Abstract
We have previously hypothesized that pentoxifylline could be beneficial for the treatment of COVID-19 given its potential to restore the immune response equilibrium, reduce the impact of the disease on the endothelium and alveolar epithelial cells, and improve the circulatory function. Serum lactate dehydrogenase (LDH) and lymphocyte count are accessible biomarkers that correlate with the severity of COVID-19, the need for hospitalization, and mortality, reflecting the host immune response’s contribution to the seriousness of SARS-CoV-2 infection.
We carried out this external pilot study on 38 patients with moderate and severe COVID-19 to test the effect pentoxifylline on parameters such as LDH, lymphocyte count, days of hospitalization, mortality, and proportion of patients requiring intubation. Twenty-six patients were randomized to receive 400 mg of pentoxifylline t.i.d. plus standard therapy (pentoxifylline group), while the rest received the standard treatment (control group). Linear regression models were built for statistically significant parameters.
Pentoxifylline treatment was associated with a 64.25% increase (CI95% 11.83, 116.68) in lymphocyte count and a 29.61% decrease (CI95% 15.11, 44.10) in serum LDH. Although a trend towards reduced days of hospitalization, mortality, and proportion of patients requiring intubation was observed, no statistically significant difference was found for these parameters.
Our findings open the possibility of pentoxifylline being repositioned as a drug for COVID-19 treatment with the advantages of a proven safety profile, availability, and no risk of immunosuppression; however, this evidence needs to be confirmed in a pragmatic randomized controlled trial.
Keywords: COVID-19, Lymphocytes, Lactate dehydrogenase, Pentoxifylline, Immunomodulatory treatment
1. Introduction
The World Health Organization (WHO) declared the coronavirus disease 2019 (COVID-19) outbreak as a global public health emergency on January 30, 2020 [1], [2]. For the week ending on August 16, 2020, the WHO reported 1.8 million new COVID-19 cases and 39,000 deaths, leading to a cumulative total of 21.2 million confirmed cases and 761,000 deaths [3].
Manifestations of COVID-19 ranges from asymptomatic infection to severe disease with various symptoms: fever, cough, myalgia, fatigue, and dyspnea; it can potentially progress to pneumonia, acute respiratory distress syndrome (ARDS), or multiple organ failure, and even death [4], [5]. Unfortunately, there is still no specific treatment for COVID-19, so management is limited to supportive therapy, which is guided by the clinical evolution of the patient and by biomarkers of disease severity that contribute to therapeutic decision making to reduce mortality rates in patients [6]. Clinical lab assessments provide information regarding disease evolution and prognosis, and response to treatment [7]. Among these assessments, those with the most significant availability and accessibility are the lymphocyte count and lactate dehydrogenase (LDH) [8], [9], [10].
The immunological reactions could partially explain the devastating consequences of COVID-19, such as lung injury, acute respiratory distress syndrome (ARDS), and multiple organ involvement within 8 to 14 days of disease onset [11]. These severe cases display pathological changes such as the formation of hyaline membranes, infiltration of inflammatory cells with multinucleated syncytial cells in the lungs, and a burst of cytokine release, leading to increased morbidity and mortality [12], [13]. These processes can result in cell death and tissue damage allowing the release of LDH into the bloodstream since this enzyme is expressed in a variety of organs, including the heart, liver, muscles, kidneys, lungs, and bone marrow; in fact, serum LDH has been useful as an indicator of poor prognosis and the need for admission to the ICU [8], [9], [10].
As an example of the prognostic value of LDH, a multi-center study involving 1099 patients showed an important correlation between the extent of tissue damage, inflammation and increased levels of LDH, that allowed the detection of severe cases of COVID-19 (LDH ≥ 250 U/L; 58.1% vs. 37.2%, P < 0.001)[4]. Furthermore, in their clinical and radiologic study, Xiong et al. found that LDH levels positively correlate with the severity of lung abnormalities quantified on CT scans [14]. Therefore, elevation of LDH levels in COVID-19 could reflect the severity of pneumonia [14]. That is congruent with previous observations that LDH levels might indicate the extent of inflammation or extensive tissue damage and are frequently high in viral pneumonia, including SARS [14]. Besides, LDH levels allowed the identification of 70% of COVID-19-positive patients with cut-off levels of 210 U/L for COVID-19 positivity (PPV: 83.3%) diagnosed by rRT-PCR [15]. These results might be explained by the fact that this enzyme is a marker of lung damage [16] and that COVID-19 primarily infects the lower respiratory tracts [15]. Several studies have shown a correlation between these lab parameters and the severity of the disease, the need for hospitalization (either in general wards or in intensive care units), and mortality [8], [9], [10].
In a recent study, Yi Han et al. described that LDH levels were significantly higher at admission in patients with severe disease compared to those with the non-severe form. Conversely, CD3+, CD4+, and CD8 + lymphocyte count were lower. They also determined that serum lymphocytes and LDH levels were independent predictive factors for the severity of COVID-19. Using a cutoff value of LDH greater than 344.5 U/L and lymphocyte count lower than 0.985x10^9/L, they were able to predict severe conditions with excellent accuracy. They also found that LDH correlated with low PaO2/FiO2 (P/F) ratio, the high pneumonia severity index, and a high Lunǵs affection evidenced by a computed tomography score. It was also relevant to APACHE II and SOFA scores, which reflected a strong correlation of LDH with lung damage and disease severity. Besides, LDH was negatively associated with lymphocyte count in severe cases during the 14-day observation period [17].
In another example of the correlation of lymphocyte count and serum LDH levels with disease severity, a study carried out by Mo et al. included 155 patients hospitalized with confirmed COVID-19. They divided them into patients with general COVID-19 if they filled the next inclusion criteria:
Alleviation of respiratory symptoms (e.g., cough, chest distress, and breath shortness) after treatment
Maintenance of body temperature without fever for three days without the use of corticosteroid or antipyretics
Improvement in radiological abnormalities on chest CT or X-ray after treatment
A hospital stay of ≤ 10 days
Otherwise, the patients were considered refractory COVID-19 [18]. Their study reported a median stay of 10.5 days (IQR: 7–16), in congruence with other studies [20]. Furthermore, they found that on admission, the majority of patients had lymphopenia. Compared with general patients, refractory patients had a higher level of LDH (P = 0.017) [19].
Increases in LDH levels and the lymphopenia that accompany more severe forms of COVID-19 may reflect a contribution of the host immune response to the pathogenic mechanisms involved in SARS-CoV-2 infection [5]. Indeed, Caricchio et al. proposed predictive criteria for cytokine storm development in patients with COVID-19; with a sensitivity of 0.85 and a specificity of 0.80 [20]. Among these criteria included in three coherent clusters were low lymphocyte count (belonging to Cluster I) and high LDH levels (belonging to Cluster II) as markers of inflammation and cell death attributable to tissue damage [20], predicting greater clinical severity of the disease reflected in a longer duration of hospital stay (15.1 ± 13 vs. 5.7 ± 6.7) and mortality (28.8% vs. 6.6%) [20].
Also, patients experiencing more severe COVID-19 and those who required admission to the intensive care unit (ICU) had higher concentrations of granulocyte colony-stimulating factor (G-CSF), gamma interferon-induced protein 10 (IP10), monocyte chemoattractant protein 1 type 1 (MCP1), macrophage inflammatory protein alpha 1 (MIP1A), and tumor necrosis factor-alpha (TNF-α) [5]. Similarly, patients who died from COVID-19 had higher levels of interleukin-2 receptor (IL-2R), interleukin-6 (IL-6), interleukin-8 (IL-8), interleukin-10 (IL-10), and TNF - α compared to survivors of the infection (11]. The presence of higher concentrations of cytokines and lymphopenia in COVID-19 patients may be a reflection of uncontrolled replication of the virus in patients with severe COVID-19 [21], as shown by the association between decreased lymphocyte count and increased severity of the disease [22], [23] and b3y the fact that patients who died from COVID-19 had lower lymphocyte counts than survivors [23].
This study aimed to test the effect Pentoxifylline (PTX) on parameters such as LDH, lymphocyte count, days of hospitalization, mortality, and the need for intubation on patients with severe and moderate COVID-19 in a public hospital in Mexico City based in our previous hypothesis that Pentoxifylline a phosphodiesterase (PDE) inhibitor with the ability to reduce the synthesis of the pro-inflammatory cytokines IL-1, IL-6, and TNF-α [24], [25], [26] could decrease the neutrophil/lymphocyte ratio while restoring Treg/Th17 lymphocyte subpopulations [27]. The rheological and anti-inflammatory properties of pentoxifylline and its effects on renin-angiotensin system (RAS) led us to suggest the repositioning of this drug as an alternative for the treatment of patients with COVID-19 and as a potential therapeutic tool to restore the immune response equilibrium, reduce the endothelial and alveolar damage, improve circulation, and avoid microvascular thrombosis [27]. This drug also affects the RAS in vitro by inhibiting the expression of angiotensin receptor 1 (AT1R). Besides, pentoxifylline can restore glutathione levels, maintain mitochondrial viability, and preserve microvascular blood flow, along with the observed improvement in endothelial function and coagulation that encouraged its use for the treatment of neonatal sepsis, where a reduction in days of hospitalization and mortality was observed. [28], [29], [30] Furthermore, in the context of COVID-19, pentoxifylline has shown improvement in ARDS experimental models [27].
Moreover, PTX was shown to inhibit TNF-α production by alveolar macrophages [31], [32], [33] Because pulmonary sarcoidosis is a chronic inflammatory disease, interactions between an antigen-presenting cell and an unknown antigen are perceived by naive CD4 + lymphocytes (Th0 cells) and alveolar macrophages, leading to the activation and proliferation of both cell types and the consequent release of IL-2, TNF- α, and IFN-γ [34]. In support of these effects, adding PTX to a systemic steroid regimen allowed for a steroid dose reduction [35]. Park et al. confirmed this steroid-sparing effect of PTX in a randomized controlled clinical trial in patients with pulmonary sarcoidosis, in which PTX improved the pulmonary diffusion of carbon monoxide and arterial blood oxygen pressure during exercise, especially in patients who were naïve to steroid treatment [36]. In addition, to these anti-inflammatory properties, PTX has also been reported to suppress tissue fibrosis by blocking TGF-β1 and preventing the deposition of type I collagen [36], [37]. Several in vitro studies have shown that PTX inhibits fibroblast proliferation and extracellular matrix production [38], [39], [40], and a clinical study demonstrated that administration of pentoxifylline to obese patients decreased the plasma levels of plasminogen activator inhibitor-1 (PAI-1) [41]. These findings motivated Lee and colleagues [42] to test the effect of PTX administration in an experimental model of radiation-induced pulmonary fibrosis in rats, and found a decrease in the expression levels of both fibronectin and PAI-1. This was considered to be particularly relevant in light of evidence that PAI-1 expression is elevated in fibrotic pathological conditions. Indeed, PAI-1 contributes to a reduction in fibrinolysis rates and a subsequent decrease in the degradation of components of the extracellular matrix, including fibronectin, leading to tissue fibrosis [43]. Since there are no clinical guidelines for the management of pulmonary fibrosis, pentoxifylline is currently recommended for the prevention and treatment of this condition [44]. Moreover, PTX was shown to prevent the development of pneumonitis in patients with breast and lung cancers [45], [46].
Therefore, Pentoxifillyne shows promise as a useful therapeutic tool for COVID- 19 because the RAS is one of the most critical systems activated during oxidative stress. Upon binding of Ang II with AT1Rs, the secondary messengers inositol triphosphate and diacylglycerol are produced, resulting in the production of reactive oxygen and vasoconstriction [47]. A meta-analysis showed that PTX had an anti-inflammatory effect in adults with a variety of disorders, including coronary artery disease, type 2 diabetes mellitus, idiopathic or ischemic cardiomyopathy, and chronic kidney disease. The statistically significant differences were corroborated by a reduction in plasma concentrations of TNF-α and CRP and no deleterious effects on blood pressure [48] Besides. Hendry et al. suggested that pentoxifylline could be effective in reducing the severity of lung injury in patients with COVID-19 primarily by its effect on TNF –α [49]. Other hypotheses suggest that pentoxifylline could be of utility in COVID-19 due to its action in platelets and related coagulopathy, and rheological effects improving the blood flow in the microcirculatory disturbances [49].
Taken together, the evidence highlighted herein strongly points to the benefits of redirecting PTX as an ethical and legal attempt in the treatment of COVID-19, which can help support patients in critical care and overwhelmed hospital resources in the face of this pandemic [27].
2. Methods
Volunteers.
An external pilot study was carried out on moderate and severe COVID-19 patients with the approval of the Research and Ethics Committee of the Mexican Institute of Social Security (Instituto Mexicano del Seguro Social, IMSS) (protocol number R-2020785-079) and the authorization of the Federal Commission for the Protection against Sanitary Risk (COFEPRIS) (number COF-002495). This research was carried out under the principles of the Declaration of Helsinki. All participants in this study signed informed consent, and their privacy rights were observed.
We did not use a formal sample size calculation according to Lancaster's suggestion that this may not be necessary for a phase III clinical trial pilot study. Therefore, we followed the recommendation of a sample size of between 24 and 50 patients to meet our objective [50].
Sixty-six Mexican patients that were hospitalized for suspected COVID-19 at the General Hospital of Zone number 27 of the IMSS in Mexico City from July to August 2020 were assessed for eligibility based on the following criteria: 1) pneumonia suspected of being caused by SARS-COV2 with clinical symptoms and signs such as fever, fatigue, dry cough, anorexia, myalgia, dyspnea, sputum production, dysgeusia, and anosmia; 2) infiltrates in chest imaging studies (plain radiography or tomography), 3) confirmation of SARS-CoV-2 infection by viral PCR, and 4) signed informed consent for receiving their primary treatment and pentoxifylline if selected to be included in the pentoxifylline group. Pregnant or lactating patients were excluded, and also those who did not wish to participate in the study or had an allergy, intolerance, or contraindication for pentoxifylline.
Initially, 54 patients met the selection criteria and were submitted to simple randomization in a 2:1 ratio [51] (pentoxifylline:conventional treatment). Pentoxifylline was administered orally at a dose of 400 mg every 8 h from admission to discharge. Concomitant medications were prescribed following the international guidelines for COVID-19 treatment.
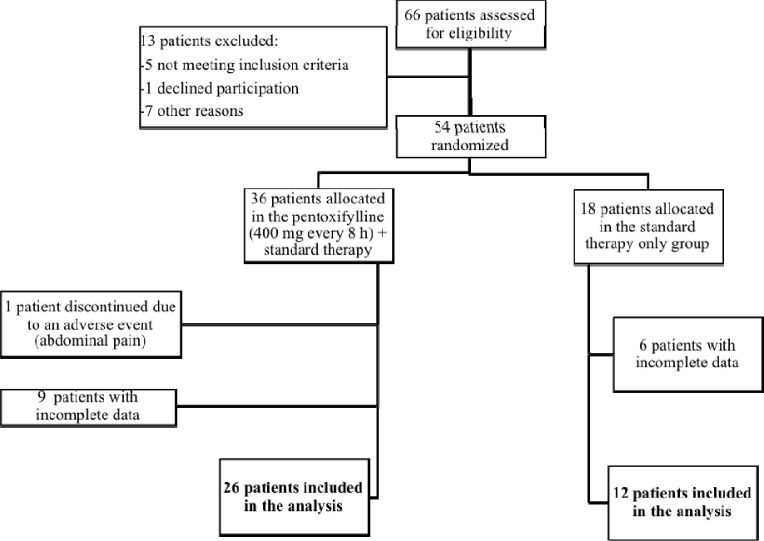
From the 54 patients initially selected, one discontinued the treatment due to abdominal pain, as a probable adverse effect due to PTX. 53 patients were enrolled in the study. Using the NEWS 2 score, 25 and 10 patients were classified as having moderate or severe disease in the treatment group, respectively. Meanwhile, using the same score in the control group, 11 and 7 patients were classified as having moderate or severe disease, respectively. At the end of the study, only 38 patients had complete data and were included in the final analysis (Fig. 1 ).
Fig 1.
Flow diagram of participants. Sixty-six patients were evaluated for eligibility, and 38 of them were included in the analysis.
3. Assessments
The vital signs (blood pressure, heart rate, respiratory rate, and oxygen saturation) assessments were performed in the admission and every 24 h. Blood tests (Complete blood cell count, blood chemistry, arterial blood gases, liver function tests) were run at the moment of admission and discretionary according to the clinical needs and attending physician’s criteria. The average number of days from basal to post-treatment lab assessments was compared between groups, obtaining no statistically significant difference (5.8 ± 4.6 days for controls vs. 5.2 ± 2.5 days for pentoxifylline treated group p = 0.654). At the same time, scales such as the acute physiology and chronic health disease classification system II (APACHE II), the sequential organ failure assessment (SOFA), the Quick sequential organ failure assessment (q SOFA) and the National Early Warning Score (NEWS) were calculated. Besides, for LDH and lymphocyte count, the percentage of change between the basal evaluation and 48–72 h after treatment was calculated.
The number of concomitant medications (ceftriaxone, azithromycin, levofloxacin, oseltamivir, enoxaparin, tocilizumab, meropenem, and/or dexamethasone) and comorbidities (diabetes mellitus, arterial hypertension, obesity, and/or tobacco smoking) for each patient was recorded. The need for intubation during hospitalization, days of hospitalization, and the outcome (discharge due to improvement or death) were documented.
3.1. Statistical analysis
Results for continuous variables were expressed as mean + SD or median (p25, p75), while for categorical variables, the results are reported as n (%). Initial comparisons between the pentoxifylline and control groups were performed as follows: Student’s t-test was used for continuous variables when the assumptions of normality and homogeneity of variances were met, and the Mann Whitney U test when they were not. Categorical variables were evaluated by employing the Fisher exact test. We performed a linear regression analysis for the association between pentoxifylline treatment and the percentages of change in LDH and lymphocyte count, adjusting by sex, age, number of concomitant medications, and number of comorbidities.
4. Results
No differences between pentoxifylline and control groups were found regarding all the basal measurements (Table 1 , Table 2 and Table 3 ). The majority of volunteers were under treatment with three or four concomitant medications and had at least one comorbidity.
Table 1.
Demographic data of the two groups.
| Characteristic | Control (n = 12) | Pentoxifylline (n = 26) | p-value | |
|---|---|---|---|---|
| Age [years(mean ± SD)] | 62.3 ± 15.3 | 55.3 ± 9.2 | 0.091 | |
| Sex [n(%)] | Women | 5 (41.7) | 12 (46.2) | 0.796 |
| Men | 7 (58.3) | 14 (53.8) | ||
| Smoking [n(%)] | No | 12 (100) | 21 (80.8) | 0.131 |
| Yes | 0 (0) | 5 (19.2) | ||
| Arterial hypertension [n(%)] | No | 8 (66.7) | 15 (57.7) | 0.599 |
| Yes | 4 (33.3) | 11 (42.3) | ||
| Diabetes mellitus 2 [n(%)] | No | 5 (41.7) | 14 (53.8) | 0.485 |
| Yes | 7 (58.3) | 12 (46.2) | ||
| Obesity [n(%)] | No | 7 (58.3) | 10 (38.5) | 0.252 |
| Yes | 5 (41.7) | 16 (61.5) | ||
| Number of comorbidities [n(%)] | 0 | 4 (33.3) | 3 (11.5) | 0.367 |
| 1 | 2 (16.7) | 10 (38.5) | ||
| 2 | 4 (33.3) | 6 (23.1) | ||
| 3 | 2 (16.7) | 6 (23.1) | ||
| 4 | 0 (0.0) | 1 (3.8) | ||
Table 2.
Baseline levels, and comparison of APACHE II, SOFA, q SOFA and NEWS scales between the two groups of intervention.
| Characteristic | Control (n = 12) | Pentoxifylline (n = 26) | p-value | |
|---|---|---|---|---|
| SOFA at admission | 2.0 (2.0, 2.5) | 2.0 (2.0, 3.0) | 0.631 | |
| qSOFA n(%) at admission | 0 | 2 (16.7) | 16 (61.5) | 0.036 |
| 1 | 8 (66.7) | 8 (30.8) | ||
| 2 | 2 (16.7) | 2 (7.7) | ||
| Apache II at admission | 9.5 ± 6.2 | 7.8 ± 4.6 | 0.340 | |
| NEWS at admission | 7.1 ± 1.8 | 5.9 ± 2.2 | 0.112 | |
Table 3.
Baseline levels of the absolute lymphocyte count and lactic dehydrogenase levels for the control group and the group treated with pentoxifylline.
| Characteristic | Control (n = 12) | Pentoxifylline (n = 26) | p-value |
|---|---|---|---|
| Lymphocyte count basal (x10/L) | 1.05 (0.87,1.16) | 1.16 (0.67,1.10) | 0.447 |
| DHL basal (U/L) | 398 (276.0, 571.0) | 399.5 (354.0,505.0) | 0.485 |
They are shown as medians and percentiles 25 and 75 because they do not meet normality.
The p indicates that the baseline values are not statistically different.
Some interesting trends were observed: fewer days of hospitalization, lower mortality, and reduced need for intubation in the pentoxifylline group; however, no statistical significance was obtained. Besides, although the pentoxifylline group displayed a trend to be older than the control group, the difference did not reach statistical significance.
Complete initial and 48–72 h data for LDH and lymphocyte count were available only for 38 patients. Both assessments resulted significantly different between pentoxifylline and control groups (table 4 ). Subsequently, linear models showed that pentoxifylline treatment was associated with a 29.61% decrease in LDH and a 64.25% increase in lymphocyte count (Table 5 ). Importantly, statistical significance was maintained even when the models were adjusted by covariates.
Table 4.
Descriptive data of the sample of patients with COVID-19 separated by groups of treatment.
| Characteristic | Control (n = 12) | Pentoxifylline (n = 26) | p-value | |
|---|---|---|---|---|
| % change in lymphocytes (basal vs. 48–72 h) | 0.5 (–22.1, 36.7) | 60.3 (28.6, 112.5) | 0.013 | |
| % change in LDH (basal vs. 48–72 h) | −7.9 ± 23.6 | −36.9 ± 20.1 | <0.001 | |
| Days of hospitalization | 13.1 ± 5.6 | 11.2 ± 5.5 | 0.325 | |
| Oxygen saturation | 82.3 ± 8.6 | 86.5 ± 10.3 | 0.227 | |
| paO2a | 49.0 (47.0, 54.0) | 55.0 (49.0, 64.7) | 0.123 | |
| paCO2a | 35.0 (29.0, 42.2) | 30.9 (25.3, 35.0) | 0.238 | |
| Number of concomitant medications n(%) | 2 | 2 (16.7) | 4 (15.4) | 0.348 |
| 3 | 4 (33.3) | 11 (42.3) | ||
| 4 | 6 (50.0) | 7 (26.9) | ||
| 5 | 0 (0.0) | 4 (15.4) | ||
| Mortality n(%) | No | 8 (66.7) | 23 (88.5) | 0.107 |
| Yes | 4 (33.3) | 3 (11.5) | ||
| Need for intubation n(%) | No | 9 (75.0) | 23 (88.5) | 0.290 |
| Yes | 3 (25.0) | 3 (11.5) | ||
For these variables, there were data for 15 patients treated with pentoxifylline and nine from the control group.
Table 5.
Linear regression models for the association between pentoxifylline treatment and the percentage of change in lymphocytes and LDH.
| Dependent Variable | Unadjusted model |
Adjusted model for covariables |
Final Model * |
||||||
|---|---|---|---|---|---|---|---|---|---|
| β (IC 95%) | βstd | p-value | β (IC 95%) | βstd | p-value | β (IC 95%) | βstd | p-value | |
| % change in lymphocytes (basal vs. 48–72 h)a | 64.25 (11.83, 116.68) | 0.383 | 0.018 | 57.07 (0.013, 114.12) | 0.340 | 0.050 | 64.25 (11.83, 116.68) | 0.383 | 0.018 |
| % change in LDH (basal vs. 48–72 h)b | −29.02 (−44.05, −13.99) | −0.547 | <0.001 | −26.51 (−41.87, −11.14) | −0.499 | 0.001 | −29.61 (−44.10, −15.11) | −0.558 | <0.001 |
95% CI, 95% confidence interval; β std, standardized beta coefficient.
The model considers the variables of the last model's variables discarding the non-significant variables by the backwards method.
No covariate reached enough statistical significance to be included in the final model.
Only the covariate sex reached enough statistical significance to be included in the final model.
5. Discussion
In this study, we evaluated the effect of pentoxifylline on some clinical parameters and biomarkers and found that pentoxifylline treatment was associated with an increase in the lymphocyte count and decreased LDH levels, although no difference was observed regarding days of hospitalization, mortality, and need for intubation.
It is known that elevated LDH and decreased lymphocyte count are hallmarks of severe COVID-19 (8–10, 12, 21–23]. In fact, progressive lymphopenia with depletion of T cells is a critical signal of COVID-19 severity [51], [52], [53]. CD4 + and CD8 + T lymphocytes are decreased in severe cases (median 177.5 and 89.0 × 106 /L, respectively), compared with the moderate ones (median 381.5 and 254.0 × 106/L, respectively), suggesting that T-cell lymphopenia may constitute a potential prognostic marker to be included in the follow-up of patients with COVID-19 [53]. Even CD4 + Th1 T cells tend to be lower in severe illness than in moderate disease (median 14.1% vs. 22.8%, respectively), possibly indicating a progressive shift from Th1 / Th2 balance towards a tolerogenic response [54). Furthermore, the percentages of memory Th cells and regulatory T cells are decreased in severe cases [52].
Given the decisive role that lymphocytes play in maintaining immune homeostasis and the inflammatory response throughout the organism [6], the fact that pentoxifylline is associated with an increase in the number of these cells could make this drug an excellent candidate to counteract the consequences that lymphopenia brings to the organism during COVID-19. The lymphopenia observed in COVID-19 could have its origin in the relationship between the RAS and immune system since the activation of the ACE/AngII/AT1R axis can lead to increased levels of inflammatory cytokines such as NF-κβ, IL-6, TNF-α, IL-1β, and IL10 [55], [56], [57], which can promote lymphopenia. There is a downregulation of ACE2 during COVID-19 with a consequent decrease in angiotensin 1–7, which plays a crucial role in the counterregulation of pathophysiological actions of AT1R [57], [58], [59]. In this respect, the evidence suggests that the use of ACE inhibitors and angiotensin receptor blockers in patients with hypertension and COVID-19 is associated with a less severe infection and a tendency to lower IL-6 levels and increase circulating CD3 + and CD4 + T lymphocytes [60].
It should be noted that Tan et al. proposed the lymphocyte count as a practical and feasible indicator to evaluate the severity of COVID-19 and as an aid to guide therapeutic decisions on admission to the general ward or the ICU and even to discharge a patient from the hospital [6]. This proposal was based on the observation that in SARS - CoV infection (this virus has a genomic similarity of 88% with SARS - CoV 2), the adaptive immune system participates in controlling the disease [21]: viral peptide antigens are presented to naïve TCD4 + lymphocytes by antigen-presenting cells, leading to the activation of TCD4 + and production of TNF- α, IL-2, and INF- γ. This process promotes the differentiation of cytotoxic T cells (CTLs) that kill infected cells [61].
Thevarajan et al. [62] found that circulating activated helper T lymphocytes and CD8 + T cells increase gradually during the first week of non-severe COVID-19; simultaneously, CD8 + T cells release perforin and granzymes A and B that induce apoptosis of infected cells. While natural killer (NK) cells and CTLs can kill infected cells, T helper cells adjust the adaptive immune response, and antibodies limit the infection and protect against re-infection [22]. In this regard, Zheng et al. demonstrated that NK and CTLs are significantly reduced in patients with COVID-19 and that the number of these lymphocytes is notably reconstituted after patients recover [63]. Another study showed that the total number of CD8 + and CD4 + T lymphocytes is decreased in patients with SARS CoV-2 infection, particularly in patients over 60 years and those who required admission to the ICU [64].
A proposed mechanism to explain the lymphopenia commonly found in COVID-19 is that the virus generates immune dysregulation, with the consequent release of inflammatory cytokines that leads to lymphocyte apoptosis, as supported by the fact that TNF-α, IL-6, and other pro-inflammatory cytokines induce lymphocyte deficiency [65]. Lymphocyte fatigue is another phenomenon that has been documented during SARS-CoV-2 infection [21], and that can be enhanced by the production of pro-inflammatory cytokines (TNF- α, IL-2, IL-10, and TNF- β) [66]. Not only cytokines could cause lymphopenia in patients with COVID-19 but also the direct attack of SARS-CoV-2 to lymphocytes since they express the ACE2 receptor [67] and lactic acid, whose levels have been reported to be increased in cases of severe COVID-19 [54], [68].
The effect that pentoxifylline had on the lymphocyte count in our study may be explained, at least partially, by its ability to decrease AT1R expression, possibly leading to a reduction of inflammatory cytokine levels and allowing the number of lymphocytes to be restored. The administration of pentoxifylline to rats with induced heart failure attenuated the expression of AT1R in the hypothalamic paraventricular nucleus [69]; a similar behavior was observed in a rat model of metabolic syndrome, where the AT1R reduced expression was accompanied by lower levels of TNF-α and higher levels of the anti-inflammatory cytokine adiponectin [70].
An imbalance between regulatory T cells (Tregs) and interleukin (IL)-17 producing T helper lymphocytes (Th17) are among the main immune alterations observed in ARDS [71], [72], [73], [74]. Li et al. demonstrated that pre-treatment with pentoxifylline attenuated lung injury and reduced mice mortality with cecal ligation and puncture (CLP)-induced ARDS while raising cAMP levels. Besides, pre-treatment with pentoxifylline partially restored the Treg/Th17 ratio by modulating the transcription of Forkhead box p3 (Foxp3) and RAR-related orphan receptor γt (RORγt) through the signal transducer and activator of transcription (STAT3) pathway favoring immune tolerance [75].
Beyond that, it is known that COVID-19 is characterized by extensive tissue damage; for instance, Ackermann et al. described the histological pattern of the lungs of patients who died from this disease, reporting diffuse alveolar damage with perivascular infiltration of T lymphocytes and characteristic vascular patterns associated with high ACE2 expression in the endothelial cells. The presence of the virus at the intracellular level disrupted endothelial cell membranes and caused intussusceptive angiogenesis [76]. This damage and cell death can result in extracellular elevation of LDH, which is a cytoplasmic enzyme expressed in several organs (lungs, kidney, liver, brain, spleen, skeletal muscle, erythrocytes, leukocytes, and platelets) [77], [78], [79], many of which are affected by SARS-CoV2, so it is considered highly sensitive although nonspecific for a particular organ [80].
The usefulness of LDH as a prognosis marker has been observed in several pulmonary disorders; for instance, ARDS, Pneumocystis jirovecii pneumonia [81], [82], [83], alveolar proteinosis [84], interstitial desquamative pneumonitis [85], [86], [87], and extrinsic allergic alveolitis [88]. Since pentoxifylline treatment was associated with decreased LDH levels, this could be a sign of reduced tissular damage.
In our study, the time between the first blood collection at hospital admission and the second blood collection showing changes in lymphocytes blood count and LDH levels of 38 patients was 48 h to 72 h.
By using this interval, we were able to observe major changes since other researchers described that the decrease in T cell count in severe COVID-19 patients reached its nadir within three days of evolution and was sustained up to two weeks after, when they began to return to non-severe COVID-19 levels [17]. Given that basal LDH and lymphocyte count were not significantly different between pentoxifylline-treated and control group, the improvements observed in the patients treated with pentoxifylline are very likely associated with the drug. Other researchers reported that the lowest lymphocyte count recorded during blood routine examination and biochemical examination was observed from 5 to 7 days after admission. In contrast, lactate dehydrogenase levels were higher in the ICU than in the general ward group within 24 h before or after admission. Additionally, biochemical examination at five to seven days after admission indicated that the lactate dehydrogenase level was still higher in the ICU group [89], [90].
Furthermore, when Caricchio et al. analyzed the disaggregated laboratory parameters to determine the time that patients required to meet the criteria for COVID-cytokine storm (CS), they found that, among the patients with the clinical consensus of CS, 43% met the criteria at hospital admission. The remaining patients reached the asymptote at ten days of hospitalization [20]. This author clarified that although the validation cohort received higher and earlier doses of steroids than the initial cohort, a higher percentage received anticoagulants. These criteria in the validation cohort are still very valuable for predicting COVID-CSwith a specificity of 0.73 (CI 0.69 to 0.78) and a sensitivity of 0.69 (CI 0.58 to 0.81), indicating that they can be applied successfully to other cohorts. As in the first cohort, the patients who met the criteria (33%) had a significantly higher LoS (15.5 ± 10.1 vs 4.7 ± 3.7, p < 0.001) and mortality (33.7% vs 4.2%, p < 0.0001) [21].
The improvements in LDH levels and lymphocyte count were not reflected on outcome variables such as mortality, days of hospitalization, and need for intubation; however, the observed trend to a reduction in these parameters deserves to be further studied on a larger sample.
We acknowledge some limitations of our study: 1) the sample size was small; however, the results have shed some light on the potential of pentoxifylline as a drug to be included in COVID-19 treatment if its efficacy can be confirmed in a larger pragmatic randomized controlled trial; 2) the levels of some biomarkers such as Ferritin, D- Dimer, cytokines, TNF- α and RAS-related molecules were not available in our hospital, and they could have provided more information on the actions of pentoxifylline, and 3) we do not have data on the behavior of different lymphocyte subpopulations, which can be useful to deepen into which are the ones that pentoxifylline affects the most. Nevertheless, it is of utmost importance to point out that all of us involved in this research are first-line physicians, so the study was designed and carried out in a clinical setting in a real and contingency situation.
Considering the global emergency that we are facing with a disease that rapidly becomes severe, which can be overwhelming for first-line physicians, the need for effective treatment alternatives is evident. Because pentoxifylline was associated with improvement in LDH and lymphocyte levels, and has a proven safety profile, it is available for the general population, and has no risk of immunosuppression [20], we propose that this drug may be an excellent candidate to be repositioned for COVID-19 treatment. However, we consider that further research on a larger sample is needed and a more in-depth analysis of important biomarkers, such as cytokines and ACE/AngII/ATR1 axis-related molecules.
Sources of support in the form of grants
None.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to thank all the General Hospital of Zone 27 Mexican Institute of Social Security doctors, nurses, inhalotherapists, nutrition service, social workers, Clinical laboratory staff, cleaning staff, stretch -bearers, and all personnel who fight against COVID-19 with empathy and love, specially those who supported this research: Dr. Mayra Perez Perez, Dr. Gabriela Escamilla, Dr. Lorena Zaragoza, Dr. Rodrigo Collado, Dr. Guillermo Cordero, Dr. Alejandra Pedro, Dr. Liliana Bustos, Dr. Tonatzin Catalan, Dr. Carlos Rodriguez, Dr. Fabian Chavez, Dr. Bruno Lopez, Dr. Raúl Trinidad, Dr. Reina Montaño, Dr. Evelyn Garcia, Dr. Jessica Sosa. Pedro Alarcón, Dr. Carlos Garcia, Dra. Veronica Durán, Dra. Claudia Martínez, Dra. Lorena Ávila, Dr. Joel Ramírez, Dr. Juan Antonio Cartagena, Dr. Yamille Fatell, Dr. Juan José Palma, Dr. Roberto Rivelino López; Ms. Leticia Flores- Rojas, Ms. Melba Elena Mendoza - Ángeles.
References
- 1.Bogoch I.I., Watts A., Thomas-Bachli A., et al. Potential for global spread of a novel coronavirus from China. J. Travel Med. 2020;27(2) doi: 10.1093/jtm/taaa011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization (2020) Coronavirus disease 2019 (COVID-19) situation report–39. World Health Organization, Geneva. Available via https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200228-sitrep-39-covid-19.pdf?sfvrsn=5bbf3e7d_2. Accessed 3 Mar 2020.
- 3.World Health Organization (2020) Coronavirus disease 2019 (COVID-19) situation report–209. World Health Organization, Geneva. Available via https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200228-sitrep-39-covid-19.pdf?sfvrsn=5bbf3e7d_2. Accessed 21 agosto 2020.
- 4.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Liu L., Shan H., Lei C.L., Hui D.S.C., Du B., Li L.J., Zeng G., Yuen K.Y., Chen R.C., Tang C.L., Wang T., Chen P.Y., Xiang J., Li S.Y., Wang J.L., Liang Z.J., Peng Y.X., Wei L., Liu Y., Hu Y.H., Peng P., Wang J.M., Liu J.Y., Chen Z., Li G., Zheng Z.J., Qiu S.Q., Luo J., Ye C.J., Zhu S.Y., Zhong N.S., China Medical Treatment Expert Group for Covid-19 Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. Epub 2020 Feb 28. PMID: 32109013; PMCID: PMC7092819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tan L., Wang Q., Zhang D., Ding J., Huang Q., Tang Y.-Q., Miao H. Lymphopenia predicts disease severity of COVID-19: A descriptive and predictive study. Signal Transduction and Targeted. Therapy. 2020;5(33) doi: 10.1038/s41392-020-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frater J.L., Zini G., d'Onofrio G., Rogers H.J. COVID-19 and the clinical hematology laboratory. Int. J. Lab. Hematol. 2020;42(Suppl 1):11–18. doi: 10.1111/ijlh.13229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lippi G., Plebani M. The critical role of laboratory medicine during coronavirus disease 2019 (COVID-19) and other viral outbreaks. Clin. Chem. Lab. Med. 2020 Jun 25;58(7):1063–1069. doi: 10.1515/cclm-2020-0240. PMID: 32191623. [DOI] [PubMed] [Google Scholar]
- 9.Lippi G., Plebani M. Laboratory abnormalities in patients with COVID-2019 infection. Clin. Chem. Lab. Med. 2020 doi: 10.1515/cclm-2020-0198. [DOI] [PubMed] [Google Scholar]
- 10.Fan B.E., Chong V.C.L., Chan S.S.W., et al. Hematologic parameters in patients with COVID-19 infection. Am. J. Hematol. 2020 doi: 10.1002/ajh.25774. [DOI] [PubMed] [Google Scholar]
- 11.Tisoncik J.R., Korth M.J., Simmons C.P., Farrar J., Martin T.R., Katze M.G. Into the eye of the cytokine storm. Microbiol. Mol. Biol. Rev. 2012 Mar;76(1):16–32. doi: 10.1128/MMBR.05015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen T., Wu D., Chen H., Yan W., Yang D., Chen G., Ma K., Xu D., Yu H., Wang H. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368 doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lan J., Ge J., Yu J., Shan S., Zhou H., Fan S., Zhang Q., Shi X., Wang Q., Zhang L. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020:1–9. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- 14.Xiong Y., Sun D., Liu Y., Fan Y., Zhao L., Li X., Zhu W. Clinical and High-Resolution CT Features of the COVID-19 Infection: Comparison of the Initial and Follow-up Changes. Invest. Radiol. 2020 Jun;55(6):332–339. doi: 10.1097/RLI.0000000000000674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrari D., Motta A., Strollo M., Banfi G., Locatelli M. Routine blood tests as a potential diagnostic tool for COVID-19. Clin. Chem. Lab. Med. 2020 Jun 25;58(7):1095–1099. doi: 10.1515/cclm-2020-0398. PMID: 32301746. [DOI] [PubMed] [Google Scholar]
- 16.Jurisic V., Radenkovic S., Konjevic G. The Actual Role of LDH as Tumor Marker, Biochemical and Clinical Aspects. Adv. Exp. Med. Biol. 2015;867:115–124. doi: 10.1007/978-94-017-7215-0_8. PMID: 26530363. [DOI] [PubMed] [Google Scholar]
- 17.Han Y., Zhang H., Mu S., Wei W., Jin C., Tong C., Song Z., Zha Y., Xue Y., Gu G. Lactate dehydrogenase, an independent risk factor of severe COVID-19 patients: a retrospective and observational study. Aging (Albany NY) 2020;12(12):11245–11258. doi: 10.18632/aging.103372. Epub 2020 Jun 24. PMID: 32633729; PMCID: PMC7343511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mo P., Xing Y., Xiao Y., Deng L., Zhao Q., Wang H., Xiong Y., Cheng Z., Gao S., Liang K., Luo M., Chen T., Song S., Ma Z., Chen X., Zheng R., Cao Q., Wang F., Zhang Y. Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa270. ciaa270. Epub ahead of print. PMID: 32173725; PMCID: PMC7184444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., Zhao Y., Li Y., Wang X., Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020 Mar 17;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caricchio R., Gallucci M., Dass C., Zhang X., Gallucci S., Fleece D., Bromberg M., Criner G.J. Temple University COVID-19 Research Group. Preliminary predictive criteria for COVID-19 cytokine storm. Ann. Rheum. Dis. 2020 doi: 10.1136/annrheumdis-2020-218323. annrheumdis-2020-218323. Epub ahead of print. PMID: 32978237. [DOI] [PubMed] [Google Scholar]
- 21.Fathi N., Rezaei N. Lymphopenia in COVID-19: Therapeutic opportunities. Cell Biol. Int. 2020;44(9):1792–1797. doi: 10.1002/cbin.11403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 2020 doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020 doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lugnier C. Cyclic nucleotide phosphodiesterase (PDE) superfamily: a new target for the development of specific therapeutic agents. Pharmacol. Ther. 2006;109:366–398. doi: 10.1016/j.pharmthera.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 25.Cheng J., Grande J.P. Cyclic nucleotide phosphodiesterase (PDE) inhibitors: novel therapeutic agents for progressive renal disease. Exp. Biol. Med. 2007;232:38–51. [PubMed] [Google Scholar]
- 26.Ward A., Pentoxifylline C.SP. A review of its pharmacodynamic and pharmacokinetic properties, and its therapeutic efficacy. Drugs. 1987 Jul;34(1):50–97. doi: 10.2165/00003495-198734010-00003. PMID: 3308412. [DOI] [PubMed] [Google Scholar]
- 27.Maldonado V., Loza-Mejía M.A., Chávez-Alderete J. Repositioning of pentoxifylline as an immunomodulator and regulator of the renin-angiotensin system in the treatment of COVID-19 [published online ahead of print, 2020 Jun 9] Med Hypotheses. 2020;144:109988. doi: 10.1016/j.mehy.2020.109988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harris E., Schulzke S.M., Patole S.K. Pentoxifylline in preterm neonates: a systematic review. Paediatric Drugs. 2010;12:301–311. doi: 10.2165/11532600-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 29.Vilcek J., Lee T.H. Tumor necrosis factor. New insights into the molecular mechanisms of its multiple actions. J. Biol. Chem. 1991;266:7313–7316. [PubMed] [Google Scholar]
- 30.Lauterbach R., Zembala M. Pentoxifylline reduces plasma tumour necrosis factor-alpha concentration in premature infants with sepsis. Eur. J. Pediatr. 1996;155:404–409. doi: 10.1007/BF01955273. [DOI] [PubMed] [Google Scholar]
- 31.Marques L.J., Zheng L., Poulakis N., Guzman J., Costabel U. Pentoxifylline inhibits TNF-alpha production from human alveolar macrophages. Am. J. Respir. Crit. Care Med. 1999;159:508–511. doi: 10.1164/ajrccm.159.2.9804085. [DOI] [PubMed] [Google Scholar]
- 32.Tong Z., Dai H., Chen B., Abdoh Z., Guzman J., Costabel U. Inhibition of cytokine release from alveolar macrophages in pulmonary sarcoidosis by pentoxifylline: comparison with dexamethasone. Chest. 2003;124:1526–1532. doi: 10.1378/chest.124.4.1526. [DOI] [PubMed] [Google Scholar]
- 33.Crommelin H.A., Vorselaars A.D., van Moorsel C.H., Korenromp I.H., Deneer V.H., Grutters J.C. Anti-TNF therapeutics for the treatment of sarcoidosis. Immunotherapy. 2014;6:1127–1143. doi: 10.2217/imt.14.65. [DOI] [PubMed] [Google Scholar]
- 34.Zabel P., Entzian P., Dalhoff K., Schlaak M. Pentoxifylline in treatment of sarcoidosis. Am. J. Respir. Crit. Care Med. 1997;155:1665–1669. doi: 10.1164/ajrccm.155.5.9154873. [DOI] [PubMed] [Google Scholar]
- 35.Park M.K., Fontana J., Babaali H., et al. Steroid-sparing effects of pentoxifylline in pulmonary sarcoidosis. Sarcoidosis. Vasc. Diffuse Lung Dis. 2009;26:121–131. [PMC free article] [PubMed] [Google Scholar]
- 36.Valente E.G., Vernet D., Ferrini M.G., et al. L-arginine and phosphodiesterase (PDE) inhibitors counteract fibrosis in the Peyronie's fibrotic plaque and related fibroblast cultures. Nitric Oxide. 2003;9:229–244. doi: 10.1016/j.niox.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 37.Raetsch C., Jia J.D., Boigk G., et al. Pentoxifylline downregulates profibrogenic cytokines and procollagen I expression in rat secondary biliary fibrosis. Gut. 2002;50:241–247. doi: 10.1136/gut.50.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin S.L., Chen R.H., Chen Y.M., et al. Pentoxifylline attenuates tubulointerstitial fibrosis by blocking Smad3/4-activated transcription and profibrogenic effects of connective tissue growth factor. J. Am. Soc. Nephrol. 2005;16:2702–2713. doi: 10.1681/ASN.2005040435. [DOI] [PubMed] [Google Scholar]
- 39.Berman B., Duncan M.R. Pentoxifylline inhibits normal human dermal fibroblast in vitro proliferation, collagen, glycosaminoglycan, and fibronectin production, and increases collagenase activity. J. Invest. Dermatol. 1989;92:605–610. doi: 10.1111/1523-1747.ep12712140. [DOI] [PubMed] [Google Scholar]
- 40.Duncan M.R., Hasan A., Berman B. Pentoxifylline, pentifylline, and interferons decrease type I and III procollagen mRNA levels in dermal fibroblasts: evidence for mediation by nuclear factor 1 down-regulation. J. Invest. Dermatol. 1995;104:282–286. doi: 10.1111/1523-1747.ep12612819. [DOI] [PubMed] [Google Scholar]
- 41.Muldowney J.A.S., III, Chen Q., Blakemore D.L., Vaughan D.E. Pentoxifylline lowers plasminogen activator inhibitor 1 levels in obese individuals: a pilot study. Angiology. 2012;63:429–434. doi: 10.1177/0003319712436755. [DOI] [PubMed] [Google Scholar]
- 42.Lee J.G., Shim S., Kim M.J., et al. Pentoxifylline regulates plasminogen activator inhibitor- 1 expression and protein kinase A phosphorylation in radiation-induced lung fibrosis. Biomed. Res. Int. 2017;2017:1279280. doi: 10.1155/2017/1279280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Loskutoff D.J., Quigley J.P. PAI-1, fibrosis, and the elusive provisional fibrin matrix. J. Clin. Invest. 2000;106:1441–1443. doi: 10.1172/JCI11765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hanania A.N., Mainwaring W., Ghebre Y.T., Hanania N.A., Ludwig M. Radiation-inducedlung injury: assessment and management. Chest. 2019;156:150–162. doi: 10.1016/j.chest.2019.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ozturk B., Egehan I., Atavci S., Kitapci M. Pentoxifylline in prevention of radiation induced lung toxicity in patients with breast and lung cancer: a double-blind randomized trial. Int. J. Radiat. Oncol. Biol. Phys. 2004;58:213–219. doi: 10.1016/s0360-3016(03)01444-5. [DOI] [PubMed] [Google Scholar]
- 46.Delanian S., Porcher R., Balla-Mekias S., Lefaix J.L. Randomized, placebo-controlled trial of combined pentoxifylline and tocopherol for regression of superficial radiation- induced fibrosis. J. Clin. Oncol. 2003;21:2545–2550. doi: 10.1200/JCO.2003.06.064. [DOI] [PubMed] [Google Scholar]
- 47.Frigolet M.E., Torres N., Tovar A.R. The renin-angiotensin system in adipose tissue and its metabolic consequences during obesity. J. Nutr. Biochem. 2013;24:2003–2015. doi: 10.1016/j.jnutbio.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 48.Brie D., Sahebkar A., Penson P.E., et al. Effects of pentoxifylline on inflammatory markers and blood pressure: a systematic review and meta-analysis of randomized controlled trials. J. Hypertens. 2016;34:2318–2329. doi: 10.1097/HJH.0000000000001086. [DOI] [PubMed] [Google Scholar]
- 49.Hendry B.M., Stafford N., Arnold A.D., Sangwaiya A., Manglam V., Rosen S.D., Arnold J. Hypothesis: Pentoxifylline is a potential cytokine modulator therapeutic in COVID-19 patients. Pharmacol. Res. Perspect. 2020 Aug;8(4) doi: 10.1002/prp2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lancaster G.A., Dodd S., Williamson P.R. Design and analysis of pilot studies: recommendations for good practice. J. Eval. Clin. Pract. 2004 May;10(2):307–312. doi: 10.1111/j.2002.384.doc.x. PMID: 15189396. [DOI] [PubMed] [Google Scholar]
- 51.Chen G., Wu D., Guo W., et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Invest. 2020;130(5):2620–2629. doi: 10.1172/JCI137244. PMID: 32217835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qin C., Zhou L., Hu Z., et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. 2020;71(15):762–768. doi: 10.1093/cid/ciaa248. PMID: 32161940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Diao B., Wang C., Tan Y., et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19) Front. Immunol. 2020;11:827. doi: 10.3389/fimmu.2020.00827. eCollection 2020. PMID: 32425950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jamaluddin M., Meng T., Sun J., Boldogh I., Han Y., Brasier A.R. Angiotensin II induces nuclear factor (NF)-κB1 isoforms to bind the angiotensinogen gene acute phase response element: a stimulus-specific pathway for NF-κB activation. Mol. Endocrinol. 2000;14(1):99–113. doi: 10.1210/mend.14.1.0400. [DOI] [PubMed] [Google Scholar]
- 55.Ruiz-Ortega M., Lorenzo O., Suzuki Y., Rupérez M., Egido J. Proinflammatory actions of angiotensins. Curr. Opin. Nephrol. Hypertens. 2001;10(3):321–329. doi: 10.1097/00041552-200105000-00005. [DOI] [PubMed] [Google Scholar]
- 56.Skurk T., van Harmelen V., Hauner H. Angiotensin II stimulates the release of interleukin-6 and interleukin-8 from cultured human adipocytes by activation of NF-κB. Arterioscler. Thromb. Vasc. Biol. 2004;24(7):1199–1203. doi: 10.1161/01.ATV.0000131266.38312.2e. [DOI] [PubMed] [Google Scholar]
- 57.Hirano T., Murakami M. COVID-19: A New Virus, but a Familiar Receptor and Cytokine Release Syndrome. Immunity. 2020 May 19;52(5):731–733. doi: 10.1016/j.immuni.2020.04.003. Epub 2020 Apr 22. PMID: 32325025; PMCID: PMC7175868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Murakami M., Kamimura D., Hirano T. Pleiotropy and specificity: insights from the interleukin 6 family of cytokines. Immunity. 2019;50(4):812–831. doi: 10.1016/j.immuni.2019.03.027. [DOI] [PubMed] [Google Scholar]
- 59.Vaduganathan M., Vardeny O., Michel T., McMurray J.J.V., Pfeffer M.A., Solomon S.D. Renin-Angiotensin-Aldosterone System Inhibitors in Patients with Covid-19. N. Engl. J. Med. 2020 Apr 23;382(17):1653–1659. doi: 10.1056/NEJMsr2005760. Epub 2020 Mar 30. PMID: 32227760; PMCID: PMC7121452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meng G., Xiao J., Zhang X., He M., Ou J., Bi R., Yang W., Di Z., Wang Z. Li, Renin-angiotensin system inhibitors improve the clinical outcomes of COVID-19 patients with hypertension. Emerg. Microbes Infect. 2020;9(1):757–760. doi: 10.1080/22221751.2020.1746200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bengsch B., Martin B., Thimme R. Restoration of HBV-specific CD8+ T cell function by PD-1 blockade in inactive carrier patients is linked to T cell differentiation. J. Hepatol. 2014;61(6):1212–1219. doi: 10.1016/j.jhep.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 62.Thevarajan I., Nguyen T.H., Koutsakos M., Druce J., Caly L., van de Sandt C.E., Kedzierska K. Breadth of concomitant immune responses prior to patient recovery: A case report of non-severe COVID-19. Nat. Med. 2020;26(4):453–455. doi: 10.1038/s41591-020-0819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zheng H.-Y., Zhang M., Yang C.-X., Zhang N., Wang X.-C., Yang X.-P., Zheng Y.-T. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID-19 patients. Cell. Mol. Immunol. 2020;17(5):541–543. doi: 10.1038/s41423-020-0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Diao B., Wang C., Tan Y., Chen X., Liu Y., Ning L., Chen Y. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019. Front. Immunol. 2020;11:827. doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liao Y.C., et al. IL-19 induces production of IL-6 and TNF-alpha and results in cell apoptosis through TNF-alpha. J. Immunol. 2002;169:4288–4297. doi: 10.4049/jimmunol.169.8.4288. [DOI] [PubMed] [Google Scholar]
- 66.Schietinger A., Greenberg P.D. Tolerance and exhaustion: defining mechanisms of T cell dysfunction. Trends Immunol. 2014;35(2):51–60. doi: 10.1016/j.it.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu H., et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int. J. Oral. Sci. 2020;12:8. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fischer K., et al. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood. 2007;109:3812–3819. doi: 10.1182/blood-2006-07-035972. [DOI] [PubMed] [Google Scholar]
- 69.Kang Y.M., Wang Y., Yang L.M., Elks C., Cardinale J., Yu X.J., Zhao X.F., Zhang J., Zhang L.H., Yang Z.M., Francis J. TNF-α in hypothalamic paraventricular nucleus contributes to sympathoexcitation in heart failure by modulating AT1 receptor and neurotransmitters. Tohoku J. Exp. Med. 2010 Dec;222(4):251–263. doi: 10.1620/tjem.222.251. PMID: 21135513. [DOI] [PubMed] [Google Scholar]
- 70.Azhar A., El-Bassossy H.M. Pentoxifylline alleviates hypertension in metabolic syndrome: effect on low-grade inflammation and angiotensin system. J. Endocrinol. Invest. 2015;38:437–445. doi: 10.1007/s40618-014-0209-z. [DOI] [PubMed] [Google Scholar]
- 71.Pierrakos C., Karanikolas M., Scolletta S., Karamouzos V., Velissaris D. Acute respiratory distress syndrome: Pathophysiology and therapeutic options. J. Clin. Med. Res. 2012;4:7–16. doi: 10.4021/jocmr761w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Adamzik M., Broll J., Steinmann J., et al. An increased alveolar CD4+CD25+Foxp3+T regulatory cell ratio in acute respiratory distress syndrome is associated with increased 30-day mortality. Intensive Care Med. 2013;39:1743–1751. doi: 10.1007/s00134-013-3036-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.D’Alessio F.R., Tsushima K., Aggarwal N.R., et al. CD4+CD25+Foxp3+ Tregs resolve experimental lung injury in mice and are present in humans with acute lung injury. J. Clin. Invest. 2009;119:2898–2913. doi: 10.1172/JCI36498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yu Z.X., Ji M.S., Yan J., Cai Y., Liu J., Yang H.F., Li Y., Jin Z.C., Zheng J.X. The ratio of Th17/Treg cells as a risk indicator in early acute respiratory distress syndrome. Crit. Care. 2015 Mar 11;19(1):82. doi: 10.1186/s13054-015-0811-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li Q., Hu X., Sun R., Tu Y., Gong F., Ni Y. Resolution acute respiratory distress syndrome through reversing the imbalance of Treg/Th17 by targeting the cAMP signaling pathway. Mol. Med. Rep. 2016;14(1):343–348. doi: 10.3892/mmr.2016.5222. [DOI] [PubMed] [Google Scholar]
- 76.Ackermann M., Verleden S.E., Kuehnel M., et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N. Engl. J. Med. 2020;383(2):120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.J.A. Lott, E. Nemensanszky, Lactate dehydrogenase, In: J.A. Lott, P.L. Wolf (Eds.), Clinical Enzymology, a case-orianted Aprroach. 1987. pp. 213–244. [Google Scholar].
- 78.Moss D.W., Henderson A.R. In: Tietz Textbook of Clinical Chemistry. 2nd edition. Burtis C.A., Ashwood E.R., editors. W. B. Saunders Company; Philadelphia: 1986. Enzymes; pp. 735–896. [Google Scholar]
- 79.Glick J.H. Serum lactate dehydrogenase isoenzyme and total lactate dehydrogenase values in health and disease, and clincal evaluation of these test by means of discriminant analysis. Am. J. Clin. Pathol. 1969;52:320–328. doi: 10.1093/ajcp/52.3.320. [DOI] [PubMed] [Google Scholar]
- 80.Drent M., Cobben N.A., Henderson R.F., Wouters E.F., van Dieijen-Visser M. Usefulness of lactate dehydrogenase and its isoenzymes as indicators of lung damage or inflammation. Eur. Respir. J. 1996;9(8):1736–1742. doi: 10.1183/09031936.96.09081736. [DOI] [PubMed] [Google Scholar]
- 81.Smith R.L., Ripps C.S., Lewis M.L. Elevated lactate dehydrogenase values in patients with Pneumocystis carinii pneumonia. Chest. 1988;93:987–992. doi: 10.1378/chest.93.5.987. [DOI] [PubMed] [Google Scholar]
- 82.Fernandez P., Torres A., Miro J.M., et al. Prognostic factors influencing the outcome in Pneumocystis carinii pneumonia in patients with AIDS. Thorax. 1995;50:668–671. doi: 10.1136/thx.50.6.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Quist J., Hill A.R. Serum lactate dehydrogenase (LDH) in Pneumocystis carinii pneumonia, tuberculosis and bacterial pneumonia. Chest. 1995;108:415–418. doi: 10.1378/chest.108.2.415. [DOI] [PubMed] [Google Scholar]
- 84.Hoffman R.M., Rogers R.M. Serum and lavage lactate dehydrogenase isoenzymes in pulmonary alveolar proteinosis. Am. Rev. Respir. Dis. 1991;143:42–46. doi: 10.1164/ajrccm/143.1.42. [DOI] [PubMed] [Google Scholar]
- 85.Krugten van M., Cobben N.A.M., Lamers R.J.S., et al. Serum LDH: a marker of disease activity and its response to therapy in idiopathic pulmonary fibrosis. Neth. J. Med. 1996;48:220–223. doi: 10.1016/0300-2977(95)00074-7. [DOI] [PubMed] [Google Scholar]
- 86.Lindy S., Kahanpää K., Karhunen P., Halme J., Uitto J. Lactate dehydrogenase isoenzymes during the development of experimental fibrosis. J. Lab. Clin. Med. 1970;76:756–760. [PubMed] [Google Scholar]
- 87.Matusiewicz S.P., Williamson I.J., Sime P.J., et al. Plasma lactate dehydrogenase: a marker of disease activity in cryptogenic fibrosing alveolitis and extrinsic allergic alveolitis? Eur. Respir. J. 1993;6:1282–1286. [PubMed] [Google Scholar]
- 88.DeRemee R.A. Serum lactic dehydrogenase activity and diffuse interstitial pneumonitis. JAMA. 1968 Jun 24;204(13):1193–1195. PMID: 5694700. [PubMed] [Google Scholar]
- 89.Clinical findings of 35 cases with novel coronavirus pneumonia outside of Wuhan, [cited 2020 Apr 29]; Available from, 2020 Apr 17. https://www.researchsquare.com/article/rs-22554/v1.
- 90.Kermali M., Khalsa R.K., Pillai K., Ismail Z., Harky A. The role of biomarkers in diagnosis of COVID-19 - A systematic review. Life Sci. 2020 Aug;1(254) doi: 10.1016/j.lfs.2020.117788. Epub 2020 May 13. PMID: 32475810; PMCID: PMC7219356. [DOI] [PMC free article] [PubMed] [Google Scholar]