In the ongoing Coronavirus disease 2019 (COVID-19) pandemic, there is a pressing need to identify clinical factors that are predictive of disease severity. The development of accurate algorithms and risk stratification models, incorporating both clinical and laboratory variables, may aid in optimizing allocation of limited resources. In general, chronic liver disease is associated with increased severity and mortality for pneumonias [1,2]. Moreover, as in the severe acute respiratory syndrome coronavirus (SARS-CoV) and in the Middle East respiratory syndrome coronavirus outbreaks, hepatic injury has been reported in cases of severe COVID-19 [3]. Due to the limited number of patients with chronic liver disease within individual studies on COVID-19 to-date, the impact of a history of hepatic pathology on COVID-19 progression and outcomes is unknown. Therefore, the aim of the present article was to analyze if co-morbid chronic liver disease in patients with laboratory-confirmed COVID-19 is associated with increased odds of the severe form of disease or mortality.
An electronic search of Medline (PubMed interface), Scopus, and Web of Science was performed, employing the keywords ‘chronic liver disease’ OR ‘cirrhosis’ OR ‘hepatitis’ AND ‘coronavirus 2019’ OR ‘COVID-19’ OR ‘2019-nCoV’ OR ‘SARS-CoV-2’ in all fields, between 2019 and present time (i.e. 18 March 2020). No language or date restrictions were applied. All documents were screened by title, abstract, and full text. Articles reporting data on the rate of chronic liver disease in adult (>18 years of age) COVID-19 with or without severe illness or mortality were finally included in a pooled analysis. A clinically validated definition of ‘severe disease’ (i.e. patients requiring mechanical ventilation, vital life support, ICU admission) was required. Liver pathology was defined as chronic liver disease, cirrhosis, steatosis, or chronic hepatitis. No exclusion criteria were applied. The reference list of all included articles was also hand-searched (through forward and backward citation tracking) to identify additional eligible studies. As the expected data set was limited and would include case series, no study risk of bias or publication bias evaluation was performed.
A pooled analysis was performed to estimate the odds ratio (OR) and 95% confidence interval (CI) of chronic liver disease in COVID-19 patients with or without severe disease and in non-survivors versus survivors. The statistical analysis was carried out using MetaXL, software Version 5.3 (EpiGear International Pty Ltd., Sunrise Beach, Australia), with inverse-variance model. The study was performed in compliance with the declaration of Helsinki and local legislation.
After removing duplicated or overlapping publication, a total number of 39 documents were initially identified. Among these, 34 were excluded because they were review articles (n = 10), did not report data on COVID-19 disease (n = 12), did not provide the rate of chronic liver disease (n = 7), or were editorial materials (n = 5). Two additional studies were identified from the reference list of included articles. A total of seven articles were selected. One further article by Guan et al. [4] was excluded as it only considered the rate of hepatitis B surface antigen positivity without assessment of chronicity. Thus, the final pooled analysis included six studies [5–10]. Four studies compared chronic liver disease in severe vs. non-severe cases, with a total sample of 702 confirmed COVID-19 patients, 371 of whom (52.8%) were classified as having severe disease [5–8]. Two studies with 202 patients compared the rate of chronic liver disease in COVID-19 patients who did not-survive vs. survived, with 100 (49.5%) classified as non-survivors [9,10].
The results of pooled analysis are presented in Fig. 1. Chronic liver disease was not found to be associated with increased odds of the severe form of COVID-19[(OR 0.96 (95% CI 0.36–2.52), I2 = 0%, Cochran’s Q, P = 0.86]. Moreover, chronic liver disease was neither significantly associated with increased odd of mortality in COVID-19 patients [OR 2.33 (95% CI 0.77–7.04), I2 = 30%, Cochran’s Q, P = 0.23].
Fig. 1.
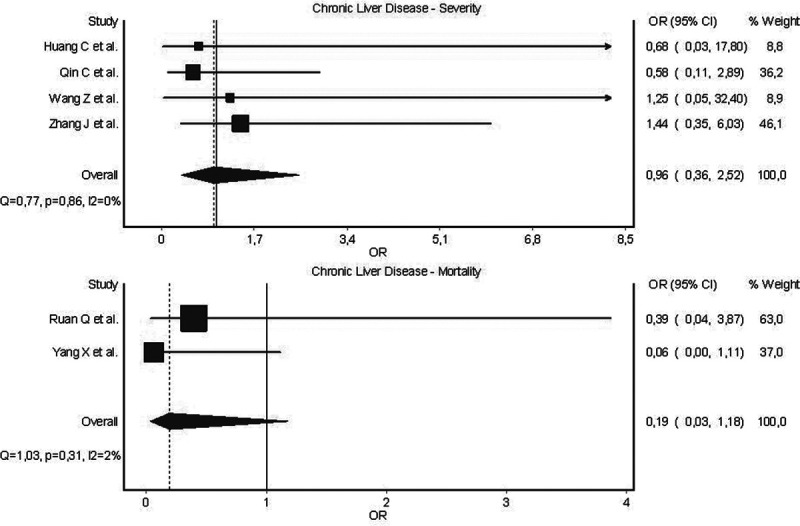
Forest plots of odds of severe disease and mortality in laboratory-confirmed COVID-19 patients with co-morbid chronic liver disease.
Based on pooled results of early COVID-19 data, chronic liver disease seems to play a minor role in influencing patient progression towards the severe form of disease. A non-significant trend towards increased odds of mortality in COVID-19 patients with chronic liver disease should continue to be evaluated in larger studies. Therefore, data to-date would not apparently support the inclusion of chronic liver disease into risk stratification models as clinical predictor of severe disease.
Acknowledgements
Conflicts of interest
There are no conflicts of interest.
References
- 1.Hung TH, Tseng CW, Hsieh YH, Tseng KC, Tsai CC, Tsai CC. High mortality of pneumonia in cirrhotic patients with ascites. BMC Gastroenterol. 2013; 13:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Pasquale M, Esperatti M, Crisafulli E, Ferrer M, Bassi GL, Rinaudo M, et al. Impact of chronic liver disease in intensive care unit acquired pneumonia: a prospective study. Intensive Care Med. 2013; 39:1776–1784 [DOI] [PubMed] [Google Scholar]
- 3.Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020; 395:497–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. 2020; pii:ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Z, Yang B, Li Q, Wen L, Zhang R. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis. 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, Yan YQ, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020 [DOI] [PubMed] [Google Scholar]
- 9.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]


