Abstract
Case series
Patients: Female, 70-year-old • Female, 88-year-old • Female, 63-year-old
Final Diagnosis: Pulmonary fibrosis
Symptoms: Cough • dyspnea • fever
Medication: Steroids
Clinical Procedure: Chest CT scan
Specialty: Immunology • Infectious Diseases • Pulmonology
Objective:
Unusual clinical course
Background:
Since December 2019, an outbreak caused by a novel coronavirus infection (severe acute respiratory syndrome coronavirus 2, SARS-CoV-2) occurred in Wuhan, China, and it rapidly spread all over the world. The clinical spectrum of coronavirus disease 2019 (COVID-19) is wide, with acute respiratory distress syndrome (ARDS) occurring in 15% of patients affected, requiring high oxygen support. Currently, there is no clearly effective antiviral therapy. Steroids and immunomodulators are under investigation for potential activity. Little is known about middle and long-term sequelae on respiratory function. According to some authors, COVID-19 could cause pulmonary fibrosis. We report 3 cases of pulmonary fibrosis detected on follow-up computed tomography (CT) imaging in 3 female patients who recovered from COVID-19 pneumonia in Italy (L’Aquila, Abruzzo).
Case Reports:
All patients were female and had no significant previous respiratory disease or history of smoke exposure, and none had received high-flow oxygen support during treatment of the disease. In all cases, late onset of mild dyspnea, slow and incomplete respiratory recovery, and early evidence of fibrous signs on chest CT scan were characteristic of the clinical course.
Conclusions:
This report focuses on a possible scenario of long-term lung damage in COVID-19 pneumonia survivors. Limitations are lack of long-term follow-up and functional data in the very early phase. It is advantageous that all COVID-19 pneumonia patients undergo serial chest CT and spirometry long-term follow-up for at least 1 year to assess residual damage. This is particularly relevant in those with slow respiratory recovery and long hospitalization.
MeSH Keywords: COVID-19, Immunomodulation, Pulmonary Fibrosis
Background
Since December 2019, a disease outbreak caused by a novel coronavirus infection (severe acute respiratory syndrome coronavirus 2, SARS-CoV-2) occurred in Wuhan, China, and it rapidly spread in many countries all over the world. Italy was the first country in Europe where the epidemic spread. As of May 26, 2020, in Italy there were 230 158 confirmed cases of SARSCoV2 infection with 32 877 related deaths and a case-fatality rate of about 14% [1]. The clinical spectrum of coronavirus disease 2019 (COVID-19) is wide and includes asymptomatic infection, mild upper-respiratory disease with fever and cough, and severe pneumonia that can lead to adult respiratory distress syndrome (ARDS). ARDS occurs in 15% of patients affected and is one of the main causes of death [2]. Many patients require low-, medium- or high-flow oxygen support. Currently, there is no validated antiviral therapy for this infection. Steroids and immunomodulators, including tocilizumab, are under investigation for potential activity. Pulmonary fibrosis (PF) is a descriptive term referring to an excess of fibrotic tissue in the lungs and is a well-known evolution of various lung diseases [3,4]. It occurs in a wide range of clinical settings and can be associated with many conditions such as ARDS, viral infections, inhaled dust, radiation, medications, gastro-esophageal reflux disease (GERD), and smoking. PF has been associated with several respiratory viral infections such as Herpesviruses EBV and CMV, adenovirus, transfusion-transmitted virus (TTV, also known as Torque Teno Virus), and hepatitis C virus (HCV) [5] influenza A (H1N1) [6]. High-flow oxygen support can also cause pulmonary fibrosis [7]. Idiopathic pulmonary fibrosis (IPF) is a fibrotic lung disease with progressive evolution. In patients with IPF, there is no known trigger for the onset of the disease, but viral infections are thought to be a co-factor [5,8]. Irrespective of the viral etiology, pulmonary fibrosis has been shown to develop after apparent recovery from the infection. According to some authors, SARS coronavirus (SARS-CoV) causes pulmonary fibrosis more frequently than other viruses [9]. We describe our experience managing 3 cases of pulmonary fibrosis that developed after SARS-CoV-2 infection in the Infectious Disease Unit of San Salvatore Hospital (L’Aquila, Abruzzo, Italy) in patients who had not received high-flow oxygen support.
Case Reports
All patients were Italian. They had no known respiratory comorbidities and reported no history of smoking, except for 1 patient who reported she had been a light smoker 40 years before. None had a history of exposure to toxic or known fibrogenic substances.
Patient 1
A 70-year-old woman developed dry cough, fever, and diarrhea on March 22. She reported no other symptoms or history of travel abroad, but she had exposure to a patient infected by SARS-CoV-2. She had been a light smoker 40 years before. A chest computer tomography (CT) scan showed bilateral inter-stitial pneumonia. SARS-CoV-2 was identified on nasopharyngeal swab. On illness day 15, she developed increasing dyspnea and cough, with low-flow oxygen required. She was treated with intravenous methylprednisolone, with significant clinical improvement, but during the following days low-level hypoxemia persisted. A CT scan at 1-month follow-up showed disease progression with increasing range of ground-glass density patches and consolidation and scant fibrous interstitial stripes. The patient was discharged 39 days after admission, eupneic in room air, with SpO2 91% and PaO2 61mmHg (P/F 290).
Clinical course of Patient 1
On hospital admission on March 27 (illness day 5), Patient 1 complained of a dry cough, fever, and diarrhea. She was awake and conversant, blood pressure 160/90 mmHg (normal value (n.v.) 120/80 mmHg), HR 94 beats per minute (n.v. 60–100 beats per minute), RR 19 breaths per minute (n.v. 12–16 breaths per minute), body temperature 38.2°C (n.v. 35.5–37.0°C armpit) and SpO2 95% (n.v. 95–100%) on room air. Physical examination was unremarkable. Her blood tests showed leucocytes 1.71×103/uL (n.v. 4.8–10.8×103/uL), hemoglobin 13.4 g/dl (n.v. 12.0–16.0 g/dl), platelet count of 241×103/uL (n.v. 130–400×103/uL), D-dimer 0.38 ng/mL (n.v. 0.0–0.5 mg/mL), C-reactive protein (CRP) 4.26 mg/dl (n.v. <0.05 mg/dl), procalcitonin 0.130 ng/mg (n.v. 0.10–0.49 ng/mL), and ferritin 209.3 ng/mL (n.v. in adult female 10 to 120 ng/mL). Arterial blood gas analysis on room air showed pH 7.44 (n.v. 7.35 to 7.45), PO2 65 mmHg (n.v. >79 mmHg), P/F ratio 310 (n.v. >300), PCO2 33 (n.v. 35–45 mmHg), and O2ST 94% (n.v. 95–100%). Table 1 summarizes the clinical laboratory results and vital signs of the patient. A chest computed tomography (CT) showed multiple patchy ground-glass opacities and consolidation shadow in the bilateral lung view, which was very highly suggestive of bilateral interstitial pneumonia (Figure 1). SARS-CoV-2 was identified on nasopharyngeal swab by RT-PCR RNA detection (TaqMan TM 2019-nCoV Assay Kit v1/v2, Thermo Fisher, qPCR) tested at Istituto Zooprofilattico Sperimentale dell’Abruzzo e del Molise G. Caporale (ISZAM). On March 29, she started oral therapy with azithromycin 500 mg once daily (OD) and antiviral therapy with lopinavir/ritonavir 200/50 mg twice a day, hydroxychloroquine 200 mg twice a day, and subcutaneous sodium enoxaparin 6000 UL daily. During illness days 9 and 10, fever continued, with occasional non-productive cough. She remained clinically stable with SpO2 ranging from 93 to 97% on 2 L/min of oxygen. On illness day 15, she developed increasing dyspnea, with SpO2 of 91% despite 4 L/min of oxygen with venturi mask (VM), and cough with mild hemoptysis, and physical examination showed bilateral chest crepitation. Intravenous methylprednisolone 250 mg was started followed by infusion of tocilizumab at a dose of 8 mg/kg; a second dose was administered after 12 h. The day after treatment, significant clinical improvement of dyspnea and respiratory parameters was observed and she was no longer febrile. Dry cough and diarrhea disappeared soon thereafter and the patient improved gradually. Methylprednisolone was tapered during the following days. Despite rapid initial improvement, during the following days low-level hypoxemia persisted and she required low-flow oxygen support, so steroidal therapy (prednisolone 25 mg orally) was reintroduced. On April 22, 1 month after the onset, a new CT scan was performed, showing disease progression with increasing range of ground-glass density patches and consolidation, and scant fibrous interstitial stripes also appeared (Figure 2). After obtaining 2 negative SARS-COV-2 nasopharyngeal swab tests, the patient was discharged from the hospital on May 4 (39 days after admission), eupneic on room air, with SpO2 91% and PaO2 61mmHg (P/F 290). Frequent clinical evaluation and serial 3-month follow-up spirometry, CT chest scan, blood tests, and arterial oxygen saturation on room air were recommended. Enoxaparin 6000 Ul was administered subcutaneously for another 14 days at home.
Table 1.
Clinical laboratory results and vital signs Patient 1.
| Hospital day | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Illness day | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Temp (°C) | 36 | 38.2 | 38.7 | 38.5 | 38 | 37.6 | 37.2 |
| BP (mmHg) | 160/60 | 125/70 | 125/75 | 110/70 | 140/70 | 110/70 | 110/70 |
| Pulse (/min) | 94 | 81 | 84 | 84 | 88 | 82 | 72 |
| O2 sat (%) | 95 | 92 | 92 | 92 | 93 | 89 | 94 |
| FiO2 (%) | 21 | 21 | 21 | 21 | 21 | 28 | 36 |
| Leukocytes (×103/µl) | 1.71 | 2.27 | 4.87 | ||||
| Neutrophils (%) | 56.7 | 67.4 | 86.8 | ||||
| Lymphocytes (%) | 29.8 | 23.8 | 10.1 | ||||
| Hemoglobin (g/dl) | 13.4 | 12.4 | 12.2 | ||||
| Platelets (×103/µl) | 241 | 247 | 272 | ||||
| NPS/OPS PCR | 25/03 + | 07/04 + | 15/04 – | 18/04 + | 20/04 + | 25/04 + |
Temp – body temperature; BP – blood pressure; Pulse – heart rate; O2 sat – oxygen saturation; FiO2 – inspiratory oxygen fraction; NPS/OPS PCR – nasopharyngeal swab.
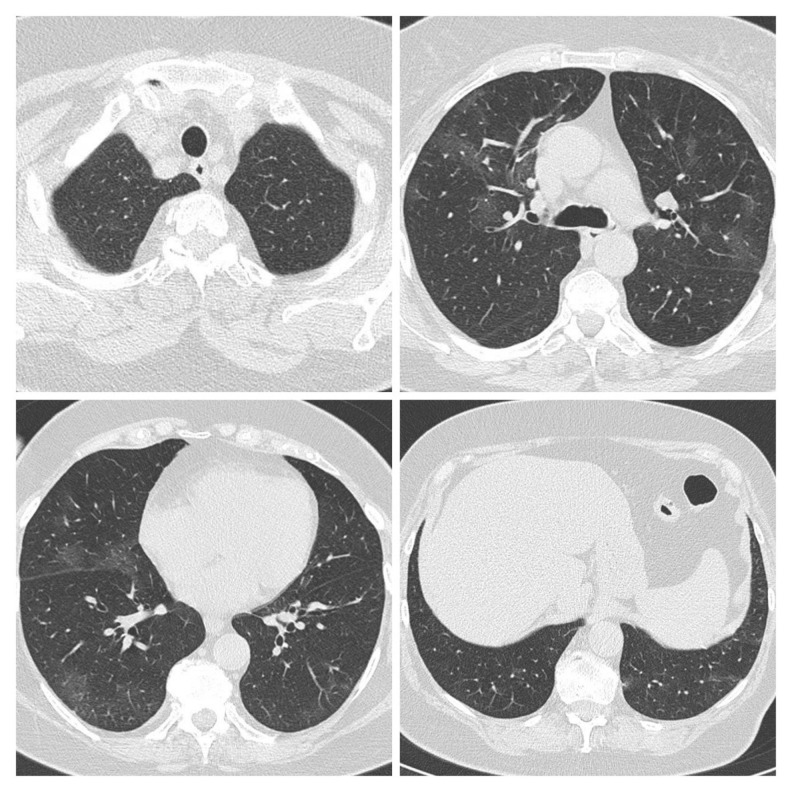
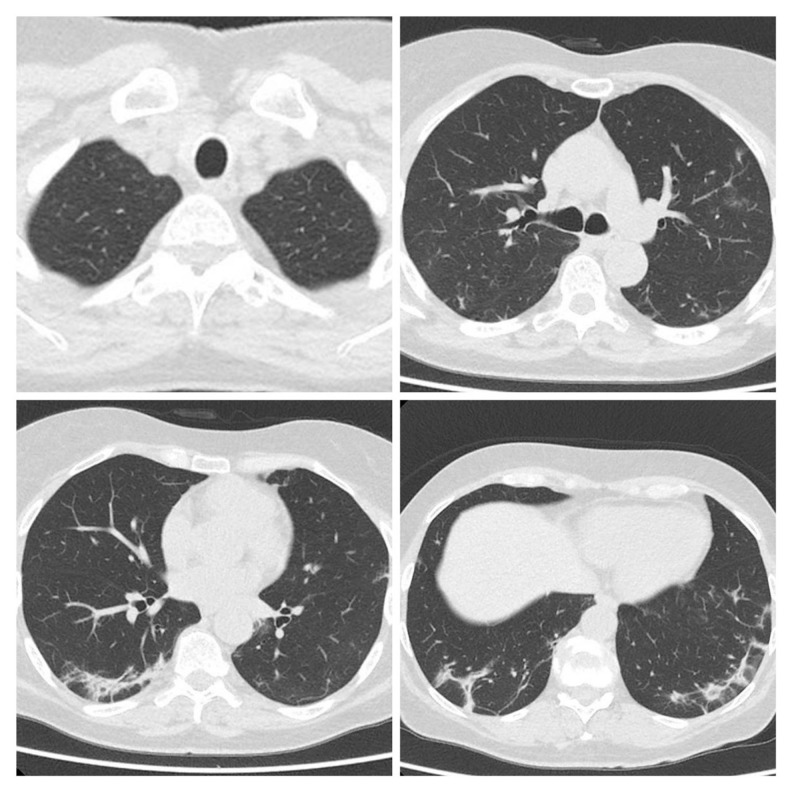
Figure 1.
First CT scan of Patient 1 performed on March 27 after hospital admission showed multiple patchy ground-glass opacity and consolidation shadow in the bilateral lung view, which was suggestive for bilateral interstitial pneumonia.
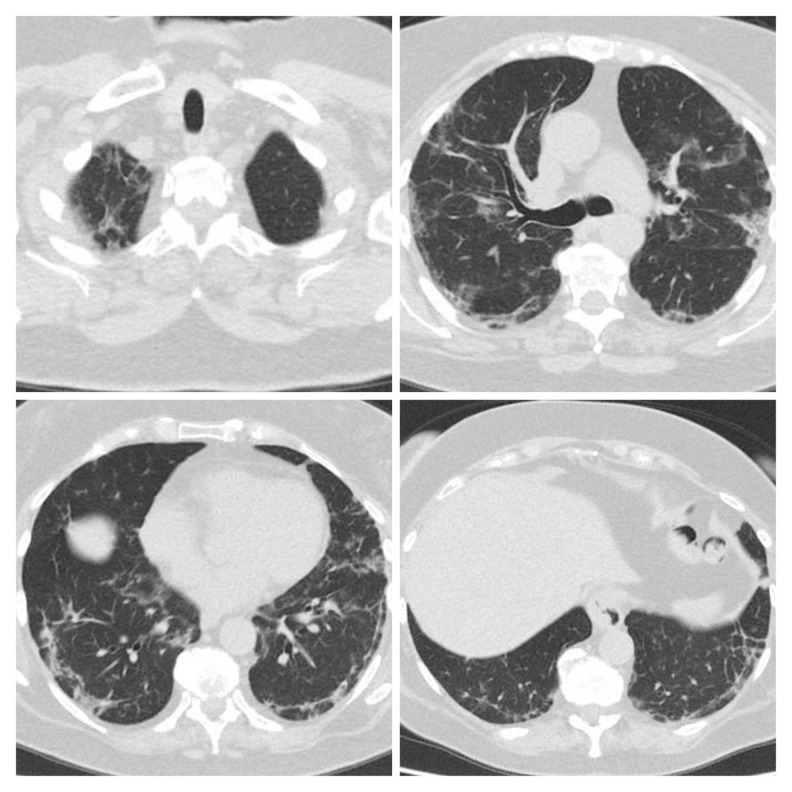
Figure 2.
Second CT scan of Patient 1 performed on April 22 showed disease progression: increasing range of ground-glass density patches and consolidation, with scant fibrous interstitial stripes.
Patient 2
An 88-year-old woman was moved from another hospital to our Infectious Disease Department on March 25 (illness day 6), complaining of fever and dry cough. She reported no other symptoms and no history of travels abroad, but she had exposure to a patient infected by SARS-CoV-2. She had high blood pressure and diabetes mellitus treated with ACE-inhibitors and insulin therapy, respectively. Chest computer tomography (CT) showed bilateral interstitial pneumonia. SARS-CoV-2 was identified on nasopharyngeal swab. On illness day 15, the patient was no longer febrile but mild dyspnea appeared, with worsening of respiratory parameters, so methylprednisolone was added together with high-flow oxygen support. The patient improved gradually, but persistent O2 support (2 L/min) by nasal cannula was needed. A 1-month follow-up CT scan showed decreasing range of ground-glass density patches and consolidation, but thin fibrous interstitial stripes appeared. The patient was discharged from our hospital 45 days after admission, with PaO2 62 mmHg on room air (P/F 295) O2ST 94%.
Clinical course of Patient 2
The patient came from another hospital and she was admitted to our Infectious Disease Department on March 25 (illness day 6) complaining of fever and dry cough. She reported no other symptoms or history of travel abroad, but she had exposure to a SARS-CoV-2-positive patient. Her body temperature was 37.0°C (n.v. 35.5–37.0°C armpit), blood pressure 130/80 mmHg (n.v. 120/80 mmHg), pulse 75 beats per minute (n.v. 60– 100 beats per minute), and blood oxygen saturation 96% (n.v. 95–100%) with VM 60% 12 L/min. Blood tests showed leucocytes 3.36×103/uL (n.v. 4.8–10.8×103/uL), hemoglobin 12.6 g/ dl (n.v. 12.0–16.0 g/dl), platelet count of 166×103/uL (n.v. 130– 400×103/uL), D-dimer 1.61 ng/ml (n.v. 0.0–0.5 mg/ml), C-reactive protein (CRP) 4.5 mg/dl (n.v. <0.05 mg/dl), and procalcitonin 0.30 ng/mg (n.v. 0.10–0.49 ng/mL). On arterial blood gas analysis, pH was 7.44 (n.v. 7.35 to 7.45), PO2 was 79 mmHg (n.v. >79 mmHg), PCO2 was 46 (n.v. 35–45 mmHg), and O2ST was 96% (n.v. 95–100%) (O2 support MV 60%). Table 2 summarizes her clinical laboratory results and vital signs. A chest CT scan showed multiple patchy ground-glass opacity and consolidation shadow in the bilateral lung view, mainly to the left, which was very highly suggestive of bilateral interstitial pneumonia (Figure 3). SARS-CoV-2 was identified on nasopharyngeal swab by RT-PCR (TaqMan TM 2019-nCoV Assay Kit v1/v2, Thermo Fisher, qPCR) tested at ISZAM, according to the current WHO testing guidelines [10]. On March 26 (illness day 7), she started oral therapy with lopinavir/ritonavir 200/50 mg twice a day, hydroxychloroquine 200 mg twice a day, and subcutaneous sodium enoxaparin 4000 UL daily. On March 30 (illness day 11), she still had fever, and a blood test showed increasing inflammation markers (CRP 8.55 mg/dl), so intravenous antibiotic therapy (levofloxacin 500 mg and ceftriaxone 2 g) was started. On illness day 15, the patient was no longer febrile, but mild dyspnea appeared, with worsening of respiratory parameters, so methylprednisolone 20 mg daily was added together with O2 support with MV 60%. On April 10 (illness day 22) O2 support was switched to low-flow nasal cannula oxygen 2 L/min, antiviral therapy was stopped, and steroid was tapered. Arterial blood gas analysis showed pH 7.42, PO2 59 mmHg, PCO2 51 mmHg, and O2ST 90% with O2 support 2 L/min by nasal cannula. Her dry cough soon disappeared, and she improved gradually, but persistent O2 support (2 L/min) by nasal cannula was needed. On April 30 (illness day 32), a CT scan showed decreasing range of ground-glass density patches and consolidation, but thin fibrous interstitial stripes appeared, mainly in the basal lung segment (Figure 4). After 2 negative SARS-COV-2 nasopharyngeal swab tests, the patient was discharged from hospital on May 9, 45 days after admission, with PaO2 62 mmHg in room air (P/F 295) and O2ST 94%.
Table 2.
Clinical laboratory results and vital signs Patient 2.
| Hospital day | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Illness day | 9 | 10 | 11 | 12 | 13 | 14 | 15 |
| Temp (°C) | 36.8 | 36.6 | 37.2 | 37.4 | 37 | 36.6 | 36.5 |
| BP (mmHg) | 130/80 | 170/80 | 160/100 | 150/80 | 150/80 | 130/70 | 150/80 |
| Pulse (/min) | 75 | 64 | 68 | 86 | 75 | 98 | 83 |
| O2 sat (%) | 96 | 94 | 97 | 96 | 95 | 93 | 92 |
| FiO2 (%) | 60 | 60 | 60 | 40 | 40 | 40 | 60 |
| Leukocytes (×103/µl) | 3.36 | 4.66 | 3.89 | ||||
| Neutrophils (%) | 58.7 | 65.1 | 62.9 | ||||
| Lymphocytes (%) | 32.1 | 25.3 | 24.9 | ||||
| Hemoglobin (g/dl) | 12.6 | 11.7 | 10.6 | ||||
| Platelets (×103/µl) | 166 | 232 | 241 | ||||
| NPS/OPS PCR | 23/03 + | 30/03 + | 06/04 – | 09/04 – | 04/05 – |
Temp – body temperature; BP – blood pressure; Pulse – heart rate; O2 sat – oxygen saturation; FiO2 – inspiratory oxygen fraction; NPS/OPS PCR – nasopharyngeal swab.
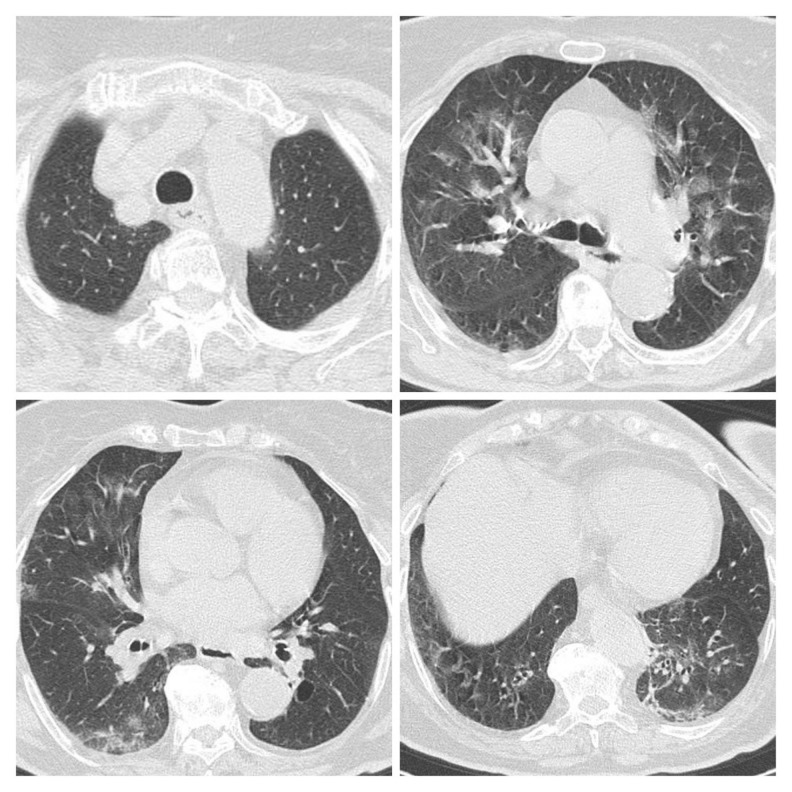
Figure 3.
First CT scan of Patient 2 performed on March 25 showed multiple patchy ground-glass opacity and consolidation shadow in the bilateral lung view, mainly to the left, highly suggestive for bilateral interstitial pneumonia.
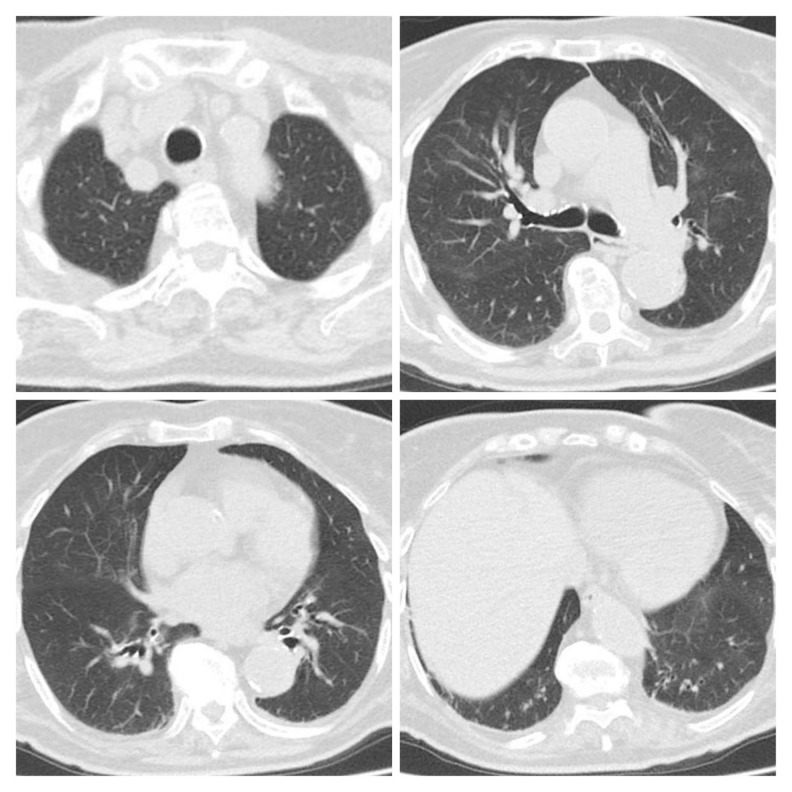
Figure 4.
Second CT scan of Patient 2 performed on April 30 showed decreasing range of ground-glass density patches and consolidation but scant fibrous interstitial stripes, mainly in the basal lung segment.
Patient 3
A 63-year-old woman had been experiencing fever, myalgia, and anorexia for 10 days. She had a history of high blood pressure treated with beta-blockers. She had never smoked or travelled abroad. She was a healthcare worker in direct contact with SARSCoV-2-infected patients. A chest X-ray showed bilateral interstitial pneumonia. SARS-CoV-2 was identified on nasopharyngeal swab. Therapy, including intravenous methylprednisolone, was started. During the following days, her fever slowly disappeared but mild dyspnea developed, and on illness day 18 a CT scan showed consolidation shadow in bilateral lung view, interlobular septal thickening with bronchiolectasis, and diffuse fibrotic evolution of the interstitial inflammation. Steroidal therapy was boosted and in the following days her clinical conditions gradually improved. The patient was discharged from hospital 17 days after admission. Arterial blood gas analysis showed PO2 71 mmHg and O2ST 95% on room air, with P/F 338.
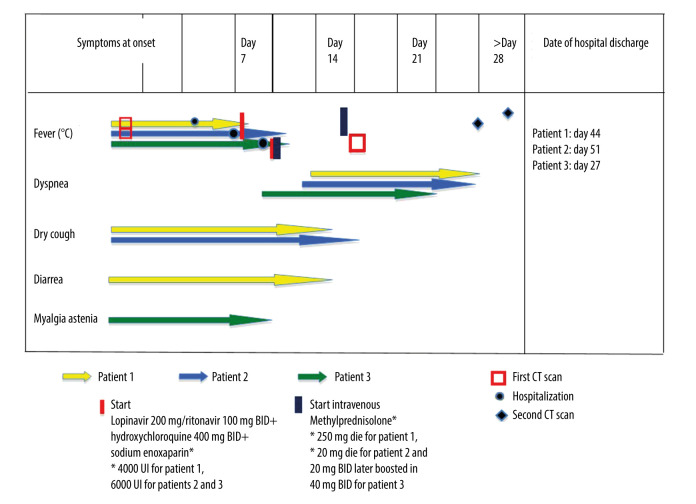
Figure 5 summarizes the timing of symptoms, hospitalization, clinical course, treatment, and follow-up.
Figure 5.
Timeline of symptoms, CT scan, treatment initiation, day of hospitalization, and day of discharge of all patients according to the day of illness. BID – bis in die (twice a day).
Clinical course of Patient 3
A 63-year-old woman admitted to our hospital reported having had fever, myalgia, and anorexia for 10 days. She had a history of high blood pressure treated with beta-blockers. She had never smoked and did not travel abroad. She was a healthcare worker in direct contact with SARS-CoV-2-infected patients. She was awake and conversant, with a blood pressure of 140/80 mmHg (n.v. 120/80 mmHg), HR 90 beats per minute (n.v. 60–100 beats per minute), RR 19 breaths per minute (n.v. 12–16 breaths per minute), temperature 37.5°C (n.v. 35.5–37.0°C armpit), and SpO2 of 94% (n.v. 95–100%) on room air. Auscultation of the lungs disclosed bilateral diffuse crackles mainly in the right medium-basal side. Blood tests showed leucocytes 4.95×103/uL (n.v. 4.8–10.8×103/uL), hemoglobin 11.1 g/dl (n.v. 12.0–16.0 g/dl), platelet count of 278×103/uL (n.v. 130–400×103/uL), D-dimer 0.71 ng/mL (n.v. 0.0–0.5 ng/mL), C-reactive protein (CRP) 14.26 mg/dl (n.v. <0.05 mg/dl), and procalcitonin 0.02 ng/mg (n.v. 0.10–0.49 ng/mL). Arterial blood gas analysis showed pH 7.45 (n.v. 7.35 to 7.45), PO2 72 mmHg (n.v. >79 mmHg), PCO2 33 (n.v. 35–45 mmHg), O2ST 95% on room air (n.v. 95–100%), and P/F was 342. Table 3 summarizes clinical laboratory results and vital signs of the patient. A chest X-ray showed bilateral increased markings at the medium-lower lung lobes, highly suggestive of bilateral interstitial pneumonia (Figure 6). Nasopharyngeal and oropharyngeal swab (NPS/ORS) specimens were collected and SARS-CoV-2 was identified by RT-PCR (TaqMan TM 2019-nCoV Assay Kit v1/v2, Thermo Fisher, qPCR) tested at ISZAM. Soon after hospital admission, she started antiviral therapy with oral lopinavir/ritonavir 200/50 mg twice a day, subcutaneous sodium enoxaparin 6000 UL daily, and intravenous methylpredniso-lone 20 mg twice a day. Blood tests revealed low G6PDH, so hydroxychloroquine was not indicated. Considering her elevated CPR value, antibiotic therapy with IV ceftriaxone 2 g and oral azithromycin 500 mg once daily were also started. During the following days, her fever slowly disappeared, but mild dyspnea developed and arterial blood gas analysis showed pH 7.42, PO2 60 mmHg, PCO2 42, and O2ST 91% on room air, with P/F 286. For this reason, on illness day 18, a CT scan was performed, revealing consolidation shadow in bilateral lung view, interlobular septal thickening with bronchiolectasis, and diffuse fibrotic evolution of the interstitial inflammation previously observed (Figure 7). Mediastinal lymphadenopathy was also found. Steroidal therapy was boosted with methylprednisolone 40 mg twice daily. In the following days, her clinical conditions gradually improved. On April 28, 17 days after the admission and after 2 negative SARS-COV-2 nasopharyngeal swab tests, the patient was discharged from hospital with no home therapy. Arterial blood gas analysis showed pH 7.42, PO2 71 mmHg, PCO2 42, and O2ST 95% on room air, with P/F 338. Frequent clinical checks and serial 3-month follow-up spirometry, CT chest scan, blood tests, and arterial oxygen saturation on room air were recommended.
Table 3.
Clinical laboratory results and vital signs Patient 3.
| Hospital day | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Illness day | 10 | 11 | 12 | 13 | 14 | 15 | 16 |
| Temp (°C) | 37 | 37.3 | 36 | 36.5 | 36 | 37.5 | 36.5 |
| BP (mmHg) | 140/80 | 120/70 | 120/80 | 120/70 | 130/90 | 120/80 | 120/70 |
| Pulse (/min) | 90 | 73 | 75 | 74 | 65 | 68 | 60 |
| O2 sat (%) | 94 | 94 | 93 | 93 | 95 | 95 | 93 |
| FiO2 (%) | 21 | 21 | 21 | 21 | 21 | 21 | 21 |
| Leukocytes (×103/µl) | 4.95 | 10.8 | 10.8 | ||||
| Neutrophils (%) | 68.3 | 83.4 | 68.4 | ||||
| Lymphocytes (%) | 20.8 | 12.7 | 21 | ||||
| Hemoglobin (g/dl) | 11.1 | 11.1 | 10.5 | ||||
| Platelets (×103/µl) | 278 | 556 | 210 | ||||
| NPS/OPS PCR | 06/04 + | 22/04 – | 25/04 – |
Temp – body temperature; BP – blood pressure; Pulse – heart rate; O2 sat – oxygen saturation; FiO2 – inspiratory oxygen fraction; NPS/OPS PCR – nasopharyngeal swab.
Figure 6.
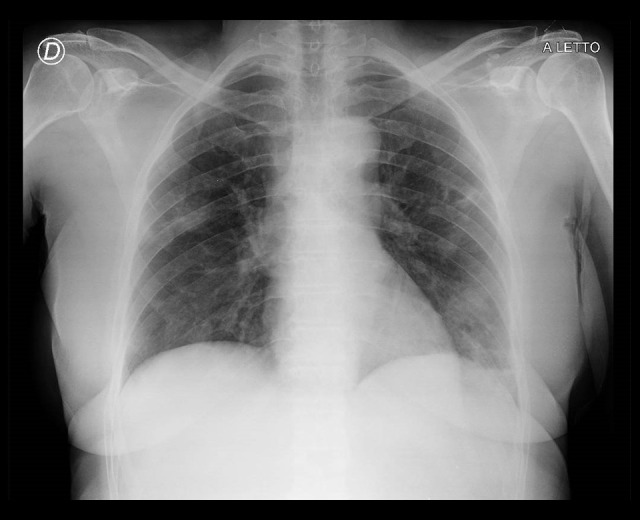
Chest radiograph of Patient 3 at presentation, 2020 (illness day 11).
Figure 7.
Follow-up CT scan of Patient 3: Consolidation shadow in bilateral lung view, interlobular septal thickening, and diffuse fibrous interstitial stripes are evident. Mediastinal lymphadenopathy can also be seen.
Discussion
This report describes 3 cases of COVID-19 pneumonia without need of intubation, with slow and incomplete respiratory recovery and evidence of fibrous signs on CT scan. The patients were all women and had no significant previous respiratory disease or history of smoke exposure. Fibrous streaks or subpleural line were described as CT signs in the follow-up of COVID patients [11]. In a recent report [12], evidence of pulmonary fibrosis as progression of SARS-COV-2 infections in CT scan was found in 42.9% of cases, and it was significantly associated with older age, longer hospitalization, and ICU admission. The clinical implications of these radiological signs are still unclear, but the incomplete respiratory recovery observed in our patients suggests the possibility of rapid evolution toward stable functional damage. This needs to be assessed by further investigations such as spirometry and chest CT scan follow-up in the next months. It should be noted that these patients were not admitted to the ICU and did not received high-flow oxygen support, had a medium or long length of hospitalization (39, 35, and 17 days, respectively) and were of different ages, all being older than 60 years. Residual pulmonary fibrosis has been reported after severe acute respiratory syndrome (SARS)-2003 in many surviving patients, and these data were confirmed by autopsy [9]. Considering SARS and Middle East respiratory syndrome (MERS) together, nearly 30% of survivors of these 2 infections showed radiological and physiological abnormalities consistent with fibrotic lung disease during their follow-up. Assuming similar repercussions in the COVID-19 pandemic, a large cohort of individuals could be diagnosed with pulmonary fibrosis associated with persistent and potentially progressive major complication, and this is supported by current epidemiological, viral, immunological, and clinical evidence [14]. Close long-term follow-up studies will be required to establish the true prevalence of post-COVID-19 fibrosis. Autopsy examination of lungs from novel coronavirus pneumonia patients showed significant pathological lesions, including alveolar exudative and interstitial inflammation, alveolar epithelium proliferation, hyaline membrane, focal hemorrhage, and pulmonary interstitial fibrosis [15]. An evolution to irreversible fatal respiratory failure has been described, even in patients in whom nucleic acid test results had turned negative, after prolonged ICU stays and despite maximal support with mechanical ventilation (MV) and extra-corporeal membrane oxygenation (ECMO) [16]; in these critical patients, prolonged intubation may also be involved. An abnormal immune mechanism that probably plays a key role in the severity of respiratory failure in acute COVID-19 pneumonia may promote pulmonary fibrosis in the same way. Through pro-inflammatory cytokines (IL-1 and IL-6), SARS-COV-2 may induce the production of active mature IL-1β, which is a mediator of lung inflammation, fever, and fibrosis [17]. In this context, it is remarkable that tocilizumab successfully inhibits the proliferation of human lung fibroblasts from IPF patients [18]. This IL-6 inhibitor is now under investigation for its possible therapeutic role in COVID-19 pneumonia and it was used in our Patient 1 due to her sudden worsening of symptoms and respiratory parameters. It is crucial to fully understand why certain patients have a shift to unchecked cellular proliferation with accumulation of fibroblasts and myofibroblasts, leading to excessive deposition of collagen and other extracellular matrix components [13]. The prevalence of post-COVID-19 fibrosis is currently unclear, but early analysis of patients with COVID-19 discharged from hospital suggests a high prevalence of fibrotic lung function abnormalities. In our 3 patients, variable doses of steroids were used, which may be advantageous in COVID-19 disease [19], but there is no clear optimal therapy, including antiviral treatments. This radiological and clinical outcome is even more impressive considering that the anti-inflammatory effect of steroids should slow the process that is possibly responsible for late pulmonary fibrosis. The role of early anti-fibrotic therapy in high-risk patients needs to be investigated [13]. Actually, pulmonary fibrosis can develop early in patients with COVID-19 pneumonia after hospital discharge. The present case series emphasizes the possible role of radiologic alterations consisting of interstitial thickening, coarse reticular pattern, and parenchymal bands in predicting pulmonary fibrosis in COVID-19 pneumonia patients. Limitations of our study are its short-term follow-up and lack of functional data from the very early observational phase. We had no previous CT scans or respiratory function test results evaluating possible pre-existing lesions before infection. Long-term follow-up is needed to confirm this diagnosis and assess the extent and persistence of functional damage or eventual its reversibility. In addition, due to lack of evidence-based guidelines in COVID-19 therapy, our 3 patients received various treatments. All 3 patients were women none had severe pneumonia, reflecting the observation that men with SARS-CoV-2 infection tend to have more severe disease. This stresses the importance of close follow-up in all COVID-19 pneumonia patients, irrespective of disease severity.
Conclusions
In conclusion, this report focuses on a possible scenario of long-term lung damage in COVID-19 pneumonia survivors who did not receive mechanical ventilation. All COVID-19 pneumonia patients, irrespective of sex, age, risk factors, and intubation or ICU stay, should undergo serial chest CT and spirometry long-term follow-up for at least 1 year to assess residual damage. This may be particularly relevant in those with slow respiratory recovery and long hospitalization. Further evidence regarding the possible therapeutic role of steroidal and immunomodulatory therapy in this infection are urgently needed in this context.
References:
- 1.World Health Organization (WHO) Coronavirus disease 2019 (COVID-19) Situation Report-92. 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200421-sitrep-92-covid-19.pdf.
- 2.Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. 2020;395(10223):470–73. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becroft DM. Bronchiolitis obliterans, bronchiectasis, and other sequelae of adenovirus type 21 infection in young children. J Clin Pathol. 1971;24:72–82. doi: 10.1136/jcp.24.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ward PA, Hunninghake GW. Lung inflammation and fibrosis. Am J Respir Crit Care Med. 1998;157(4 Pt 2):S123–29. doi: 10.1164/ajrccm.157.4.nhlbi-10. [DOI] [PubMed] [Google Scholar]
- 5.Naik PK, Moore BB. Viral infection and aging as cofactors for the development of pulmonary fibrosis. Expert Rev Respir Med. 2010;4(6):759–71. doi: 10.1586/ers.10.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wen Y, Deng BC, Zhou Y, et al. Immunological features in patients with pneumonitis due to influenza A H1N1 infection. Investig Allergol Clin Immunol. 2011;21(1):44–50. [PubMed] [Google Scholar]
- 7.Crapo JD. Morphologic changes in pulmonary oxygen toxicity. Annu Rev Physiol. 1986;48:721–31. doi: 10.1146/annurev.ph.48.030186.003445. [DOI] [PubMed] [Google Scholar]
- 8.Vannella KM, Moore BB. Viruses as co-factors for the initiation or exacerbation of lung fibrosis. Fibrogenesis Tissue Repair. 2008;1(1):2. doi: 10.1186/1755-1536-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Venkataraman T, Frieman MB. The role of epidermal growth factor receptor (EGFR) signaling in SARS coronavirus-induced pulmonary fibrosis. Antiviral Res. 2017;143:142–50. doi: 10.1016/j.antiviral.2017.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization (WHO) Use of laboratory methods for SARS diagnosis. 2020. https://www.who.int/csr/sars/labmethods/en/
- 11.Wu J, Pan J, Teng D, et al. Interpretation of CT signs of 2019 novel corona-virus (COVID-19) pneumonia. Eur Radiol. 2020;30(10):5455–62. doi: 10.1007/s00330-020-06915-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lei P, Fan B, Mao J, et al. The progression of computed tomographic (CT) images patients with coronavirus disease (COVID-19) pneumonia. J Infect. 2020;80(6):e30–31. doi: 10.1016/j.jinf.2020.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peter MG, Athol UW, Gisli J. Pulmonary fibrosis and COVID-19: The potential role for antifibrotic therapy. Lancet Respir Med. 2020;8(8):807–15. doi: 10.1016/S2213-2600(20)30225-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang J, Wang BJ, Yang JC, et al. [Advances in the research of mechanism of pulmonary fibrosis induced by Corona Virus Disease 2019 and the corresponding therapeutic measures.] Zhonghua Shao Shang Za Zhi. 2020 doi: 10.3760/cma.j.cn501120-20200307-00132. [in Chinese] [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 15.Carsana L, Sonzogni A, Nasr A, et al. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: A two-centre descriptive study. Lancet Infect Dis. 2020;20(10):1135–40. doi: 10.1016/S1473-3099(20)30434-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen JY, Qiao K, Liu F, et al. Lung transplantation as therapeutic option in acute respiratory distress syndrome for coronavirus disease 2019-related pulmonary fibrosis. Chin Med J (Engl) 2020;133(12):1390–96. doi: 10.1097/CM9.0000000000000839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conti P, Ronconi G, Caraffa A, et al. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARSCoV-2): Anti-inflammatory strategies. J Biol Regul Homeost Agents. 2020;34(2):327–31. doi: 10.23812/CONTI-E. [DOI] [PubMed] [Google Scholar]
- 18.Epstein SG, Brook E, Bardenstein-Wald B, Shitrit D. TGF-β pathway activation by idiopathic pulmonary fibrosis (IPF) fibroblast derived soluble factors is mediated by IL-6 trans-signaling. Respir Res. 2020;21(1):56. doi: 10.1186/s12931-020-1319-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ñamendys-Silva SA, Domínguez-Cherit G. Recommendations for the management of critically ill adult patients with COVID-19. Gac Med Mex. 2020 doi: 10.24875/GMM.M20000375. [Online ahead of print] [DOI] [PubMed] [Google Scholar]