Abstract
Background
Brain metastases of HER2+ breast cancer persist as a clinical challenge. Many therapeutics directed at human epidermal growth factor receptor 2 (HER2) are antibodies or antibody-drug conjugates (ADCs), and their permeability through the blood–tumor barrier (BTB) is poorly understood. We investigated the efficacy of a biparatopic anti-HER2 antibody-tubulysin conjugate (bHER2-ATC) in preclinical models of brain metastases.
Methods
The compound was evaluated in 2 hematogenous HER2+ brain metastasis mouse models, SUM190-BR and JIMT-1-BR. Endpoints included metastasis count, compound brain penetration, cancer cell proliferation, and apoptosis.
Results
Biparatopic HER2-ATC 3 mg/kg prevented metastasis outgrowth in the JIMT-1-BR model. At 1 mg/kg bHER2-ATC, a 70% and 92% reduction in large and micrometastases was observed. For the SUM190-BR model, an 85% and 53% reduction, respectively, in large and micrometastases was observed at 3 mg/kg, without statistical significance. Proliferation was reduced in both models at the highest dose. At the endpoint, bHER2-ATC uptake covered a median of 4–6% and 7–17% of metastasis area in the JIMT-1-BR and SUM190-BR models, respectively. Maximal compound uptake in the models was 19% and 86% in JIMT-1-BR and SUM190-BR, respectively. Multiple lesions in both models demonstrated ADC uptake in the absence or low diffusion of Texas Red Dextran, a marker of paracellular permeability. Using in vitro BTB assays, the ADC was endocytosed into brain endothelial cells, identifying a potentially new mechanism of antibody permeability.
Conclusions
Biparatopic HER2-ATC significantly prevented JIMT-1-BR brain metastasis outgrowth and showed activity in the SUM190-BR model. The bHER2-ATC penetration into metastases that are impermeable to fluorescent dye suggested an endocytic mechanism of brain penetration.
Keywords: antibody-drug conjugate, antibody-tubulysin conjugate, blood-brain barrier, blood-tumor barrier, brain, breast cancer, central nervous system, metastasis, permeability
Key Points.
An anti-HER2 antibody-tubulysin conjugate demonstrated significant metastasis preventive efficacy in one hematogenous mouse model of brain metastasis of breast cancer, and a strong trend in a second model.
ADC uptake into brain metastases was low and heterogeneous at the experimental endpoint.
ADC uptake did not correlate with uptake of 3 kDa Texas Red Dextran, a marker of paracellular permeability. In vitro data suggest an endocytic mechanism of entry.
Importance of the Study.
HER2+ brain metastases of breast cancer continue to be a significant clinical challenge. Both new preventives and therapeutics, and an understanding of their penetration into metastatic tissue are needed. We report brain metastasis preventive efficacy of bHER2-ATC in 2 hematogenous brain metastasis model systems. Tumor-bound ADC was clearly detectable but was overall low and variable. Interestingly, it did not correlate with uptake of Texas Red Dextran, a marker of paracellular permeability. In vitro data suggest that an ADC may use endocytic mechanisms for ingress.
Monoclonal antibodies are the backbone of therapy for human epidermal growth factor receptor 2 (HER2+) breast cancer. These include monoclonal antibodies to HER2 (trastuzumab and pertuzumab) and the antibody-drug conjugates (ADCs) trastuzumab emtansine (T-DM1) and more recently trastuzumab deruxtecan. Brain metastases are common in metastatic HER2+ breast cancer (30.6‒37.7%) and are associated with neurocognitive decline and poor survival. Observational cohorts of HER2+ breast cancer patients have recorded a 37.7% incidence of brain metastases over a median of 29 months1 and 30.6% over 27.8 months2 from metastatic diagnosis. While active in systemic disease, monoclonal antibody therapy has shown limited efficacy for established brain metastases as monotherapy3–5 or in combinations.6,7 T-DM1 was recently reported to extend invasive disease-free survival as a continuation of neoadjuvant trastuzumab-based therapy when pathological complete response was not achieved. However, despite a significant decrease in distant recurrence, brain metastasis development was similar with or without T-DM1.8
A major limitation of monoclonal antibody therapy for brain metastases is drug penetration. Brain capillaries are encased by a blood–brain barrier (BBB), consisting of endothelial cells with continuous tight junctions and efflux transporters, pericytes, 2 basement membranes, and the feet of astrocytes, all of which provide barrier function. Metastases that break through the BBB to colonize the parenchyma interact with an altered structure, termed the blood–tumor barrier (BTB).9,10 Pharmacokinetic data for multiple drugs indicate that most brain lesions have a BTB permeability higher than that of the BBB, but drug uptake is heterogeneous and only a minority of lesions have sufficient permeability to allow drugs to accumulate to therapeutic levels capable of inducing an apoptotic response.11–13
Data for antibody therapeutics in the brain are limited. It was estimated that 0.1% of circulating antibodies reach the brain.14 However, it is not known how far into the brain antibodies penetrate: tissue concentrations may represent the steady state endothelial ingress and egress back into the bloodstream of antibodies via their fragment crystallizable (Fc) domains by the endothelial neonatal receptor (FcRN), never reaching the parenchyma.15 Antibody levels in the brain are also a function of ingress and clearance, the latter pathways only recently reported in the brain.16–18 The Lockman lab quantified iodine-125 (125I)-trastuzumab distribution in a hematogenous preclinical model of brain metastases.19 Approximately 3% of injected dose reached uninvolved brain versus 5% to brains with metastases. In human studies, trastuzumab levels in the cerebrospinal fluid (CSF), an imperfect measure of brain parenchymal distribution, were 420:1 serum:CSF, improving to 76:1 after radiation therapy.20 Imaging of a small number of brain metastasis patients with zirconium-89 (89Zr) antibody demonstrated uptake in lesions.21,22 Pharmacokinetic data for the cytotoxic warheads of ADCs are even more limited, and unavailable in brain metastases.23
Herein, we report preclinical data in 2 hematogenous brain metastasis models using a biparatopic anti-HER2 antibody conjugated to 2 tubulysin warheads to form biparatopic HER2-antibody-tubulysin conjugate (bHER2-ATC). This antibody binds to 2 non-overlapping HER2 domains conjugated to a tubulysin payload. This conjugation eliminated Fc-mediated antibody functions (Supplementary Figure 1). We report the metastasis preventive effects of this ADC, its uptake into lesions, and evidence for a non-paracellular entry mechanism.
Materials and Methods
See the Supplementary Methods for additional details.
Antibody Drug Conjugates
Biparatopic HER2-ATC is similar to the previously described MEDI4276.24 The version used herein had 2 tubulysin warheads conjugated instead of 4 (Supplementary Figure 1). The isotype control is also conjugated to 2 tubulysin warheads but does not target HER2, designated control-ATC. The ADCs were provided by MedImmune/AstraZeneca.
Cell Line Origin and In Vitro Viability Assays
Experiments were performed using brain tropic variants of 2 human breast cancer cell lines: the JIMT-1-BR and the SUM190-BR.9 Both lines endogenously overexpress HER2. The JIMT-1 line is hormone receptor negative.25 The SUM190 line expresses estrogen receptor (ER)β and an alternative splicing variant of ERα.26
Animal Experiments
Animal experiments were conducted following the regulation and approval of the Animal Care and Use Committee of the National Cancer Institute. Tumor cells were injected into the left cardiac ventricle of 5- to 7-week old mice, as previously described.10 In each experiment, Group 1 received control-ATC, which did not target HER2, at 3 mg/kg, and Groups 2 and 3 received bHER2-ATC at 3 and 1 mg/kg, respectively, i.p., qw. The first dose was administrated at 10 days post-injection for the JIMT-1-BR model to maximize time for all tumor cells to extravasate. For the SUM190-BR model, weekly treatment began on day 21. Treatment started when the cancer cells had extravasated but had not proliferated into metastatic lesions. Both models measure a prevention of metastasis outgrowth. To evaluate paracellular permeability of metastatic clusters, Texas Red Dextran (TRD) injection and perfusion were performed just before euthanasia as previously described.9
Brain Tissue Processing and Analysis
Brains were dissected, frozen, sectioned, and analyzed. Five mice per group, one slide per mouse, were chosen to perform staining for Ki67, cleaved caspase-3 (CC3), cluster of differentiation (CD)31, and anti-human immunoglobulin (Ig)G. Those mice harboring a metastasis number closest to the median of the group were selected for staining in the SUM190-BR model. For the JIMT-1-BR model, the compounds being very efficacious, only the few mice displaying metastases could be utilized for staining.
The analysis of permeability (ie, TRD diffusion) was performed on 3 mice per group.
Statistical analyses are described in Supplementary Materials and Methods.
Results
Tumor Cell Viability In Vitro
The efficacy of the bHER2-ATC was first evaluated in an in vitro viability assay. JIMT-1-BR cells were sensitive to the bHER2-ATC, with half-maximal inhibitory concentrations (IC50) of 10.9, 10.86, and 19.92 picomolar (pM) in 3 independent biological replicates after 4 days of drug incubation. SUM190-BR cells were similarly sensitive, with IC50 of 9.49, 12.21, and 15.49 pM in 3 independent biological replicates. Control-ATC had no effect on cell viability in the dose ranges tested. Supplementary Figure 2 illustrates a representative viability curve for each cell line.
Biparatopic HER2-ATC Prevented Brain Metastasis Outgrowth in the JIMT-1-BR Model
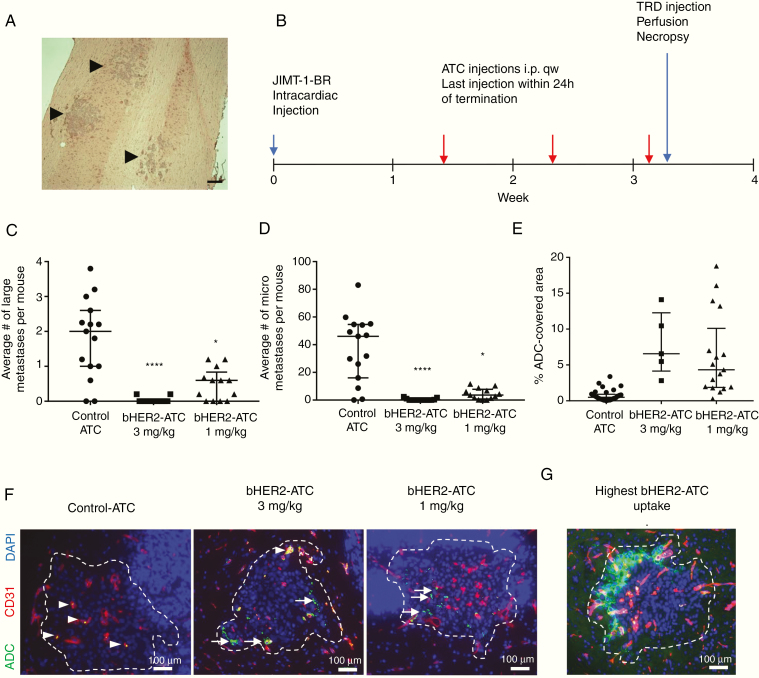
Intracardiac injection of JIMT-1-BR brain-tropic tumor cells produced clusters of micro and large metastases in approximately 3 weeks (Fig. 1A). Treatment with bHER2-ATC (1 or 3 mg/kg) or an isotype control-ATC (non-HER2 targeting 3 mg/kg) started 10 days after cell injection and continued weekly for a total of 3 doses (Fig. 1B). Necropsy occurred within 24 hours of the final dose. This schedule mimics a hypothetical prevention of brain metastasis formation trial by starting therapy after tumor cell extravasation and prior to colonization to a detectable mass. Before euthanasia, mice received TRD, a fluorescent dye, and were then perfused to visualize paracellular permeability into tissue. Metastasis counts were dichotomized into large versus micro metastases as previously reported27 (Fig. 1C, D; Supplementary Tables 1 and 2).
Fig. 1.
bHER2-ATC efficacy in the JIMT-1-BR hematogenous mouse brain metastasis model. (A) Representative hematoxylin and eosin (H&E) staining photograph of JIMT-1-BR metastatic clusters (black arrows) in a mouse brain tissue section. Scale bar: 200 µm. (B) Schematic of the experiment. Mice harboring JIMT-1-BR experimental brain metastases started to receive treatment at day 10 after cancer cell injection. The non-HER2 targeting control-ATC (3 mg/kg) and bHER2-ATC (3 and 1 mg/kg) were administrated intraperitoneally (i.p.), once a week. A last dose was given 24 h before euthanasia. On the day of euthanasia, mice received Texas Red Dextran (TRD) and were subsequently perfused for 10 min. Brain was dissected at necropsy. (C, D) Metastasis count was reported per mouse as large (C) vs micro (D) metastases. Statistical significance was determined using Kruskal–Wallis test and Dunn’s multiple comparison test. *P < 0.05, ****P < 0.0001. (E) The graph indicates the percent of metastasis area covered by ADC. One dot represents one cluster. (F) Representative immunofluorescent photographs of a metastatic cluster (DAPI [4′,6′-diamidino-2-phenylindole] cluster circled by a white dashed line; DAPI-saturated areas represent cell nuclei in cerebellum). The vasculature is visualized by CD31 staining (red). The bHER2-ATC is detected by anti-human IgG antibody staining (green). Each photograph illustrates the ADC coverage near the median for each group: control-ATC median control = 0.5%, bHER2-ATC 3 mg/kg median = 6.5%, bHER2-ATC 1 mg/kg median = 4%. (G) The highest ADC coverage among all the treatment groups (19% of bHER2-ATC coverage). White arrow heads indicate ADC associated with the vasculature; white arrows indicate ADC associated with the metastatic cells. Scale bar: 100 µm.
Treatment with control-ATC resulted in a median of 46 micro and 2 large metastases per brain. The highest dose of bHER2-ATC, 3 mg/kg, significantly prevented the outgrowth of both large and micro metastases, to a point close to complete eradication (P < 0.001) (Fig. 1C, D). Among the 13 mice evaluated in the 3 mg/kg group, only 4 had detectable metastases, with an average of 0.8 large and 2.4 micro metastases per brain. At the lowest dose, 1 mg/kg, bHER2-ATC remained efficacious, with 70% and 92% decreases in the median number of large and micro metastases, respectively (P = 0.03 and 0.02 for large and micro metastases, respectively) (Fig. 1D and Supplementary Tables 1 and 2). Animal weights were unaffected by treatment with bHER2-ATC (Supplementary Figure 3).
ADC presence in the brain was quantified at the experimental endpoint using anti-human IgG staining (Fig. 1E, F and Supplementary Table 3). Each metastatic cluster was encircled (Fig. 1F, white dashes), its area measured, and the area of human IgG staining within the metastasis determined. Representative photographs of the median uptake in the 3 groups are shown in Fig. 1F. Human IgG staining was homogeneously low using the control-ATC, under 4% of the metastatic area, with a median of 0.49%. The control-ATC appeared near the vasculature (Fig. 1, left photograph, and Supplementary Figure 4). For the 3 mg/kg bHER2-ATC group, the median metastatic area stained was 6.6%, varying 2.81–14.11%. Human IgG staining in the 1 mg/kg bHER2-ATC group was lower, with a median of 4.33% metastatic area stained, ranging 0.28–18.78%. Nine out of 17 lesions showed <5% of area stained, and no lesion had >20% area stained (Fig. 1G). HER2 staining of JIMT-BR metastases showed homogeneous staining (Supplementary Figure 5A), so lack of ADC decoration was not due to lack of HER2 expression. Supplementary Figure 6 presents images of ADC penetration into micrometastatic lesions. Little if any staining was observed in uninvolved brain. Overall, the data suggest that, in the JIMT-1-BR model, limited permeability to an ADC exists in hematogenous brain metastases, at a level sufficient to confer metastasis-preventive efficacy of a tubulysin-containing HER2-ADC.
Biparatopic HER2-ATC Demonstrated Penetration into SUM190-BR Brain Metastases and Showed a Trend of Decreased Large Metastases
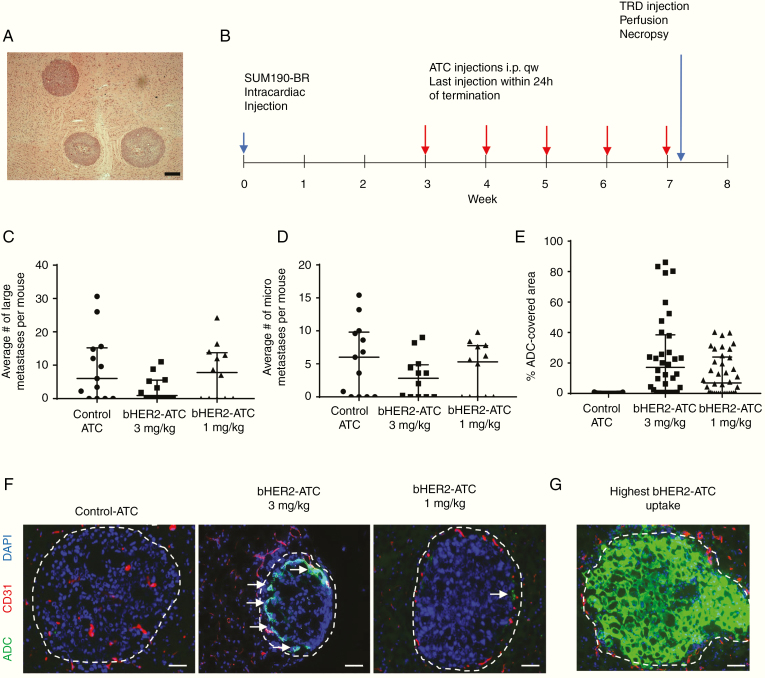
A second HER2+ hematogenous model was tested. This model presents a distinct histopathology, displaying round solid lesions, with some developing a necrotic core (Fig. 2A). Treatment started at day 21 after cell injection and the experiment lasted 50 days (Fig. 2B).
Fig. 2.
bHER2-ATC efficacy in the SUM190-BR hematogenous mouse model of brain metastasis. (A) Representative H&E staining photograph of 3 SUM190-BR metastatic clusters in a mouse brain tissue section. Scale bar: 200 µm. (B) Schematic of the mouse experiment. Mice received the first treatment at day 21 after injection of the SUM190-BR cell line: i.p. injection of non-HER2 targeting control-ATC (3 mg/kg) or bHER2-ATC (3 and 1 mg/kg). The treatment was continued once a week for 4 weeks. A last dose was given 24 h before euthanasia. Before euthanasia, mice received Texas Red Dextran (TRD), followed by perfusion for 10 min. Brain was dissected at necropsy. (C, D) Metastasis count was reported per mouse as large (C) vs micro (D) metastases. Statistical significance was determined using Kruskal–Wallis test and Dunn’s multiple comparison test. ns: non-significant. (E) The graph indicates the percent of metastasis area covered by ADC. One dot represents one metastatic lesion. (F) Representative immunofluorescent photograph of a metastatic lesion (DAPI cluster delineated by a white dashed line). The vasculature is visualized by CD31 staining (red). The bHER2-ATC is detected by anti-human IgG antibody staining (green). Each photograph illustrates the ADC coverage near the median for each group: control-ATC median control = 0.05%, bHER2-ATC 3 mg/kg median = 17%, bHER2-ATC 1 mg/kg median = 7%. (G) The highest ADC coverage among all the treatment groups (86% of ADC coverage). White arrows indicate ADC staining. Scale bar: 50 µm.
Fig. 2C, D summarizes the brain metastasis data from histologic counts. Mice treated with control-ATC produced a median of 6 large and 6 micro metastases per brain. Both types of metastases were variably present (Supplementary Tables 4–6). Although not statistically significant (P = 0.18), the 3 mg/kg bHER2-ATC dosing resulted in a notable 85% reduction in large metastases to a median of 0.9. A reduction of 53% in the micrometastases was also observed in the 3 mg/kg bHER2-ATC group, with a median of 2.8/brain (P = 0.41). The 1 mg/kg bHER2-ATC dosing showed no effect. Thus, metastasis prevention was observed, but only at the highest dose tested and with a high variability, precluding statistical significance. Animal weight data are presented on Supplementary Figure 7.
Presence of ADC in SUM190-BR lesions was assessed by anti-human IgG staining (Fig. 2E, F and Supplementary Table 7). Staining for HER2 in this model showed homogeneous tumor cell expression (Supplementary Figure 5B). Control-ATC was almost undetectable in this model. Heterogeneous human IgG staining was found in the samples from mice treated with bHER2-ATC, with a median of 17.1% metastatic area in the 3 mg/kg group (range, 0.15–86.1%) and 6.93% metastatic area in the 1 mg/kg group (range, 0.003–40.22%). Representative images at the median of each group are shown in Fig. 2F. Fig. 2G presents the lesion with the highest ADC presence (86% of surface area). Micrometastases in this model system also stained for human IgG (Supplementary Figure 8). Little if any staining was observed in uninvolved brain. In summary, ADC decoration of endpoint bHER2-ATC lesions was higher overall than in the JIMT-1-BR model system but was associated with only a trend in metastasis prevention. The apparent discrepancy between drug uptake and drug efficacy between both models is probably multifactorial, combining inherent characteristics of each model and differences in treatment starting schedule.
Proliferation and Apoptotic Endpoints
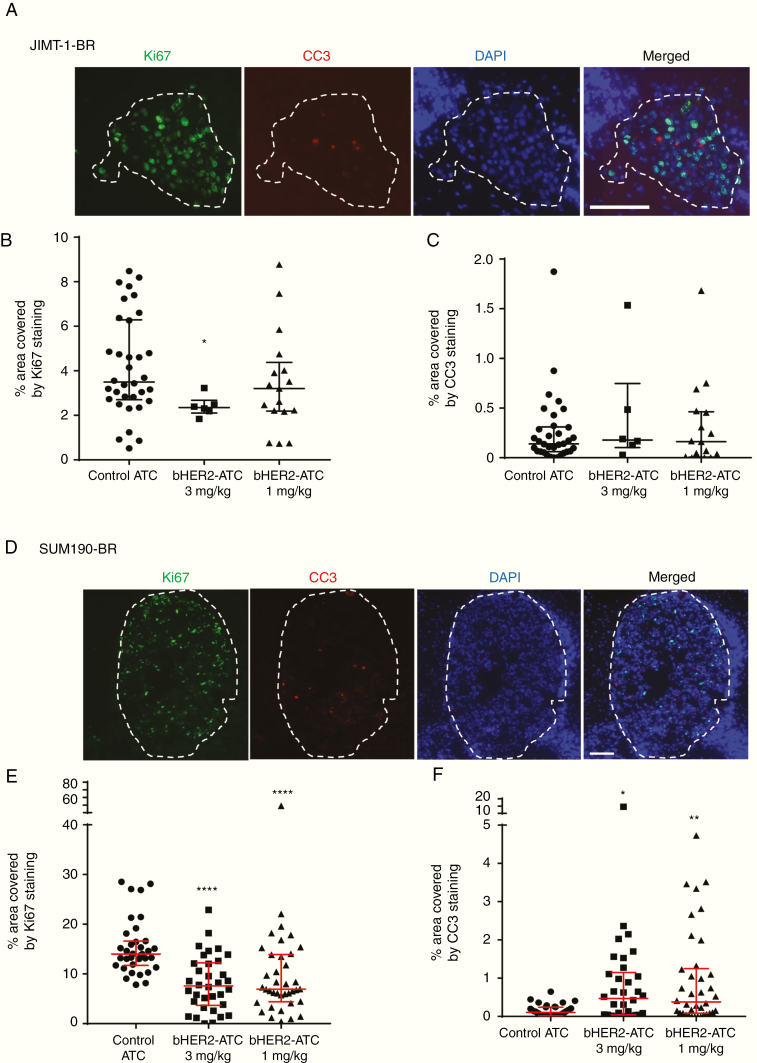
To determine if the ADCs altered tumor proliferation or apoptosis at the experimental endpoint, Ki67 and CC3 stainings were performed (Fig. 3 and Supplementary Tables 8‒10). Five mice per group were randomly selected for analysis, with the exception of the JIMT-1-BR model at 3 mg/kg, where only 4 mice had lesions. The 3 mg/kg bHER2-ATC decreased Ki67 staining in the few remaining JIMT-1-BR lesions by 33%, from 3.49 to 2.35% (P = 0.04; Fig. 3A, B). The lowest dose did not affect proliferation. For SUM190-BR, the median percentage of metastatic area covered with Ki67 decreased by approximately 50% for both doses; from 14% in the control to 7.6% and 6.9% in the 3 mg/kg and 1 mg/kg groups, respectively (P < 0.0001 for both doses).
Fig. 3.
bHER2-ATC effect on proliferation and apoptosis. Panels A–C display the analyses of proliferation and apoptosis in the JIMT-1-BR model. Panels D–F illustrate proliferation and apoptosis quantitation in the SUM190-BR model. (A, D) Representative immunofluorescent photograph of a metastatic lesions (delineated with a dashed line) stained with antibodies to detect Ki67+ proliferative cells (green) and cleaved caspase-3 (CC3) antibodies (red) to detect apoptotic cells. Cell nuclei were stained with DAPI (blue). Scale bar bottom left: 100 µm. (B, C, E, F) The graphs represent percent of the metastatic surface area covered by Ki67 staining as an indicator of proliferative state (B and E) or cleaved caspase-3 staining as an indicator of apoptosis (C and F). Graphs represent the median and the interquartile range, with one dot illustrating one metastatic cluster. Statistical significance was determined using Kruskal–Wallis test and Dunn’s multiple comparison test. *P < 0.05, **P < 0.01, ****P < 0.0001, ns; non-significant.
Apoptosis (CC3) was low in both models, with most lesions having <1% of metastatic area stained. In the SUM190-BR model, significant increased CC3 staining was observed in both bHER2-ATC arms. No significant changes were observed in the JIMT-1-BR model.
Correlates of ADC Penetration
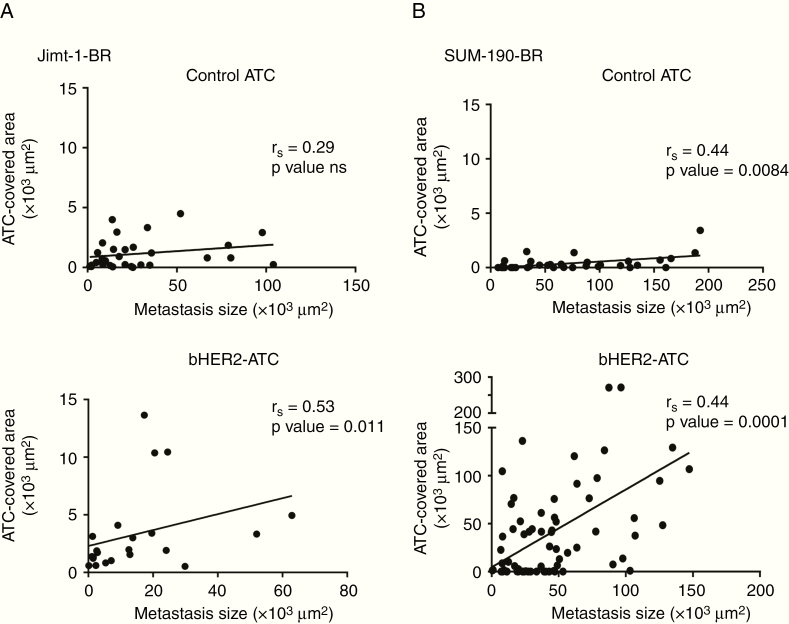
Given the potential clinical importance of ADCs in brain metastases, potential factors that could influence ADC penetration were evaluated. We tested for the correlation of 3 factors with human IgG staining (ADC presence), grouping data from both bHER2-ATC doses. Fig. 4 examines the correlation of metastatic lesion size and ADC presence. In all of the experimental arms a direct relationship of increasing metastasis size with increased ADC presence was observed, though it was not significant in the control-ATC treated JIMT-1-BR model. The positive correlation was lost when the percent of metastasis ADC-covered area was examined instead of the total ADC surface area (Supplementary Figure 9). Thus, bHER2-ATC penetrated smaller metastases at levels comparable to those for the larger metastases in both models.
Fig. 4.
Correlative studies of ADC brain presence with metastatic burden. Correlation between ADC presence (per human IgG staining) and metastatic size in the JIMT-1-BR (A) and the SUM190-BR (B) models. The size of the metastases is represented as a surface area (µm2) of tumor DAPI staining, in the circled metastatic cluster/lesion (x-axis). ADC brain uptake is measured by the area (µm2) of the circled metastatic cluster/lesion covered by anti-human antibody staining (y-axis). The correlation with the control-ATC is presented on the top graphs of panels A and B, and bHER2-ATC on the bottom. One dot represents one cluster (JIMT-1-BR: panel A) or one lesion (SUM190-BR, panel B). Spearman correlation coefficient was calculated, and linear regression analyses were performed. P < 0.05 indicates significant correlation.
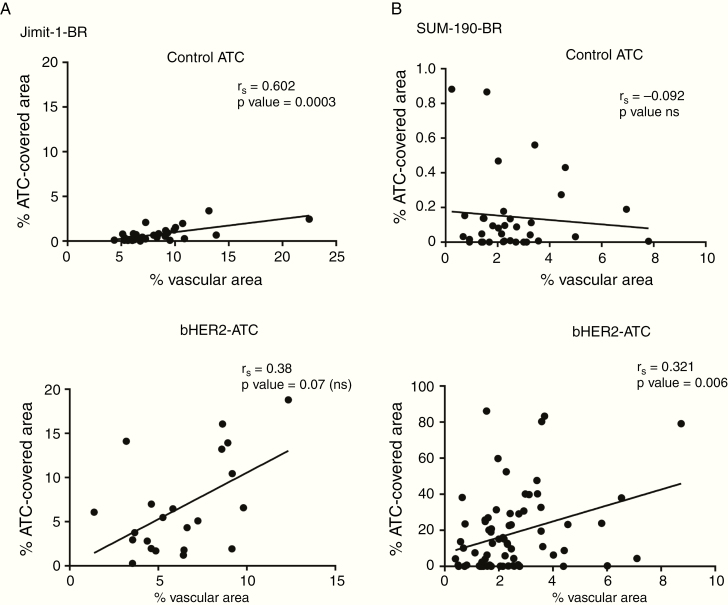
Fig. 5 presents a similar analysis of ADC presence and vascular area (CD31 staining). Two of 4 experimental arms showed a statistical correlation of vascular density with ADC presence. While the correlation between vascular density and percent ADC-covered area did not reach statistical significance in the bHER2-ATC treated JIMT-1-BR group, it remains a positive correlative trend. The percent of area covered by control-ATC in the one nonsignificant experimental arm is infinitesimal, not exceeding 0.9% of the metastatic lesion area.
Fig. 5.
Correlative studies of ADC brain presence with vascular area. Correlation between ADC presence and vascular density in the JIMT-1-BR (A) and the SUM190-BR (B) models. Vascular density is evaluated using the surface area (µm2) covered by CD31 staining (x-axis). ADC uptake is represented as the percentage of the metastatic area (y-axis). The top panels represent the control-ATC uptake and the bottom ones the bHER2-ATC uptake. One dot represents one cluster (JIMT-1-BR) or one lesion (SUM190-BR). Spearman correlation coefficient was calculated, and linear regression analyses were performed. P < 0.05 indicates significant correlation.
Of the standard chemotherapeutic drugs tested in the brain-tropic model systems, all have shown a close correlation with TRD uptake, suggesting a paracellular permeability.28 The 2 models displayed distinct baseline characteristics of TRD diffusion, quantified in the control-ATC arm (Fig. 6A, B). For the JIMT-1-BR model, the percentage of metastatic area with TRD permeability never exceeded 6% at endpoint. The SUM190-BR model showed drastically greater TRD permeability, ranging from 0 to nearly 100% of metastasis area.
Fig. 6.
Assessment of paracellular permeability. Paracellular permeability was evaluated measuring metastatic area covered by TRD diffusion in the JIMT-1-BR (A and C) and SUM190-BR (B and D) metastasis-bearing mice. (A, B) Percent of TRD diffusion was reported by model (JIMT-1-BR in A and SUM190-BR in B) and by treatment group (control-ATC, bHER2-ATC 1 mg/kg, bHER2-ATC 3 mg/kg). Graphs represent the median and the interquartile range, with one dot indicating one metastatic cluster/lesion. (C and D) Percent of metastatic area covered by TRD (x-axis) was correlated to the percent of metastatic area covered by ADC brain uptake (y-axis). The control-ATC group is graphed in the top panel C and D, and bHER2-ATC (both doses combined) in the bottom. Spearman correlation coefficient was calculated, and linear regression analyses performed. P < 0.05 indicates significant correlation. *P < 0.05.
The potential correlations of TRD permeability and ADC permeability are graphed in Fig. 6C, D. The 2 models showed distinct trends for TRD and ADC permeability with bHER2-ATC treatment. Control-ATC produced a positive and significant correlation with TRD in the JIMT1-BR model, while no correlation was observed in the SUM190-BR. Biparatopic HER2-ATC uptake appeared inversely correlated to TRD in the JIMT-1 model and was positively correlated with TRD diffusion in the SUM190-BR model. Of these, only SUM190-BR was statistically significant.
The most consistent aspect of these graphs are the ADC permeability data points at ~0 TRD permeability (Fig. 6). Some metastatic clusters in each experimental arm did not harbor any TRD diffusion while ADC presence was observed (Supplementary Figure 10, panels A and B). These data demonstrate that ADC penetrated the BTB, despite an intact paracellular connection, suggesting a transcellular route. In vitro BTB assays were conducted and demonstrated the ability of bHER2-ATC to penetrate directly into the endothelial cells.10 ATC penetration started at 1.5 h of incubation, increasing at 3 h and persisting at 6 h (Supplementary Figure 11). Confocal imaging confirmed the presence of bHER2-ATC inside the endothelial cells. The data were compared with the general transcytosis marker albumin,29 which endocytosed at 30 min in more endothelial cells. The data suggest an endocytic mechanism of ADC entrance with distinct kinetics (Supplementary Figure 12).
Discussion
The ability of ADCs to either treat or prevent brain metastases is an important clinical topic. T-DM1 has been the clinical model of ADCs in HER2-targeted therapy, but no clinical trial has yet to demonstrate a therapeutic effect on brain metastases.4,5,30 In a phase III clinical trial (EMILIA), T-DM1 improved clinical outcomes for HER2+ breast cancer metastatic patients, but not the rate of CNS progression. CNS metastases were the first site of relapse in 2% of T-DM1 treated patients and in 0.7% of patients treated with lapatinib plus capecitabine.31 Trastuzumab deruxtecan recently became the second ADC to be added to standard clinical use, FDA approved as third-line therapy for metastatic patients,32 although the extension of its effect in brain metastases is still unclear.
Several reports have used hematogenous brain metastasis model systems preclinically, and most demonstrate heterogeneous uptake.19,33,34 Unlike intracranial models,35–41 the experiments reported here utilize a model in which tumor cells transmigrate from the blood into the brain parenchyma. Our results show that when utilizing an ADC with a potent warhead, sufficient level of ADC are able to enter the brain parenchyma to control tumor growth.
Biparatopic HER2-ATC demonstrated significant control of brain metastases in the JIMT-1-BR model, and a positive trend in the SUM190-BR model. The initiation of ADC treatment was delayed in the latter model to adapt to this slow-growing model and to raise confidence that cancer cells were out of the vasculature before treatment began. We cannot rule out the possibility that starting dosing earlier would have been more beneficial. Decreased brain metastases were accompanied by decreased tumor cell proliferation in both models.
It was possible to examine drug effects on micrometastases, the target of brain metastasis prevention, at the experimental endpoint. These small lesions may be comparable to occult lesions in a human brain based on size in a single dimension. Biparatopic HER2-ATC showed heterogeneous human IgG staining in micrometastases comparable to most of the larger metastases, suggesting that a tubulysin-containing HER2-ADC may have brain metastasis preventive capability. The balance of efficacy in the brain and overall toxicity will be an important consideration going forward in optimizing potency versus toxicity of ADCs. Secondary brain metastasis prevention trials are now open to find a possible strategy against brain metastasis development. In the case of NCT03190967, patients with limited HER2+ brain metastases treated with local therapy are enrolled, with a primary endpoint of increasing freedom of a new brain metastasis at the one-year mark.
An interesting dataset from these experiments concerns correlates of ADC presence in the brain. For these experiments, data from both doses of bHER2-ATC were combined to provide statistical power, as fewer metastases developed. A clear enhancement of ADC presence was observed in all models when the HER2 binding ADC was compared with control-ATC. The data suggest that, whatever the level of antibody penetration of the BTB, binding to a cell surface target likely prevents clearance from the CNS.
Despite a low percentage of metastatic area covered by ADC staining, the drug was efficacious in the JIMT-1-BR group. It is likely that the ADC interacted with and killed cancer cells at the micrometastatic stage, and therefore only had to eliminate relatively few tumor cells to achieve prevention. Once in the cells, the cleavage of the toxin and its subsequent release from the targeted cells may create a bystander effect, killing adjacent cancer cells.24
Brains from bHER2-ATC–treated mice showed a positive correlation of ADC presence with metastasis size and CD31 area in both models. Both correlations make intuitive sense: the larger the metastases, the more cancer cells are available for bHER2-ATC binding; and the higher the area covered by vasculature, the more surface area for drug access. This correlation with the vasculature suggests that a combination with vascular endothelial growth factor‒targeted therapy could potentiate bHER2-ATC uptake and efficacy.42
Most interesting was the correlation of ADC presence with that of TRD. TRD is a 3 kDa fluorescent marker that has significantly correlated with the uptake of 4 small molecule drugs (paclitaxel, doxorubicin, vinorelbine, lapatinib) in multiple models.11–13 It is thought to reflect paracellular/passive permeability—ie, drug entry between capillary endothelial cells due to loss of continuous tight junctions and cell:cell adhesions. The overall relationship of TRD diffusion to ADC presence varied by experimental arm and model. Many lesions with no apparent TRD uptake had observable bHER2-ATC bound to tumor cells. This trend was observed in both models. The molecular weight of bHER2-ATC is 198 kDa compared with 3kDa TRD, so the smaller TRD should more easily diffuse through an opening. ADC uptake levels in the TRD-low lesions were comparable to those in TRD-permeable lesions. In vitro BTB assays strongly suggested an endocytosis/transcytosis route; bHER2-ATC penetrated inside the BTB endothelial cells. Comparison with albumin endocytosis suggests a unique form of transcytosis. Uptake into the BTB endothelial cells was restricted to a small percentage of cells, suggesting that there may be specific subsets of endothelial cells capable of this endocytic mechanism. Discovery and mechanistic validation of contributing factors to ADC permeability may facilitate translational development of this important field.
Supplementary Material
Acknowledgments
The authors thanks the Antibody Development/Protein Engineering group of MedImmune/AZ for generating and providing the ADC reagents and Nazzareno Dimasi for discussions and help preparing illustrations.
References
Funding
This work was supported by a Collaborative Research and Development Agreement between MedImmune/AstraZeneca and the NCI, and by the Intramural program of the National Cancer Institute. This project has been funded in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract no. 75N91019D00024. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
Conflict of interest statement. PSS received research funding from MedImmune/AstraZeneca. MGO is an employee of AstraZeneca and has stock and/or stock options or interests in AstraZeneca.
Authorship statement. Conceptualized and designed the experiments: BG, MGO, PSS. Performed experiments: BG, DW, CR, SD, IK. Analyzed experiments: BG. Coordinated and supervised animal experiments: SD. Data interpretation: BG, PSS. Writing of the manuscript: BG, PSS. Manuscript review and edits: MGO, SD, ASZ.
References
- 1. Brufsky AM, Mayer M, Rugo HS, et al. Central nervous system metastases in patients with HER2-positive metastatic breast cancer: incidence, treatment, and survival in patients from registHER. Clin Cancer Res. 2011;17(14):4834–4843. [DOI] [PubMed] [Google Scholar]
- 2. Hurvitz SA, O’Shaughnessy J, Mason G, et al. Central nervous system metastasis in patients with HER2-positive metastatic breast cancer: patient characteristics, treatment, and survival from SystHERs. Clin Cancer Res. 2019;25(8):2433–2441. [DOI] [PubMed] [Google Scholar]
- 3. Bartsch R, Berghoff AS, Vogl U, et al. Activity of T-DM1 in Her2-positive breast cancer brain metastases. Clin Exp Metastasis. 2015;32(7):729–737. [DOI] [PubMed] [Google Scholar]
- 4. Jacot W, Pons E, Frenel JS, et al. Efficacy and safety of trastuzumab emtansine (T-DM1) in patients with HER2-positive breast cancer with brain metastases. Breast Cancer Res Treat. 2016;157(2):307–318. [DOI] [PubMed] [Google Scholar]
- 5. Fabi A, Alesini D, Valle E, et al. T-DM1 and brain metastases: clinical outcome in HER2-positive metastatic breast cancer. Breast. 2018;41:137–143. [DOI] [PubMed] [Google Scholar]
- 6. Murthy R, Borges VF, Conlin A, et al. Tucatinib with capecitabine and trastuzumab in advanced HER2-positive metastatic breast cancer with and without brain metastases: a non-randomised, open-label, phase 1b study. Lancet Oncol. 2018;19(7):880–888. [DOI] [PubMed] [Google Scholar]
- 7. Van Swearingen AED, Siegel MB, Deal AM, et al. LCCC 1025: a phase II study of everolimus, trastuzumab, and vinorelbine to treat progressive HER2-positive breast cancer brain metastases. Breast Cancer Res Treat. 2018;171(3):637–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. von Minckwitz G, Huang CS, Mano MS, et al. ; KATHERINE Investigators Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380(7):617–628. [DOI] [PubMed] [Google Scholar]
- 9. Lyle LT, Lockman PR, Adkins CE, et al. Alterations in pericyte subpopulations are associated with elevated blood-tumor barrier permeability in experimental brain metastasis of breast cancer. Clin Cancer Res. 2016;22(21):5287–5299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gril B, Paranjape AN, Woditschka S, et al. Reactive astrocytic S1P3 signaling modulates the blood-tumor barrier in brain metastases. Nat Commun. 2018;9(1):2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lockman PR, Mittapalli RK, Taskar KS, et al. Heterogeneous blood-tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer. Clin Cancer Res. 2010;16(23):5664–5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Taskar KS, Rudraraju V, Mittapalli RK, et al. Lapatinib distribution in HER2 overexpressing experimental brain metastases of breast cancer. Pharm Res. 2012;29(3):770–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Samala R, Thorsheim HR, Goda S, et al. Vinorelbine delivery and efficacy in the MDA-MB-231BR preclinical model of brain metastases of breast cancer. Pharm Res. 2016;33(12):2904–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Boado RJ, Zhou QH, Lu JZ, Hui EK, Pardridge WM. Pharmacokinetics and brain uptake of a genetically engineered bifunctional fusion antibody targeting the mouse transferrin receptor. Mol Pharm. 2010;7(1):237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang Y, Pardridge WM. Mediated efflux of IgG molecules from brain to blood across the blood-brain barrier. J Neuroimmunol. 2001;114(1-2):168–172. [DOI] [PubMed] [Google Scholar]
- 16. Abbott NJ. Evidence for bulk flow of brain interstitial fluid: significance for physiology and pathology. Neurochem Int. 2004;45(4):545–552. [DOI] [PubMed] [Google Scholar]
- 17. Carare RO, Bernardes-Silva M, Newman TA, et al. Solutes, but not cells, drain from the brain parenchyma along basement membranes of capillaries and arteries: significance for cerebral amyloid angiopathy and neuroimmunology. Neuropathol Appl Neurobiol. 2008;34(2):131–144. [DOI] [PubMed] [Google Scholar]
- 18. Da Mesquita S, Louveau A, Vaccari A, et al. Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease. Nature. 2018;560(7717):185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Terrell-Hall TB, Nounou MI, El-Amrawy F, Griffith JIG, Lockman PR. Trastuzumab distribution in an in-vivo and in-vitro model of brain metastases of breast cancer. Oncotarget. 2017;8(48):83734–83744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stemmler HJ, Schmitt M, Willems A, Bernhard H, Harbeck N, Heinemann V. Ratio of trastuzumab levels in serum and cerebrospinal fluid is altered in HER2-positive breast cancer patients with brain metastases and impairment of blood-brain barrier. Anticancer Drugs. 2007;18(1):23–28. [DOI] [PubMed] [Google Scholar]
- 21. Dijkers EC, Oude Munnink TH, Kosterink JG, et al. Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin Pharmacol Ther. 2010;87(5):586–592. [DOI] [PubMed] [Google Scholar]
- 22. Ulaner GA, Lyashchenko SK, Riedl C, et al. First-in-human human epidermal growth factor receptor 2-targeted imaging using 89Zr-Pertuzumab PET/CT: dosimetry and clinical application in patients with breast cancer. J Nucl Med. 2018;59(6):900–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mou S, Huang Y, Rosenbaum AI. ADME considerations and bioanalytical strategies for pharmacokinetic assessments of antibody-drug conjugates. Antibodies. 2018;7(4). doi: 10.3390/antib7040041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li JY, Perry SR, Muniz-Medina V, et al. A biparatopic HER2-targeting antibody-drug conjugate induces tumor regression in primary models refractory to or ineligible for HER2-targeted therapy. Cancer Cell. 2016;29(1):117–129. [DOI] [PubMed] [Google Scholar]
- 25. Tanner M, Kapanen AI, Junttila T, et al. Characterization of a novel cell line established from a patient with Herceptin-resistant breast cancer. Mol Cancer Ther. 2004;3(12):1585–1592. [PubMed] [Google Scholar]
- 26. Ohshiro K, Schwartz AM, Levine PH, Kumar R. Alternate estrogen receptors promote invasion of inflammatory breast cancer cells via non-genomic signaling. PLoS One. 2012;7(1):e30725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gril B, Palmieri D, Bronder JL, et al. Effect of lapatinib on the outgrowth of metastatic breast cancer cells to the brain. J Natl Cancer Inst. 2008;100(15):1092–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Anderson JM, Van Itallie CM. Physiology and function of the tight junction. Cold Spring Harb Perspect Biol. 2009;1(2):a002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Knowland D, Arac A, Sekiguchi KJ, et al. Stepwise recruitment of transcellular and paracellular pathways underlies blood-brain barrier breakdown in stroke. Neuron. 2014;82(3):603–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bartsch R, Berghoff AS, Vogl U, et al. Activity of T-DM1 in Her2-positive breast cancer brain metastases. Clin Exp Metastasis. 2015;32(7):729–737. [DOI] [PubMed] [Google Scholar]
- 31. Krop IE, Lin NU, Blackwell K, et al. Trastuzumab emtansine (T-DM1) versus lapatinib plus capecitabine in patients with HER2-positive metastatic breast cancer and central nervous system metastases: a retrospective, exploratory analysis in EMILIA. Ann Oncol. 2015;26(1):113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Modi S, Saura C, Yamashita T, et al. ; DESTINY-Breast01 Investigators Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med. 2020;382(7):610–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Connell JJ, Chatain G, Cornelissen B, et al. Selective permeabilization of the blood-brain barrier at sites of metastasis. J Natl Cancer Inst. 2013;105(21):1634–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Baker JHE, Kyle AH, Reinsberg SA, et al. Heterogeneous distribution of trastuzumab in HER2-positive xenografts and metastases: role of the tumor microenvironment. Clin Exp Metastasis. 2018;35(7):691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Arvanitis CD, Askoxylakis V, Guo Y, et al. Mechanisms of enhanced drug delivery in brain metastases with focused ultrasound-induced blood-tumor barrier disruption. Proc Natl Acad Sci U S A. 2018;115(37):E8717–E8726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Park EJ, Zhang YZ, Vykhodtseva N, McDannold N. Ultrasound-mediated blood-brain/blood-tumor barrier disruption improves outcomes with trastuzumab in a breast cancer brain metastasis model. J Control Release. 2012;163(3):277–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kobus T, Zervantonakis IK, Zhang Y, McDannold NJ. Growth inhibition in a brain metastasis model by antibody delivery using focused ultrasound-mediated blood-brain barrier disruption. J Control Release. 2016;238:281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Askoxylakis V, Ferraro GB, Kodack DP, et al. Preclinical efficacy of ado-trastuzumab emtansine in the brain microenvironment. J Natl Cancer Inst. 2016;108(2). doi: 10.1093/jnci/djv313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Regina A, Demeule M, Tripathy S, et al. ANG4043, a novel brain-penetrant peptide-mAb conjugate, is efficacious against HER2-positive intracranial tumors in mice. Mol Cancer Ther. 2015;14(1):129–140. [DOI] [PubMed] [Google Scholar]
- 40. Hu J, Ljubimova JY, Inoue S, et al. Phosphodiesterase type 5 inhibitors increase Herceptin transport and treatment efficacy in mouse metastatic brain tumor models. PLoS One. 2010;5(4):e10108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lewis Phillips GD, Nishimura MC, Lacap JA, et al. Trastuzumab uptake and its relation to efficacy in an animal model of HER2-positive breast cancer brain metastasis. Breast Cancer Res Treat. 2017;164(3):581–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kodack DP, Chung E, Yamashita H, et al. Combined targeting of HER2 and VEGFR2 for effective treatment of HER2-amplified breast cancer brain metastases. Proc Natl Acad Sci U S A. 2012;109(45):E3119–E3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.