Abstract
Background
Hippocampus is considered to be the seat for neurocognitive functions. Avoidance of hippocampus during radiotherapy to brain may serve to preserve various domains of neurocognition. We aimed to derive radiotherapy dose constraints to hippocampi for preserving neurocognition in young patients with brain tumors by measuring various neurocognitive parameters.
Methods
Forty-eight patients with residual/progressive benign or low-grade brain tumors treated with stereotactic conformal radiotherapy (SCRT) to a dose of 54 Gy in 30 fractions underwent prospective neuropsychological assessments at baseline before SCRT and at 6 months and 2, 3, 4, and 5 years. Hippocampi were drawn as per the Radiation Therapy Oncology Group atlas. Longitudinal change in intelligence quotient scores was correlated with hippocampal doses.
Results
Mean volume of bilateral hippocampi was 4.35 cc (range: 2.12–8.41 cc). Craniopharyngioma was the commonest histologic subtype. A drop of >10% in mean full-scale intelligence quotient (FSIQ) scores at 3 and 5 years post SCRT was observed in patients in whom left hippocampus received a mean dose of 30.7 Gy (P = 0.04) and 31 Gy (P = 0.04), respectively. Mean performance quotient (PQ) scores dropped > 10% at 5 years when the left hippocampus received a dose of > 32 Gy (P = 0.03). There was no significant correlation of radiotherapy doses with verbal quotient, or with doses received by the right hippocampus. Multivariate analysis revealed young age (<13 y) and left hippocampus dose predicted for clinically relevant decline in certain neurocognitive domains.
Conclusions
A mean dose of ≤30 Gy to the left hippocampus as a dose constraint for preserving intelligence quotient is suggested.
Key Points
1. Children and young adults with benign and low-grade gliomas survive long after therapy.
2. Higher dose to the hippocampi may result in long-term neurocognitive impairment.
3. Mean dose of <30 Gy to left hippocampus could be used as a pragmatic dose constraint to prevent long-term neurocognitive decline.
Keywords: neurocognitive function, hippocampus, conformal radiotherapy, dose constraints, brain tumors
Importance of the Study.
Preclinical and a few prospective clinical studies have shown hippocampal avoidance as a strategy to preserve certain neurocognitive functions like memory. Most of the clinical work comes from adult patients who undergo whole brain radiation or adult patients of gliomas who undergo conformal radiotherapy where various subdomains of memory were studied. However, children who undergo radiation to the brain are more prone to develop neurocognitive impairment. The interplay between radiation and the evolving IQ in children is not very clearly understood. The present study looks at neurocognitive subdomains like full scale (FSIQ), Verbal (VQ), Performance (PQ) as well as memory (MQ) in children and adolescents with diverse brain tumors hitherto not studied. Our observations showed that hippocampal dose is associated with the above neurocognitive functions. Greater hippocampal dose was associated with greater decline in the above subdomains. Therefore, reducing hippocampal dose is of paramount importance. Based on the hippocampal dose constraints obtained, prospective studies should aim at validating these results in the era of contemporary radiotherapy practice.
Minimizing therapy-induced long-term sequelae remains an important goal to institute optimal management protocols in cancer patients. Preserving long-term neurocognitive functions (NCF) has been long recognized to be an extremely relevant endpoint in cancer patients including in brain tumor survivors.
Although causation of neurocognitive impairment in brain tumor patients is multifactorial, radiotherapy (RT) is considered a critical factor. However, RT has been shown to be integral in treatment protocols to improve local control in various cancers, including brain tumors.1,2 Modern high-precision highly conformal RT techniques used in the contemporary clinical practices have the capability to reduce long-term neurocognitive morbidity in young brain tumor patients.3
In the recent era, several attempts have been made to identify certain critical structures in the brain responsible for preserving neurocognition.4–8 Among the structures identified, preclinical evidence has shown hippocampus to be the niche for glial neurogenesis and therefore preservation of neurocognition.9–11 Evolving clinical evidence further corroborates the preclinical findings about the importance of hippocampus in preserving neurocognition, especially memory function, during therapeutic radiation.12,13 The evidence regarding hippocampal dosimetry is, however, sparse and is limited to adult brain tumor patients with absence of any data in children and young adolescents.14 Moreover, the exact dose-response relationship and the threshold hippocampal doses causing impairment in neurocognition in this cohort is not well established.
We therefore sought to study the relationship between hippocampal dose on various neurocognitive domains by delineating the hippocampi in a cohort of young and adolescent patients with benign or low-grade brain tumors prospectively treated with fractionated stereotactic conformal (SC)RT and correlating hippocampal dose-volume histogram (DVH) data with long-term NCF impairment.
Materials and Methods
Patient Cohort
Children and adolescent patients with pathologically confirmed or clinically suspected benign or low-grade intracranial neoplasms enrolled in a single institutional prospective trial (Clinical trial identifier; NCT:00517959) approved by the institutional review and ethics board of Tata Memorial Centre were accrued in this study. Eligible patients were <25 years of age with no history of prior chemotherapy or RT. All the patients underwent high-precision focal conformal RT with stereotactic guidance.
Hippocampus Delineation
The hippocampi were delineated by using contouring guidelines currently in use and published in a cooperative-group phase II trial of hippocampal avoidance during whole-brain (WB)RT for brain metastasis (Radiation Therapy Oncology Group [RTOG] 0933).12 The hippocampus was contoured on T1-weighted 3D-FSPGR (fast spin gradient) MRI axial sequence with a slice thickness of 1 mm and fused to the treatment planning contrast enhanced CT of the entire head region with a slice thickness of 2.5 mm. Given the preponderance of gray matter in the hippocampus, we focused on contouring the T1-hypointense signal medial to the temporal horn. Anatomically, we focused on delineating the dentate gyrus and cornu ammonus of the hippocampal region, which is assumed to be the niche for the neural progenitor cells required for memory-related and other neurocognitive functions (Fig. 1). The delineation of the hippocampus was performed retrospectively after the completion of RT. No effort was made to spare the hippocampus during the treatment planning stage.
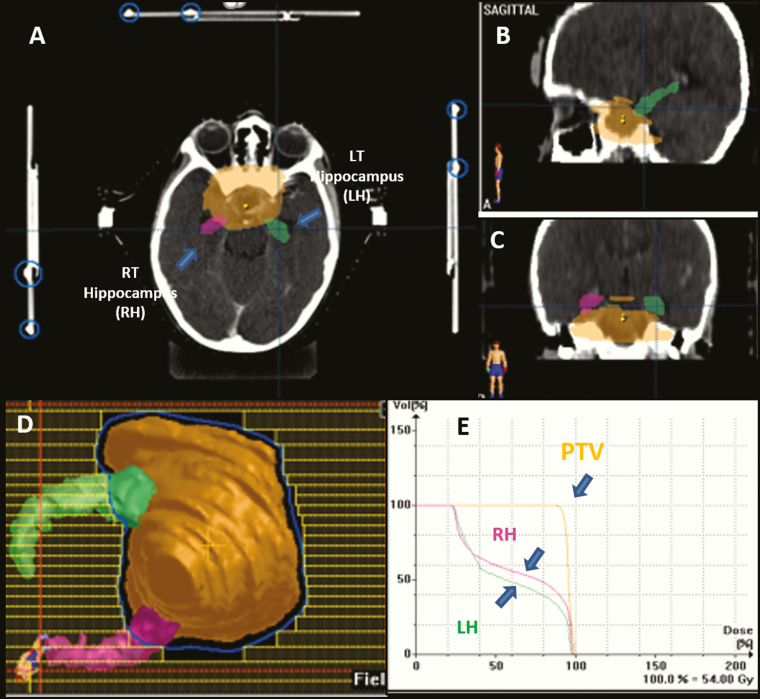
Fig. 1.
Hippocampus as delineated according to the RTOG atlas shown in (A) axial, (B) coronal, and (C) sagittal planes in a representative patient with diagnosis of craniopharyngioma receiving SCRT, (D) beams eye view depicting the prescribed dose wash encompassing the tumor and sparing of the hippocampi, and (E) dose volume histogram showing the dose to the planning target volume and the hippocampus.
Neurocognitive Assessment
All neurocognitive assessments were done by a specialist neuropsychologist. Baseline neuropsychological evaluation was done before start of RT and then at 6 months; thereafter yearly assessments were carried out. An age-appropriate neuropsychological battery of tests was administered for each patient. Standardized norms of each of these tests are available for Indian population. Intelligence quotient was measured by an age-adjusted and validated Wechsler Intelligence Score Chart (WISC-III) to give the verbal quotient (VQ), performance quotient (PQ), and full-scale intelligence quotient (FSIQ). WISC-III is a collection of 13 distinct subtests divided into 2 scales: a Verbal and Performance Scale. Five of the subtests in each scale produce scale-specific IQs and the 10 subtest scores produce a full-scale IQ. For patients less than 16 years old, we used the verbal quotient to measure the memory quotient. For patients older than 16 years, instead of verbal quotient, the memory quotient (MQ) was measured by the Wechsler Memory Scale (WMS). The WMS-I is an individually administered measure of memory for verbal and figural stimuli, memory for meaningful and abstract material, and delayed and immediate recall. This is an individually administered measure of memory for verbal and figural stimuli. It measures orientation, registration, immediate recall, logical memory, short-term working memory, attention, and concentration. Scores from the 7 subtests were added up to obtain a raw score, to which a constant is added based on the subject’s age group. This weighted score is then converted to MQ.
Because of the unavailability of the Wechsler Adult Intelligence Scale, Bhatia’s test was used for assessing PQ in these patients. Bhatia’s battery of performance test was developed to test intelligence of Indian population which has been validated. It consists of nonverbal assessment of intelligence, executive functioning, attention, spatial ability, and motor skills. For patients who were blind, the Vithoba Paknikar performance tests for the blind were used. The neurocognitive battery of tests is depicted in Supplementary Table 1. A 10% decline in any of the neurocognitive functions was considered to be clinically significant and considered a cutoff value. We analyzed the neurocognitive domains for 48 patients who have had at least 5 years follow-up neurocognitive assessments.
DVH Analysis of Hippocampus
Dose volume histograms were generated for the left and right hippocampi individually. For the purpose of analysis, the dosimetric parameters and the mean, maximum, and minimum doses received by each hippocampus were calculated. Other dosimetric parameters which were analyzed were D80 (RT dose received by 80% volume of the hippocampus), D40 (RT dose received by 40% volume of hippocampus), D30 (RT dose received by 30% volume of hippocampus), and D20 (RT dose received by 20% volume of hippocampus) from the DVH.
Stereotactic Conformal Radiotherapy
All patients were treated with fractionated SCRT by using a high-precision optically guided intracranial RT system. The immobilization procedures, MR and CT imaging protocol, target volume delineation, RT treatment planning, and plan implementation have been described extensively previously.5
Statistical Analysis
Radiotherapy doses and fraction sizes were based on current clinical practices. All low-grade gliomas and craniopharyngiomas enrolled in the trial were treated to a dose of 54 Gy in 30 fractions at 1.8 Gy per fraction.
Descriptive statistics were generated for patient characteristics and neuropsychological measures. For the purpose of analysis, a drop in FSIQ of 5% and 10% from baseline values at the last available follow-up was considered a significant drop, so that a minor fall in IQ due to chance was not taken into consideration. A drop of 5% was equivalent to a 7-point drop in absolute terms and a drop of 10% was equivalent to a drop of 12 points in absolute terms. A 10% drop was considered clinically significant. Tests for normality in the dose-volume characteristics were performed using the Kolmogorov‒Smirnov test. Age- and dose-related groups were compared using Fisher’s exact test. Binary logistic regression was used to determine the independent effects of age and dose groups on IQ. A P-value of <0.05 was considered significant.
Results
Forty-eight patients prospectively enrolled in the SCRT trial (NCT00517959) and treated with conformal RT were enrolled in the study. The patient demographic, tumor, and clinical and treatment characteristics at baseline are reported in Table 1. Near total excision was done in 26 patients (54%), while partial excision or biopsy was done in 22 patients (46%). Median follow-up for the entire cohort was 86 months (interquartile range [IQR]: 61.4–98.1 mo). At last follow-up, all 48 patients remained free of local disease progression. Only 1 patient (with diagnosis of hemangiopericytoma) had systemic skeletal disease even though he was locally controlled. The 5-year local control and overall survival was 98% and 100%, respectively.
Table 1.
Patient and tumor and treatment characteristics (n = 48)
| Patient Characteristics | Frequency (%) |
|---|---|
| Age, y | |
| Mean | 13.02 (range, 4–25) |
| Median | 13.00 (IQR, 8–17) |
| ≤13 y | 26 (54) |
| >13 y | 22(46) |
| Sex | |
| Male | 37 (77) |
| Female | 11 (23) |
| Hydrocephalus | |
| Mild/moderate | 29 (60) |
| Severe | 19 (40) |
| Tumor Type | |
| Craniopharyngioma | 18 (38) |
| Glioma | 28 (58) |
| Others* | 2 (4) |
| Tumor Location | |
| Sellar/suprasellar | 31 (65) |
| Cerebellar | 11 (23) |
| Cerebral | 6 (12) |
| Distance from Hypothalamic Pituitary Axis | |
| <2 cm | 35 (73) |
| >2 cm | 13 (27) |
*Pineocytoma, n = 1; hemangiopericytoma, n = 1.
Median age of the entire cohort of patients was 13 years (IQR: 8–17 y). Mean scores of all neurocognitive subdomains assessed over a period of 5 years for all the 48 patients are reported in Table 2. Mean bilateral hippocampus volume was 4.35 cc (range: 2.12–8.41 cc). Mean right and left hippocampi volumes were 2. 24 cc and 2.11 cc, respectively. Various dosimetric parameters such as the mean, maximum, and minimum hippocampus doses, D80, D40, D30, and D20 received by each hippocampus analyzed from the DVH are reported in Table 3.
Table 2.
IQ parameters over 5-year follow-up following radiotherapy
| IQ Parameters | Mean IQ Level Before and After Radiotherapy | |||||
|---|---|---|---|---|---|---|
| Baseline | 6 Mo | 2 Y | 3 Y | 4 Y | 5 Y | |
| Full-Scale Intelligence Quotient /Global Quotient (FSIQ/GQ) | 83.96 | 87.1 | 88.98 | 89.49 | 96.19 | 94.81 |
| Performance IQ | 86.53 | 93.00 | 98.89 | 102.50 | 109.50 | 112.28 |
| Verbal IQ | 87.59 | 89.43 | 85.39 | 85.22 | 83.94 | 87.62 |
| Memory IQ | 92.00 | 93.16 | 99.29 | 107.75 | 113.56 | 116.61 |
Table 3.
Summary of hippocampal parameters (dosimetry metrics volumes)
| Bilateral Hippocampus Volume: Mean: 4.35 cc (range, 2.12–8.41 cc) | |||
|---|---|---|---|
| Left Hippocampus | Right Hippocampus | ||
| Volume | Mean 2.11 cc (range, 1.12–4.25 cc) | Volume | Mean 2.24 cc (range, 1.00–4.16 cc) |
| Mean dose | 29.1 Gy (3.6–54.0) | Mean dose | 33.2 Gy (5.4–54.3) |
| Maximum dose | 47.3 Gy (15.0–56.7) | Maximum dose | 48.8 Gy (17.7–56.7) |
| Minimum dose | 10.9 Gy (1.2–51.3) | Minimum dose | 11.6 Gy (1.5–42.0) |
| D80% | 20.2 Gy (3.0–53.1) | D80% | 21.4 Gy (3.0–49.8) |
| D40% | 33.1 Gy (6.0–53.4) | D40% | 36.6 Gy (6.6–54.6) |
| D30% | 36.0 Gy (8.7–54.0) | D30% | 38.3 Gy (9.6–55.2) |
| D20% | 38.3 Gy (12.9–55.5) | D20% | 41.7 Gy (12.6–55.8) |
The mean of all the dosimetry parameters were computed; values in parentheses indicate range.
Predictive associations of each of the neurocognitive subdomains with hippocampal dose parameters were evaluated in detail for each of the individual hippocampi. Univariate analysis showed a statistical significance for FSIQ and PQ subdomains with mean left hippocampal dose. Mean left hippocampus dose of >30.7 Gy predicted for >10% decline in FSIQ at 3 years and a radiation dose of >31 Gy were associated with >10% FSIQ decline at 5 years. The mean left hippocampal dose of 36 Gy was associated with PQ decline at 2 years, while doses of 53.4 and 36 Gy were associated with PQ decline of >10% at 3 years and 5 years, respectively. However, the mean left hippocampal dose was not predictive of outcome in the VQ subdomain over the entire follow-up period. In contrast, the mean right hippocampus dose did not predict for the neurocognitive decline in any of the subdomains (Table 4). Multivariate analysis using a binary logistic regression model showed age <13 years and mean left hippocampal dose of >30 Gy to be independent predictive factors for decline in the FSIQ subdomain at 5 years (P = 0.03, P = 0.04). Children and young adults diagnosed with craniopharyngiomas as opposed to gliomas had greater decline in FSIQ, although not statistically significant (P = 0.06) (Table 5). Age <13 years was the only parameter associated with >10% drop in the VQ subdomain (P = 0.02). The mean left hippocampus dose of >25 Gy was predictive of >10% drop in the PQ subdomain (P = 0.03) (Table 5).
Table 4.
Association between mean hippocampal dose and longitudinal neurocognitive outcome at 6 months and 2, 3, and 5 years estimated by regression model
| Left Hippocampus | Cognitive Decline | 6 Mo | 2 Y | 3 Y | 5 Y | ||||
|---|---|---|---|---|---|---|---|---|---|
| % drop | Mean Dose, Gy | P-value | Mean Dose, Gy | P-value | Mean Dose, Gy | P-value | Mean Dose, Gy | P- value | |
| FSIQ | >10% drop | 22.6 | 0.15 | 29 | 0.21 | 30.7 | 0.043 | 31.0 | 0.040 |
| <10% drop | 30.3 | 27.4 | 25.4 | 26.5 | |||||
| PQ | >10% drop | 25.6 | 0.61 | 36 | 0.03 | 53.4 | 0.023 | 32.0 | 0.037 |
| <10% drop | 29.4 | 28.5 | 26.0 | 26.0 | |||||
| VQ/ MQ | >10% drop | 34.5 | 0.12 | 27.6 | 0.92 | 28.5 | 0.99 | 32.0 | 1.00 |
| <10% drop | 27.4 | 27.4 | 24.6 | 25.6 | |||||
| Right Hippocampus | Cognitive Decline | 6 Mo | 2 Y | 3 Y | 5 Y | ||||
| % drop | Mean Dose, Gy | P-value | Mean Dose, Gy | P-value | Mean Dose, Gy | P-value | Mean Dose, Gy | P-value | |
| FSIQ | >10% drop | 30.5 | 0.54 | 31.4 | NS | 32.7 | 0.17 | 39.9 | 0.40 |
| <10% drop | 33.7 | 31.9 | 28.8 | 7 | 21.4 | ||||
| PQ | >10% drop | 32.8 | 0.95 | 38.8 | NS | 42.9 | 0.06 | 35.6 | 0.29 |
| <10% drop | 33.3 | 32.5 | 31 | 8 | 30.7 | ||||
| VQ/MQ | >10% drop | 38.3 | 0.14 | 28.8 | NS | 29.4 | 0.33 | 35.6 | 0.99 |
| <10% drop | 31.7 | 31.7 | 36.8 | 5 | 22.6 |
FSIQ: Full-Scale Intelligence Quotient; VQ: Verbal; PQ: Performance Quotient; MQ: Memory Quotient.
Table 5.
Binary logistic regression analysis for factors associated with risk of impairment in various IQ domains at 5 years of longitudinal follow-up
| Parameter | Mean Improvement in IQ Scores at 5 Years from Baseline | ||||||
|---|---|---|---|---|---|---|---|
| FSIQ/GQ | P-value | VQ/MQ | P-value | PQ | P-value | ||
| Age | <13 y | 3.5 | 0.03* | −5.0 | 0.02* | 24.0 | 0.11 |
| >13 y | 16.1 | 36.0 | 15.8 | ||||
| Sex | Male | 10.1 | 0.80 | −1.6 | 0.97 | 18.3 | 0.87 |
| Female | 8.5 | −2.0 | 19.7 | ||||
| Histology | Glioma | 9.2 | 0.06 | −2.8 | 0.25 | 17.7 | 0.11 |
| Craniopharyngioma | 8.1 | 0.75 | 20.6 | ||||
| Site | Cerebral/cerebellar | 11.8 | 0.63 | 0.0 | 0.71 | 14.2 | 0.30 |
| Sellar/suprasellar | 8.9 | 4.0 | 21.6 | ||||
| Hydrocephalus | Mild/moderate | 11.7 | 0.24 | 3.6 | 0.26 | 22.7 | 0.29 |
| Severe | 7.8 | −12.2 | 12.6 | ||||
| Mean left hippocampus dose | <30 Gy | 14.8 | 0.04* | 3 | 1.00 | 25.0 | 0.03* |
| >30 Gy | 1.25 | −6.3 | 7.8 | ||||
| Mean right hippocampus dose | <33 Gy | 11.4 | 0.4 | 7.8 | 0.986 | 19.6 | 0.29 |
| >33 Gy | 8.4 | −20.7 | 17.2 |
Note: Minus sign ndicates decline in IQ at 5 years from baseline.
Discussion
The present study reiterates the importance of hippocampus as a seat of glial neurogenesis and therefore long-term preservation of cognition similar to the observations of past studies.7,10,13–16 Our group is perhaps among the few to show that the mean doses to left hippocampus is relevant in preserving FSIQ in children and young adults with brain tumors requiring therapeutic radiation for local control. Other groups have shown a correlation in RT dose to hippocampus and memory recall.12–14 The results of our study have shown that the mean dose of <30 Gy to left hippocampus preserves clinically relevant long-term FSIQ over 5 years. This dose to the hippocampus could be used as a possible dose constraint for planning conformal RT in children and young adults with benign and/or low-grade brain tumors. We also observed that apart from the hippocampal dose, age and tumor histology were important prognostic factors governing the neurocognitive outcome. Our group had previously published a study in a similar cohort of patients receiving SCRT that looked at the association of left temporal lobe dose with respect to neurocognitive decline.5 This study showed a significant association of >10% decline in FSIQ when left temporal lobe received higher radiation doses. Since the left temporal lobe and left hippocampus reside in the dominant cerebral hemisphere, the neurocognitive impact on the left hippocampus and left temporal lobe is more profound than the right. This finding leads to a plausible hypothesis of differential radiosensitivity of the hippocampi that warrants further preclinical and clinical evaluation. Given the importance and attention hippocampus has attracted in the recent past, our present study focused only on hippocampus. It may also be possible the relationship of left hippocampus with neurocognitive decline demonstrated in the present work was responsible in the earlier reported work on left temporal lobes as hippocampus occupies a considerable portion within the left temporal lobe volumes and indirectly validating the present results as well. Nevertheless, evaluation of hippocampi, temporal lobes, and indeed other parts of the brain should be ideally tested independently in future such studies. Similar to our observations the association of dosimetric parameters of left hippocampus with immediate recall of verbal memory decline has been highlighted in a study from Taiwan where hippocampal-sparing WBRT was given.17
The spectrum of neurocognitive dysfunction also varies in children and adolescents as opposed to adults and elderly. In children and adolescents, indices such as intelligence quotient are considered important surrogate parameters, while in adults, memory is an important measurable index associated with neurocognitive function. These deficits negatively affect patients’ health-related quality of life and well-being. Therefore, measuring these indices of cognitive function has become increasingly important in patients with brain tumors especially in clinical trials evaluating the efficacy of modern radiotherapeutic techniques.
Although various structures in the brain are important in maintaining long-term NCFs, the strongest evidence points toward hippocampus and the temporal lobes, especially the left temporal lobe, and studies conducted prospectively have shown that radiation doses to both these structures correlate significantly with evolution of neurocognitive dysfunctions.5,12,13 Our group had initially observed a dose volume correlation (13% volume of left temporal lobe receiving >43.2 Gy) with FSIQ impairment in patients receiving high-precision conformal RT with stereotactic guidance.5 To explore this hypothesis, we retrospectively investigated the dose response relationship between radiation dose to the hippocampus and long-term neurocognitive impairment in certain domains. We contoured the hippocampi of 48 patients who were prospectively accrued in the SCRT trial (NCT:00517959) and longitudinally assessed for their neurocognitive functions in various domains.
We now demonstrate from our results that a mean dose of >30 Gy to left hippocampus results in impairment of FSIQ and PQ domains not only at 3 years but also at 5 years. Left hippocampus dose did not correlate with dysfunction in VQ domain, which was quite surprising considering that hippocampus is the seat of memory recall. In this context, it is to be reiterated that apart from memory functions, hippocampus controls other neurological functions, like flexible cognition, which is often discussed in the context of executive function, supporting the ability to switch between competing goals, as well as contributing to high-level human behavior, such as planning, organizing, and decision making. The hippocampus is also critical for performance in complex situations that unfold over time.18Given that hippocampus is associated with various other complex executive and behavioral functions, apart from memory functions, our findings of FSIQ and PQ domain being affected by hippocampal dose may not be completely paradoxical.
Additionally, none of the dosimetric parameters of the right hippocampus correlated with any of the NCF domain impairment. The results of this study and our previous study5 demonstrate that the left hippocampus and temporal lobe are paramount to preserving certain domains of NCF and are extremely important for constraining radiation doses to these structures while planning contemporary RT treatment in the setting of modern radiation delivery techniques and treatment planning platforms like intensity-modulated (IM)RT with image guidance,19–21 or IM proton beam therapy.22–24 Apart from modern delivery methods, studies have been looking at reducing the clinical target volume margins in pediatric brain tumors without compromising efficacy.25 Therefore, the results of our study allow a more pragmatic approach to achieve the dose constraint of 30 Gy mean dose to the left hippocampus. Additionally, utilizing the above techniques along with reduced margin may have a more favorable hippocampal dosimetry profile that may extrapolate to long-term preservation of NCF. A study conducted on a cohort of 70 pediatric brain tumor patients receiving proton therapy at Massachusetts General Hospital and with a median follow-up of 3 years found that V20GyE (volume of a particular anatomic region receiving ≥20 Gy equivalent) to the left hippocampus correlated with decline in immediate verbal memory.26
The results of our study add to the evolving evidence about the importance of hippocampus in preserving the intelligence quotient in children and adolescents. In the context of the conduct of our study, it is worth mentioning certain distinctions from the published literature on hippocampal sparing in low-grade gliomas. Past studies have evaluated the role of hippocampal doses for preserving memory, especially short-term recall memory, using the Hopkins Verbal Learning Test or long-term memory using WMS-III in adults undergoing either WBRT for brain metastasis13 or fractionated conformal RT for gliomas.13 A prospective clinical study by Gondi et al in adult gliomas looked at delayed memory recall as measured by WMS-III in a relatively small cohort of 29 adult low-grade glioma patients at a cross-sectional time point of 18 months and has shown that a dose greater than 7.3 Gy to 40% volume of bilateral hippocampi correlated with delayed memory recall.13 However, in young patients with brain tumors, it’s not only the memory that is important but also the intelligence quotient that defines long-term neurocognitive outcome. In contrast, we measured different subdomains of NCF (FSIQ, PQ, VQ, and MQ) longitudinally as function of time (pre-RT, at 6 mo, and at 2, 3, and 5 y) in a relatively larger cohort of 48 patients. The dose constraints achieved from the results of the above study appear to be quite stringent and may not be very easy to plan and achieve. The second and a more recent study from St Jude hospital focused on different domains of short-term and long-term memory in a cohort of 80 childhood and adolescent low-grade gliomas, with a longitudinal follow-up of up to 10 years. On univariate regression analysis, it was observed that decline in total memory recall was associated with volume of right hippocampus receiving >40 Gy, whereas multivariate regression analysis showed a statistically significant decline in short delay memory recall when both the hippocampi received doses >40Gy (V40Gy).14 The results from the St Jude study gave a more pragmatic dose constraint required to be achieved to maintain long-term and short-term memory. Both the above studies looked at hippocampal dose in a homogeneous cohort of low-grade gliomas as compared with our study, which had diverse histologies. In the St Jude study, all the children aged 6–21 years (median 9.5 y) had centrally placed tumors, which could have resulted in irradiation exposure to both the hippocampi to a critical threshold, resulting in both the hippocampi being significantly associated with decline in various domains of memory recall. On the contrary, the cohort of children in our study had a higher median age (13 y) and had diverse tumor histologies. Additionally, the lesions were located at both central and peripheral areas of the brain that could have resulted in significant differences in the radiation dose levels to both the hippocampi. We therefore hypothesize that, in view of the peripherally placed lesions (approximately 60% in our cohort of patients), radiation doses to only one of the hippocampi was significantly associated with profound neurocognitive decline, which in our case was the left hippocampus dose.
It may be argued that the mean left hippocampal dose of 30 Gy seems to be high in predicting the neurocognitive outcomes in our cohort of children, given the data on hippocampal-sparing WBRT (D100 of <9 Gy; Dmax of 16 Gy) based on the RTOG 0933 study.31 However, the differences in the hippocampal dose constraints between our study and the RTOG study could be attributed to the fact that the RTOG study had brain metastasis patients in whom the baseline neurocognition is itself low due to poor performance status of the patients. Moreover, all the brain metastasis patients received hypofractionated WBRT (3 Gy/fraction) in the RTOG 0933 study as opposed to our study where focal conformal RT was given at conventional dose per fraction (1.8 Gy per fraction), which could have allowed for a more relaxed hippocampal dose threshold as opposed to stringent hippocampal dose constraints obtained while treating brain metastasis patients with hypofractionated whole-brain radiation.
Multivariate analysis using binary logistic regression showed age <13 years and mean dose of the left hippocampus and patients with diagnosis of craniopharyngiomas to be independent predictors for clinically significant long-term FSIQ impairment, while mean left hippocampus dose was an independent predictor for PQ decline (Table 5). Age at radiation exposure is considered to be an important factor as children and elderly are prone to develop neurocognitive decline as opposed to adults.27 The fact that craniopharyngiomas had greater NCF impairment than gliomas could be due to the greater proximity of these centrally placed tumors as opposed to the gliomas that were located both in the supra and infratentorial region away from the hippocampi. Various literature studies have reported a decline in neurocognition in patients with hydrocephalus.28–30 Hydrocephalus may affect measures of various domains of NCF via increased intracranial pressure stretching and distorting various neural pathways. Post RT, we observed a greater improvement in IQ scores across all the measured NCF domains in patients with mild to moderate hydrocephalus as opposed to severe hydrocephalus, although the difference in the scores was not statistically significant (Table 5).
The present study is unique in the sense that the RT planning was done without giving any constraints to hippocampus and a post-hoc statistical analysis revealed the dose levels required to prevent the neurocognitive decline in terms of FSIQ using WISC. These promising results warrant further validation within the phase III setting, in part because of the expected limitations of a retrospective study wherein complete data were not available at every time point. Summing up our clinical observations, therefore, may provide a rationale for exploring the hypothesis that conformal avoidance of the hippocampus using IMRT may spare patients some of the cognitive sequelae of cranial irradiation.
Conclusion
Doses to hippocampi appear to correlate significantly with IQ decline (>30 Gy mean dose to the left hippocampus). We propose a mean dose of ≤30 Gy to the left hippocampus as a dose constraint for predicting IQ decline, based on long-term prospective data. Contemporary RT techniques like IMRT, volumetric arc RT, and IM proton therapy may have the potential to achieve these relatively simple constraint models in routine clinical practice.
Funding
This work was supported by the Tata Memorial Centre and Terry Fox India Committee.
Conflict of interest statement. None of the authors have any conflicts of interests to declare.
Supplementary Material
Acknowledgments
We are grateful to Tata Memorial Centre, Terry Fox India Committee, and the Brain Tumor Foundation (BTF) India for the financial support. We would also like to thank our colleagues from neurosurgery, pediatric oncology, and medical physics for patient referral and management. Special thanks to Nayana Golambade for the administrative and secretarial assistance for the smooth implementation of the trial.
References
- 1. Merchant TE, Conklin HM, Wu S, Lustig RH, Xiong X. Late effects of conformal radiation therapy for pediatric patients with low-grade glioma: prospective evaluation of cognitive, endocrine, and hearing deficits. J Clin Oncol. 2009;27(22):3691–3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Marcus KJ, Goumnerova L, Billett AL, et al. Stereotactic radiotherapy for localized low-grade gliomas in children: final results of a prospective trial. Int J Radiat Oncol Biol Phys. 2005;61(2):374–379. [DOI] [PubMed] [Google Scholar]
- 3. Jalali R, Gupta T, Goda JS, et al. Efficacy of stereotactic conformal radiotherapy vs conventional radiotherapy on benign and low-grade brain tumors: a randomized clinical trial. JAMA Oncol. 2017;3(10):1368–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Eekers DBP, In ‘t Ven L, Deprez S, et al. The posterior cerebellum, a new organ at risk? Clin Transl Radiat Oncol. 2018;8:22–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jalali R, Mallick I, Dutta D, et al. Factors influencing neurocognitive outcomes in young patients with benign and low-grade brain tumors treated with stereotactic conformal radiotherapy. Int J Radiat Oncol Biol Phys. 2010;77(4):974–979. [DOI] [PubMed] [Google Scholar]
- 6. Noll KR, Ziu M, Weinberg JS, Wefel JS. Neurocognitive functioning in patients with glioma of the left and right temporal lobes. J Neurooncol. 2016;128(2):323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gondi V, Tomé WA, Mehta MP. Why avoid the hippocampus? A comprehensive review. Radiother Oncol. 2010;97(3):370–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hoffmann M. The human frontal lobes and frontal network systems: an evolutionary, clinical, and treatment perspective. ISRN Neurol. 2013;2013:892459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Peissner W, Kocher M, Treuer H, Gillardon F. Ionizing radiation-induced apoptosis of proliferating stem cells in the dentate gyrus of the adult rat hippocampus. Brain Res Mol Brain Res. 1999;71(1):61–68. [DOI] [PubMed] [Google Scholar]
- 10. Raber J, Rola R, LeFevour A, et al. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat Res. 2004;162(1):39–47. [DOI] [PubMed] [Google Scholar]
- 11. Monje ML, Mizumatsu S, Fike JR, Palmer TD. Irradiation induces neural precursor-cell dysfunction. Nat Med. 2002;8(9):955–962. [DOI] [PubMed] [Google Scholar]
- 12. Gondi V, Hermann BP, Mehta MP, Tomé WA. Hippocampal dosimetry predicts neurocognitive function impairment after fractionated stereotactic radiotherapy for benign or low-grade adult brain tumors. Int J Radiat Oncol Biol Phys. 2012;83(4):e487–e493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gondi V, Pugh SL, Tome WA, et al. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol. 2014;32(34):3810–3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Acharya S, Wu S, Ashford JM, et al. Association between hippocampal dose and memory in survivors of childhood or adolescent low-grade glioma: a 10-year neurocognitive longitudinal study. Neuro Oncol. 2019;April 12. doi: 10.1093/neuonc/noz068. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302(5651):1760–1765. [DOI] [PubMed] [Google Scholar]
- 16. Ihunwo AO, Tembo LH, Dzamalala C. The dynamics of adult neurogenesis in human hippocampus. Neural Regen Res. 2016;11(12):1869–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tsai PF, Yang CC, Chuang CC, et al. Hippocampal dosimetry correlates with the change in neurocognitive function after hippocampal sparing during whole brain radiotherapy: a prospective study. Radiat Oncol. 2015;10:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rubin RD, Watson PD, Duff MC, Cohen NJ. The role of the hippocampus in flexible cognition and social behavior. Front Hum Neurosci. 2014;8:742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kazda T, Jancalek R, Pospisil P, et al. Why and how to spare the hippocampus during brain radiotherapy: the developing role of hippocampal avoidance in cranial radiotherapy. Radiat Oncol. 2014;9:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kothavade V, Jamema SV, Gupta T, et al. Which is the most optimal technique to spare hippocampus? Dosimetric comparisons of SCRT, IMRT, and tomotherapy. J Cancer Res Ther. 2015;11(2):358–363. [DOI] [PubMed] [Google Scholar]
- 21. Gondi V, Tolakanahalli R, Mehta MP, et al. Hippocampal-sparing whole-brain radiotherapy: a “how-to” technique using helical tomotherapy and linear accelerator-based intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010;78(4):1244–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stoker J, Vora S, Patel A, et al. Advantages of intensity modulated proton therapy during hippocampal avoidance whole brain radiation therapy. Phys Imag Radiat Oncol. 2018;8:28–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ding X, Zhou J, Li X, et al. Improving dosimetric outcome for hippocampus and cochlea sparing whole brain radiotherapy using spot-scanning proton arc therapy. Acta Oncol. 2019;58(4):483–490. [DOI] [PubMed] [Google Scholar]
- 24. Patrice AF, Taylor R, Hugtenburg R, Lambert J, Powell J. Hippocampal sparing radiotherapy in adults with primary brain tumors: a comparative planning and dosimetric study using IMPT, IMRT and 3DCRT. Neuro Oncol. 2019;21(4):2–3. [Google Scholar]
- 25. Cherlow JM, Shaw DWW, Margraf LR, et al. Conformal radiation therapy for pediatric patients with low-grade glioma: results from the Children’s Oncology Group phase 2 study ACNS0221. Int J Radiat Oncol Biol Phys. 2019;103(4):861–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zureick AH, Evans CL, Niemierko A, et al. Left hippocampal dosimetry correlates with visual and verbal memory outcomes in survivors of pediatric brain tumors. Cancer. 2018;124(10):2238–2245. [DOI] [PubMed] [Google Scholar]
- 27. Taphoorn MB, Martin K. Cognitive deficits in adult patients with brain tumors. Lancet Neurol. 2004;3(3):159–168. [DOI] [PubMed] [Google Scholar]
- 28. Aarsen FK, Willem FMA, Van Veelen-Vincent MLC, Lequin MH, Coriene E, Catsman-Berrevoets CE. Long-term outcome in children with low grade tectal tumors and obstructive hydrocephalus. Eur J Paediatr Neurol. 2014;18(4):469–474. [DOI] [PubMed] [Google Scholar]
- 29. Di Rocco C, Chieffo D, Pettorini BL, Massimi L, Caldarelli M, Tamburrini G. Preoperative and postoperative neurological, neuropsychological and behavioral impairment in children with posterior cranial fossa astrocytomas and medulloblastomas: the role of the tumor and the impact of the surgical treatment. Childs Nerv Syst. 2010;26(9):1173–1188. [DOI] [PubMed] [Google Scholar]
- 30. Scott MA, Fletcher JM, Brookshire BL, et al. Memory functions in children with early hydrocephalus. Neuropsychology. 1998;12(4):578–589. [DOI] [PubMed] [Google Scholar]
- 31. Gondi V, Pugh SL, Tome WA, et al. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol. 2014;32(34):3810–3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.