Abstract
Background
Successful management of pediatric low-grade glioma (pLGG) can be complicated by eloquent anatomical location, as well as specific pathologic and molecular features. Some authors have proposed using the VEGF inhibitor bevacizumab to improve disease control, but its safety and efficacy are poorly defined. Correspondingly, our aim was to pool systematically identified clinical data in the literature to assess the clinical utility of bevacizumab for pLGG at progression.
Methods
A systematic search of 7 electronic databases from inception to June 2019 was conducted following PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) guidelines. Articles were screened against prespecified criteria. Outcomes were then pooled by random-effects meta-analyses of proportions.
Results
Seven pertinent studies described the outcomes of 110 progressive pLGG patients managed with bevacizumab in largely multiagent regimens. While on treatment, the rate of clinical response was 58% (95% CI, 43%-72%), and the rate of response on imaging was 80% (95% CI, 58%-96%). The rate of grade 3 or higher toxicity was 8% (95% CI, 2%-17%), with proteinuria the most commonly described. In the off-treatment period up to median 1 year, the rate of progression was estimated to be 51% (95% CI, 28%-74%).
Conclusions
Bevacizumab has the potential to control clinical and radiographic disease with relatively low grade 3 or higher toxicity risk in progressive pLGG patients. However, the long-term off-treatment benefits of this therapy are not yet well defined. Heterogeneity in the literature precludes any formal recommendations regarding its use until larger, more standardized investigations can be performed.
Keywords: Avastin, bevacizumab, low-grade glioma, pediatric, safety
Pediatric low-grade gliomas (pLGGs) are a diverse group of World Health Organization (WHO) grades I and II gliomas in children that comprise about one-third of all pediatric brain tumors.1 Although surgical resection is the mainstay primary treatment for many of these tumors, pLGGs that are refractory or inaccessible to surgical resection can recur or progress, leading to increased risk of long-term morbidity and mortality.2–4 Owing to tumor location and neurodevelopmental concerns, surgery and radiation therapy respectively are not universally indicated for pLGGs, highlighting the need to explore novel drug agents to continue to optimize disease control.5–8
There have been multiple chemotherapeutic agents tested in the setting of progressive pLGG to understand whether surgical limitations and radiation restrictions can be circumnavigated for long-term control.9–12 However, there is a lack of high-quality evidence suggesting these interventions confer universal long-term disease control.13 More recently, the efficacy of the VEGFA inhibitor bevacizumab (Genentech, Avastin) has been demonstrated to increase progression-free survival in adult high-grade glioma,14–17 with emerging anecdotal series about its use in the setting of pLGG13,18–23 conferring both tumor volume reduction and clinical symptomatology resolution. Clinically, improved visual field and acuity, improved motor function, weight gain in diencephalic syndrome, and reversal of psychomotor retardation have all been reported to occur in pLGG patients with bevacizumab treatment specifically, highlighting it as a “novel” therapy that warrants serious consideration to further improve the prognosis of pLGG patients.22,24
However, a recent trial investigating bevacizumab in the setting of pediatric high-grade glioma has raised concerns about safety in this specific age group—all participants experienced at least 1 adverse event, the most common being proteinuria and thromboembolic events, and the majority of these patients required dose modification, if not discontinuation, in response.25 Correspondingly, the aim of this study was to assess the efficacy and safety of bevacizumab in the setting of progressive pLGG as reported in the literature to better inform clinical practice.
Methods
Search Strategy
The search strategy was designed using the Population, Intervention, Comparison, Outcome and Study type (PICOS) format. Specifically, the research question was, Among patients with progressive pLGG (Population) treated with bevacizumab (Intervention and Comparator), what are clinical and survival outcomes (Outcome) based on observational studies (Study type) during and after treatment? A systematic review of the literature was conducted in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines and recommendations.26 Electronic searches were performed using Ovid Embase, PubMed, SCOPUS, and Cochrane databases from inception through June 2019. Records were screened independently by 2 investigators (V.M.L. and J.P.W.) using the following string of terms: (bevacizumab OR Avastin) AND (pediatric OR children) AND (glioma), with sample query and term translations provided in Supplementary Table 1.
Selection Criteria
Included articles reported patient cohorts 1) with confirmed progressive LGG (WHO grades I and II), 2) age 18 years or younger at time of initial bevacizumab treatment, 3) with bevacizumab as part of their treatment, and 4) with at least response or adverse event outcome reported. Patients presenting with recurrent disease were considered progressive in this context. No restrictions were made regarding prior treatment or concomitant use of other agents in combination with bevacizumab. Studies were excluded from analysis if they assessed 1) adult patients, 2) patients with high-grade glioma, 3) patients with nonglioma pathology, and 4) outcomes for fewer than 3 patients. For institutions publishing serial overlapping cohorts, the larger, more clinically complete study was included for quantitative assessment. Case reports, editorials, reviews, and abstracts were likewise excluded from analysis. Only publications in English were considered for review.
Data Extraction
In accordance with PRISMA guidelines, outcomes were extracted directly from article text, tables, and figures independently by 2 investigators (V.M.L. and J.P.W.). The primary end points were clinical response, response on imaging, adverse events while on treatment, and radiographic progression off treatment. Because there is no clear standard number of bevacizumab treatment cycles for pLGG, the actual number of cycles administered was allowed to be study specific, indicative of patient-dependent tolerability. Clinical response was defined as a favorable change in at least 1 clinical symptom, such as improved visual acuity, hemiparesis, or gait instability. A response on imaging was defined as a greater than 25% reduction in tumor volume from baseline dimensions on MR imaging in the largest bidirectional area as reported by study investigators. This included complete, partial, and minor responses as based on the previously used scale used to study bevacizumab in pLGG patients specifically.24 Similarly, radiographic progression was defined as a greater than 25% sustained increase in tumor volume off treatment compared to last known volume on treatment.24 Grade 3 or higher toxicities were defined according to Common Terminology Criteria for Adverse Events 4.0.27 In brief, grade 3 complications are so disabling, severe, or medically significant but not immediately life-threatening that admission or prolongation of hospitalization is indicated. Grade 4 complications have potentially life-threatening consequences mandating urgent medical intervention.
Meta-Analysis
The incidence rates of the previously stated outcomes were the primary summary statistics of this study. Incidence was calculated with initial variance by Fisher exact test for binomial data, and then transformed by Freeman-Tukey transformation to stabilize the variances.28 All statistics were pooled by meta-analysis of proportions using the random-effects model described by DerSimonian and Laird29 to provide the overall study statistic. Heterogeneity was assessed using I2 for random-effects modeling, with values greater than 50% indicating substantial heterogeneity.30 Meta-analytic data were presented as forest plots. All P values were 2-sided, and significance was defined using the alpha threshold .05. All statistical analyses were conducted with STATA 14.1 (StataCorp).
Certainty, Quality, and Bias Assessment
The certainty of each outcome was evaluated using the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) approach and presented as a summary of findings to identify the certainty of pooled outcomes.31 The quality of evidence for each study was then evaluated using a modified Newcastle-Ottawa scale32 for assessment of single-arm cohort studies.33 Overall methodologic quality was then summarized based on the quality of trends observed. In terms of bias for each outcome, when the number of studies exceeded 10, publication bias was assessed using funnel plots, and small-study biases were evaluated using the Egger linear regression test and Begg correlation test.34,35
Results
Search Results
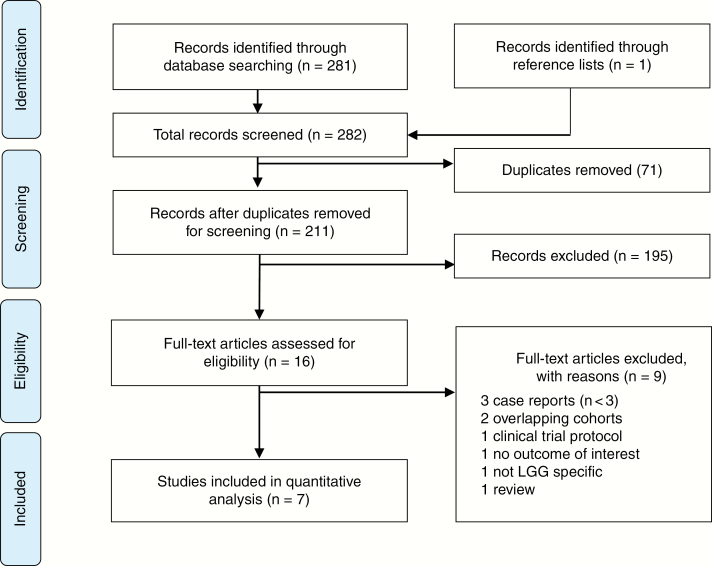
The primary search produced 282 articles. Following removal of 71 duplicate citations, the titles and abstracts of 211 articles were screened against the selection criteria (Fig. 1). Sixteen abstracts were deemed relevant to the study question and met the criteria, without any discrepancies. Six retrospective studies,13,18,19,21–23 and 1 prospective (Pediatric Brain Tumor Consortium, PBTC) phase 2 trial20 published between 2012 and 2019 satisfied all selection criteria after full-text screening (Table 1). Avery et al36 and Packer and colleagues24 were excluded because of cohort overlap with Hwang et al.13
Fig. 1.
Search Results According to PRISMA Guidelines LGG indicates low-grade glioma; PRISMA, Preferred Reporting Items for Systematic reviews and Meta-Analyses.
Table 1.
Study Characteristics and Basic Cohort Demographics
| Study | Primary Location | Designa | Study Period | Cohort Size, No. | Female, No., % | Median Age at First Treatment, y | LGG Included | NF1, No., % | Mean Follow-Up After Bevacizumab, mo |
|---|---|---|---|---|---|---|---|---|---|
| Zhukova et al, 201923 | Melbourne, Australia | R OCS (2) | 2014-2017 | 15 | 6, 40% | 7 | 9 PA, 1 PXA, 1 GG, 4 RC | 2, 13% | 11 |
| Gorsi et al, 201819 | San Diego, CA, US | R OCS (1) | 2012-2017 | 15 | 10, 67% | 7 | 10 PA (2 OPG), 4 LGG, 1 RC | 1, 7% | 5 |
| Kalra et al, 201522 | Sydney, Australia | R OCS (1) | 2009-2013 | 16 | 7, 44% | 8.6 | 10 PA (6 OPG), 1 PXA, 1 LGG, 4 RC | 5, 31% | 9.2 |
| Hsu et al, 201521 | Stanford University, Palo Alto, CA, US | R OCS (1) | 2009-2013 | 8 | 2, 25% | 4.8 | 4 OPG (1 RC), 3 PA, 1 GG | 2, 25% | 49 |
| Gururangan et al, 201420 | Duke University, Durham, NC, US | P OCS (multiple) | 2008-2010 | 35 | NR | 8.4 | 16 PA, 12 OPG (12 RC), 1 GG, 1 LGG | 5, 15% | 5.1 |
| Hwang et al, 201213 | Washington, DC, US | R OCS (4) | 2006-2009 | 14 | NR | 5.3 | 4 PA, 4 LGG, 6 RC (4 OPG) | 3, 21% | >12 |
| Couec et al, 201218 | Nantes, France | R OCS (7) | 2007-2010 | 7 | NR | 10 | 7 PA (2 OPG) | NR | 12 |
Abbreviations: GG, ganglioglioma; LGG, low-grade glioma; NF1, neurofibromatosis 1; NR, not reported; OCS, observational cohort study; OPG, optic pathway glioma; P, prospective; PA, pilocytic astrocytoma; PXA, pleomorphic xanthroastrocytoma; R, retrospective; RC, radio-clinical; US, United States of America.
aNumber of institutions involved in parentheses.
Demographics and Clinical Features
In total, the outcomes of 110 pLGG patients treated by bevacizumab at progression were included in our analysis. The proportion of female patients ranged from 25% to 67% where reported, and median age at first treatment was 7 years (range, 4.8-10 years) (Table 1). The most common histopathology reported was pilocytic astrocytoma, with 7% to 31% of included patients having neurofibromatosis type 1. Median follow-up time after bevacizumab therapy was 11 months (range, 5-49 months).
In terms of prior treatment, surgical resection was performed in 0% to 70% of patients, radiation therapy in 0% to 27%, and previous chemotherapy in 75% to 100% (Table 2). All studies dosed bevacizumab at 10 mg/kg every 2 weeks, with the median number of doses ranging from 10 to 24 doses equating to 5 to 48 months on therapy. Only 2 studies19,21 reported monotherapy administration of bevacizumab, with the most common combination otherwise being dual therapy with irinotecan.
Table 2.
Clinical Features of Included Studies
| Study | Cohort Size, No. | Previous Debulking, No., % | Previous Radiation, No., % | Previous Chemotherapy, No., % | Bevacizumab Regimen (Median No. of Doses) | Other Agents | Clinical Responses | Serious Adverse Events With Bevacizumab | Progression, No., % |
|---|---|---|---|---|---|---|---|---|---|
| Zhukova et al, 201923 | 15 | 1, 7% | 1, 7% | 15, 100% | 10 mg/kg every 2 wks (16) | Multiplea | 4 improved vison, 2 improved motor, 1 improved endocrine | 2 proteinuria | 5, 33% |
| Gorsi et al, 201819 | 15 | 0 | 4, 27% | 12, 80% | 10 mg/kg every 2 wks (10) | No | 7 improved motor, 4 improved vision | 1 bone pain, 1 hyperglycemia, 1 hyperkalemia | 11, 73% |
| Kalra et al, 201522 | 16 | 11, 70% | 3, 19% | 16, 100% | 10 mg/kg every 2 wks (24) | Irinotecan | 3 improved headache, 2 improved vision, 2 improved motor | 2 nauseab | 4, 25% |
| Hsu et al, 201521 | 8 | 0 | 0 | 6, 75% | 10 mg/kg every 2 wks (16) | No | NR | 0 | NR |
| Gururangan et al, 201420 | 35 | 0 | 2, 6% | 35, 100% | 10 mg/kg every 2 wks (12) | Irinotecan | NR | 3 proteinuria, 4 neutropeniab, 2 diarrheab | 20, 57% |
| Hwang et al, 201213 | 14 | 4, 30% | 3, 21% | 13, 93% | 10 mg/kg every 2 wks (24) | Irinotecan | 4 improved vision, 4 improved endocrine, 2 improved ataxia, 1 improvement motor | 1 proteinuria, 1 fatigue, 1 gastrointestinal, 1 synovitis | 13, 93% |
| Couec et al, 201218 | 7 | NR | NR | NR | 10 mg/kg every 2 wks (16) | Irinotecan | NR | 0 | 1, 14% |
Abbreviation: NR, not reported.
aIrinotecan, carboplatin, vinblastine, vincrinstine, and lomustine.
bIrinotecan-associated event.
On-Treatment Outcomes
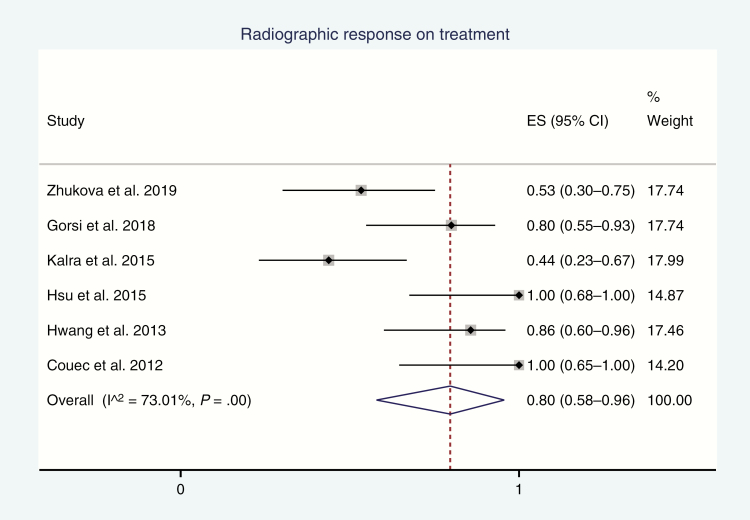
The 6 retrospective studies reported responses on imaging to bevacizumab,13,18,19,21–23 and the pooled rate was 80% (95% CI, 58%-96%; I2 = 73%; P-heterogeneity < .01; Fig. 2). All studies except 1 used a threshold of 25% tumor volume reduction for response, with Couec et al18 using a 50% threshold value. Notably, the prospective PBTC study by Gururangan and colleagues20 reported sustained response on imaging in only 2 of 29 (7%) of their eligible cohort; however, it was not pooled with the other studies because of concerns about its retrospective design given that they considered responses occurring only within the first 4 courses of bevacizumab and lasted for at least 8 weeks.
Fig. 2.
Forest Plot of Incidence of Favorable Responses on Imaging (Regression or Stabilization) on Treatment Effect size (ES) of incidence, its 95% CI, and relative weightings are represented by the middle of the square, the horizontal line, and the relative size of the square, respectively.
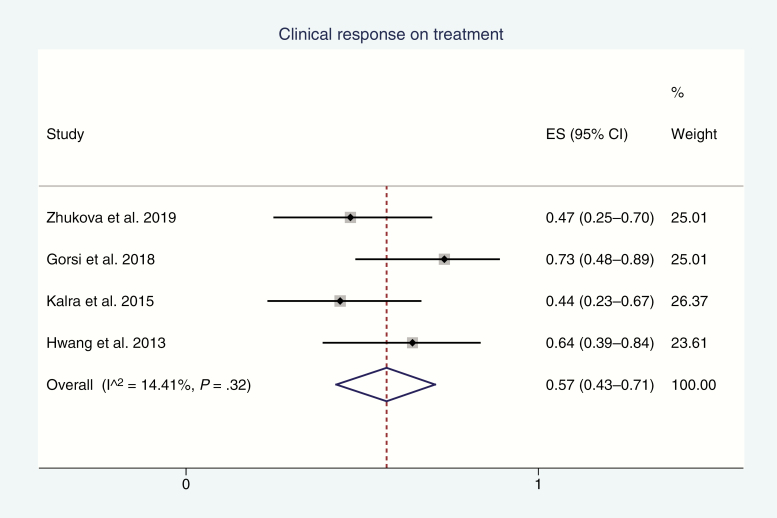
Clinical response was reported by 4 studies13,19,22,23 for a pooled response rate of 57% (95% CI, 43%-71%; I2 = 14%; P-heterogeneity = .32; Fig. 3). The most common clinical response was improvement in vision (visual field), with improvements in motor and endocrinologic function, and headaches also noted (Table 2).
Fig. 3.
Forest Plot of Incidence of Favorable Clinical Response (ie, Symptom Resolution or Improvement) on Treatment
Grade 3 or higher toxicity was evaluated by all 7 studies.13,18–23 Pooled incidence of the 6 retrospective studies yielded an incidence of 12% (95% CI, 5–22%; I2 = 10%; P-heterogeneity = .35) (Supplementary Fig. 1). The most common toxicity was proteinuria, with other relevant complications including bone pain, hyperglycemia, hyperkalemia, fatigue, gastrointestinal toxicity, and synovitis (Table 2). The prospective PBTC study by Gururangan et al20 reported grade 3 or higher toxicity in only 3 of 35 (9%) of their eligible cohort. No significant hemorrhagic events were reported in any study.
Off-Treatment Outcomes
At last clinical follow-up, pLGG progression or recurrence after bevacizumab treatment was reported by 5 retrospective studies13,18,19,22,23 at an estimated rate of 50% (95% CI, 20%-80%; I2 = 84%; P-heterogeneity < .01), with a median follow-up time of 11 months (range, 5-49 months) after cessation of treatment (Supplementary Fig. 2). The prospective PBTC study by Gururangan et al20 reported off-treatment progression in 12 of 35 (44%) of their eligible cohort at a median 5 months after cessation of treatment. No significant off-treatment bleeding events were reported during this period in any study.
Certainty Assessment
All outcomes were assessed for certainty using the GRADE criteria (Table 3). The certainty of response on imaging was deemed very low because of quality and consistency concerns. The certainty of clinical response was deemed low because of generalizability and sparsity of data concerns. The certainty of grade 3 or higher toxicity was deemed moderate. Finally, the certainty of progression was deemed very low because of the heterogeneous nature of recurrence and follow-up concerns.
Table 3.
GRADE Assessment for Reported Outcomes
| Certainty Assessment | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcome | Pooled Incidence (95% CI) | No. of Cohort Studies | Type of Evidence | Quality | Consistency | Directness | Effect Size | Overall Quality | Certainty |
| Radiographic response | 80% (58%-96%) | 6 | +2 | –1 | –1 | 0 | +1 | +1 | Very low |
| Clinical response | 57% (43%-71%) | 4 | +2 | –2 | +1 | –1 | +2 | +2 | Low |
| Serious adverse event | 8% (2%-17%) | 7 | +2 | –1 | +1 | 0 | +1 | +3 | Moderate |
| Progression | 51% (28%-74%) | 6 | +2 | –2 | –1 | 0 | +2 | +1 | Very low |
Abbreviation: GRADE, Grading of Recommendations, Assessment, Development and Evaluations.
Overall quality score is determined based on the sum of included domains. Type of evidence is based on design of included studies (range, +2 to +4). Study quality reflects blinding and allocation, follow-up and withdrawals, sparsity of data, and methodological concerns (range, –3 to 0). Consistency is graded based on heterogeneity of included population and study end points with respect to one another (range, –1 to +1). Directness is graded based on generalizability of included results (range, –2 to 0). Effect size is graded based on the overlap of 95% CI estimates within 10% of either 0% or 100% incidence (range, 0 to 2). The overall quality of results for each outcome can be considered high (≥ 4 points), moderate (3 points), low (2 points), or very low (≤ 1 point).
Quality and Bias Assessments
For the purposes of this study, the quality of evidence from the included studies ranged from high to moderate according to the modified Newcastle-Ottawa Scale criteria (Supplementary Table 2). The primary reason for quality deficiency was the lack of reporting of all our 4 outcomes of interest. The risks of publication and small-study biases could not be reliably performed because of limited cohort numbers (≤ 10) and so were not conducted.
Discussion
The optimal management for progressive pLGG is not well defined. Our study aimed to summarize the efficacy and safety of the anti-angiogenic agent bevacizumab in this setting, either in single-agent or multiagent intervention protocols. Based on the studies pooled in the current literature, clinical and imaging improvements were observed in approximately 3 in 5 and 4 in 5 patients while on treatment, respectively, with 1 in 10 patients experiencing a grade 3 or higher toxicity. Following treatment cessation, progression was estimated to occur in half the cases at a median time of 1-year posttherapy. These data will assist clinicians in prognosticating patients with progressive or recurrent pLGG considering therapy with bevacizumab as part of management.
The propensity for many types of pLGG to progress or recur mandates the exploration for effective interventions that afford favorable disease control. Repeat surgery and radiation therapy are effective modalities, but their clinical utility is limited to a subset of patients because of involvement of eloquent regions and the pediatric brain’s vulnerability to radiation.37,38 For instance, optic pathway involvement is often a contraindication to further resection and radiation, and the improvement in vision in an appreciable proportion of pLGGs presented here supports bevacizumab as an alternative intervention in these patients. Zhukova and colleagues23 reported that all pLGG patients with visual deficits had either improved or stabilized after the commencement of bevacizumab. Other reported clinical improvements, such as motor and endocrine responses, likely reflect the alleviation of glioma-associated tumor burden and peritumoral edema, as in the setting of recurrent glioblastoma.39,40 Our analysis indicates that not all pLGG patients will have a clinical response to bevacizumab; however, not all indications to use bevacizumab in progressive pLGG require a clinical symptom to target vs prevent further radiographic progression, for example. Greater granularity as to specific clinical indications and contraindications is needed to better frame its application for this aspect.
In terms of imaging, bevacizumab resulted in either regression or stability in a majority of pLGG patients. When the clinical use of bevacizumab was first conceived for glioblastoma, its antiglioma effects were envisaged to be more amenable to radiographic than clinical detection, which appears to also apply to pLGG based on our results.41 However, at least in the context of pLGG, response on imaging alone may not prove the most useful metric of success given the large variance in defining success. In particular, the prospective PBTC study by Gururangan et al20 observed only a 2 of 29 (7%) response rate when requiring responses to occur within the first 4 courses of bevacizumab and be sustained for 8 weeks. This rate was drastically lower than all other retrospective pooled studies, although none of those studies mandated an 8-week period of response maintenance. Ultimately, the combination of the relatively insidious course of LGG and the longer life expectancy potential of the pediatric demographic implies that other aspects, such as quality of life and cognitive function, posttherapy may also be just as insightful for the prognosis of pLGG patients.2,3,42,43 Nevertheless, the reported rates of regression and stabilization of pLGG by imaging while on treatment is an encouraging therapeutic result to appreciate.
The overall incidence of grade 3 or higher toxicity following bevacizumab therapy in pLGG was 12% in our study, with no cases of intracranial hemorrhage reported. In fact, the overall complication rate was significantly lower than the rate of similarly graded events in adult glioblastoma,14 as well as pediatric high-grade glioma.25 The most common serious complication requiring medical intervention and/or therapy discontinuation was hyperproteinuria, a known associated adverse event with bevacizumab thought to result from VEGF and other growth factors disrupting the podocytes and increasing permeability.44–47 Although manageable by bevacizumab discontinuation, we posit temporizing measures such as therapy “holidays” to prevent therapy discontinuation may one day be validated for pLGG cohorts based on the experience of other pediatric tumors, such as those in neurofibromatosis type 2,48 to allow for prolonged therapy and further optimized clinical efficacy and safety. This highlights further that greater experience with bevacizumab in other pediatric tumors may assist in identifying pLGG patients most amenable to treatment in the future because anecdotal reports in vestibular schwannoma cohorts suggest that efficacy is maximal following partial surgical, not complete, resection.49 Nevertheless, the relatively low incidence overall of grade 3 or higher toxicity in the current literature appears to support the judicious use of bevacizumab for pLGG management.50–52
The impact of bevacizumab on long-term progression-free and overall survival of patients with pLGG is unclear. Although on-treatment outcomes were generally favorable, about half of patients progressed following cessation of bevacizumab, albeit with a high degree of statistical heterogeneity. One possible explanation for this is the variation in on-therapy duration, on which there is currently no consensus and that varied greatly among the included studies. Hwang and colleagues13 tested the hypothesis of repeated bevacizumab courses in pLGG affecting long term-control and noted that early favorable efficacy was not attenuated on subsequent readministrations. Therefore, although the optimal duration of bevacizumab therapy is unclear, the response of pLGG to bevacizumab appears reproducible between cycles in well-selected pLGG patients. At a basic science level, this is supported by the theory that repeat induction of telomere senescence results in superior proliferation control in vitro, with a transcriptional synergy posited between telomere reverse transcriptase activity and VEGFA activity.53,54 In the PBTC study by Gururangan et al,20 approximately 80% of all pLGG patients were able to be sustained on bevacizumab therapy for at least 6 months, suggesting such a time frame as a plausible goal for future studies to investigate.
Strengths and Limitations
By pooling the literature, we have been able to accumulate a cohort size sufficient for analysis, which to date has been a severe limitation in the statistical evaluation of bevacizumab in the pLGG setting. By limiting the cohort to pediatric patients and lower-grade tumors only, interstudy heterogeneity and external validity have been maximized as much as practically possible.
There are limitations of the current literature that affect the findings of our study. First, given the mostly retrospective nature of the included studies, we cannot completely disregard intrinsic publication and selection biases within each study affecting how true our pooled outcomes are. We recognize that the prospective PBTC study by Gururangan et al20 separated itself from most studies in terms of an appreciably lower response on the imaging rate on therapy, which we posit may be due to their more stringent definition of response and ability to monitor response in a prospective fashion. Therefore, there is a possibility that the outcomes rates may be underestimated in the current retrospective literature, and only more prospective studies in the future will be able to validate this concern without quantitatively pooling alongside retrospective metadata.
Next, because of the limitations of retrospective data, clinical heterogeneity between the studies is another important consideration. These studies included multiple pathologic entities encompassed under the umbrella of “pLGG,” tumor predisposition syndromes, tumor locations, and follow-up intervals, all of which may have influenced the reported clinical and imaging outcomes of this study. In addition, the duration of bevacizumab therapy was patient dependent and institution dependent in all included studies, which severely limits the accuracy of the reported outcomes and the generalizability of the current literature. Therefore, the greater certainty in the adverse events outcome compared to response outcomes should be taken into consideration when considering applying these results to practice.
The incidence of multimodal chemotherapy could have also affected the clinical results to an uncertain degree. For instance, chemotherapy-induced proteinuria is greater when bevacizumab is combined with another chemotherapy agent.55 Until a more standardized regimen is tested in trial, it is unclear how the short-term and long-term efficacy of bevacizumab are modulated by time and use with another agent. Additionally, as we continue to broaden our understanding of molecular and biologic markers of different pLGG types, it is not improbable that bevacizumab may be shown to confer more persistent efficacy in a subgroup yet to be identified.6,56
Response and follow-up data are currently lacking in the literature. More robust analysis in the future of different response strata on imaging will assist us in understanding what constitutes a therapeutically relevant response because the required granularity in response thresholds to do so is lacking because of low statistical power in the small cohorts to date. Details regarding clinical symptom outcomes can also be enhanced—vision outcomes could summarize numerical measures of visual field and acuity, and neurological outcomes were not uniformly commented on in the current literature but would provide an additional dimension for clinicians in the future to consider. However, this should be pursued with caution because clinical assessment in particularly young children may not generate the most reproducible data given highly variable abilities to follow commands in that age group. Finally, longer-term surveillance of these patients with robust molecular workup will facilitate greater understanding of off-therapy outcomes, primarily that of time to and true incidence of progression as well as the duration of benefits this therapy confers in various pLGG types once treatment is ceased. Other parameters as previously mentioned, such as quality of life and cognition, may prove the difference in advocating for bevacizumab therapy for pLGG given the relatively high progression rate off therapy.
Conclusion
Bevacizumab has the potential to demonstrate imaging and clinical responses in 3 in 5 and 4 in 5 patients while on treatment, respectively, whereas 1 in 10 patients are estimated to experience grade 3 or higher toxicity. Despite these encouraging findings, it appears that 1 in 2 patients will progress following cessation of bevacizumab within the first year off therapy, limiting its utility as a long-term treatment using current dosing regimens based on the current metadata. The use of bevacizumab in the context of progressive pLGG requires a higher degree of clinical and temporal granularity before any recommendations can be made because of the high degree of variance in current reported management.
Supplementary Material
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement. None declared.
References
- 1. Ostrom QT, Gittleman H, Truitt G, Boscia A, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2011-2015. Neuro Oncol. 2018;20(suppl 4):iv1–iv86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Beebe DW, Ris MD, Armstrong FD, et al. Cognitive and adaptive outcome in low-grade pediatric cerebellar astrocytomas: evidence of diminished cognitive and adaptive functioning in National Collaborative Research Studies (CCG 9891/POG 9130). J Clin Oncol. 2005;23(22):5198–5204. [DOI] [PubMed] [Google Scholar]
- 3. Fouladi M, Wallace D, Langston JW, et al. Survival and functional outcome of children with hypothalamic/chiasmatic tumors. Cancer. 2003;97(4):1084–1092. [DOI] [PubMed] [Google Scholar]
- 4. Qaddoumi I, Sultan I, Broniscer A. Pediatric low-grade gliomas and the need for new options for therapy: why and how? Cancer Biol Ther. 2009;8(1):4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bouffet E, Jakacki R, Goldman S, et al. Phase II study of weekly vinblastine in recurrent or refractory pediatric low-grade glioma. J Clin Oncol. 2012;30(12):1358–1363. [DOI] [PubMed] [Google Scholar]
- 6. Gajjar A, Bowers DC, Karajannis MA, Leary S, Witt H, Gottardo NG.. Pediatric brain tumors: innovative genomic information is transforming the diagnostic and clinical landscape. J Clin Oncol. 2015;33(27):2986–2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gajjar A, Sanford RA, Heideman R, et al. Low-grade astrocytoma: a decade of experience at St. Jude Children’s Research Hospital. J Clin Oncol. 1997;15(8):2792–2799. [DOI] [PubMed] [Google Scholar]
- 8. Schmandt SM, Packer RJ. Treatment of low-grade pediatric gliomas. Curr Opin Oncol. 2000;12(3):194–198. [DOI] [PubMed] [Google Scholar]
- 9. Dodgshun AJ, Maixner WJ, Heath JA, Sullivan MJ, Hansford JR.. Single agent carboplatin for pediatric low-grade glioma: a retrospective analysis shows equivalent efficacy to multiagent chemotherapy. Int J Cancer. 2016;138(2):481–488. [DOI] [PubMed] [Google Scholar]
- 10. Gnekow AK, Kortmann RD, Pietsch T, Emser A. Low grade chiasmatic-hypothalamic glioma-carboplatin and vincristin chemotherapy effectively defers radiotherapy within a comprehensive treatment strategy—report from the multicenter treatment study for children and adolescents with a low grade glioma—HIT-LGG 1996—of the Society of Pediatric Oncology and Hematology (GPOH). Klin Padiatr. 2004;216(6):331–342. [DOI] [PubMed] [Google Scholar]
- 11. Gnekow AK, Walker DA, Kandels D, et al. ; of the Low Grade Glioma Consortium and the participating centers A European randomised controlled trial of the addition of etoposide to standard vincristine and carboplatin induction as part of an 18-month treatment programme for childhood (≤16 years) low grade glioma—a final report. Eur J Cancer. 2017;81:206–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lassaletta A, Scheinemann K, Zelcer SM, et al. Phase II weekly vinblastine for chemotherapy-naive children with progressive low-grade glioma: a Canadian Pediatric Brain Tumor Consortium study. J Clin Oncol. 2016;34(29):3537–3543. [DOI] [PubMed] [Google Scholar]
- 13. Hwang EI, Jakacki RI, Fisher MJ, et al. Long-term efficacy and toxicity of bevacizumab-based therapy in children with recurrent low-grade gliomas. Pediatr Blood Cancer. 2013;60(5):776–782. [DOI] [PubMed] [Google Scholar]
- 14. Diaz RJ, Ali S, Qadir MG, De La Fuente MI, Ivan ME, Komotar RJ.. The role of bevacizumab in the treatment of glioblastoma. J Neurooncol. 2017;133(3):455–467. [DOI] [PubMed] [Google Scholar]
- 15. Musella A, Vertechy L, Romito A, et al. Bevacizumab in ovarian cancer: state of the art and unanswered questions. Chemotherapy. 2017;62(2):111–120. [DOI] [PubMed] [Google Scholar]
- 16. Rosen LS, Jacobs IA, Burkes RL. Bevacizumab in colorectal cancer: current role in treatment and the potential of biosimilars. Target Oncol. 2017;12(5):599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gilbert MR, Dignam JJ, Armstrong TS, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Couec ML, André N, Thebaud E, et al. ; Comité Pharmacologie of the SFCE Bevacizumab and irinotecan in children with recurrent or refractory brain tumors: toxicity and efficacy trends. Pediatr Blood Cancer. 2012;59(1):34–38. [DOI] [PubMed] [Google Scholar]
- 19. Gorsi HS, Khanna PC, Tumblin M, et al. Single-agent bevacizumab in the treatment of recurrent or refractory pediatric low-grade glioma: a single institutional experience. Pediatr Blood Cancer. 2018;65(9):e27234. [DOI] [PubMed] [Google Scholar]
- 20. Gururangan S, Fangusaro J, Poussaint TY, et al. Efficacy of bevacizumab plus irinotecan in children with recurrent low-grade gliomas—a Pediatric Brain Tumor Consortium study. Neuro Oncol. 2014;16(2):310–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hsu CH, Lober RM, Li MD, et al. Decreased tumor apparent diffusion coefficient correlates with objective response of pediatric low-grade glioma to bevacizumab. J Neurooncol. 2015;122(3):491–496. [DOI] [PubMed] [Google Scholar]
- 22. Kalra M, Heath JA, Kellie SJ, et al. Confirmation of bevacizumab activity, and maintenance of efficacy in retreatment after subsequent relapse, in pediatric low-grade glioma. J Pediatr Hematol Oncol. 2015;37(6):e341–e346. [DOI] [PubMed] [Google Scholar]
- 23. Zhukova N, Rajagopal R, Lam A, et al. Use of bevacizumab as a single agent or in adjunct with traditional chemotherapy regimens in children with unresectable or progressive low-grade glioma. Cancer Med. 2019;8(1):40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Packer RJ, Jakacki R, Horn M, et al. Objective response of multiply recurrent low-grade gliomas to bevacizumab and irinotecan. Pediatr Blood Cancer. 2009;52(7):791–795. [DOI] [PubMed] [Google Scholar]
- 25. Grill J, Massimino M, Bouffet E, et al. Phase II, Open-Label, Randomized, Multicenter Trial (HERBY) of bevacizumab in pediatric patients with newly diagnosed high-grade glioma. J Clin Oncol. 2018;36(10):951–958. [DOI] [PubMed] [Google Scholar]
- 26. Moher D, Liberati A, Tetzlaff J, et al. ; PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. U.S. Department of Human Health Services. Common Terminology Criteria for Adverse Events (CTCAE) v4.0 2010. http://www.eortc.be/services/doc/ctc/ctcae_4.03_2010-06-14_quickreference_5x7.pdf. Accessed March 2019.
- 28. Freeman MF, Tukey JW. Transformations related to the angular and the square root. Annals of Mathematical Statistics. 1950;21(4):607–611. [Google Scholar]
- 29. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. [DOI] [PubMed] [Google Scholar]
- 30. Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Atkins D, Best D, Briss PA, et al. ; GRADE Working Group Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa, ON: Ottawa Hospital Research Institute; 2009. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp Accessed June 13, 2019. [Google Scholar]
- 33. Murad MH, Sultan S, Haffar S, Bazerbachi F.. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med. 2018;23(2):60–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. [PubMed] [Google Scholar]
- 35. Egger M, Davey Smith G, Schneider M, Minder C.. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Avery RA, Hwang EI, Jakacki RI, Packer RJ.. Marked recovery of vision in children with optic pathway gliomas treated with bevacizumab. JAMA Ophthalmol. 2014;132(1):111–114. [DOI] [PubMed] [Google Scholar]
- 37. Bandopadhayay P, Bergthold G, London WB, et al. Long-term outcome of 4,040 children diagnosed with pediatric low-grade gliomas: an analysis of the Surveillance Epidemiology and End Results (SEER) database. Pediatr Blood Cancer. 2014;61(7):1173–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Krishnatry R, Zhukova N, Guerreiro Stucklin AS, et al. Clinical and treatment factors determining long-term outcomes for adult survivors of childhood low-grade glioma: a population-based study. Cancer. 2016;122(8):1261–1269. [DOI] [PubMed] [Google Scholar]
- 39. Ananthnarayan S, Bahng J, Roring J, et al. Time course of imaging changes of GBM during extended bevacizumab treatment. J Neurooncol. 2008;88(3):339–347. [DOI] [PubMed] [Google Scholar]
- 40. Keunen O, Johansson M, Oudin A, et al. Anti-VEGF treatment reduces blood supply and increases tumor cell invasion in glioblastoma. Proc Natl Acad Sci U S A. 2011;108(9):3749–3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Reardon DA, Turner S, Peters KB, et al. A review of VEGF/VEGFR-targeted therapeutics for recurrent glioblastoma. J Natl Compr Canc Netw. 2011;9(4):414–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. van den Bent MJ, Wefel JS, Schiff D, et al. Response assessment in neuro-oncology (a report of the RANO group): assessment of outcome in trials of diffuse low-grade gliomas. Lancet Oncol. 2011;12(6):583–593. [DOI] [PubMed] [Google Scholar]
- 43. Turner CD, Rey-Casserly C, Liptak CC, Chordas C.. Late effects of therapy for pediatric brain tumor survivors. J Child Neurol. 2009;24(11):1455–1463. [DOI] [PubMed] [Google Scholar]
- 44. Azizi AA, Schouten-van Meeteren AYN. Current and emerging treatment strategies for children with progressive chiasmatic-hypothalamic glioma diagnosed as infants: a web-based survey. J Neurooncol. 2018;136(1):127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Morris KA, Golding JF, Blesing C, et al. ; UK NF2 research group Toxicity profile of bevacizumab in the UK Neurofibromatosis type 2 cohort. J Neurooncol. 2017;131(1):117–124. [DOI] [PubMed] [Google Scholar]
- 46. Izzedine H, Rixe O, Billemont B, Baumelou A, Deray G.. Angiogenesis inhibitor therapies: focus on kidney toxicity and hypertension. Am J Kidney Dis. 2007;50(2):203–218. [DOI] [PubMed] [Google Scholar]
- 47. Plotkin SR, Duda DG, Muzikansky A, et al. Multicenter, prospective, phase II and biomarker study of high-dose bevacizumab as induction therapy in patients with neurofibromatosis type 2 and progressive vestibular schwannoma. J Clin Oncol. 2019;37(35):3446–3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Slusarz KM, Merker VL, Muzikansky A, Francis SA, Plotkin SR.. Long-term toxicity of bevacizumab therapy in neurofibromatosis 2 patients. Cancer Chemother Pharmacol. 2014;73(6):1197–1204. [DOI] [PubMed] [Google Scholar]
- 49. Gugel I, Kluwe L, Zipfel J, et al. Minimal effect of bevacizumab treatment on residual vestibular schwannomas after partial resection in young neurofibromatosis type 2 patients. Cancers. 2019;11(12):E1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Benesch M, Windelberg M, Sauseng W, et al. Compassionate use of bevacizumab (Avastin) in children and young adults with refractory or recurrent solid tumors. Ann Oncol. 2008;19(4):807–813. [DOI] [PubMed] [Google Scholar]
- 51. Millan NC, Poveda MJ, Cruz O, Mora J.. Safety of bevacizumab in patients younger than 4 years of age. Clin Transl Oncol. 2016;18(5):464–468. [DOI] [PubMed] [Google Scholar]
- 52. Reismüller B, Azizi AA, Peyrl A, et al. Feasibility and tolerability of bevacizumab in children with primary CNS tumors. Pediatr Blood Cancer. 2010;54(5):681–686. [DOI] [PubMed] [Google Scholar]
- 53. Tabori U, Vukovic B, Zielenska M, et al. The role of telomere maintenance in the spontaneous growth arrest of pediatric low-grade gliomas. Neoplasia. 2006;8(2):136–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hartwig FP, Nedel F, Collares TV, Tarquinio SB, Nör JE, Demarco FF.. Telomeres and tissue engineering: the potential roles of TERT in VEGF-mediated angiogenesis. Stem Cell Rev Rep. 2012;8(4):1275–1281. [DOI] [PubMed] [Google Scholar]
- 55. Zhu X, Wu S, Dahut WL, Parikh CR.. Risks of proteinuria and hypertension with bevacizumab, an antibody against vascular endothelial growth factor: systematic review and meta-analysis. Am J Kidney Dis. 2007;49(2):186–193. [DOI] [PubMed] [Google Scholar]
- 56. Jones DTW, Kieran MW, Bouffet E, et al. Pediatric low-grade gliomas: next biologically driven steps. Neuro Oncol. 2018;20(2):160–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.