason for this may be expanded reach of Ayurveda in terms of educational, research & clinical institutions. As a matter of fact, Yoga has more global popularity and acceptance than Ayurveda. Though, acclaimed throughout the world for its benefits, not only at physical but even at psychological level, very little contribution of Yoga was reflected in terms of registered studies (n = 12/197). There is big opportunity for studies using Yoga intervention for mitigation of psychological stress caused due to fear of disease and consequences of lockdown. Interestingly, we observed nine studies involving collaboration among AYUSH systems probably due to integration and pluralism of medicines recommended in National Health Policy, 2017 [15].
4.2. General characteristics
During the initial period (February and March, 2020), no studies were registered from AYUSH systems for COVID-19. The months of April and May witnessed maximum studies with a declination in number of registered trials after month of June. This was contradictory to gradually increasing numbers of cases [16]. Since the outbreak of COVID-19 pandemic, maximum number of cases have been found in Maharashtra followed by Andhra Pradesh and Tamil Nadu [17]. This was reflected in state-wise distribution of study sites.
A substantial number of studies (n = 83) were planned to be conducted by non-AYUSH institutions/personnel. Though this is a welcome step towards integrative medicine, it is unclear from the available details, whether personnel from AYUSH system have been involved in their design and conduct as per the directives for AYUSH clinical trials [18]. Majority of studies (N = 105) have reported financial support from the government agencies like Ministry of AYUSH or its councils viz. CCRAS, CCRS, CCRUM, CCRH and their allied institutes and colleges. This highlights the phenomenal efforts by the Ministry to fight against COVID-19. The geographical mapping of study sites also points towards the same as evident from greater number of study sites from the states or union territories where allied institutes of AYUSH councils are situated. Only two studies have mentioned support from CSIR (Council of Scientific and Industrial Research), New Delhi. Although the Ministry of AYUSH is responsible for research in these systems in our country, research on traditional medicinal systems should be considered National priority requiring involvement of other ministries and departments as well. A call for proposals from DBT-BIRAC inviting research on traditional formulations was an apt and timely initiative [19].
4.3. Study types and designs
Of the 197 total AYUSH studies, only six studies were observational in nature. There has been a scope to understand COVID-19 from AYUSH epistemological perspective through simple observations like Ayurvedic clinical profiling reported by Rammanohar et al. [20] Unfortunately, such studies were not seen in registered studies.
The number of recovery studies were much higher (n = 112) than the prevention studies (n = 66). The strength of AYUSH systems lies in their preventive approach and management of chronic diseases. On this background, it is an optimistic gesture that AYUSH systems are being explored for treatment of COVID-19, an infectious disease.
The important characteristics of prevention studies revealed maximum number of double arm studies (n = 40), AYUSH interventions compared with conventional care (n = 23), sample size ranging from 100 to 1000 (n = 27), inclusion of high risk individuals (n = 57), equal distribution of single drug & multi-drug formulations and multi-formulation regimen, preferred duration of treatment between 15 and 30 days (n = 19), overall study duration less than six months (n = 56) and incidence of infection as a primary outcome (n = 61). The interesting feature observed in one preventive study was use of multi-arm, multi-stage design, suggestive of platform study design [21]. This study could be compared to WHO’s ‘Solidarity Trial for COVID-19’, that compares multiple options against conventional care to find an effective treatment [22]. The use of Ayurvedic assessment criteria is another feature observed in four preventive studies.
The recovery studies have also had maximum number of double arm studies (n = 84). There were 38 studies comparing AYUSH treatments against conventional care. This design indicates the efforts to prove AYUSH interventions as stand-alone treatment modality. In 13 recovery studies, placebo or no treatment was used as a comparator. Though the use of placebo is deemed controversial in infectious diseases [23], participants in such studies would have been advised to follow COVID-19 specific safety measures. In maximum (n = 62) studies, sample size is between 31 and 100. The patients with mild to moderate symptoms were recruited in 74 studies with equal number of studies having clearance from infection as an outcome measure. In 101 recovery studies, the overall duration was less than six months, while in 54 studies the treatment duration was seven to 15 days. This time period has been found satisfactory considering the clinical recovery period of the disease [24].
In both preventive and recovery studies, geriatric population was involved (n = 110). COVID-19 majorly affects the elderly population [25]. They are also considered as high-risk population due to other age-related comorbidities. Children as young as one year of age and elders as old as 99 years of age, have also been included in 26 studies. The approval from Ethics Committee is a pre-requisite for registering any study with CTRI. Hence, it is presumed that the ethical concerns related to these age groups must have been properly taken care of, by the respective committees. Interestingly, there has not been a single study involving patients with comorbid conditions or immunocompromised populations.
4.4. Interventions
In case of interventions, more preference is given to multi-drug formulations and multi-formulation regimen probably due to mention of multi-formulation regimen in AYUSH advisories for prevention [26]. The use of therapeutic procedures like Nasya (n = 7), targeting mucosal barrier strengthening, need to be encouraged as this approach is closer to doctrines of the AYUSH systems. The unique feature of AYUSH systems is its individualized treatment modalities. This person-specific selection of medicines was followed in two Ayurveda (one prevention & one recovery) and three Homeopathy recovery studies.
From the interventions used in Ayurveda studies, Guduchi (T. cordifolia) remains the most frequently used drug (n = 29 preventive and n = 16 recovery studies). Unani Joshanda (Unani), Kabasura Kudineer (Siddha) and Arsenicum album (Homeopathy) were preferred formulations from among the other AYUSH systems.
Along with herbal medicines, use of herbo-mineral formulations has also been noted irrespective of the system. The inclusion of herbo-minerals has been a bold step considering their limited acceptance especially by other-than the Ayurveda scientific community.
4.5. Limitations
The present analysis was carried out based on the data available at CTRI. Being a primary registry of WHO registry network; CTRI follows the ICTRP’s Trial Registration Data Set (TRDS) consisting of essential 21 items [27]. This set does not cover all the items of protocol listed in the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) checklist [28]. This may be due to regulatory/administrative nature of CTRI more than a technical/scientific document. We therefore had limitations in assessing rationale behind certain characteristics as well as assessment of quality of the registered studies. This issue has been also raised by Pillamarupu et al. [29].
4.6. Implications
The first case of COVID-19 was detected in early December 2019 in China. Since then, over a period of almost 10 months, the knowledge and evidence regarding prevention and management of COVID-19 remains limited. Against this background, the initiatives of the AYUSH sector are remarkable. Our review has succinctly summarized all possible characteristics of the AYUSH studies using the data available on CTRI. It is expected that some of these characteristics will facilitate designing of future studies. Since most of these studies are of short duration, their results will be available in near future. It would be important to know how many and how correctly these studies were executed. Further, the outcomes of these studies will state more about their impact and contribution of AYUSH systems towards the fight against the pandemic. Nonetheless, the number of AYUSH studies registered with CTRI definitely indicates a progressive change towards evidence-based medicine.
Sources of funding
None declared.
Conflicts of interest
Dr. Supriya Bhalerao, Corresponding Author, is on the Editorial Board of JAIM. The author has not been involved in the peer review process of this submission.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.Overview of the current COVID-19 situation. https://www.who.int/countries/ind/
- 2.Coronavirus disease (COVID-19) advice for the public: MythBusters. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public/myth-busters
- 3.World health organization. Q&A on coronaviruses. 2nd February,2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/question-and-answers-hub/q-a-detail/q-a-coronaviruses Available at:
- 4.WHO supports scientifically-proven traditional medicine. 4th May,2020. https://www.afro.who.int/news/who-supports-scientifically-proven-traditional-medicine
- 5.Wang Shi-xin, Wang Yan, Lu Yu-bao, Li Jie-yun, Song Yu-jun, Nyamgerelt M., Wang Xue-xi. Diagnosis and treatment of novel coronavirus pneumonia based on the theory of traditional Chinese medicine. J Integr Med. 2020;18(4):275–283. doi: 10.1016/j.joim.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ministry of AYUSH Ayurveda’s immunity boosting measures for self-care during COVID 19 crisis. https://www.ayush.gov.in/docs/123.pdf
- 7.Constitution of an "Interdisciplinary AYUSH research and development Task Force" in the Ministry of AYUSH for initiating, coordinating and monitoring the R & D activities in the AYUSH sector related to SARS-Cov-2 virus and the COVID-19 disease, Notification no. A.17020/1/2020/E.1, Govt. of India. Ministry of AYUSH; 2020. https://icssr.org/sites/default/files/Notification/on/task/force002.pdf [Google Scholar]
- 8.About CTRI. http://ctri.nic.in/Clinicaltrials/cont1.php
- 9.AYUSH health promotion product for commercial manufacturing by Ayurveda, Siddha and Unani drug manufacturers- reg, F. No. Z 25023/09/2018-2020-DCC (AYUSH), Govt. of India. Ministry of AYUSH; 24th April, 2020. http://www.ccras.nic.in/sites/default/files/Notices/25042020_Letter_to_States_UTs_for_Ayush_Kwath.pdf [Google Scholar]
- 10.Yang M., Shang Ya-xi, Tian Zi-yu, Xiong Min, Lu Chun-li, Jiang Yue, et al. Characteristics of registered studies for Coronavirus disease 2019 (COVID-19): a systematic review. Int Med Res. 2020;9:100426. doi: 10.1016/j.imr.2020.100426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu Rui-fang, Gao Yu-lu, Robert Sue-Ho, Gao Jin-ping, Yang Shi-gui. Chang-tai Zhu, Systematic review of the registered clinical trials for coronavirus disease 2019 (COVID-19) J Transl Med. 2020;18:274. doi: 10.1186/s12967-020-02442-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Charan Jaykaran, Kaur Rimplejeet, Bhardwaj Pankaj, Kanchan Tanuj, Mitra Prasenjit, Yadav Dharmveer, et al. Snapshot of COVID-19 related clinical trials in India. Indian J Clin Biochem. 2020 doi: 10.1007/s12291-020-00918-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deepak J Londhe, Kumar Shobhit, Chiluveri Ashwin C., Goel Sumeet, Sudha K Chiluveri, Singh Rajeshwari, et al. Ayurveda research studies on COVID-19 registered in clinical trials registry of India: a critical appraisal. J Res Ayurvedic Sci. 2020;4(3):128–134. doi: 10.5005/jras-10064-0113. [DOI] [Google Scholar]
- 14.Vishnu Vardhana Rao M., Juneja Atul, Maulik Mohua, Adhikari Tulsi, Sharma Saurabh, Gupta Jyotsna, et al. Emerging trends from COVID-19 research registered in the clinical trials registry – India. Indian J Med Res. 2020 doi: 10.4103/ijmr.IJMR_2556_20. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Key policy principles of national health policy. 2017. https://www.nhp.gov.in/nhpfiles/national_health_policy_2017.pdf
- 16.Overview of coronavirus disease (COVID-19) by date. https://www.who.int/countries/ind
- 17.COVID-19 state-wise status. https://www.mygov.in/corona-data/covid19-statewise-status/
- 18.Scientific studies and publication of research papers on AYUSH drugs and treatments by Non-AYUSH researchers/scientists-reg., F. No. Z.25023/09/2018/-DCC(AYUSH), Govt. of India. Ministry of AYUSH; 2nd April,2019. https://main.ayush.gov.in/sites/default/files/Advisory.pdf [Google Scholar]
- 19.DBT and BIRAC announce call for proposals on anti- sars-cov-2/ncov-2 virus studies using botanical ingredients and traditional formulations. https://birac.nic.in/cfp_view.php?id=54&scheme_type=34
- 20.Puthiyedath R., Kataria S., Payyappallimana U., Mangalath P., Nampoothiri V., Sharma P., et al. Ayurvedic clinical profile of COVID-19 - a preliminary report. J Ayurveda Integr Med. 2020 Jun 12;S0975–9476(20):30039–30045. doi: 10.1016/j.jaim.2020.05.011. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.H Park Jay J., Siden Ellie, Zoratti Michael J., Dron Louis, Harari Ofir, Singer Joel, et al. Systematic review of basket trials, umbrella trials, and platform trials: a landscape analysis of master protocols. Trials. 2019;20:572. doi: 10.1186/s13063-019-3664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Solidarity clinical trial for COVID-19 treatments. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov/solidarity-clinical-trial-for-covid-19-treatments
- 23.Aronson Jeffrey K., DeVito Nicholas, Ferner Robin E., Mahtani Kamal R., Nunan David, Plüddemann Annette. Nuffield Department of Primary Care Health Sciences, University of Oxford; June 30, 2020. The ethics of COVID-19 treatment studies: too many are open, too few are double-masked, Centre for Evidence-Based Medicine.https://www.cebm.net/covid-19/the-ethics-of-covid-19-treatment-studies-too-many-are-open-too-few-are-double-masked/ [Google Scholar]
- 24.Report of the WHO-China joint mission on coronavirus disease 2019 (COVID-19) https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf
- 25.Yin Wong Lydia Su, Ling Loo Evelyn Xiu, Hui Kang Alicia Yi, Lau Hui Xing, Tambyah Paul Anantharajah, Huiwen Tham Elizabeth. Age-related differences in immunological responses to SARS-CoV-2. J Allergy Clin Immunol Pract. 2020 Aug 27 doi: 10.1016/j.jaip.2020.08.026. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Advisory from ministry of AYUSH for meeting the challenge arising out of spread of corona virus (COVID-19) in India. 6th March,2020. https://www.ayush.gov.in/docs/125.pdf
- 27.International standards for clinical trial registries, version 1.2.1. https://apps.who.int/iris/bitstream/handle/10665/76705/9789241504294_eng.pdf;jsessionid=3E302A0ED1674D1E558C6D9FA28E903D?sequence=1
- 28.Chan A.W., Tetzlaff J.M., Altman D.G., Laupacis A., Gøtzsche P.C., Krleža-Jerić K., et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013 Feb 5;158(3):200–207. doi: 10.7326/0003-4819-158-3-201302050-00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pillamarapu Mounika, Mohan Abhilash, Saberwal Gayatri. An analysis of deficiencies in the data of interventional drug trials registered with Clinical Trials Registry - India. Trials. 2019;20:535. doi: 10.1186/s13063-019-3592-0. [DOI] [PMC free article] [PubMed] [Google Scholar]