Abstract
Objective
Limited information is available regarding barriers to breastfeeding during the COVID-19 lockdown.
Study design
This study was designed as a non-concurrent case-control study on breastfeeding initiation practices, defined according to WHO, in women giving birth during lockdown, between March 8 and May 18, 2020, in the COVID-19 ‘hotspot’ in Northeastern Italy (study group), with an antecedent puerperae-matched group (control group). Exclusive, complementary, and formula feeding practices were collected from maternal charts at hospital discharge, on the second day post-partum, when puerperae filled out the Edinburg Postnatal Depression Scale (EPDS).
Results
The COVID-19 study group presented significantly lower exclusive breastfeeding rates than the control group who members gave birth the previous year (−15%, p = 0.003), as a consequence of the significantly higher prevalence of complementary feeding practices in the former (+20%, p = 0.002). Conversely, the COVID-19 study group showed significantly higher EPDS scores (8.03 ± 4.88 vs. 8.03 ± 4.88, p < 0.005) and higher anhedonia (0.56 ± 0.65 vs. 0.18 ± 0.38, p < 0.001) and depression (0.62 ± 0.60 vs. 0.39 ± 0.44, <0.001) subscale scores. In the general linear model analysis, women practicing exclusive breastfeeding showed significantly lower EPDS scores in comparison with those practicing complementary (p = 0.003) and formula feedings (p = 0.001). Furthermore, the highest EPDS scores were observed in women adopting formula feeding, mainly during the COVID-19 quarantine (p = 0.019).
Conclusion
This study indicates that hospital containment measures adopted during lockdown in the ‘hotspot’ COVID-19 epidemic area of Northeastern Italy have a detrimental effect on maternal emotions and on breastfeeding exclusivity practices.
Keywords: Breastfeeding initiation, COVID-19, Lockdown, Psychoemotional distress
Highlights
-
•
Of the various barriers to breastfeeding, limited information is available on the COVID-19 lock down.
-
•
Covid-19 lock down was associated with significantly lower exclusive breastfeeding practices.
-
•
COVID-19 lock down had detrimental effect on maternal emotions and on breastfeeding exclusivity practices.
1. Introduction
Optimal breastfeeding initiation and exclusivity contribute to significant short- and long-term health benefits for both mother and baby [1,2]. Current professional associations, including the WHO, recommend starting breastfeeding, ideally, within 1 h after delivery and recommend that it last for at least 6 months [3,4]. While breastfeeding is a protective factor against physical illness during childhood and later life and may reduce negative mood in mothers [5], a growing body of research indicates that maternal stress in gestation, known to be associated with other adverse perinatal outcomes (i.e. infants small for their gestational age, preterm birth, and maternal depression…), is also a risk factor for impaired lactogenesis [6]. In fact, several studies have documented maternal psychoemotional vulnerability and impaired lactation in natural and man-made disasters [7,8]. After the SARS outbreak in 2003, both healthcare workers and people who were self-quarantined exhibited symptoms of post-traumatic stress disorder [9]. Of note, the findings of Crew C.'s recent systematic review identified complex relationships between maternal stress and infant feeding practices in the context of natural calamities [8]. Therefore, the effect of COVID-19-related calamity-induced stress on breastfeeding women cannot be ignored.
In February 2020, Northern Italy became the epicentre for COVID-19 in Europe, and widespread community transmission occurred, with many exportations to other countries [10]. The COVID-19 ‘hotspot’ in Northeastern Italy was Vò, a municipality in the Euganean Hills, about 40 km from Venice, where the death of the first European from COVID-19 was recorded on February 21st, 2020 [11]. As a public health response to limit viral transmission, on February 22nd, 2020 Italy imposed a lockdown with shutdown of public places in addition to physical distancing in ‘hotspot’ towns close to Venice and Milan. The lockdown was subsequently expanded nationwide and lasted until May 17th, 2020, when Italy's ‘phase two’ stage began in which Italians would learn to coexist with the virus. Since the beginning of the COVID-19 epidemic, the Italian Central Government implemented primary prevention actions (masks, gloves, and social distancing) and tailored several restrictive measures to contain the spread of the infection, including case isolation, contact tracing, and quarantine mitigation measures, modelled after China's successful strategy against COVID-19 [9]. In this context, hospitals changed policies and protocols around perinatal care, replacing office visits with remote checkups, sending them to an offsite laboratory for blood draws, cancelling birth center tours and other nonessential visits, and barring extra people from the laboring mom, the delivery room, and the postpartum units. In an effort to keep moms and babies safe, mothers-to-be were routinely tested by rapid antibody test and by nasopharyngeal swab on admission. Symptomatic women and those who tested positive for the virus were nursed in isolation, in separate facilities within the hospital. Skin-to-skin contact in the first hour after birth, breastfeeding, and rooming-in were encouraged in maternity units. Finally, the hospital stay was minimised, depending on circumstances.
The hospital where this study took place, the Policlinico Abano Terme is located in an industrialized area of Northeastern Italy, which borders the municipalities of the COVID-19 Vò ‘hotspot’. It supports about 1000 births per year [12]. Pregnant women managed in the maternity ward for delivery present low and late fertility, good socio-economic status, occupation, and with advanced educational levels [12]. Nevertheless, the public health measures adopted as an effort to keep moms and babies safe [2] would increase stress of isolation, anxiety over disease status, and apprehension in response to new maternal responsibilities, making lockdown in the hospital challenging for maternal mental health [13] and breastfeeding initiation [14].
In the present study, therefore, we explored psychoemotional distress, tested by the Edinburg Postnatal Depression Scale (EPDS) in early postpartum [15,16] and breastfeeding initiation practices, defined according to WHO [14], among quarantined women who gave birth in a COVID-19 ‘hotspot’ in Northeastern Italy.
2. Patients and methods
This study was designed as a non-concurrent case-control study on breastfeeding initiation and psychoemotional distress in the immediate postpartum period in women who gave birth at Policlinico Abano Terme during the COVID-19 quarantine (study group) and an antecedent group of matched postpartum women (control group), who gave birth during 2019.
Data collection was approved by the Institutional Review Board of Policlinico Abano Terme. All participants were given an information sheet and were only included in the study if they had signed the consent form.
Women aged over 18 years who could read and understand Italian, who had delivered a singleton, healthy neonate at term at Policlinico Abano Terme between February 22nd (start of quarantine in ‘hotspot’ towns close to Milan and Venice) and May 18th (quarantine measures eased, ‘phase two’), 2020, were consecutively asked to participate. A control group of women was also recruited, comprising women aged over 18 years (able to read and understand Italian) who lived in the same geographic area and had delivered at the hospital in the same time period as the study group but in the previous year (2019). This was possible because mothers had provided written permission for us to access their obstetric records, which included basic personal data, education, medical history, pre-discharge feeding modalities [4], and EPDS screening results [12].
In accordance with the hospital's standard practice [12], following an uneventful delivery, infants are placed on the mother's chest for about 15 min during which time the midwife assists with the first suckling episode. Infants are then dried, they receive umbilical care, and they are weighed before their first warm water bath. During the subsequent 2 days in our ward, the neonates room-in with their mothers, who are encouraged to feed them on demand (with no more than 3-hr interfeeding intervals). The infants received complementary or formula milk if breast milk intake was judged insufficient by the midwives. According to standard maternity routines, in the absence of obstetric or neonatal complications, length of hospital stay was scheduled for 48 h for both vaginal and cesarean delivery. Proactive telephone support provided by health professionals was offered after discharge for breastfeeding support and for the general well-being of mothers.
During the study period (February 22nd to May 18th), the EPDS was distributed prior to discharge to 163 women (study group) on the second day postpartum. During the corresponding period in 2019, the EPDS had been distributed to 154 women (control group). The EPDS [15] is a self-administered questionnaire composed of 10 items scored in a four-point Likert scale (0–3) designed to screen for postpartum depression symptoms. Postpartum depression represents the end of a continuum of severity of symptoms, and the present study used a cutoff point for depressive symptomatology risk of >12 [17]. Following Tuohy and McVey [ 16], we also extracted three EPDS subscales: anhedonia subscale (items 1 and 2), anxiety subscale (items 3–6), and depression subscale (items 7–10).
A total of 5 women were excluded from the study group: 1 whose length of hospital stay was prolonged, 1 who underwent general anesthesia, 1 under psychological treatment, and 2 whose infants had jaundice. Three women were excluded from the control group: 1 whose length of hospital stay was prolonged, and 2 whose infant had jaundice. Among eligible mothers, 7 subsequently declined to participate (3 in the study group and 4 in the control group). In addition, 3 women in the study group were excluded owing to incomplete data. Thus, data from 152 women in the study group and 147 in the control group were analyzed.
SPSS version 26 for MAC (IBM, Armonk, NY, USA) was used for statistical analysis. Data are expressed as mean ± SD or frequencies (percentage). Continuous variables were analyzed by independent sample t-test, while the Chi-squared test was used to analyze qualitative variables. In more detail, frequencies of the three feeding modalities – exclusive, complementary, and formula feeding – were compared in the study group women during COVID-19 lock down and in the non-concurrent control group women, using the Chi-squared test. Moreover, the odds ratio for alternative adopted feeding modalities other than the exclusive one was calculated in the study and control group women. A general linear model analysis with Bonferroni-corrected post-hoc comparisons was applied to test differences in global EPDS and subscales scores (anhedonia, anxiety, and depression) between the study group and the control group, and among the three feeding modalities practiced by the women. Finally, the association between the EPDS global score of >12 and the feeding modalities practiced by study group and control group women was analyzed using the Chi-squared test. p < 0.05 was set as statistical significance.
3. Results
The sociodemographic characteristics of 152 study group mothers, who gave birth during the quarantine, from March 8th to May 18th, in the COVID-19 ‘hotspot’ in Northeastern Italy and 147 control group mothers, who delivered in the previous year, together with breastfeeding practices and EPDS scores collected at discharge on the second day postpartum, are shown in Table 1 .
Table 1.
Sociodemographic characteristics, breastfeeding modality initiation, and EPDS scores collected at hospital discharge on the second day postpartum, of COVID-19 study group and non-concurrent control group mothers.
| Control group | Study group | p | |
|---|---|---|---|
| N = 299 | 147 (49.16) | 152 (50.84) | |
| Age, year | 33.18 ± 5.10 | 33.47 ± 4.93 | 0.618 |
| Gestational age, week | 39.73 ± 1.13 | 39.68 ± 1.19 | 0.713 |
| Neonatal birth weight, g | 3446.16 ± 395.69 | 3355.90 ± 419.25 | 0.058 |
| Nulliparae | 69 (46.94) | 72 (47.37) | 1.000 |
| Smokers | 10 (6.80) | 9 (5.92) | 1.000 |
| Pre-pregnancy obesity | 5 (3.40) | 4 (7.69) | 0.746 |
| Gestational obesity | 27 (18.37) | 22 (14.47) | 0.435 |
| Level of instruction: | |||
| Elementary | 17 (6.93) | 8 (5.26) | 0.060 |
| High | 76 (61.38) | 76 (50.00) | 0.817 |
| Degree | 54 (36.73) | 67 (44.08) | 0.238 |
| Civil status: | |||
| Single | 2 (1.36) | 1 (0.66) | 0.617 |
| Married | 80 (54.42) | 84 (55.26) | 0.907 |
| Cohabitating | 64 (43.54) | 67 (44.08) | 1.000 |
| Occupation: | |||
| Student | 0 (0.00) | 1 (0.66) | 1.000 |
| Housewife | 15 (10.20) | 21 (13.82) | 0.377 |
| Unemployed | 9 (6.12) | 11 (7.24) | 0.818 |
| Working | 123 (83.67) | 118 (77.63) | 0.192 |
| Cesarean delivery: | 19 (12.93) | 27 (17.76) | 0.265 |
| Elective | 12 (10.88) | 16 (10.53) | 0.553 |
| Emergency | 7 (7.48) | 11 (7.24) | 0.468 |
| Breastfeeding at discharge: | |||
| Formula | 4 (2.72) | 5 (3.29) | 1.000 |
| Complementary | 18 (12.24) | 40 (26.32) | 0.002 |
| Exclusive | 123 (86.39) | 107 (70.39) | 0.003 |
| EPDS, score | 6.58 ± 4.08 | 8.03 ± 4.88 | 0.005 |
| EPDS subscales: | |||
| Anhedonia | 0.18 ± 0.38 | 0.56 ± 0.65 | <0.001 |
| Anxiety | 1.15 ± 0.62 | 1.17 ± 0.65 | 0.821 |
| Depression | 0.39 ± 0.44 | 0.62 ± 0.60 | <0.001 |
| EPDS score >12 | 17 (11.56) | 35 (23.03) | < 0.001 |
There were no significant differences between the study group and control group women for all sociodemographic variables. However, the study group showed significantly lower exclusive breastfeeding initiation rates than the control group (107 (70.39%) vs, 123 (86.39%), p = 0.003), due to a significantly higher prevalence of complementary feeding practices in the former (18 (12.24%) vs, 40 (26.32%), p = 0.002).
At the same time, the study group showed significantly higher EPDS scores (6.58 ± 4.08vs, 8.03 ± 4.88, p < 0.005) and significantly higher anhedonia (0.18 ± 0.38 vs, 0.56 ± 0.65, p < 0.001) and depression (0.39 ± 0.44 vs, 0.62 ± 0.60, p < 0.001) subscale scores than the control group. Furthermore, in the study group, women with EPDS levels >12, indicating the threshold for a higher probability of depression, were more frequently represented than in the control group [12 (11.56%) vs. 35 (23.03%), respectively; p < 0.001].
The relationship between exclusive, complementary, and formula feeding modalities, defined according to WHO, and EPDS scores at hospital discharge on the second day postpartum, are shown in Table 2 .
Table 2.
EPDS and anhedonia, anxiety, and depression subscale scores in COVID-19 study group and control group women across the breastfeeding practices, defined according to WHO.
| N (%) or Mean ± SD |
Breastfeeding practices |
||||
|---|---|---|---|---|---|
| Exclusive | Complementary | Formula | p | ||
| EPDS: | |||||
| Total score | Study group | 7.53 ± 4.23 | 8.57 ± 5.61 | 14.40 ± 7.63 | 0.006† |
| Control group | 6.58 ± 4.17 | 5.72 ± 3.35 | 10.50 ± 2.38 | 0.107† | |
| >12 score | Study group | 19/107 (18%) | 13/40 (32%) | 3/5 (60%) | 0.023†† |
| Control group | 14/123 (11%) | 2/18 (11%) | 1/4 (25%) | 0.696†† | |
| EPDS subscales: | |||||
| Anhedonia score | Study group | 0.453 ± 0.596 | 0.713 ± 0.659 | 1.701 ± 0.671 | <0.001† |
| Control group | 0.184 ± 0.389 | 0.194 ± 0.303 | 0.250 ± 0.501 | 0.940† | |
| Anxiety score | Study group | 1.155 ± 0.637 | 1.181 ± 0.709 | 1.551 ± 0.755 | 0.427† |
| Control group | 1.151 ± 0.620 | 0.986 ± 0.531 | 2.001 ± 0.408 | 0.112† | |
| Depression score | Study group | 0.569 ± 0.554 | 0.693 ± 0.649 | 1.351 ± 0.993 | 0.014† |
| Control group | 0.398 ± 0.468 | 0.347 ± 0.333 | 0.500 ± 0.204 | 0.809† | |
Statistical significance at p < 0.05.
Statistical significance by the general linear model analysis.
Statistical significance by Chi-squared test.
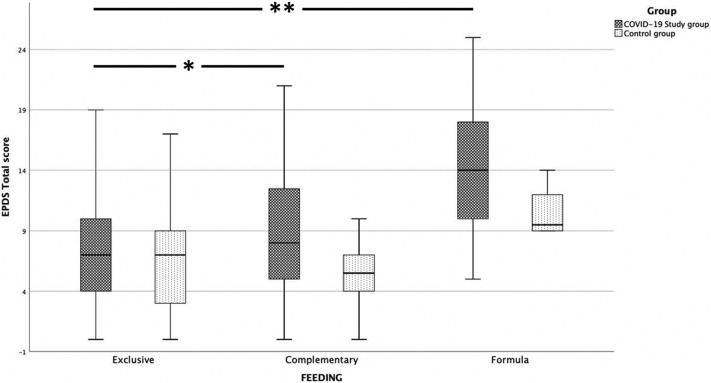
The general linear model analysis revealed significant differences in EPDS scores among the three feeding modalities practiced by women. In particular, women practicing exclusive breastfeeding showed a significantly lower EPDS score in comparison with those practicing complementary (p = 0.003) and formula feedings (p = 0.001). However, the highest EPDS scores were observed in women adopting formula, mainly during the COVID-19 quarantine (p = 0.019) (Fig. 1 ).
Fig. 1.
Clustered boxplot of EPDS total scores and feeding practices in COVID-19 study group nd control group women at hospital discharge.
Higher EPDS scores are present in women giving birth during the COVID-19 lock down compared to controls. In exclusive breastfeeding women, EPDS scores were significantly lower in comparison to those who practiced complementary (* p = 0.003) and formula feeding (** p = 0.001).
In addition, women with an EPDS score of >12 more frequently practiced complementary and formula feeding modalities. However, this association was statistically significant only in the study group (p = 0.023), not in the control group (p = 0.696). Of note, the risk of exclusive breastfeeding failure upon hospital discharge during the COVID-19 quarantine in women with an EPDS score of >12 increased from 1.252 to 1.844 when compared with non-concurrent control group women giving birth in 2019.
Finally, women who practiced exclusive breastfeeding in both the study and control groups presented significantly lower anhedonia and depression subscale scores than those practicing complementary (p = 0.017 and p = 0.046, respectively) and formula feeding practices (p = 0.001 and p = 0.001, respectively). Anxiety levels were instead comparable between study group and control group puerperae (p = 0.586). Nevertheless, women practicing formula feeding showed higher anxiety scores than those practicing complementary (p = 0.009) and exclusive (p = 0.014) breastfeeding.
4. Discussion
In the present study, women giving birth during the COVID-19 quarantine in the ‘hotspot’ area of Northeastern Italy, between February 22nd and May 18th, 2020, presented lower exclusive breastfeeding rates (−15%, p = 0.003) than the control-matched group who gave birth the previous year, due to a significantly higher prevalence of complementary feeding practices in the former (+20%, p = 0.002). Conversely, the COVID-19 study group showed significantly higher EPDS scores, higher anhedonia and depression subscale scores, and a higher prevalence of EPDS levels over the threshold (> 12) for an enhanced probability of postpartum depression. The general linear model analysis confirmed that women who practiced complementary and formula feedings had higher EPDS and anhedonia and depression subscales scores. However, the higher EPDS scores were present in women who practiced formula feeding during the COVID-19 lockdown.
These finding suggest that early maternal postpartal psychological responses to the COVID-19 pandemic may be mediated by postpartum EPDS symptoms, severe enough to predict a higher postpartal depression risk with depression occurring in up to one in four puerperae, and breastfeeding initiation failure occurring in up to 15% puerperae upon hospital discharge. This seems in accordance with previous studies that have documented maternal psychoemotional vulnerability in calamities, catastrophic events [4] and natural and man-made disasters (e.g., terrorist attacks, earthquakes, tsunamis, Chernobyl), all predictors of postpartum depressive symptoms in the general population [6,9] and associated with a wide range of adverse perinatal outcomes, including formula feeding practices and a shorter duration of any and exclusive breastfeeding. In the studies, the nadir of exclusive breastfeeding prevalence among infants below six months was 20.0% [8].
To our knowledge, no previous studies have, however, examined infant feeding practices of women giving birth in the context of the COVID-19 lockdown. Learning from China's successful battle against COVID-19, the Italian Central Government implemented primary preventive actions and tailored several restrictive, containing measures in the hospital to contain the spread of the infection. Such measures involved cancelling antenatal classes, birth centre tours, open days, and other nonessential visits, and included barring extra people (fathers, doulas, relatives, and visitors…) from the mothers in labor, the delivery room, and the postpartum units in an effort to keep moms and babies safe. Such measures however had potential stressful consequences and detrimental psychological effects on a vulnerable population [18], which possibly interfered with achieving the goal of breastfeeding [8,14].
Therefore, these data may have some clinical relevance. Pregnancy can be a stressful time for many expecting mothers [19], but now the COVID-19 crisis is adding a new layer of worry and stress about how the pandemic will impact the feeding practices of their baby [12,14,18]. While it seems reasonable that mothers experiencing stress would affect breastfeeding initiation and/or stop breastfeeding earlier, other findings are less intuitive. The relationship between stressful life events and risk of depression symptoms is likely complex and is perhaps bidirectional (i.e. peripartal depressive symptomatology might causally contribute to failed breastfeeding goal and/or breastfeeding might contribute to reduce postpartum depressive symptomatology) [5,20].
Although some literature suggests an indirect relationship between postpartum depression and breastfeeding outcomes, our findings reaffirm the unique impact that stress associated with the COVID-19 lockdown may have on breastfeeding initiation. A further interpretation of this significant relationship is supported in our study by the analysis of the three-dimensional structure of EPDS [16]. Specific to our findings, the higher anhedonia and depression subscale scores in COVID-19 study group women were significantly associated with complementary and formula feeding practices, also suggesting that EPDS three-factor structure analysis may represent an additional good tool to better understand the spectrum of negative psychological issues that the COVID-19 lockdown might give rise to among pregnant women.
Postpartum depression is the result of a dynamic interplay of biological, psychological, and social risk factors [19], all of which can be amplified by the current COVID-19 pandemic. As previous studies have addressed in the devastation caused by natural calamities [8], pregnant women giving birth during the COVID-19 pandemic represent a high risk vulnerable population that needs to be carefully followed to minimize postpartal mental dysfunction [21], upon which the establishment of successful breastfeeding initiation depends. Concerns about exposure to COVID-19, combined with physical distancing and containment recommendations, may adversely affect the thoughts, emotions, and functioning of new mothers, worsening depressive symptoms and decreasing the mother's ability to achieve her breastfeeding and mothering plan. Therefore, medical and mental care interventions should be carried out concurrently to prevent adverse maternal perinatal outcomes. For this reason, guidance for infant feeding in emergencies requires that emergency plans include provisions for breastfeeding counselling, as well as other components of breastfeeding support, like emotional support and reassurance, this being especially significant to the wellbeing of mothers who were isolated because of COVID-19 [22].
We recognize several limitations to this study. Firstly, being limited to postpartal breastfeeding practices in the hospital, this study may not have had sufficient statistical power to demonstrate a significant effect in the achievement of lasting WHO breastfeeding goals by mothers. Secondly, a non-concurrent case-control study such as this cannot guarantee that the observed relationships represent causal factors. Thirdly, given that the EPDS was used to screen the psychoemotional distress in puerperae giving birth during COVID-19 pandemic, we did not confirm the diagnosis of postpartum depression using the unique criteria defined in the medical literature [23]. However, this should not invalidate our results, because the general geographic, demographic, and clinical variables in the studied sample were similar among non-concurrent study groups. However, literature on this subject is sparse. Thus, the present study makes an important contribution to the understanding of the impact of the COVID-19 life-threatening pandemic upon women's postpartal well-being and their ability to successfully initiate exclusive breastfeeding.
In conclusion, the results of our study indicate that containment and distancing measures around labor and delivery adopted during lockdown in the COVID-19 ‘hotspot’ in Northeastern Italy had detrimental effects on maternal breastfeeding initiation exclusivity, due to a significantly higher prevalence of complementary feeding practices. Women who practiced complementary and formula feedings had higher EPDS and anhedonia and depression subscale scores, especially those who practiced formula feeding during the COVID-19 quarantine. Concerns about exposure to COVID-19 risk, combined with changed policies around prenatal care in hospitals, negatively affected the emotions and the functioning of women, impairing their ability to achieve breastfeeding exclusivity.
CRediT authorship contribution statement
Dr. Vincenzo Zanardo had primary responsibility for protocol development and writing the manuscript. Dr. Domenico Tortora performed the statistical analysis. Prof. Pietro Guerrini and Dr. Gianpaolo Garani participated in the design and coordination of the study. Dr. Lorenzo Severino helped to draft the manuscript. Dr. Gino Soldera and Dr. Gianluca Straface participated in the development of the protocol.
Declaration of competing interest
The authors disclose any conflict of interest.
Acknowledgments
Acknowledgements
The authors wish to acknowledge Andrea Sandri of UC San Diego School of Medicine for proofreading and editing the paper.
Formatting of funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Ip S., Chung M., Raman G., Chew P., Magula N., DeVine D. Evidence Report/Technology Assessment. Vol. 153. 2007. Breastfeeding and maternal and infant health outcomes in developed countries; pp. 1–186. [PMC free article] [PubMed] [Google Scholar]
- 2.Kramer M.S., Kakuma R. Optimal duration of exclusive breastfeeding. Cochrane Database Syst. Rev. 2002;1 doi: 10.1002/14651858.CD003517. [DOI] [PubMed] [Google Scholar]
- 3.DHHS U . Office of Disease Prevention and Health Promotion; 2010. Healthy People 2020. [Google Scholar]
- 4.AAP Section on Breastfeeding. Breastfeeding and the use of human milk. Pediatrics. 2012;129:e827–e841. doi: 10.1542/peds.2011-3552. [DOI] [PubMed] [Google Scholar]
- 5.Mezzacappa E.S., Katkin E.S. Breast-feeding is associated with reduced perceived stress and negative mood in mothers. Health Psychol. 2002;21:187–193. [PubMed] [Google Scholar]
- 6.Dozier A.M., Nelson A., Brownell E. The relationship between life stress and breastfeeding outcomes among low-income mothers. Adv. Prev. Med. 2012;2012:902487. doi: 10.1155/2012/902487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crew C. 2019. Infant and Young Child Feeding Practices In the Context of Natural Disasters: A Systematic Review. [DOI] [Google Scholar]
- 8.Maunder R. The experience of the 2003 SARS outbreak as a traumatic stress among frontline healthcare workers in Toronto: lessons learned. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2004;29(359):1117–1125. doi: 10.1098/rstb.2004.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2015;20(395):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sjödin H., Wilder-Smith A., Osman S., Farooq Z., Rocklöv I. Only strict quarantine measures can curb the coronavirus disease (COVID-19) outbreak in Italy, 2020. Euro Surveill. 2020;25:2000280. doi: 10.2807/1560-7917.ES.2020.25.13.2000280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zanardo V., Manghina V., Vettore M., Severino L., Straface G. Psychological impact of COVID-19 quarantine measures in northeastern Italy on mothers in the immediate postpartum period. Int. J. Gynaecol. Obstet. 2020 May 31 doi: 10.1002/ijgo.13249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brooks S.K., Webster R.K., Smith L.E., Woodland L., Wessely S. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. www.thelancet.com [DOI] [PMC free article] [PubMed]
- 13.Liu X., Chen M., Wang Y. 24 June 2020. Prenatal Anxiety and Obstetric Decisions Among Pregnant Women in Wuhan and Chongqing During the COVID-19 Outbreak: A Cross-sectional Study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cox J.L., Holden J.M., Sagovsky R. Detection of postnatal depression: development of the 10-item Edinburgh Postnatal Depression Scale. Br. J. Psychiatry. 1987;150:782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- 15.Tuohy A., CMcVey C. Subscales measuring symptoms of non-specific depression, anhedonia, and anxiety in the Edinburgh Postnatal Depression Scale. Br. J. Clin. Psychol. 2008;47:153–169. doi: 10.1111/j.2044-8260.2008.tb00463.x. [DOI] [PubMed] [Google Scholar]
- 16.Matthey S. Using the Edinburgh Postnatal Depression Scale to screen for anxiety disorders. Depress. Anxiety. 2008;25:926–931. doi: 10.1002/da.20415. [DOI] [PubMed] [Google Scholar]
- 17.Wu Y., Zhang C., Liu H., Duan C., Li C., Fan J. Perinatal depressive and anxiety symptoms of pregnant women along with COVID-19 outbreak in China. Am J Obstet Gynecol. 2020;10 doi: 10.1016/j.ajog.2020.05.009. (S0002-9378(20)30534-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parker V.J., Douglas A.J. Stress in early pregnancy: maternal neuro-endocrine-immune responses and effects. J. Reprod. Immunol. 2010;85:86–92. doi: 10.1016/j.jri.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 19.Zubaran C., Foresti K. The correlation between breastfeeding self-efficacy and maternal postpartum depression in southern Brazil. Sex. Reprod. Healthc. 2013;4:9–15. doi: 10.1016/j.srhc.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Jager E., Skouteris H., Mello K. Psychosocial correlates of exclusive breastfeeding: a systematic review. Midwifery. 2013;29:506–518. doi: 10.1016/j.midw.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 21.Harville E., Xiong X., Buekens P. Disasters and perinatal health: a systematic review. Obstet. Gynecol. Surv. 2010;65:713–728. doi: 10.1097/OGX.0b013e31820eddbe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fadden A., Siebelt L., Marshall J.L., Gavine A., Lisa-Christine Girard L., Symon A. Counselling interventions to enable women to initiate and continue breastfeeding: a systematic review and meta-analysis. Int Breastfeed J. 2019;21(14):42. doi: 10.1186/s13006-019-0235-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghaedrahmati M., Kazemi A., Kheirabadi G., Ebrahimi A., Bahrami M. Postpartum depression risk factors: a narrative review. J. Educ. Health Promot. 2017;6:60. doi: 10.4103/jehp.jehp_9_16. [DOI] [PMC free article] [PubMed] [Google Scholar]