Abstract
Background
Glioblastoma (GBM) is the most aggressive primary brain tumor and has a dismal prognosis. Previously, we identified that junctional adhesion molecule A (JAM-A), a cell adhesion molecule, is highly elevated in human GBM cancer stem cells and predicts poor patient prognosis. While JAM-A is also highly expressed in other cells in the tumor microenvironment, specifically microglia and macrophages, how JAM-A expression in these cells affects tumor growth has yet to be determined. The goal of this study was to understand the role of microenvironmental JAM-A in mediating GBM growth.
Methods
Male and female wild-type (WT) and JAM-A–deficient mice were transplanted intracranially with the syngeneic glioma cell lines GL261 and SB28 and were assessed for differences in survival and microglial activation in tumors and in vitro. RNA-sequencing was performed to identify differentially regulated genes among all genotypes, and differences were validated in vitro and in vivo.
Results
We found that JAM-A–deficient female mice succumbed to GBM more quickly compared with WT females and JAM-A–deficient and male WT mice. Analysis of microglia in the tumors revealed that female JAM-A–deficient microglia were more activated, and RNA-sequencing identified elevated expression of Fizz1 and Ifi202b specifically in JAM-A–deficient female microglia.
Conclusions
Our findings suggest that JAM-A functions to suppress pathogenic microglial activation in the female tumor microenvironment, highlighting an emerging role for sex differences in the GBM microenvironment and suggesting that sex differences extend beyond previously reported tumor cell–intrinsic differences.
Keywords: glioblastoma, junctional adhesion molecule A, microglia, sex differences
Key Points.
1. Sex differences in the GBM microenvironment impact tumor growth.
2. Cell adhesion molecules have context- and sex-dependent roles in GBM.
3. Microglial sex differences can drive differential GBM growth.
Importance of the Study.
GBM remains refractory to current standard of care. In addition to the cellular and molecular heterogeneity present in GBM, epidemiological studies indicate the presence of additional complexity associated with biological sex. GBM is more prevalent and aggressive in male patients, suggesting the existence of sex-specific growth, invasion, and therapeutic resistance mechanisms. While sex-specific molecular mechanisms have been reported at a tumor cell–intrinsic level, sex-specific differences in the tumor microenvironment have not been investigated. Using transgenic mouse models, we demonstrate that deficiency of JAM-A in female mice enhances microglia activation, GBM cell proliferation, and tumor growth. Mechanistically, JAM-A suppresses anti-inflammatory/pro-tumorigenic gene activation via Ifi202b and Fizz1 in female microglia. These findings highlight a sex-specific role for JAM-A and represent the first evidence of sexual dimorphism in the GBM microenvironment.
Glioblastoma (GBM) is the most common primary malignant brain tumor and, despite aggressive therapies, has a median survival of 15‒20 months.1 There are multiple barriers to the development of more effective therapies, including inter- and intratumoral heterogeneity at the cellular and molecular levels, a high degree of invasion into the surrounding brain, mechanisms of intrinsic resistance to radiation and chemotherapy, and an immune-suppressive tumor microenvironment. The GBM microenvironment consists of 30–50% microglia, the resident immune cells of the brain, and infiltrating tumor-associated macrophages (TAMs).2 The interaction between GBM cells and microglia/TAMs is principally mediated through direct cell-cell contact and a series of secreted factors, with GBM cells amplifying the immune-suppressive phenotypes of microglia/TAMs and microglia/TAMs concomitantly driving GBM cell growth and tissue infiltration.3,4 An understudied barrier to effective treatment is the inherent sex differences that exist within GBM.5–7 These differences are supported at the epidemiological level, with a male-to-female incidence ratio of 1.6:1.8,9 Furthermore, these differences manifest clinically, with females showing a more dispersive phenotype radiographically10 and males experiencing a poorer prognosis.11 There is supporting evidence in the literature to suggest that sexual dimorphism in GBM is mediated through sex-specific differences in tumor cell–intrinsic oncogenic signaling pathways,5,7,12,13 epigenetic states, and metabolic profiles.6 While sex differences might induce differential GBM cell–intrinsic responses, leading to differences in survival, sex-specific differences in the tumor microenvironment have not yet been elucidated.
Microglia are a major cell population in the tumor microenvironment with inherent sex signatures that impact their development, maintenance, activation, and overall function in homeostatic and disease states.14 Microglia-mediated sex differences are prominent and have been well characterized in neurological disorders such as autism and Alzheimer’s disease.15 Microglia continuously survey their surrounding environment16 through a variety of cell adhesion mechanisms,17 and this extends to interactions with adjacent tumor cells. These interactions are mediated in part by tight junction proteins, including junctional adhesion molecule A (JAM-A, also known as F11r), which was shown to be highly expressed by tumor-associated microglia and TAMs.18 Beyond this role in microglia and TAMs, we previously demonstrated that JAM-A was necessary and sufficient for GBM cancer stem cell (CSC) maintenance and correlated with poor patient prognosis.19,20 However, it is unclear as to which cell type(s) are driving the human GBM survival data, given the expression on TAMs, microglia, and CSCs. These data address a knowledge gap regarding the function of JAM-A in the tumor microenvironment. Here, we investigated whether JAM-A mediates sex differences in tumor progression and found that JAM-A deficiency in microglia impacts GBM pathogenesis in a sex-specific manner.
Materials and Methods
Additional details are provided in the Supplementary Material. JAM-A–deficient (−/−) mice were generated by gene trap technology.21 Wild-type (WT) C57BL/6 mice were purchased from Jackson Laboratories. All animal experiments were performed under Cleveland Clinic–approved Institutional Animal Care and Use Committee protocols.
For intracranial implantation of tumor cells, 10 000 GL261 or 5000 SB28 cells were resuspended in 5 μL of RPMI media and injected intracranially into 6-week-old male and female JAM-A−/− and WT mice.
Mouse brains containing tumors were dissected from JAM-A−/− and WT mice; fixed sections were stained with anti–ionized calcium binding adaptor molecule 1 (Iba1) antibody, and microglia counting was performed using ImageJ software, with rounded or amoeboid microglia with no ramifications considered as activated.
For primary microglia cultures, mixed cortical cultures from newborn JAM-A−/− and WT mice (days 0–3) were generated using standard protocols.22 Microglia phagocytosis assays were performed according to previously published protocols.23 For the co-culture cell proliferation assay, a ratio of 2.5 microglia to each tumor cell (2.5:1) was used as previously published.24
GL261 cells were injected into male and female JAM-A−/− and WT mice; tumor tissues were isolated at endpoint, and RNA-sequencing analysis was performed. RNA was extracted using an RNeasy Mini Kit from JAM-A−/− and WT male and female tumors (n = 3 per each group).
For quantitative reverse transcription PCR, RNA from cells of interest was extracted using an RNeasy Mini Kit, and cDNA was synthesized using qSCRIPT cDNA SuperMix (Quanta Biosciences).
For sex-determination PCR, the sex of microglia was confirmed by expression of the X- and Y-encoded paralogs Jarid1c and Jarid1d using previously published protocols.25
For JAM-A blocking antibody treatment of microglia, a total of 3–4 × 105 microglia were plated in a 6-well plate and treated with 10 µg/mL immunoglobulin (Ig)G1 control or JAM-A–blocking (J10.4) antibody.
Statistical Analysis
Graphs were created using GraphPad Prism 6.0. Results are expressed as mean ± standard deviation. Information regarding the numbers of experimental replicates, statistical tests performed, and significance values are provided in the figure legend for each figure panel.
Results
JAM-A Deficient Female Mice Display an Aggressive GBM Phenotype
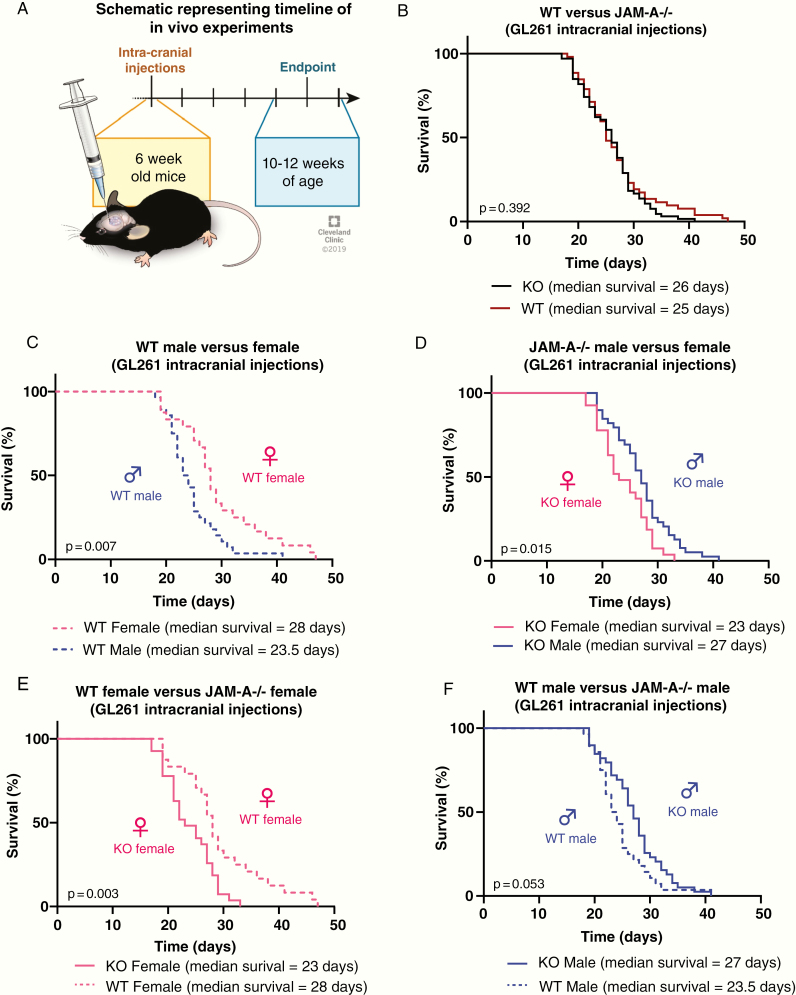
To assess the function of JAM-A in the tumor microenvironment, we took advantage of JAM-A–deficient mice in combination with a transplantable syngeneic orthotopic mouse glioma model. We transplanted an equal number of GL261 cells into 6-week-old male and female WT and JAM-A–deficient mice and assessed survival based on the development of neurological signs, which reflected the experimental endpoint (Fig. 1A). Using this approach, no differences were observed in the survival of WT and JAM-A–deficient mice when both sexes were combined together (Fig. 1B). Given the sex differences in survival observed in human patients, we compared the groups based on sex of the mice. When comparing the WT groups, we observed a similar trend with GL261 cells as seen in human population, where females survived significantly longer compared with males (Fig. 1C). In the context of JAM-A deficiency, however, disease severity was significantly increased in females compared with males (Fig. 1D), reversing the survival difference observed between male and female GBM patients. When comparing genotypes by sex, JAM-A–deficient females had significantly poorer survival compared with WT females in both the GL261 and SB28 models (Fig. 1E, Supplementary Figure 1A). However, this difference was the opposite between JAM-A–deficient and WT males, although it did not reach statistical significance (Fig. 1F). Importantly, a single copy of JAM-A was sufficient to improve the survival of female mice, as heterozygous JAM-A mice had a significantly better survival than JAM-A–deficient mice (Supplementary Figure 1B). However, this was not observed in the context of male heterozygous and JAM-A–deficient mice (Supplementary Figure 1C). It is important to note that the syngeneic mouse glioma cells used for intracranial transplants express JAM-A (Supplementary Figure 1D) and thus the differences in survival observed are likely due to JAM-A deficiency in the tumor microenvironment. Taken together, these data suggest a tumor-suppressive role for JAM-A specifically in the female tumor microenvironment.
Fig. 1.
Female JAM-A–deficient mice exhibit poor survival upon tumor implantation. (A) Schematic showing the timeline of intracranial implantation of tumor cells and manifestation of endpoint symptoms. Survival curves upon intracranial implantation of GL261 cells into (B) WT (n = 52, median survival = 25 days) and JAM-A–deficient mice (n = 66, median survival = 26 days); P = 0.392. (C) Wild-type male (n = 28, median survival = 23.5 days) and WT female mice (n = 24, median survival = 28 days); **P = 0.007. (D) JAM-A–deficient male (n = 39, median survival = 27 days) and JAM-A–deficient female mice (n = 27, median survival = 23 days); *P = 0.015. (E) JAM-A–deficient (n = 27, median survival = 23 days) and WT females (n = 24, median survival = 28 days); **P = 0.003. (F) JAM-A–deficient (n = 39, median survival = 27 days) and WT male mice (n = 28, median survival = 23.5 days); P = 0.053. Kaplan–Meier survival curves were assessed using log-rank (Mantel–Cox) test.
Female JAM-A Deficient Mice Have Increased Activation of Microglia in the Tumor Microenvironment Compared with Other Genotypes
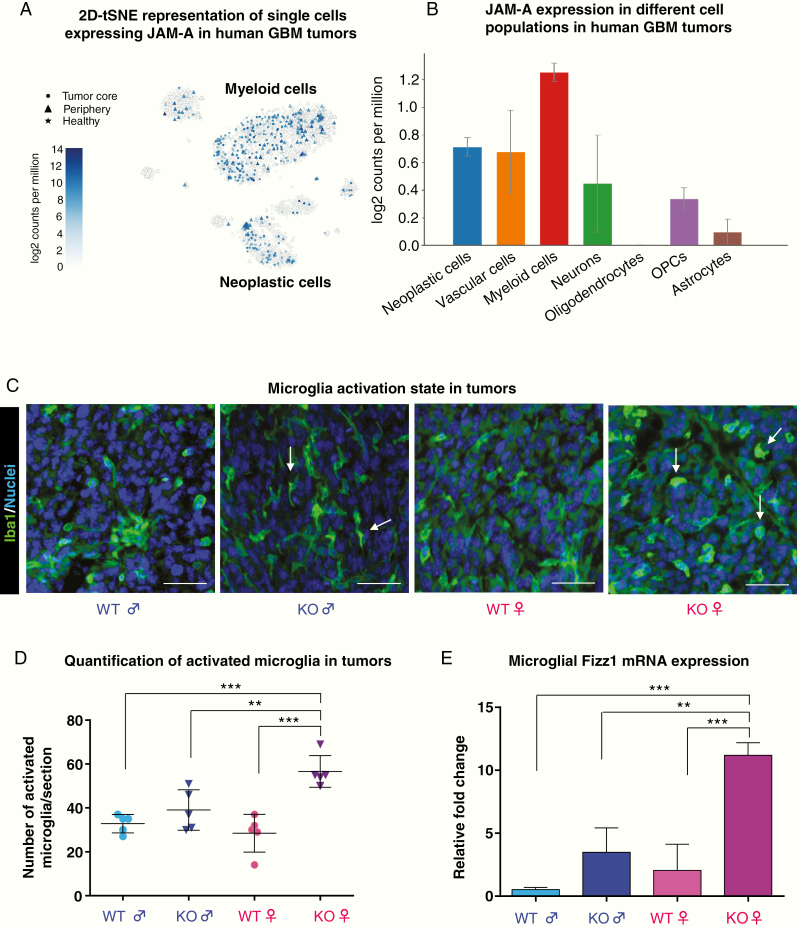
These sex differences in survival of mice implanted with identical cells suggest that the host sex and microenvironment are likely to be the mediators of the observed effect. We next assessed the potential for cells in the tumor microenvironment to drive these differences. We first focused on microglia given their reported sex differences in developmental, homeostatic, and disease states, including optic pathway glioma,26 and that we and others have previously reported that GBM-associated microglia express JAM-A.18,19 To determine the expression of JAM-A in various cell populations, we assessed the Brain RNA-Seq database that provides expression levels for purified cell types from the healthy adult mouse brain,27,28 but unfortunately cannot be subdivided based on sex. This analysis revealed that JAM-A expression was highest in microglia, followed by endothelial cells in the brain in both mice and humans (Supplementary Figure 2A), consistent with the previous report of JAM-A expression in various types of endothelial cells.29 Single cell RNA-sequencing data from GBM patients also confirmed an elevation of JAM-A in myeloid cells30 (Fig. 2A, B, Supplementary Figure 2B). To determine whether there were baseline differences in microglial number in adult WT and JAM-A–deficient mice, we assessed the number of microglia via immunofluorescence for Iba1-positive cells in normal mice without tumors. We found that JAM-A–deficient mice had significantly more Iba1+ cells compared with WT controls, but there was no difference between males and females (Supplementary Figure 2C). To determine whether there were differences in activation state, we assessed adult WT and JAM-A–deficient mice transplanted with GBM cells by immunostaining analysis and found that female JAM-A–deficient mice had a significant increase in amoeboid/rounded Iba1+ cells, a morphological surrogate of microglial activation,31 compared with all other genotypes (Fig. 2C, D). We also assessed WT and JAM-A–deficient microglia for activation markers and found that JAM-A–deficient female microglia acquired a more anti-inflammatory phenotype (Supplementary Figure 3). Primary cultures of microglia from JAM-A–deficient female mice also showed higher expression of the microglia activation marker found in inflammatory zone (Fizz1) at baseline (Fig. 2E). Fizz1 expression in microglia and TAMs correlates with an anti-inflammatory and pro-tumorigenic state in the tumor microenvironment.32,33 We also assessed female JAM-A–deficient and WT mice for other immune cells by flow cytometry and did not observe differences in immune populations in the blood or the brain (Supplementary Figure 4). These data indicate that JAM-A deficiency results in enhanced microglia activation specifically in JAM-A–deficient female mice, which experience aggressive GBM growth and poor survival compared with other genotypes.
Fig. 2.
JAM-A deficiency promotes activation of female tumor-associated microglia. (A) Two-dimensional t-distributed stochastic neighbor embedding plot representing JAM-A expression across different cell populations in human GBM patients assessed by single-cell GBM RNA-seq (http://www.gbmseq.org/). (B) Graphical representation of JAM-A expression across all the cell populations assessed in human GBM tumors. (C) Tumor sections of JAM-A–deficient and WT mice were stained with the microglia marker Iba1, and amoeboid/rounded morphology was used as an indicator of microglia activation status. Scale: 10 μM. Arrows indicate activated microglia in JAM-A–deficient female and ramified microglia in JAM-A–deficient male tumors. (D) Quantification of rounded microglia in tumor sections indicating JAM-A–deficient female mice have more activated microglia compared with other genotypes (n = 5 tumor images per each group). Rounded Iba1+ cells were counted using ImageJ software, and the P-value was assessed by one-way ANOVA (JAM-A–deficient female vs WT male, ***P < 0.001; JAM-A–deficient female vs JAM-A–deficient male, **P < 0.01; JAM-A–deficient female vs WT female, ***P < 0.001). (E) mRNA expression of Fizz1 in microglia (n = 4 mice per each group); P-value was assessed by one-way ANOVA (JAM-A–deficient female vs WT male and female, ***P < 0.001 and JAM-A–deficient female vs JAM-A–deficient male, **P < 0.01).
JAM-A Deficient Female Microglia Are Phagocytic and Enhance Glioma Cell Proliferation In Vitro
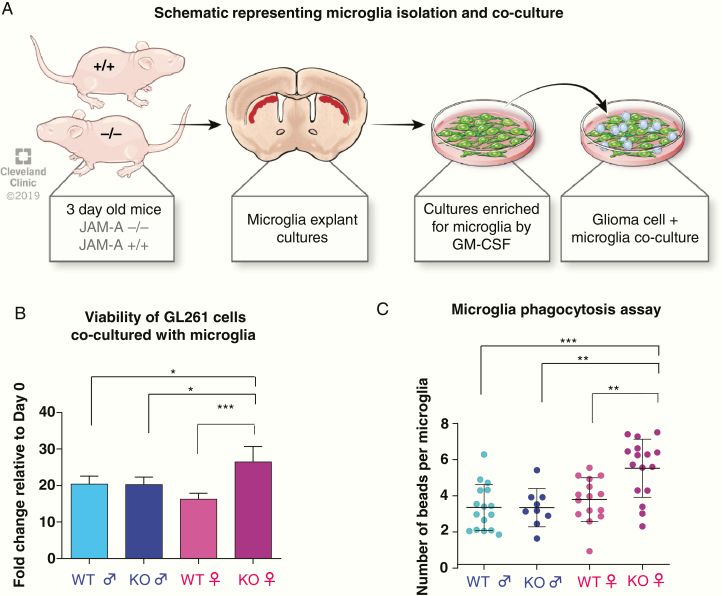
While these data indicate the presence of increased microglial activation in JAM-A–deficient, tumor-bearing female mice, we next employed in vitro functional assessments to further evaluate these differences in genotype- and sex-specific microglial activation. Microglia were isolated from mixed cortical cultures from young mice, according to the experimental workflow shown in Fig. 3A. To directly assess whether microglial activation impacts GBM cell growth, we co-cultured GBM cells with microglia isolated from each genotype and found that JAM-A–deficient female microglia significantly enhanced GBM cell number (Fig. 3B). As JAM-A functions to mediate cell-cell contact, we assessed whether direct contact between microglia and GBM cells was necessary for the enhanced growth observed in JAM-A–deficient female microglia. When GBM cells were treated with microglia conditioned media, no significant difference was observed (Supplementary Figure 5), suggesting that the JAM-A–deficient female microglia-mediated enhancement of GBM cell growth is contact dependent. Using phagocytosis assays, we observed that JAM-A–deficient female microglia had a significantly higher capacity to phagocytose particles compared with JAM-A–deficient male microglia (Fig. 3C). However, this difference was not observed between sexes for WT microglia (Fig. 3B). The elevated phagocytic ability of JAM-A–deficient female microglia was also recapitulated using a flow cytometry–based analysis (Supplementary Figure 6). Taken together, these data suggest that JAM-A–deficient female microglia are more activated and drive GBM cell growth, which underlies the aggressive phenotype observed in vivo. These data are consistent with previously published data demonstrating that activated microglia drive glioma proliferation.34–36
Fig. 3.
Female JAM-A–deficient microglia are functionally different and drive tumor growth in vitro. (A) Schematic representing microglia isolation from 3-day-old JAM-A–deficient and WT mice and co-culture with glioma cells. (B) Microglia from JAM-A–deficient and WT males and females were co-cultured with GL261 cells, and proliferation of GL261 cells was measured using CellTiter-Glo proliferation assay, adjusted to a microglia alone control from each genotype. Co-culture of GL261 glioma cells with JAM-A–deficient female microglia enhanced glioma cell proliferation compared with other genotypes (JAM-A–deficient female vs WT male, **P < 0.01; JAM-A–deficient female vs JAM-A–deficient male, **P < 0.01; JAM-A–deficient female vs WT female, ***P < 0.001 assessed by one-way ANOVA). Mice were n = 3 per each group, cells plated in triplicate. (C) Quantitation of microglial uptake of fluorescent beads in an in vitro phagocytosis assay, using primary microglia cultures from WT and JAM-A–deficient male and female mice. Images (20x magnification) were taken using a Keyence BZ-X fluorescent microscope and quantified using ImageJ software (15–16 images per group for WT female/male and JAM-A–deficient female, 9 images for JAM-A–deficient male). For statistical analysis, one-way ANOVA. Data represent mean ± SD. **P < 0.01; ***P < 0.001.
As higher levels of estrogen have been reported to increase microglial activation and lead to poorer survival in female mice with optic pathway glioma26 and as estrogen has been previously reported to regulate levels of JAM-A,37 we tested the role of estrogen in mediating the aggressive tumor phenotype and microglial activation observed in JAM-A–deficient female mice. To directly test this possibility, we assessed in vivo tumor growth in female mice after ovariectomy and observed that JAM-A–deficient mice experienced poorer survival compared with WT mice (Supplementary Figure 7A). In fact, ovariectomy increased the aggressiveness of tumors in JAM-A–deficient mice (Supplementary Figure 7B). However, ovariectomy did not impact the survival of WT mice (Supplementary Figure 7C). These data suggest that loss of JAM-A renders female mice dependent upon a protective effect of ovarian hormones.
JAM-A Deficiency in the Female Tumor Microenvironment Induces a Pro-Tumorigenic Gene Signature
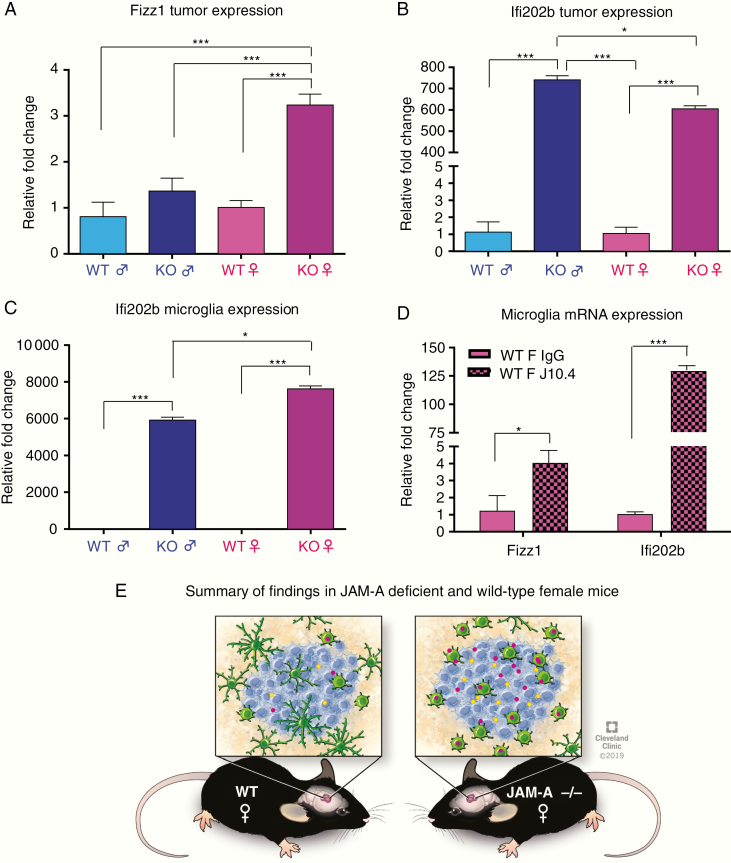
To identify the mechanism through which JAM-A deficiency mediates an aggressive GBM phenotype, we employed an RNA-sequencing approach and interrogated the GL261 model transplanted into JAM-A–deficient and WT male and female mice with the rationale that alterations in gene networks would likely be a result of cells within the tumor microenvironment, including microglia. RNA-sequencing analysis of these tumors identified that JAM-A–deficient female tumors have a distinct gene expression pattern compared with the other 3 groups (Supplementary Figure 8A), and clustering analysis revealed that tumors in female JAM-A–deficient mice have distinct gene signatures and cluster together compared with the other 3 groups (Supplementary Figure 8B). Given the sex-specific differences in microglia activation and survival, we focused on genes identified via RNA-sequencing analysis, a mouse-specific microglia activation marker (Retnla or Fizz1), and a mouse-specific interferon activated gene 202b (Ifi202b), which has known sex differences in systemic lupus erythematosus.38
Upon further validation we detected an upregulation of Fizz1 in JAM-A–deficient female tumors (Fig. 4A). Ifi202b expression was elevated in both male and female JAM-A–deficient tumors compared with WT tumors (Fig. 4B), but we observed a significant increase in Ifi202b in JAM-A–deficient female microglia compared with microglia from the other genotypes (Fig. 4C). The expression pattern was similar between the adult mice used for the RNA-sequencing and the microglia generated from young mice (data not shown). Therefore, there appears to be no major age-related difference in Fizz1 and Ifi202b in any of the genotypes. To directly test whether JAM-A suppresses the expression of Ifi202b and Fizz1, we employed a function-blocking antibody to JAM-A that prevents its dimerization and subsequent downstream signaling. When JAM-A was blocked in WT female microglia, we observed a significant increase in Ifi202b and Fizz1 (Fig. 4D). These data demonstrate that JAM-A deficiency in females changes the activation status of microglia via induction of Ifi202b and Fizz1 (Fig. 4E).
Fig. 4.
Female JAM-A–deficient mice have elevated expression of Ifi202b and Fizz1 in tumors compared with female WT mice. (A) Fizz1 mRNA expression in JAM-A–deficient and WT tumors. JAM-A–deficient female tumors had higher expression compared with other groups; ***P < 0.001 as assessed by one-way ANOVA. (B) Ifi202b mRNA expression in JAM-A–deficient and WT tumors (n = 4 per each group). P-value was assessed by two-way ANOVA (JAM-A–deficient male vs WT male and female, **P < 0.001; JAM-A–deficient male vs JAM-A–deficient female, *P < 0.05; JAM-A–deficient female vs WT male and female, P < 0.001***). (C) Ifi202b mRNA expression in JAM-A–deficient and WT male and female microglia (n = 4 or greater per each group). JAM-A–deficient female microglia had higher expression compared with all the other 3 genotypes. P-value was assessed by two-way ANOVA (JAM-A–deficient female vs JAM-A–deficient male, WT male, WT female, ***P < 0.001; JAM-A–deficient male vs WT male and WT female, ***P < 0.001). (D) Wild-type female microglia treated with control IgG or J10.4 JAM-A blocking antibody. Expression by mRNA of Ifi202b and Fizz1 was upregulated in WT female microglia (n = 3 per each group) upon blocking downstream signaling of JAM-A (WT female IgG vs J10.4 Ifi202b expression, ***P < 0.001; WT female IgG vs J10.4 Fizz1 expression, *P < 0.05. P-values were assessed by two-way ANOVA. (E) Schematic representing summary of findings, JAM-A–deficient mice have more activated microglia in the tumor, express higher amounts of Ifi202b and Fizz1 compared with WT female mice, and have poor overall survival.
Discussion
While sex differences are emerging as an area of study in GBM and some key molecular alterations between male and female GBM cells have been identified, our data provide evidence that sex differences are also present in the tumor microenvironment and can impact GBM growth. This observation is consistent with sex differences observed between male and female microglia in healthy and disease states.39 Our data suggest that JAM-A deficiency can induce an anti-inflammatory/pro-tumorigenic phenotype specifically in the female microenvironment, thereby supporting GBM aggressiveness. While it appears that these differences are driven more by microglia and less by TAMs or other infiltrating immune cells based on the number of cells present in the tumor microenvironment, future studies specifically assessing the function of JAM-A in microglia and TAMs are warranted. These studies could take advantage of intravital imaging approaches that have been informative in the interrogation of the dynamic responses of microglia in models of neuro-inflammation and have recently also been adapted to GBM models.40 Moreover, our findings also suggest that JAM-A functions as a tumor suppressor in the female microenvironment, while our previous work demonstrated that JAM-A is a cell-intrinsic tumor promoter in CSCs.19,20 These differences highlight the context-dependent role for JAM-A and provide a paradigm for the assessment of additional mechanisms that may function differently in a cell-intrinsic versus cell-extrinsic manner. The mouse glioma cell lines that we used in this study also express JAM-A (Supplementary Figure 1D). While we did not consider the cell-intrinsic effect of JAM-A signaling in tumor cells in this study, it is important to understand how JAM-A expression on CSCs/tumor cells affects tumor-associated microglia/macrophage proliferation and activation via direct interaction. Future studies that include genetic manipulation of JAM-A either by knockdown or by overexpression specifically in tumor cells could be used to assess the importance of tumor cell–microglia/macrophage interactions with the tumor microenvironment mediated by JAM-A.
While our observations demonstrate a sex-specific role for JAM-A in the tumor microenvironment in mouse models, there are some limitations to this work. First, the annotation of biological sex in many publicly available genomic databases is absent and therefore prevents assessment of data in a sex-specific manner easily. This can be overcome through bioinformatics approaches such as joint and individual variation explained (JIVE) analysis, which was recently leveraged to identify sex differences in human GBM patient transcriptional profiles.10 However, source data are required for these JIVE assessments, which may not be readily available. We were able to mine published sex-specific single cell RNA-sequencing data of human GBM specimens, and found there was no difference in JAM-A expression in tumor-associated microglia and macrophages30,41 (Supplementary Figure 9). These results suggest that the functional differences are likely not due to expression differences, but could rather be due to different downstream signaling or activation thresholds between males and females.10
Another limitation is the difference in immune genes between humans and mouse models, which limits the ability to directly compare inflammation signatures from mouse models with human GBM data. An additional caveat is that the majority of human genomic data from GBM is based on RNA-sequencing of bulk tissue and not individual cell lineages, so observations made in mice may be difficult to reproduce in human GBM patients. Given these limitations, we were able to identify a group of microglia- and myeloid-specific genes in The Cancer Genome Atlas and the Chinese Glioma Genome Atlas that showed a sex difference (Supplementary Figure 10). While no difference in gene expression for CD68, CD163, and GPR84 was observed between males and females, elevation of any of these genes did not impact male GBM patient survival. However, elevation of all 3 genes in females portended a poor prognosis. These findings support our observations in a mouse GBM model whereby an increase in microglial numbers and activation in female mice compared with males results in more aggressive GBM tumors and disease progression.
These findings also raise a series of questions that represent the starting point for future inquiry. Why does JAM-A deficiency have a stronger phenotype in females than males? In terms of activation, tumor cell proliferation, and Fizz1 and Ifi202b expression, our data demonstrate that female microglia are impacted by JAM-A deficiency to a greater extent than male microglia. It is conceivable that these findings indicate sex-specific thresholds to gene activity and function, as there were also less-pronounced differences between WT and JAM-A–deficient male mice. This notion is supported by previous reports demonstrating that male astrocytes had a lower threshold for transformation than females,5,7 and future studies assessing WT and JAM-A–deficient male mice and microglia may provide additional insight into key pathways driving these sex differences in activation thresholds. Ovariectomy data indicate that JAM-A–deficient female mice received a protective effect from ovarian hormones. Some outstanding questions including the role of JAM-A and its effect on female hormones remain to be addressed. Additional studies should determine the effect of the estrous cycle and the age of mice, as this could potentially alter the level of female hormones and tumor growth. How the levels of JAM-A and sex hormones in post-menopausal women affect tumor progression also needs to be examined.
Moreover, there is evidence in the literature supporting the notion that immune activation status differs between males and females.42 We identified that JAM-A deficiency in female microglia enhances the expression of the anti-inflammatory genes Fizz1 and Ifi202b, Ifi202b was previously reported to be upregulated in mice with lupus, where females are more susceptible to the disease.43 IFI16, the human orthologue of Ifi202b, is involved in mediating an anti-inflammatory phenotype by suppressing inflammasome activation in blood monocytes.44 Another outstanding question is the role of sex differences in cell adhesion programs. We previously demonstrated that adhesion is a CSC hallmark,45 but these studies were focused on cell-intrinsic mechanisms and devoid of sex-specific assessments. It is worth noting that the GL261 mouse model of GBM has a single copy of the X chromosome. Like multiple other cultured cancer cells, GL261 likely jettisoned its sex chromosomes as a result of the selective pressure of long-term culture.46 It would be interesting to revisit this hypothesis in the context of sex differences to determine whether male and female tumor cells have differential cell adhesion capacity. Recent work suggests that this may be the case, as long-term female GBM survivors have specific alterations in the integrin signaling network and more diffuse tumors, which may be driven by differential integrin signaling.10
Our findings suggest that JAM-A–deficient microglia have greater microglial activation, phagocytic ability, and aggressive tumor growth. Despite the greater phagocytic ability of JAM-A–deficient microglia, the tumors show aggressive growth, which could be due to the expression of anti-phagocytic receptors by tumor cells that protect them from phagocytosis.47 A recent study has suggested that tumor-associated microglia have increased expression of phagocytic receptors that promote phagocytic clearance of debris and apoptotic cells that would further lead to increased migratory capacity of tumor cells and tumor growth.48 These studies offer a possible explanation of why JAM-A female microglia might not phagocytose tumor cells. We have also demonstrated that female JAM-A–deficient microglia are more activated but express classic anti-inflammatory markers such as those expressed in M2 macrophages, and studies have shown that M2 TAMs have immune suppressive roles and thus promote tumor growth.33,49 In order to better understand the pro-tumorigenic signals mediated by JAM-A–deficient microglia, future assessments would require in vivo imaging studies to directly interrogate the interactions between tumor cells and microglia and help to understand how activated microglia interact with tumor cells and thereby influence GBM growth. As our observations demonstrate an unexpected and striking sex difference based on an adhesion mechanism in the tumor microenvironment, it becomes critically important that future studies take into account sex differences beyond cell-intrinsic alterations. These assessments are likely to yield sex-specific discoveries that can then be leveraged for the development of more personalized GBM therapies.
Supplementary Material
Acknowledgments
We thank the members of the Lathia and Davalos laboratories for insightful discussion and constructive comments on the manuscript. We thank the Thesis Committee members of SMT: Drs Crystal Weyman, Thomas McIntyre, Alexandru Almasan and Hannelore Heemers for their valuable and constructive comments on the manuscript. We thank Amanda Mendelsohn and the Center for Medical Art and Photography at the Cleveland Clinic for providing illustrations and Dr Erin Mulkearns-Hubert for editorial assistance.
Funding
This work was funded by the National Institutes of Health (R01 NS083629 to JDL) and (R01 NS112526 to DD), Sontag Foundation (JDL), Cleveland Clinic Brain Tumor Center of Excellence (JDL), Cleveland Clinic VeloSano Bike Race (DD, JDL), Case Comprehensive Cancer Center (JSB-S, JDL), American Brain Tumor Association Research Collaboration Grant (JBR, JDL), Cleveland State University Graduate Student Research Award (ST), Case Comprehensive Cancer Center T32 CA059366 (DB), NIH fellowships F32CA243314 (DB) and 1F32CA213727 (DJS), and the Bodossaki Foundation (EP).
Conflict of interest statement. The authors declare no potential conflicts of interest.
Authorship statement. All authors have significantly contributed to this work. Authors were involved in experimental design (SMT, DJS, DB, EP, DD, JDL), implementation (SMT, DB, DJS, EP, SP, AL, TJA, NB, SS), data analysis and interpretation (SMT, DJS, DB, EP, SP, AL, TJA, NB, UPN, RAK, JRC, JSB-S, JBR, MB, DD, JDL), writing of the manuscript (SMT, JDL), and approval of the final version (all authors).
References
- 1. Stupp R, Taillibert S, Kanner A, et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA. 2017;318(23):2306–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Roesch S, Rapp C, Dettling S, Herold-Mende C. When immune cells turn bad-tumor-associated microglia/macrophages in glioma. Int J Mol Sci. 2018;19(2):436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guadagno E, Presta I. Role of macrophages in brain tumor growth and progression. Int J Mol Sci. 2018;19(4):1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sørensen MD, Dahlrot RH, Boldt HB, Hansen S, Kristensen BW. Tumour-associated microglia/macrophages predict poor prognosis in high-grade gliomas and correlate with an aggressive tumour subtype. Neuropathol Appl Neurobiol. 2018;44(2):185–206. [DOI] [PubMed] [Google Scholar]
- 5. Kfoury N, Sun T, Yu K, et al. Cooperative p16 and p21 action protects female astrocytes from transformation. Acta Neuropathol Commun. 2018;6(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ippolito JE, Yim AK, Luo J, Chinnaiyan P, Rubin JB. Sexual dimorphism in glioma glycolysis underlies sex differences in survival. JCI Insight. 2017;2(15):e92142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sun T, Warrington NM, Luo J, et al. Sexually dimorphic RB inactivation underlies mesenchymal glioblastoma prevalence in males. J Clin Invest. 2014;124(9):4123–4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ostrom QT, Gittleman H, Xu J, et al. CBTRUS Statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2009–2013. Neuro-oncology. 2016;18(suppl_5):v1–v75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sun T, Plutynski A, Ward S, Rubin JB. An integrative view on sex differences in brain tumors. Cell Mol Life Sci. 2015;72(17):3323–3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yang W, Warrington NM, Taylor SJ. Sex differences in GBM revealed by analysis of patient imaging, transcriptome, and survival data. Sci. Transl. Med. 2019;11(473):eaao5253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ostrom QT, Rubin JB, Lathia JD, Berens ME, Barnholtz-Sloan JS. Females have the survival advantage in glioblastoma. Neuro Oncol. 2018;20(4):576–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ostrom QT, Coleman W, Huang W, et al. ; GliomaScan consortium Sex-specific gene and pathway modeling of inherited glioma risk. Neuro Oncol. 2019;21(1):71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ostrom QT, Kinnersley B, Wrensch MR, et al. ; GliomaScan consortium Sex-specific glioma genome-wide association study identifies new risk locus at 3p21.31 in females, and finds sex-differences in risk at 8q24.21. Sci Rep. 2018;8(1):7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lenz KM, McCarthy MM. A starring role for microglia in brain sex differences. Neuroscientist. 2015;21(3):306–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hanamsagar R, Bilbo SD. Sex differences in neurodevelopmental and neurodegenerative disorders: focus on microglial function and neuroinflammation during development. J Steroid Biochem Mol Biol. 2016;160:127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Davalos D, Grutzendler J, Yang G, et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8(6):752–758. [DOI] [PubMed] [Google Scholar]
- 17. Meller J, Chen Z, Dudiki T, et al. Integrin-kindlin3 requirements for microglial motility in vivo are distinct from those for macrophages. JCI Insight. 2017;2(11):e93002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pong WW, Walker J, Wylie T, et al. F11R is a novel monocyte prognostic biomarker for malignant glioma. PLoS One. 2013;8(10):e77571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lathia JD, Li M, Sinyuk M, et al. High-throughput flow cytometry screening reveals a role for junctional adhesion molecule A as a cancer stem cell maintenance factor. Cell Rep. 2014;6(1):117–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Alvarado AG, Turaga SM, Sathyan P, et al. Coordination of self-renewal in glioblastoma by integration of adhesion and microRNA signaling. Neuro Oncol. 2016;18(5):656–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cooke VG, Naik MU, Naik UP. Fibroblast growth factor-2 failed to induce angiogenesis in junctional adhesion molecule-A-deficient mice. Arterioscler Thromb Vasc Biol. 2006;26(9):2005–2011. [DOI] [PubMed] [Google Scholar]
- 22. Marshall GP 2nd, Demir M, Steindler DA, Laywell ED. Subventricular zone microglia possess a unique capacity for massive in vitro expansion. Glia. 2008;56(16):1799–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lian H, Roy E, Zheng H. Microglial phagocytosis assay. Bio Protoc. 2016;6(21):e1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhai H, Heppner FL, Tsirka SE. Microglia/macrophages promote glioma progression. Glia. 2011;59(3):472–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Clapcote SJ, Roder JC. Simplex PCR assay for sex determination in mice. BioTechniques. 2005;38(5):702, 704, 706. [DOI] [PubMed] [Google Scholar]
- 26. Toonen JA, Solga AC, Ma Y, Gutmann DH. Estrogen activation of microglia underlies the sexually dimorphic differences in Nf1 optic glioma-induced retinal pathology. J Exp Med. 2017;214(1):17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang Y, Sloan SA, Clarke LE, et al. Purification and characterization of progenitor and mature human astrocytes reveals transcriptional and functional differences with mouse. Neuron. 2016;89(1):37–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang Y, Chen K, Sloan SA, et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. 2014;34(36):11929–11947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Naik MU, Vuppalanchi D, Naik UP. Essential role of junctional adhesion molecule-1 in basic fibroblast growth factor-induced endothelial cell migration. Arterioscler Thromb Vasc Biol. 2003;23(12):2165–2171. [DOI] [PubMed] [Google Scholar]
- 30. Darmanis S, Sloan SA, Croote D, et al. Single-cell RNA-seq analysis of infiltrating neoplastic cells at the migrating front of human glioblastoma. Cell Rep. 2017;21(5):1399–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jonas RA, Yuan TF, Liang YX, Jonas JB, Tay DK, Ellis-Behnke RG. The spider effect: morphological and orienting classification of microglia in response to stimuli in vivo. PLoS One. 2012;7(2):e30763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Grimaldi A, Serpe C, Chece G, et al. Microglia-derived microvesicles affect microglia phenotype in glioma. Front Cell Neurosci. 2019;13:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhou W, Ke SQ, Huang Z, et al. Periostin secreted by glioblastoma stem cells recruits M2 tumour-associated macrophages and promotes malignant growth. Nat Cell Biol. 2015;17(2):170–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Daginakatte GC, Gianino SM, Zhao NW, Parsadanian AS, Gutmann DH. Increased c-Jun-NH2-kinase signaling in neurofibromatosis-1 heterozygous microglia drives microglia activation and promotes optic glioma proliferation. Cancer Res. 2008;68(24):10358–10366. [DOI] [PubMed] [Google Scholar]
- 35. Zhu W, Carney KE, Pigott VM, et al. Glioma-mediated microglial activation promotes glioma proliferation and migration: roles of Na+/H+ exchanger isoform 1. Carcinogenesis. 2016;37(9):839–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ellert-Miklaszewska A, Wisniewski P, Kijewska M, et al. Tumour-processed osteopontin and lactadherin drive the protumorigenic reprogramming of microglia and glioma progression. Oncogene. 2016;35(50):6366–6377. [DOI] [PubMed] [Google Scholar]
- 37. Choi YS, Baek K, Choi Y. Estrogen reinforces barrier formation and protects against tumor necrosis factor alpha-induced barrier dysfunction in oral epithelial cells. J Periodontal Implant Sci. 2018;48(5):284–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cao Y, Wang L, Wang CY, et al. Sex differences in correlation with gene expression levels between Ifi200 family genes and four sets of immune disease-relevant genes. J Immunol Res. 2018;2018:1290814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Guneykaya D, Ivanov A, Hernandez DP, et al. Transcriptional and translational differences of microglia from male and female brains. Cell reports. 2018;24(10):2773–2783.e2776. [DOI] [PubMed] [Google Scholar]
- 40. Davalos D, Ryu JK, Merlini M, et al. Fibrinogen-induced perivascular microglial clustering is required for the development of axonal damage in neuroinflammation. Nat Commun. 2012;3:1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Neftel C, Laffy J, Filbin MG, et al. An integrative model of cellular states, plasticity, and genetics for glioblastoma. Cell. 2019;178(4):835–849.e821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16(10):626–638. [DOI] [PubMed] [Google Scholar]
- 43. Choubey D, Panchanathan R. Interferon-inducible Ifi200-family genes in systemic lupus erythematosus. Immunol Lett. 2008;119(1-2):32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Veeranki S, Duan X, Panchanathan R, Liu H, Choubey D. IFI16 protein mediates the anti-inflammatory actions of the type-I interferons through suppression of activation of caspase-1 by inflammasomes. PLoS One. 2011;6(10):e27040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lathia JD, Gallagher J, Heddleston JM, et al. Integrin alpha 6 regulates glioblastoma stem cells. Cell Stem Cell. 2010;6(5):421–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Xu J, Peng X, Chen Y, et al. Free-living human cells reconfigure their chromosomes in the evolution back to uni-cellularity. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hutter G, Theruvath J, Graef CM, et al. Microglia are effector cells of CD47-SIRPα antiphagocytic axis disruption against glioblastoma. Proc Natl Acad Sci U S A. 2019;116(3):997–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Maas SLN, Abels ER, Van De Haar LL, et al. Glioblastoma hijacks microglial gene expression to support tumor growth. J Neuroinflammation. 2020;17(1):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Komohara Y, Ohnishi K, Kuratsu J, Takeya M. Possible involvement of the M2 anti-inflammatory macrophage phenotype in growth of human gliomas. J Pathol. 2008;216(1):15–24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.