Abstract
Simple Summary
The childhood obesity epidemic is impacting tens of millions of children globally. While obesity causes several cancers in adults, its potential role in causing pediatric cancers remains unclear. In this review, we assess the potential contribution of obesity to the development of acute lymphoblastic leukemia (ALL), the most common pediatric cancer. We review the possible mechanisms by which the adipose tissue attracts and protects leukemia cells and how it interferes with the actions of chemotherapies used in ALL treatment. We also examine adipose tissue-secreted molecules and fuels that may support leukemia development. While there are no current definite causal links between obesity and ALL, there are plausible mechanisms that need further investigation to explore the impact of obesity on causing ALL and on impacting treatment outcomes.
Abstract
Childhood obesity is a growing epidemic with numerous global health implications. Over the past few years, novel insights have emerged about the contribution of adult obesity to cancer risk, but the evidence base is far more limited in children. While pediatric patients with acute lymphoblastic leukemia (ALL) are at risk of obesity, it is unclear if there are potential causal mechanisms by which obesity leads to ALL development. This review explores the endocrine, metabolic and immune dysregulation triggered by obesity and its potential role in pediatric ALL’s genesis. We describe possible mechanisms, including adipose tissue attraction and protection of lymphoblasts, and their impact on ALL chemotherapies’ pharmacokinetics. We also explore the potential contribution of cytokines, growth factors, natural killer cells and adipose stem cells to ALL initiation and propagation. While there are no current definite causal links between obesity and ALL, critical questions persist as to whether the adipose tissue microenvironment and endocrine actions can play a causal role in childhood ALL, and there is a need for more research to address these questions.
Keywords: childhood obesity, acute lymphoblastic leukemia, adipocyte, natural killer cells, cytokines, adipose stem cells
1. Introduction
The rates of childhood obesity have started to plateau in high-income countries, yet they continue to rise in low- and middle-income countries [1]. On a global scale, the obesity epidemic impacts one in three people, including tens of millions of children. These staggering figures are coupled with evidence that obesity management programs have modest success rates, and prevention remains the best cure [2,3,4,5]. Managing and preventing obesity may help lower its cardiometabolic and psychological comorbidities that, at times, start during childhood and persist into adulthood [6,7,8,9,10,11,12,13,14].
In adults, obesity has been linked to the increased risk of several malignancies, including esophageal adenocarcinoma, colon, renal cell, uterine and postmenopausal breast cancers [15,16,17,18]. On the other hand, a normal body mass index (BMI, <25 kg/m2) is associated with reduced rates of gastric cardia, gallbladder, pancreatic, ovarian, and thyroid cancer, as well as meningiomas, hepatocellular carcinoma and multiple myeloma [15,19,20,21,22,23,24,25]. Notably, obesity during childhood is associated with an increased risk of adult pancreatic and colon cancers, especially in women [26,27,28]. The evidence for the association of childhood obesity with other adult and pediatric cancers is scarce.
The most common pediatric cancer is acute lymphoblastic leukemia (ALL) accounting for almost 25% of all childhood cancers. ALL is also responsible for 26% of cancer-related mortality, making it the second most common driver of cancer-related deaths after brain tumors [15,26,29].
While there is no current definite causal link between obesity and ALL, there are several plausible mechanisms and parallels whereby obesity may contribute to ALL development and outcomes.
Several models have demonstrated that the adipose tissue supports the rapid progression of certain cancers. For example, leptin and resistin, two adipose tissue products, promoted the growth of melanoma cells and reduced the therapeutic efficacy of dacarbazine, the chemotherapeutic agent used to treat it. Diet-induced obesity promoted melanoma development and reduced dacarbazine ability to access melanoma cells [30,31]. These lines of evidence point to the potential contribution of the adipose tissue and its products to carcinogenesis.
In this review, we evaluate the evidence for the association of obesity with ALL at diagnosis and end of therapy, and its links to the risk of relapse and mortality. We also review potential Immunometabolic and endocrine mechanisms that may promote ALL development and propagation in obese children. Finally, we propose future research priorities to further the understanding of the impact of obesity on ALL genesis and outcomes.
2. The Association of Childhood Obesity & Birth Weight with ALL
2.1. Obesity at Initial ALL Diagnosis
While the rates of pediatric obesity and ALL have risen over the past few decades [32,33], most patients with ALL have normal BMI values at diagnosis. However, weight increases are common during and after ALL treatment (Table 1) [34,35,36,37,38,39,40,41].
Table 1.
Rates of Obesity at Acute Lymphoblastic Leukemia (ALL) Diagnosis, End of Treatment, and During Follow-up.
| Author (Reference) | Study Design | Populations | Protocols | Findings |
|---|---|---|---|---|
| Van Dongen-Melman et al. [34] | RCR |
n = 113 Age: 0.5–15 years |
Overweight/obese patients (>90th BMI percentile) constituted 7.9% (n = 9) of the sample at diagnosis. At the end of therapy, 30% (n = 34) were overweight/obese. At four years after treatment completion, 23.9% (n = 27) were overweight/obese. Radiotherapy was not associated with obesity. Patients who received a combination of dexamethasone and prednisone were at the highest risk of being obese (44%). Higher cumulative steroid dose did not contribute to more obesity. | |
| Withycombe et al. [35] | RCR |
n = 1638 Age: 2–20 years |
COG (CCG 1961) | Obesity rates in children with high risk ALL were 14% at baseline and 23% at the end of treatment. Females, Black or Hispanic, and age 5–9-year-old, but not cranial irradiation, were risk factors. The highest increase in BMI% was between maintenance phases 1–3, 9–12 months postdiagnosis. (Induction BMI% 7.2, BMI% 12.6 by maintenance phase 3). |
| Breene et al. [36] | RCR |
n = 77 Age: 1–16 years |
MRC UKALL97 protocol | Whole group: Patients received only chemotherapy and no CRT. Thirty patients (39%) received prednisolone, and 47 (61%) got dexamethasone. Weight gain was not linked to steroids. There was a significant rise in BMI-SDS from diagnosis (0.35, 95% CI 0.20–0.50) to the end of treatment (1.29, 95% CI 1.13–1.45, p < 0.0001), and at three-year follow-up (1.04, 95% CI 0.85–1.22, p <0.0001). More survivors were overweight or obese at three years post treatment (25/53, 47.20%) when compared to diagnosis (23/77, 29.90%), (p-value 0.01) Female subgroup: Significant rise in BMI-SDS from diagnosis (0.46, 95% CI 0.27–0.64), at end of treatment (1.46, 95% CI 1.26–1.66, p < 0.0001) and at three-year follow-up (1.24, 95% CI 1.03–1.45, p < 0.0001) Male subgroup: Significant rise in BMI-SDS from diagnosis (0.24, 95% CI 0.01–0.46), to end of treatment (1.11, 95% CI 0.85–1.36, p < 0.0001) and at three-year follow-up (0.77, 95% CI 0.43–1.10, p < 0.0001) |
| Razzouk et al. [37] | RCS |
n = 248 Age < 19 years At diagnosis, 13.2–30 years at the latest assessment |
Chemotherapy-Total Therapy Study X protocol | The prevalence of overweight/obesity in 0–6-year-old (6%) was lower than that in the 13–19-year-old group (19%) at diagnosis. At adult height attainment, the prevalence of overweight/obese in 0-6 years of age at diagnosis was 41%, versus 13–19 years of age at 35%. These rates are close to the USA’s general population. Those <6 years of age (OR 2.3, 95% CI 1.2–4.2, p-value 0.01), male (OR 0.50, 95% CI 0.28–0.91, p-value 0.02), and being overweight/obese at diagnosis (OR 14.00, 95% CI 5.00–39.00, p-value < 0.0001) were predictors of obesity at adult height attainment. CRT (24 Gy) led to an increase in BMI trajectory, but this was not different from the 18 Gy CRT group. |
| Ghosh et al. [38] * | RCS |
n = 4775 Age: 2–30 years Age, sex, and ethnicity-matched controls from NHANES |
COG AALL17D2 |
Newly diagnosed had overweight rates of 17% and obesity rates of 20%. 58% had an average weight, and 5% were underweight. Males, Hispanics, and B-cell ALL were associated with obesity. Obesity was associated with CNS disease. |
| Foster et al. [39] | RCS |
n = 121 Age: 2–15 years |
COG AALL0232, AALL0331, AALL0932, AALL1131, POG 9904, POG9905 | 15% of patients were overweight and 15% of patients were obese at the time of diagnosis of ALL. At 5-year follow-up, 22% of patients were overweight and 35% of patients were obese. Start of treatment BMI z-score 0.25 (95% CI 0.01–0.49) Five-year follow-up BMI z-score 0.99 (95% CI 0.79–1.19; p-value < 0.0001) |
| Didi et al. [40] | PCS |
n = 114 Age: 2–16 years |
MRC UKALL protocol | 23/51 male (45%) and 30/63 female (47%) patients were obese at final height attainment. Female patients: Girls’ obesity occurred from start to end of treatment then plateaued. Start of treatment BMI z-score 0.05 (95% CI −2.2, 2.0) End of treatment BMI z-score 1.2, 95% CI 0.3–2.8; p-value 0.0002) Male patients: Boys gained weight from the start of treatment, and weight gain continued post-treatment completion. Start of treatment BMI z -score 0.10 (95% CI −1.1, 1.3); End of treatment BMI z-score 0.6 (95% CI −0.4, 4.9; p-value 0.001) |
| Craig et al. [41] # | CSS |
n = 213 radiotherapy, n = 85 no radiotherapy Age: 0–16 years |
Unirradiated: MRC UKALL XI or infant ALL protocol Irradiated:
|
Female 18–20 Gy CRT: BMI z-score at diagnosis −0.24 ± 0.15 BMI z-score at end of treatment 0.46 ± 0.11 (p-value < 0.0001) 22–24 Gy CRT: BMI z-score at diagnosis −0.70 ± 0.16 BMI z-score at the end of treatment −0.17 ± 0.12 (p-value 0.0005) Male 18–20 Gy CRT: BMI z-score at diagnosis −0.40 ± 0.16 BMI z-score at end of treatment 0.37 ± 0.16 (p-value < 0.0001) 22–24 Gy CRT: BMI z-score at diagnosis −0.17 ± 0.28 BMI z-score at end of treatment 0.48 ± 0.16 (p-value 0.02) BMI z-scores in patients with no history of CRT: Female BMI z-score at diagnosis −0.12 ± 0.19 BMI z-score at end of treatment 0.70 ± 0.17 (p-value < 0.0001) Male BMI z-score at diagnosis 0.23 ± 0.26 BMI z-score at end of treatment 0.81 ± 0.18 (p-value 0.01) |
Abbreviations: RCR, retrospective chart review; BMI, body mass index; COG, Children’s Oncology Group; CCG, Children’s Cancer Group; BMI%, body mass index percentile; MRC, Medical Research Council; UKALL, United Kingdom Acute Lymphoblastic Leukemia Regimen; NR, not reported; CI, confidence interval; SDS, standard deviation score; NS, not significant; RCS, retrospective cohort study; CRT, cranial irradiation; OR, odds ratio; Gy, Gray; COG AALL, Children’s Oncology Group Acute Lymphoblastic Leukemia protocols; POG, Pediatrics Oncology Group; z-score, standard score; PCS prospective cohort study; CCS, cross-sectional study. # The p-values include a comparison of BMI z-scores from baseline to end of treatment. * This abstract reported a study that compared ALL patients to the National Health and Nutrition Examination Survey (NHANES) control subjects (n = 30,107). This abstract also validated the already known association of being underweight at ALL diagnosis.
In addition, a few patient subgroups may be at risk of obesity when compared to the whole ALL cohort. Early evidence suggested that obesity rates are increased among children aged 5–9 years and females at diagnosis of ALL when compared to other age groups and males, respectively [34,35,42]. More recent studies demonstrated higher obesity rates in males, children with B-cell ALL, and in those with central nervous system involvement [38]. Importantly, survivors of childhood ALL who were obese at diagnosis were more likely to be obese as adults [37]. In summary, body mass increases from diagnosis to the end of therapy and beyond are common in survivors of childhood ALL. A deeper understanding of why prepubertal children, ALL subtype, and central involvement are associated with increased obesity risk is needed. The impact of sex on ALL is unclear and requires further study. Regardless, obesity during childhood is likely to propagate into adult life which may drive type 2 diabetes and cardiovascular disease in survivors.
2.2. Birth Weight and ALL
An elevated birth weight has been linked to an increased risk of ALL in children (Table 2).
Table 2.
Birth Weight and the Risk of Development of ALL.
| Author (Reference) | Study Design | Populations | Findings |
|---|---|---|---|
| Hjalgrim et al. [43] | Meta-analysis | 18 studies n = 10,282 Age: 0–29 years |
BWt > 4000 was associated with a trend of higher risk of ALL (OR 1.26, 95% CI 1.17–1.37) |
| Caughey et al. [44] | Meta-analysis | 32 studies n = 16,501 Leukemias (n = 10,974 ALL) Age: ≤ 30 years |
Significant odds for the association of high BWt with ALL risk (OR 1.23, 95% CI 1.15–1.32) |
| Milne et al. [45] | CCS |
n = 519 patients Age: 0–14 years |
OR for 1 SD increase in proportion to optimal BWt 1.18 (95% CI 1.04–1.35, p < 0.05). However, faster fetal growth, instead of BWt, was the factor associated with ALL risk |
| Jiménez-Hernández et al. [46] | CCS |
n = 2910 children Age: 0–18 years |
ALL is associated with a birth weight ≥ 2500 g (OR 2.06, 95% CI 1.59–2.66) Birth weight ≥ 3500 g was also associated with ALL OR 1.19 (95% CI: 1.00–1.41) |
| Sprehe et al. [47] | RCR |
n = 13,988 children Age < 5 years at cancer diagnosis + age-matched controls |
Increased risk of ALL was associated with LGA compared to AGA LGA (<4000g) OR 1.5 (95% CI: 0.97–2.52, p = 0.0005) LGA (>4000g) OR 1.67 (95% CI: 1.29–2.16, p = 0.0005) |
Abbreviations: BWt, birth weight; OR, odds ratio; CI, confidence interval; SD, standard deviation; CCS, case-control study; LGA, large for gestational age; AGA, appropriate for gestational age; RCR, retrospective chart review.
Chromosomal translocations such as t(12:21), t(4:11) or t(8:21) contribute to the uncontrolled proliferation of lymphoblasts, and high birth weight may drive this proliferation with high levels of growth factors [43,44,46,48,49,50,51]. Furthermore, having a high birth weight when corrected for gestational age and rapid fetal growth rates predict ALL risk during childhood [45,47]. In summary, birth weight, a variable determined by multiple prenatal influences, may play a role in the development of ALL, and the exact mechanisms by which birth weight affects ALL risk require further interrogation.
3. Obesity in Childhood ALL Survivors and Its Effects on Outcomes
3.1. Obesity is Common Among Survivors of Childhood ALL
Treatment of childhood ALL increases the risk of obesity in survivors when compared to levels noted at diagnosis (Table 1). The weight gain starts during treatment, and 40–50% of young adult survivors remain obese at follow-up [36,42]. The most significant weight gain occurs between maintenance phases 1–3, approximately 9–12 months into treatment protocols [35,39,52].
While some studies suggested that age, sex, cranial irradiation (CRT) and steroids may play a role in obesity development in ALL survivors [35,37,40,41,53,54,55], a recent meta-analysis of 20 studies (n = 1742) demonstrated that the mean BMI z-score was 0.83 (95% CI 0.60–1.06). This z-score corresponds to the 80th percentile, which is significantly higher than reference populations, including the National Health and Nutrition Examination Survey (NHANES; BMI z-score 0.4–0.6). While studies had high heterogeneity, subgroup analyses revealed a high prevalence of obesity in ALL survivors (29–69% 5–9 years post-treatment; 34–46% >10 years post-treatment) but obesity risk was not consistently linked to age, female sex, CRT, and steroid type or dose [56].
The obesogenic impact of chemotherapeutic agents has not been widely assessed. However, high-intensity induction chemotherapy (the addition of anthracycline ± cyclophosphamide) does not seem to affect BMI z-scores above standard induction chemotherapy (steroids, vincristine and l-asparaginase) [55]. In summary, there are likely factors beyond age, sex, and treatments that drive obesity risk in ALL survivors, and these factors require identification and further analysis.
3.2. The Association of Obesity with Relapse Risk and Mortality in ALL
Obesity may impact treatment outcomes in ALL. Overweight and obese children have worse event-free survival and a higher rate of morbidities than non-obese children during the induction phase [57,58,59,60]. Furthermore, obesity during the premaintenance chemotherapy phase is a risk factor for hypertension (OR 3.27; 95% CI 1.10–10.00; p = 0.05), hyperglycemia (OR 2.62; 95% CI 1.04–6.56; p = 0.04), and febrile neutropenia (incident rate ratio 1.53; 95% CI 1.10–2.12; p = 0.01) [60]. Obesity is also a risk factor for persistent minimal residual disease following induction chemotherapy (OR 2.57; 95% CI 1.19–5.54; p = 0.016) [59].
Early evidence suggested that an elevated body mass was a risk factor for ALL relapse [61,62]. However, recent meta-analyses demonstrated a nonsignificant trend in overweight and obese children [58,63] (Table 3). These data suggest that obesity does not drive relapse risk in ALL.
Table 3.
Meta-Analyses of Relapse Risk with Obesity in ALL Patients.
| Author | Study Design | Population | Protocol | Findings |
|---|---|---|---|---|
| Saenz et al. [57] | RCS + Meta-analysis |
n = 181 Age: 2–17 years |
COG | A trend of relapse risk for obese/overweight patients (HR 2.89, 95% CI 0.89–9.36, p = 0.08) in age-and sex-adjusted patients ≥ 10 years old. While meta-analyses reported increased mortality in the overweight/obese group (HR 1.39, 95% CI 1.16–1.46, p < 0.05), this was not confirmed in adjusted analyses |
| Orgel et al. [58] | Meta-analysis | 11 articles n = 8680 (ALL), Age: 0–21 years |
Included all treatment regimens that reported the effect of weight on treatment outcomes, specifically EFS mortality, overall survival, the cumulative incidence of relapse, and treatment-related toxicity |
When compared to a lower BMI, higher BMI was associated with a statistically nonsignificant trend of risk of relapse (RR 1.17; 95% CI: 0.99, 1.38) Patients with a higher BMI had lower EFS (RR 1.35, 95% CI 1.20, 1.51) than those with lower BMI |
Abbreviations: RCS, retrospective cohort study; COG, Children’s Oncology Group; HR, hazard ratio; CI, confidence interval; ALL, Acute Lymphoblastic Leukemia; EFS, event-free survival; RR, risk ratio; BMI, body mass index.
On a mechanistic level, in-vitro and in-vivo evidence suggests that adipocytes may affect leukemia treatment efficacy by attracting and supporting the proliferation of lymphoblasts, and there are several protective types of machinery against leukemia cell destruction [64].
4. Potential Mechanisms by Which Obesity May Contribute to ALL Pathogenesis
The adipose tissue is regarded as one of the most metabolically active organs in the body [65,66,67,68], and some of its endocrine actions are mediated via compounds called adipocytokines. Adipose tissue expansion in obesity is associated with immune system activation and inflammation that promotes several cardiometabolic disorders such as atherosclerosis, hyperglycemia and dyslipidemia [69,70,71].
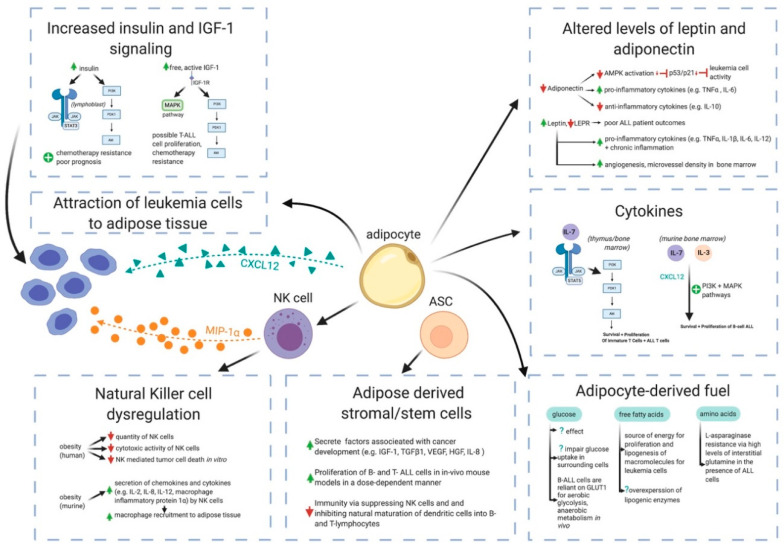
While there are data that link obesity as a causal factor to certain adult cancers [15], there is no direct evidence to suggest that obesity causes ALL in children. However, a recent study demonstrated that high-fat-fed mice experience a more rapid progression of ALL than lean mice, offering a potential link between obesity and ALL evolution [72]. Obesity is also associated with the production of several adipocytokines hypothesized to promote oncogenesis, including leptin, tumor necrosis factor-alpha (TNFα), interleukin-6 (IL-6), IL-7, and IL-8 [73,74,75,76,77]. Below, we discuss some putative pathways in which the adipose tissue may contribute to ALL pathogenesis (Figure 1).
Figure 1.
Potential mechanisms for obesity associations with ALL. The adipocytes secrete chemokines that can attract leukemia cells, including cysteine-X-cysteine (C-X-C) motif chemokine 12 (CXCL12). Adipose tissue-infiltrating Natural Killer (NK) cells secrete macrophage inflammatory protein-1 alpha (MIP-1α), another chemokine for leukemia cells. Obesity is also associated with reduced numbers and cytotoxic activity of NK cells. Adipose-derived stromal/stem cells (ASC) secrete factors that promote the proliferation of lymphoblasts.
The adipocytokine secretion by the obese adipose tissue is altered, with increased secretion of leptin and reduced production of adiponectin. The changes in these adipokines alter leukemia cell activity, cytokine secretion and bone marrow angiogenesis. Increased secretion of cytokines, including IL-7 and IL-3, promotes the survival and proliferation of leukemia cells through the activation of the phosphatidylinositol 3-kinase (PI3K) and mitogen-activated protein kinase (MAPK) pathways. The upregulated production of growth factors such as insulin and insulin-like growth factor-1 (IGF-1) contributes to enhanced chemotherapy resistance and drives poor prognosis through the overexpression of Janus kinase/signal transducer and activator of transcription protein 3 (JAK/STAT3) and activation of the PI3K and MAPK pathways. Obesity also contributes to increased adipocyte-derived fuel production of glucose, free fatty acids and amino acids, which causes leukemia cell proliferation (Figure developed in BioRender, https://biorender.com/).
4.1. White Adipose Tissue is Far More Than a Passive Calorie Sink in Obesity
The adipose tissue is comprised of three main subtypes, including white adipose tissue (WAT), beige adipose tissue and brown adipose tissue [78]. WAT can be further subdivided based on depot location into visceral or subcutaneous compartments. The former depot secretes inflammatory cytokines in obesity while the latter depot stores excess triglycerides and releases free fatty acids (FFAs) throughout periods of starvation, fasting or exercise. While brown adipose tissue is most prominent in infants and decreases with age, WAT increases with aging and obesity. The expansion of visceral WAT is associated with the risk of cardiovascular diseases, metabolic syndrome, and cancer [79,80,81,82,83].
In obesity, adipose tissue expansion leads to relative tissue hypoxia as the tissue demands exceed vascular supply and oxygenation [84]. In turn, hypoxia upregulates angiogenesis and the production of chemokines that attract immune cells into the adipose tissue [85]. The main source of cytokines in the obese adipose tissue are infiltrating immune cells, including macrophages, and these cytokines drive local tissue inflammation and insulin resistance. The ability of the adipose tissue to expand and act as a reservoir for cytokines and FFAs is finite. These molecules exit the adipose tissue, enter the circulation, and arrive at skeletal muscle and liver where they drive inflammation and insulin resistance in these metabolic organs [86,87,88,89,90].
4.2. Adipocytes Interference in Pharmacokinetics of ALL Chemotherapies
There is evidence that excess adipose tissue may lower ALL treatment efficacy by attracting lymphoblasts, supporting their proliferation and protecting them from destruction through several mechanisms (Figure 1) [64]. Lymphoblasts have been identified within the adipose tissue of mice with ALL, suggesting that these cells migrate into the adipose compartment [91]. The retro-orbital transplantation of syngeneic leukemia cells into lean and high-fat-fed obese mice resulted in the migration of these cells to the adipose tissue in-vivo [64]. Cells were also detected in the bone marrow, spleen, liver and visceral and perirenal fat. Obese mice had more leukemia cells per milligram of visceral fat than nonobese mice. The adipocyte stromal cell-derived factor 1-α (SDF-1α), also known as cysteine-X-cysteine (C-X-C) motif chemokine 12 (CXCL12), promoted leukemia cell migration into the adipose tissue [64].
Adipose tissue also alters the pharmacokinetics of chemotherapeutic agents via a combination of mechanisms including the increased accumulation of lipid-soluble drugs, increased binding of basic chemotherapeutic agents due to elevated levels of α1-acid glycoproteins and increased secretion of water-soluble drugs through activation of cytochrome P450 2E1 (Figure 1) [92]. For example, leukemia cells have an impaired response to vincristine, a lipophilic anti-leukemia agent, in obese mice. While blood and tissue vincristine levels were similar in obese and lean mice, the leukemia cells, when cocultured with adipocytes in vitro, migrated beneath the adipocyte layer, which led to reduced vincristine exposure [91].
A lower serum concentration of 6-mercaptopurine (6-MP), a drug used to maintain remission in ALL patients, was noted with a BMI >75th percentile when compared to children with a BMI <75th percentile [93]. Adipocytes also sequester and metabolize other chemotherapeutic agents, including anthracyclines, daunorubicin and doxorubicin, through the deactivating enzymes aldo-keto reductases and carbonyl reductases [94]. The adipocytes also upregulate pro-survival signals, including B-cell lymphoma 2 (BCL-2) and serine/threonine-protein kinase Pim-2 (PIM2). Furthermore, adipocytes inactivate pro-apoptotic proteins such as the B-cell lymphoma 2 protein-associated agonist cell death protein (BAD).
Overall, obesity impacts the efficacy of multiple chemotherapeutic agents used in ALL therapy. Further research is needed to understand how the expanding adipose tissue in obesity disrupts chemotherapies, as these mechanisms can be used as potential therapeutic entry points to enhance treatment success.
4.3. Cytokines
Cytokines provide stimuli for the survival, differentiation, and proliferation of hematopoietic cells [95]. In obesity, the secretion of multiple cytokines including IL-3, IL-7, IL-8, TNFα and chemokines including monocyte chemotactic protein-1 (MCP-1, also called Chemokine C-C motif ligand 2 (CCL2)), are upregulated [67,96,97]. However, the potential role of obesity-driven cytokine upregulation in ALL pathogenesis is currently unclear.
While elevated IL-6, IL-17, and IL-18 levels at birth have been associated with increased risk of developing B-cell ALL later in life, their exact role in ALL development, if any, is unknown [67,96]. Elevated levels of TNFα and CCL2 in the cerebrospinal fluid of patients with ALL were initially linked to a higher risk of relapse, but these levels were later found to be related to intrathecal chemotherapy [98,99,100,101].
IL-7 is an essential cytokine in the maturation of T-cell ALL [102,103]. While IL-7 is a critical molecule in the development of both B- and T-cells in mice [104], human studies have revealed its impact on T-cell development only [105]. Notably, gain-of-function mutations of the IL-7 receptor gene (IL-7R) have been reported in children with T-cell ALL [102].
Both T-cells and IL-7 expression are most prominent in the thymus and bone marrow. IL-7 signaling through the IL-7 receptor activates the Janus kinase/signal transducer and activator of transcription protein 5 (JAK/STAT5) and the phosphatidylinositol 3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/Akt/mTOR) pathways. The activation of the JAK/STAT5 pathway lowers naïve CD4 and CD8 T-cell numbers through unknown mechanisms [106]. PI3K catalyzes the production of phosphatidylinositol 3,4,5 triphosphate (PIP3), which allows for Akt and mTOR activation (Figure 1). In vitro studies with human T-cells demonstrate that this pathway is activated by IL-7 and is required for the proliferation and survival of both normal immature T-cells and ALL cells [103].
In the murine bone marrow, IL-3 and IL-7 promote B-cell proliferation and survival [107,108] and CXCL12 is essential for expanding leukemia cells [109]. In vitro studies revealed that IL-3 and IL-7 have clear synergistic responses to B-cell ALL proliferation when CXCL12 is present with either or both cytokines. These studies have also shown the upregulation of the PI3K and the p38 mitogen-activated protein kinase (p38MAPK) pathways and the promotion of B-cell ALL proliferation [107].
In summary, the role of cytokines in leukemia cell survival and proliferation makes IL-3 and IL-7 potential therapeutic targets for obesity-driven B-cell ALL, while IL-7 alone may be a target for obesity-driven T-cell ALL.
4.4. Adiponectin, Leptin, and Leptin Receptor
The most abundant adipokines that may influence cancer development and progression include adiponectin and leptin (Figure 1) [110,111].
Adiponectin circulates in an inverse proportion to adiposity and modulates several metabolic processes, including glucose homeostasis [96,112]. Hypoadiponectinemia is a phenomenon that was noted in ALL development and relapse [111,113]. Adiponectin activities are, in part, mediated by AMP-activated protein kinase (AMPK) that suppresses leukemia cell activity by stimulating tumor suppressors p53 and p21. The low levels of adiponectin in obesity may reduce the tumor suppressors’ activity, which may theoretically promote ALL development [113].
Adiponectin also exerts anti-inflammatory actions by downregulating the secretion of proinflammatory cytokines TNFα and IL-6 from activated macrophages, while stimulating the production of IL-10, an anti-inflammatory cytokine [114,115,116]. More research is needed to discern if this adipokine is associated explicitly with ALL development.
Leptin is a peptide hormone primarily produced by the adipose tissue [117]. Leptin impacts a wide range of biological activities, including the modulation of immune responses and angiogenesis [67,118]. In patients with obesity, while the leptin levels are increased, leptin receptor (LEPR) signaling is attenuated [119,120,121]. Leptin stimulates the production of several proinflammatory cytokines, including TNFα, IL-1β, IL-6, and IL-12, thus promoting chronic inflammation [67,78,122]. Furthermore, attenuated LEPR signaling has been linked to poor patient outcomes [120]. Specifically, B- and T-cell ALL have demonstrated faster progression and lower overall survival rates in LEPR-deficient mice models [120]. The mechanisms responsible for this association remain unclear.
Leptin also promotes angiogenesis in hematologic cancers. There is an increase in microvessel density within the bone marrow of patients with ALL, which suggests that hematological malignancies depend on angiogenesis for development and progression [123,124]. Competitive inhibition of leptin binding markedly decreases bone marrow microvessel density in rat models of leukemia [124]. The inhibition of leptin signaling in the bone marrow in ALL, and its impact on outcomes, needs further evaluation.
4.5. Insulin and Insulin-Like Growth Factor-1 (IGF-1)
Obesity drives the rise in insulin resistance and hyperinsulinemia [125], which is associated with hyperglycemia and dyslipidemia [96,126].
Insulin has mitogenic effects via its signaling pathway that is dysregulated in ALL (Figure 1) [127]. This signaling includes the overexpression of PI3K/Akt and STAT3 in lymphoblasts that is associated with chemotherapy resistance and poor prognosis [96,128,129,130,131,132]. For example, insulin reduces daunorubicin-induced toxicity and minimizes daunorubicin, vincristine, and L-asparaginase-mediated apoptosis of ALL cells in vitro [130].
Insulin-like growth factor-1 (IGF-1) is a growth factor that has a role in the progression of solid and hematologic malignancies [133]. In obese individuals, total IGF-1 levels are often in the normal to low range. However, free and active IGF-1 levels are generally higher than in nonobese individuals [96,134]. IGF-1 signaling activates many of the same pathways as insulin, including PI3K/Akt and MAPK. In vitro studies have shown that the stimulation of the IGF-1 receptor results in the proliferation of T-ALL cells and leads to chemotherapy resistance [135,136]. The role of IGF-1 in ALL pathogenesis and prognosis requires further evaluation.
4.6. Natural Killer Cell Dysregulation
Natural Killer (NK) cells are a lymphocyte subset that are vital for metabolic regulation and protection against infection and malignancy [137]. NK cells are implicated in innate immune responses and provide rapid reactions to environmental threats.
NK cell mechanisms linked directly to ALL are not reported in the literature to date. However, NK cells from obese humans are reduced in numbers [138] with lower cytotoxic activity than those from non-obese controls [139,140,141,142].
In high-fat-fed obese ALL mice, NK cells upregulate the secretion of cytokines and chemokines, including IL-2, IL-8, IL-12, and the chemokine macrophage inflammatory protein-1α (MIP-1α), which recruits macrophages to the adipose tissue (Figure 1) [137,143]. Human studies have also demonstrated that NK cells from obese individuals kill fewer tumor cells in vitro [139]. The role of NK cells in childhood ALL development requires further investigation.
4.7. Adipose-Derived Stem Cells
Adipose-derived stromal/stem cells (ASCs) are a type of mesenchymal stem cell found in the stromal vascular fraction of the adipose tissue [144,145,146]. ASCs secrete factors that are potentially associated with cancer development and progression, including IGF-1, transforming growth factor beta 1 (TGFβ1), vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF), and IL-8 [147,148,149,150,151].
Emerging evidence indicates that ASCs may promote the progression of hematologic cancers. Both B- and T-ALL cell lines expand in the presence of co-injected ASCs in mouse models, suggesting that ASCs promote the proliferation of ALL cells, and this proliferation occurs in a dose-dependent manner [78,152,153,154,155,156,157]. ASCs have also been investigated for their immunosuppressive properties, which may promote hematologic malignancies [153]. ASCs suppress immunity via interactions with innate immune cells including NK cells and dendritic cells as well as adaptive immune cells such as B- and T-lymphocytes. ASCs reduce NK cell proliferation and cytotoxic effects and inhibit the natural maturation of dendritic cells into B- and T-lymphocytes [78,158,159,160]. ASCs may be potentially important players in the initiation and propagation of ALL, and this area is in need of further research.
4.8. Glucose, Free Fatty Acids, and Amino Acids as Fuels for Leukemia Cells
The rapid proliferation of cancer cells demands large amounts of energy and nutrients. Adipocytes play an essential role in triglyceride storage and may fuel ALL cells with FFAs and amino acids (AAs).
The bone marrow provides the primary microenvironment for the development of leukemia. Increased expression of adipogenic genes CCAAT/enhancer-binding protein (CEBP) and peroxisome proliferator-activated receptor γ (PPARγ) have been observed in isolated mesenchymal stem cells from bone marrow aspirates of pediatric ALL patients, suggesting that the bone marrow is an active site of adipose tissue action [161].
In obesity, there is an excess of fuel storage within the expanded adipose tissue, both peripherally and in the bone marrow adipose depots. The higher glucose, FFA, and AA availability in both locations provides fuel substrates for nearby leukemia cells and may, in theory, support more rapid cell proliferation (Figure 1) [96,162].
ALL cells comply with the Warburg effect, with increased glucose uptake and glycolysis [163]. A study that targeted glucose transport found that B-ALL cells rely on GLUT1 for aerobic glycolysis and anaerobic metabolism [164]. Conditional GLUT1 gene knockout reduced cell proliferation, promoted apoptosis and increased sensitivity to Dasatinib in vivo [164]. Another possible mechanism reported in AML is the disruption of glucose metabolism by the induction of adipose tissue insulin-like growth factor binding Protein-1 production that impairs insulin sensitivity. Also, the depletion of gut serotonin and glucagon-like peptide-1 reduces insulin secretion, thus increasing the glucose available for leukemia cells to enhance their proliferation [165]. Although the same effect has yet to be demonstrated in ALL, this may be a plausible mechanism that requires further assessment.
Although complete leukemia remission rates were lower with hyperglycemia in adults, post-induction hyperglycemia in pediatric patients did not significantly influence outcomes [166]. Explanations for the difference in hyperglycemia effects between the groups include a higher incidence of comorbidities, increased systemic exposure to dexamethasone, and increased insulin resistance in the adult ALL population. Overall, the impact of higher glucose levels with obesity in pediatric ALL remains unclear.
Leukemia cells use FFAs as a source of energy for their proliferation and lipogenesis [96]. Overexpression of lipogenic enzymes by bone marrow adipocytes, such as hormone-sensitive lipase, and fatty acid transporters, such as fatty acid-binding protein-4 [167] and lipoprotein lipase [168], have been shown when the adipocytes were cocultured with acute myeloid and chronic lymphoblastic leukemia cells [167,168,169]. However, this overexpression and its effects have not yet been demonstrated with ALL cells.
Of note, when adipocytes were cocultured with human and murine ALL cells, the leukemia cells stimulated adipocytes to release FFAs, and ALL cells then incorporated the FFAs into phospholipid membranes and lipid droplets. [170]. A mechanism is proposed whereby adipocytes contribute to ALL development by increasing available FFAs for proliferation, though further studies would need to demonstrate this effect in vivo.
AAs are a vital source of fuel for cells that are also produced by adipocytes. ALL cells lack asparagine synthetase, which is necessary to synthesize essential AAs, including glutamate and aspartate from glutamine and asparagine, respectively. ALL cells depend on exogenous sources of AAs for cell cycle progression. Asparaginase treatments, such as L-asparaginase, hydrolyze and reduce the amount of asparagine, and to a lesser extent glutamine, available to ALL cells [171]. The bone marrow adipocytes may contribute to L-asparaginase resistance by producing high levels of glutamine in the interstitial fluid in the presence of ALL cells [172].
5. Targeted Therapy for Obesity-Associated Acute Lymphoblastic Leukemia
While obesity may be associated with the harboring of leukemia cells in the adipose tissue and circumventing chemotherapeutic efficacy, yet most of these effects are likely reversible if the adipose mass is decreased. For example, obese mice with ALL had an enhanced response to vincristine and improved survival when switched to a low-fat diet before chemotherapy initiation [173]. Therefore, targeting obesity in children at diagnosis, as well as during and post-therapy may improve treatment outcomes.
Several attempts to prevent and manage obesity during and after ALL therapy have been undertaken. However, there is a lack of high-evidence, and no effective lifestyle interventions to treat and prevent obesity in children with ALL are currently available [174]. There is a need for rigorous evidence to manage obesity in survivors and assess its impact on outcomes.
One crucial pathway in ALL is the mTOR pathway, which plays a critical role in the development and progression of both B- and T-cell ALL [128]. Significantly, mTOR is activated through several mechanisms in obesity. Multiple factors, including insulin, IGF-1, IL-1β, IL-6, IL-17 and TNFα can activate the PI3K/Akt/mTOR pathway and promote oncogenesis. There are several mechanisms through which this pathway impacts ALL development, including increased genomic instability via blocking of checkpoint kinase 1, increased mammalian target of rapamycin complex 1 (mTORC1) activity, enhancing protein synthesis, activating hypoxia-inducible factor-1α and increasing VEGF for angiogenesis promotion [97].
Several available compounds target mTOR and can provide potential therapeutic benefits. One such compound is metformin [96]. This medication, traditionally used to improve insulin sensitivity in patients with type 2 diabetes, stimulates AMPK which is a potent inhibitor of mTOR [175]. In T-cell ALL specifically, metformin induces autophagy and apoptosis of leukemia cells [176]. In vitro studies have shown that metformin is effective in downregulating signaling, with dephosphorylation and marked inhibition of mRNA translation in T-ALL cells [114]. Metformin triggers cell growth arrest and apoptosis by inducing endoplasmic reticulum stress with unfolded protein response-mediated cell death pathways. The mTOR pathway is impacted explicitly by an increase in unfolded and misfolded proteins within the endoplasmic reticulum, which results in increased proteotoxicity and cell death. While the utility of metformin in relapsed pediatric ALL patients has undergone phase 1 studies for review of toxicity, with tolerability at an appropriate dose of 1000 mg/m2/day [177], the effects of metformin on responses to therapy and outcomes are not yet established. This is an important area of further study.
6. Conclusions
Childhood obesity is an ongoing epidemic, and there is extensive evidence to support its health risks throughout the lifespan. Our current understanding of the potential mechanisms through which obesity may initiate and promote ALL development and propagation is limited. However, there are conceivable pathways whereby a general increase in body mass, or specific expansion of the adipose tissue, may contribute to ALL genesis. There is more definite evidence for the long-term implications of ALL treatment on pediatric obesity in survivors.
Future research efforts should use in-vitro and in-vivo approaches to understand the impact of the upregulation of inflammatory and mitogenic pathways, such as mTOR, in obesity on leukemia genesis and propagation. Furthermore, the role of the adipose microenvironment and adipocytokines in proliferation and protection of leukemia cells needs further analysis to see if the adipose tissue provision of a sanctuary for leukemia cells can be prevented. The role of other tissue compartments, such as skeletal muscle and muscle-based intermuscular adipose tissue in protecting leukemia cells from chemotherapies, and providing a protective microenvironment for lymphoblasts to propagate, is also unknown, and this area of research has not yet been explored. The interrogation of potential cytokines as therapeutic targets, including IL-3 and IL-7, is also an area of further potential investigation.
Although multiple potential mechanisms may be implicated in driving ALL in obesity, more evidence is urgently needed to establish if obesity can directly cause ALL. For now, there is no clear proof that obesity is causal in pediatric ALL.
Author Contributions
M.C.S. is the guarantor. The conceptualization of this review was done by M.C.S., C.P., M.J.D., S.N. and A.M. Data searches, summary, and presentation were performed by M.J.D., S.N., A.M., C.P., and M.C.S. M.J.D., S.N., A.M., and M.C.S. drafted the first version of the manuscript. All authors reviewed the manuscript and provided comments. All authors have read and agreed to the published version of the manuscript.
Funding
This work received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wabitsch M., Moss A., Kromeyer-Hauschild K. Unexpected plateauing of childhood obesity rates in developed countries. BMC Med. 2014;12:1–5. doi: 10.1186/1741-7015-12-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lipnowski S., LeBlanc C.M.A., Bridger T.L., Houghton K., Philpott J.F., Templeton C.G., Warshawski T.J., Purcell L.K. Healthy active living: Physical activity guidelines for children and adolescents. Paediatr. Child Health (Oxf.) 2012;17:209–210. doi: 10.1093/pch/17.4.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skinner A.C., Ravanbakht S.N., Skelton J.A., Perrin E.M., Armstrong S.C. Prevalence of obesity and severe obesity in US children, 1999-2016. Pediatrics. 2018;141 doi: 10.1542/peds.2017-3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown C.L., Halvorson E.E., Cohen G.M., Lazorick S., Skelton J.A. Addressing Childhood Obesity. Opportunities for Prevention. Pediatr. Clin. N. Am. 2015;62:1241–1261. doi: 10.1016/j.pcl.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ho M., Garnett S.P., Baur L., Burrows T., Stewart L., Neve M., Collins C. Effectiveness of lifestyle interventions in child obesity: Systematic review with meta-analysis. Pediatrics. 2012;130:e1647–e1671. doi: 10.1542/peds.2012-1176. [DOI] [PubMed] [Google Scholar]
- 6.The N.S., Suchindran C., North K.E., Popkin B.M., Gordon-Larsen P. Association of adolescent obesity with risk of severe obesity in adulthood. JAMA. 2010;304:2042–2047. doi: 10.1001/jama.2010.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith J.D., Fu E., Kobayashi M.A. Prevention and Management of Childhood Obesity and Its Psychological and Health Comorbidities. Annu. Rev. Clin. Psychol. 2020;16:351–378. doi: 10.1146/annurev-clinpsy-100219-060201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singhal A. Early Life Origins of Obesity and Related Complications. Indian J. Pediatrics. 2018;85:472–477. doi: 10.1007/s12098-017-2554-3. [DOI] [PubMed] [Google Scholar]
- 9.Sagar R., Gupta T. Psychological Aspects of Obesity in Children and Adolescents. Indian J. Pediatrics. 2018;85:554–559. doi: 10.1007/s12098-017-2539-2. [DOI] [PubMed] [Google Scholar]
- 10.Koves I.H., Roth C. Genetic and Syndromic Causes of Obesity and its Management. Indian J. Pediatrics. 2018;85:478–485. doi: 10.1007/s12098-017-2502-2. [DOI] [PubMed] [Google Scholar]
- 11.Vikram N.K. Cardiovascular and Metabolic Complications–Diagnosis and Management in Obese Children. Indian J. Pediatrics. 2018;85:535–545. doi: 10.1007/s12098-017-2504-0. [DOI] [PubMed] [Google Scholar]
- 12.Afshin A., Forouzanfar M.H., Reitsma M.B., Sur P., Estep K., Lee A., Marczak L., Mokdad A.H., Moradi-Lakeh M., Naghavi M., et al. Health effects of overweight and obesity in 195 countries over 25 years. N. Engl. J. Med. 2017;377:13–27. doi: 10.1056/NEJMoa1614362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zolotarjova J., ten Velde G., Vreugdenhil A.C.E. Effects of multidisciplinary interventions on weight loss and health outcomes in children and adolescents with morbid obesity. Obes. Rev. 2018;19:931–946. doi: 10.1111/obr.12680. [DOI] [PubMed] [Google Scholar]
- 14.Aggarwal B., Jain V. Obesity in Children: Definition, Etiology and Approach. Indian J. Pediatrics. 2018;85:463–471. doi: 10.1007/s12098-017-2531-x. [DOI] [PubMed] [Google Scholar]
- 15.Lauby-Secretan B., Scoccianti C., Loomis D. Body Fatness and Cancer—Viewpoint of the IARC Working Group. N. Engl. J. Med. 2016;375:794–798. doi: 10.1056/NEJMsr1606602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murphy T.K., Calle E.E., Rodriguez C., Kahn H.S., Thun M.J. Body mass index and colon cancer mortality in a large prospective study. Am. J. Epidemiol. 2000;152:847–854. doi: 10.1093/aje/152.9.847. [DOI] [PubMed] [Google Scholar]
- 17.Li S.D., Mobarhan S. Association between body mass index and adenocarcinoma of the esophagus and gastric cardia. Nutr. Rev. 2000;58:54–59. doi: 10.1111/j.1753-4887.2000.tb07811.x. [DOI] [PubMed] [Google Scholar]
- 18.Chow W.-H., Gridley G., Fraumeni J.F., Järvholm B. Obesity, Hypertension, and the Risk of Kidney Cancer in Men. N. Engl. J. Med. 2000;343:1305–1311. doi: 10.1056/NEJM200011023431804. [DOI] [PubMed] [Google Scholar]
- 19.Chen Y., Liu L., Wang X., Wang J., Yan Z., Cheng J., Gong G., Li G. Body mass index and risk of gastric cancer:a meta-analysis of a population with more than ten million from 24 prospective studies. Cancer Epidemiol. Biomark. Prev. 2013;22:1395–1408. doi: 10.1158/1055-9965.EPI-13-0042. [DOI] [PubMed] [Google Scholar]
- 20.World Cancer Research Fund Diet, Nutrition, Physical Activity and Gallbladder Cancer, 2015 (Revised 2018) [(accessed on 1 January 2020)];World Cancer Research Fund International. Available online: https://www.wcrf.org/sites/default/files/Gallbladder-cancer-report.pdf.
- 21.Genkinger J.M., Spiegelman D., Anderson K.E., Bernstein L., Van Den Brandt P.A., Calle E.E., English D.R., Folsom A.R., Freudenheim J.L., Fuchs C.S., et al. A pooled analysis of 14 cohort studies of anthropometric factors and pancreatic cancer risk. Int. J. Cancer. 2011;129:1708–1717. doi: 10.1002/ijc.25794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beral V., Hermon C., Peto R., Reeves G., Brinton L., Marchbanks P., Negri E., Ness R., Peeters P.H.M., Vessey M., et al. Ovarian cancer and body size: Individual participant meta-analysis including 25,157 women with ovarian cancer from 47 epidemiological studies. PLoS Med. 2012;9 doi: 10.1371/journal.pmed.1001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kitahara C.M., Mccullough M.L., Franceschi S., Rinaldi S., Wolk A., Neta G., Adami H.O., Anderson K., Andreotti G., Freeman L.E.B., et al. Anthropometric factors and thyroid cancer risk by histological subtype: Pooled analysis of 22 prospective studies. Natl. Cancer Inst. 2015:1–50. doi: 10.1089/thy.2015.0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arnold M., Renehan A.G., Colditz G.A. Excess weight as a risk factor common to many cancer sites: Words of caution when interpreting meta-analytic evidence. Cancer Epidemiol. Biomark. Prev. 2017;26:663–665. doi: 10.1158/1055-9965.EPI-16-0940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teras L.R., Kitahara C.M., Birmann B.M., Hartge P.A., Wang S.S., Robien K., Patel A.V., Adami H.O., Weiderpass E., Giles G.G., et al. Body size and multiple myeloma mortality: A pooled analysis of 20 prospective studies. Br. J. Haematol. 2014;166:667–676. doi: 10.1111/bjh.12935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Colditz G.A., Lindsay L. Obesity and cancer: Evidence, impact, and future directions. Clin. Chem. 2018;64:154–162. doi: 10.1373/clinchem.2017.277376. [DOI] [PubMed] [Google Scholar]
- 27.Genkinger J.M., Kitahara C.M., Bernstein L., Berrington de Gonzalez A., Brotzman M., Elena J.W., Giles G.G., Hartge P., Singh P.N., Stolzenberg-Solomon R.Z., et al. Central adiposity, obesity during early adulthood, and pancreatic cancer mortality in a pooled analysis of cohort studies. Ann. Oncol. 2015;26:2257–2266. doi: 10.1093/annonc/mdv355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang X., Wu K., Giovannucci E.L., Ma J., Colditz G.A., Fuchs C.S., Willett W.C., Stampfer M.J., Nimptsch K., Ogino S., et al. Early life body fatness and risk of colorectal cancer in U.S. women and men-results from two large cohort studies. Cancer Epidemiol. Biomark. Prev. 2015;24:690–697. doi: 10.1158/1055-9965.EPI-14-0909-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hunger S.P., Mullighan C.G. Acute lymphoblastic leukemia in children. N. Engl. J. Med. 2015;373:1541–1552. doi: 10.1056/NEJMra1400972. [DOI] [PubMed] [Google Scholar]
- 30.Malvi P., Chaube B., Singh S.V., Mohammad N., Vijayakumar M.V., Singh S., Chouhan S., Bhat M.K. Elevated circulatory levels of leptin and resistin impair therapeutic efficacy of dacarbazine in melanoma under obese state. Cancer Metab. 2018;6:1–14. doi: 10.1186/s40170-018-0176-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malvi P., Chaube B., Singh S.V., Mohammad N., Pandey V., Vijayakumar M.V., Radhakrishnan R.M., Vanuopadath M., Nair S.S., Nair B.G., et al. Weight control interventions improve therapeutic efficacy of dacarbazine in melanoma by reversing obesity-induced drug resistance. Cancer Metab. 2016;4 doi: 10.1186/s40170-016-0162-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shields M. Overweight and obesity among children and youth. Health Rep. 2006;17:27–42. [PubMed] [Google Scholar]
- 33.Smith M.A., Seibel N.L., Altekruse S.F., Ries L.A.G., Melbert D.L., O’Leary M., Smith F.O., Reaman G.H. Outcomes for children and adolescents with cancer: Challenges for the twenty-first century. J. Clin. Oncol. 2010;28:2625–2634. doi: 10.1200/JCO.2009.27.0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Dongen-Melman J.E.W.M., Hokken-Koelega A.C.S., Hâhlen K., De Groot A., Tromp C.G., Egeler R.M. Obesity after successful treatment of acute lymphoblastic leukemia in childhood. Pediatric Res. 1995;38:86–90. doi: 10.1203/00006450-199507000-00015. [DOI] [PubMed] [Google Scholar]
- 35.Withycombe J.S., Post-White J.E., Meza J.L., Hawks R.G., Smith L.M., Sacks N., Seibel N.L. Weight patterns in children with higher risk ALL: A report from the Children’s Oncology Group (COG) for CCG 1961. Bone. 2009;53:1249–1254. doi: 10.1002/pbc.22237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Breene R.A.L., Williams R.M., Hartle J., Gattens M., Acerini C.L., Murray M.J. Auxological changes in UK survivors of childhood acute lymphoblastic leukaemia treated without cranial irradiation. Br. J. Cancer. 2011;104:746–749. doi: 10.1038/bjc.2011.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Razzouk B.I., Rose S.R., Hongeng S., Wallace D., Smeltzer M.P., Zacher M., Pui C.H., Hudson M.M. Obesity in survivors of childhood acute lymphoblastic leukemia and lymphoma. J. Clin. Oncol. 2007;25:1183–1189. doi: 10.1200/JCO.2006.07.8709. [DOI] [PubMed] [Google Scholar]
- 38.Ghosh T., Richardson M., Ryder J., Spector L., Turcotte L. Obesity as a Risk Factor for Pediatric Acute Lymphoblastic Leukemia: A Report from the Children’s Oncology Group. American Association for Cancer Research; Philadelphia, PA, USA: 2019. [Google Scholar]
- 39.Foster K.L., Kern K.D., Chambers T.M., Lupo P.J., Kamdar K.Y., Scheurer M.E., Brown A.L. Weight trends in a multiethnic cohort of pediatric acute lymphoblastic leukemia survivors: A longitudinal analysis. PLoS ONE. 2019;14:1–11. doi: 10.1371/journal.pone.0217932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Didi M., Didcock E., Davies H.A., Ogilvy-Stuart A.L., Wales J.K.H., Shalet S.M. High incidence of obesity in young adults after treatment of acute lymphoblastic leukemia in childhood. J. Pediatrics. 1995;127:63–67. doi: 10.1016/S0022-3476(95)70258-X. [DOI] [PubMed] [Google Scholar]
- 41.Craig F., Leiper A.D., Stanhope R., Brain C., Meller S.T., Nussey S.S. Sexually dimorphic and radiation dose dependent effect of cranial irradiation on body mass index. Arch. Dis. Child. 1999;81:500–504. doi: 10.1136/adc.81.6.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Odame I., Reilly J.J., Gibson B.E.S., Donaldson M.D.C. Patterns of obesity in boys and girls after treatment for acute lymphoblastic leukaemia. Arch. Dis. Child. 1994;71:147–149. doi: 10.1136/adc.71.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hjalgrim L.L., Westergaard T., Rostgaard K., Schmiegelow K., Melbye M., Hjalgrim H., Engels E.A. Birth weight as a risk factor for childhood leukemia: A meta-analysis of 18 epidemiologic studies. Am. J. Epidemiol. 2003;158:724–735. doi: 10.1093/aje/kwg210. [DOI] [PubMed] [Google Scholar]
- 44.Caughey R.W., Michels K.B. Birth weight and childhood leukemia: A meta-analysis and review of the current evidence. Int. J. Cancer. 2009;124:2658–2670. doi: 10.1002/ijc.24225. [DOI] [PubMed] [Google Scholar]
- 45.Milne E., Royle J.A., De Klerk N.H., Blair E., Bailey H., Cole C., Attia J., Scott R.J., Armstrong B.K. Fetal growth and risk of childhood acute lymphoblastic leukemia: Results from an australian case-control study. Am. J. Epidemiol. 2009;170:221–228. doi: 10.1093/aje/kwp117. [DOI] [PubMed] [Google Scholar]
- 46.Jiménez-Hernández E., Fajardo-Gutiérrez A., Núñez-Enriquez J.C., Martín-Trejo J.A., Espinoza-Hernández L.E., Flores-Lujano J., Arellano-Galindo J., Medina-Sanson A., Paredes-Aguilera R., Merino-Pasaye L.E., et al. A greater birthweight increases the risk of acute leukemias in Mexican children—experience from the Mexican Interinstitutional Group for the Identification of the Causes of Childhood Leukemia (MIGICCL) Cancer Med. 2018;7:1528–1536. doi: 10.1002/cam4.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sprehe M.R., Barahmani N., Cao Y., Wang T., Forman M.R., Bondy M., Okcu M.F. Comparison of Birth Weight Corrected for Gestational Age and Birth Weight Alone in Prediction of Development of Childhood Leukemia and Central Nervous System Tumors. Pediatrics Blood Cancer. 2010;54:242–249. doi: 10.1002/pbc.22308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wiemels J.L., Cazzaniga G., Daniotti M., Eden O.B., Addison G.M., Masera G., Saha V., Biondi A., Greaves M.F. Prenatal origin of acute lymphoblastic leukaemia in children. Lancet. 1999;354:1499–1503. doi: 10.1016/S0140-6736(99)09403-9. [DOI] [PubMed] [Google Scholar]
- 49.Hjalgrim L.L., Madsen H.O., Melbye M., Jørgensen P., Christiansen M., Andersen M.T., Pallisgaard N., Hokland P., Clausen N., Schmiegelow K., et al. Presence of clone-specific markers at birth in children with acute lymphoblastic leukaemia. Br. J. Cancer. 2002;87:994–999. doi: 10.1038/sj.bjc.6600601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Greaves M.F., Wiemels J. Origins of chromosome translocations in childhood leukaemia. Nat. Rev. Cancer. 2003;3:639–649. doi: 10.1038/nrc1164. [DOI] [PubMed] [Google Scholar]
- 51.Eden T. Aetiology of childhood leukaemia. Cancer Treat. Rev. 2010;36:286–297. doi: 10.1016/j.ctrv.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 52.Zee P., Chen C.-H. Prevalence of Obesity in Children After Therapy for Acute Lymphoblastic Leukemia. J. Pediatrics Hematol. Oncol. 1986;8:294–299. doi: 10.1097/00043426-198624000-00005. [DOI] [PubMed] [Google Scholar]
- 53.Garmey E.G., Liu Q., Sklar C.A., Meacham L.R., Mertens A.C., Stovall M.A., Yasui Y., Robison L.L., Oeffinger K.C. Longitudinal changes in obesity and body mass index among adult survivors of childhood acute lymphoblastic leukemia: A report from the childhood cancer survivor study. J. Clin. Oncol. 2008;26:4639–4645. doi: 10.1200/JCO.2008.16.3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baillargeon J., Langevin A.-M., Lewis M., Estrada J., Grady J.J., Mullins J., Pitney A., Pollock B.H. Demographic correlates of body size changes in children undergoing treatment for acute lymphoblastic leukemia. Pediatrics Blood Cancer. 2007;49:793–796. doi: 10.1002/pbc.21063. [DOI] [PubMed] [Google Scholar]
- 55.Chow E.J., Pihoker C., Hunt K., Wilkinson K., Friedman D.L. Obesity and hypertension among children after treatment for acute lymphoblastic leukemia. Cancer. 2007;110:2313–2320. doi: 10.1002/cncr.23050. [DOI] [PubMed] [Google Scholar]
- 56.Zhang F.F., Kelly M.J., Saltzman E., Must A., Roberts S.B., Parsons S.K. Obesity in pediatric ALL survivors: A meta-analysis. Pediatrics. 2014;133:e704–e715. doi: 10.1542/peds.2013-3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saenz A.M., Stapleton S., Hernandez R.G., Hale G.A., Goldenberg N.A., Schwartz S., Amankwah E.K. Body Mass Index at Pediatric Leukemia Diagnosis and the Risks of Relapse and Mortality: Findings from a Single Institution and Meta-analysis. J. Obes. 2018;2018:1–8. doi: 10.1155/2018/7048078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Orgel E., Genkinger J.M., Aggarwal D., Sung L., Nieder M., Ladas E.J. Association of body mass index and survival in pediatric leukemia: A meta-analysis. Am. J. Clin. Nutr. 2016;103:808–817. doi: 10.3945/ajcn.115.124586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Orgel E., Tucci J., Alhushki W., Malvar J., Sposto R., Fu C.H., Freyer D.R., Abdel-Azim H., Mittelman S.D. Obesity is associated with residual leukemia following induction therapy for childhood B-precursor acute lymphoblastic leukemia. Blood. 2014;124:3932–3938. doi: 10.1182/blood-2014-08-595389. [DOI] [PubMed] [Google Scholar]
- 60.Meenan C.K., Kelly J.A., Wang L., Ritchey A.K., Maurer S.H. Obesity in pediatric patients with acute lymphoblastic leukemia increases the risk of adverse events during pre-maintenance chemotherapy. Pediatr. Blood Cancer. 2019;66:1–7. doi: 10.1002/pbc.27515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gelelete C.B., Pereira S.H., Azevedo A.M.B., Thiago L.S., Mundim M., Land M.G.P., Costa E.S. Overweight as a prognostic factor in children with acute lymphoblastic leukemia. Obesity. 2011;19:1908–1911. doi: 10.1038/oby.2011.195. [DOI] [PubMed] [Google Scholar]
- 62.Butturini A.M., Dorey F.J., Lange B.J., Henry D.W., Gaynon P.S., Fu C., Franklin J., Siegel S.E., Seibel N.L., Rogers P.C., et al. Obesity and outcome in pediatric acute lymphoblastic leukemia. J. Clin. Oncol. 2007;25:2063–2069. doi: 10.1200/JCO.2006.07.7792. [DOI] [PubMed] [Google Scholar]
- 63.Amankwah E.K., Saenz A.M., Hale G.A., Brown P.A. Association between body mass index at diagnosis and pediatric leukemia mortality and relapse: A systematic review and meta-analysis. Leuk. Lymphoma. 2016;57:1140–1148. doi: 10.3109/10428194.2015.1076815. [DOI] [PubMed] [Google Scholar]
- 64.Pramanik R., Sheng X., Ichihara B., Heisterkamp N., Mittelman S.D. Adipose tissue attracts and protects acute lymphoblastic leukemia cells from chemotherapy. Leuk Res. 2013;37:503–509. doi: 10.1016/j.leukres.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fasshauer M., Blüher M. Adipokines in health and disease. Trends Pharmacol. Sci. 2015;36:461–470. doi: 10.1016/j.tips.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 66.Polyzos S.A., Kountouras J., Mantzoros C.S. Adipokines in nonalcoholic fatty liver disease. Metabolism. 2016;65:1062–1079. doi: 10.1016/j.metabol.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 67.Himbert C., Delphan M., Scherer D., Bowers L.W., Hursting S., Ulrich C.M. Signals from the adipose microenvironment and the obesity-cancer link-a systematic review. Cancer Prev. Res. 2017;10:494–506. doi: 10.1158/1940-6207.CAPR-16-0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weihrauch-Blüher S., Schwarz P., Klusmann J.H. Childhood obesity: Increased risk for cardiometabolic disease and cancer in adulthood. Metabolism. 2019;92:147–152. doi: 10.1016/j.metabol.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 69.Kershaw E.E., Flier J.S. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 2004;89:2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 70.Skurk T., Alberti-Huber C., Herder C., Hauner H. Relationship between adipocyte size and adipokine expression and secretion. J. Clin. Endocrinol. Metab. 2007;92:1023–1033. doi: 10.1210/jc.2006-1055. [DOI] [PubMed] [Google Scholar]
- 71.Kern p.A., Saghizadeh M., Ong J.M., Bosch R.J., Deem R., Simsolo R.B. The expression of tumor necrosis factor in human adipose tissue: Regulation by obesity, weight loss, and relationship to lipoprotein lipase. J. Clin. Invest. 1995;95:2111–2119. doi: 10.1172/JCI117899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yun J.P., Behan J.W., Heisterkamp N., Butturini A., Klemm L., Ji L., Groffen J., Müschen M., Mittelman S.D. Diet-induced obesity accelerates acute lymphoblastic leukemia progression in two murine models. Cancer Prev. Res. 2010;3:1259–1264. doi: 10.1158/1940-6207.CAPR-10-0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hotamisligil G.S., Arner P., Caro J.F., Atkinson R.L., Spiegelman B.M. Increased adipose tissue expression of tumor necrosis factor-α in human obesity and insulin resistance. J. Clin. Invest. 1995;95:2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bruun J.M., Pedersen S.B., Richelsen B. Regulation of interleukin 8 production and gene expression in human adipose tissue in vitro. J. Clin. Endocrinol. Metab. 2001;86:1267–1273. doi: 10.1210/jc.86.3.1267. [DOI] [PubMed] [Google Scholar]
- 75.Hauner H., Bender M., Haastert B., Hube F. Plasma concentrations of soluble TNF-alpha receptors in obese subjects. Int. J. Obes. 1998;22:1239–1243. doi: 10.1038/sj.ijo.0800773. [DOI] [PubMed] [Google Scholar]
- 76.Mohamed-Ali V., Goodrick S., Rawesh A., Katz D.R., Miles J.M., Yudkin J.S., Klein S., Coppack S.W. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-α, in vivo. J. Clin. Endocrinol. Metab. 1997;82:4196–4200. doi: 10.1210/jc.82.12.4196. [DOI] [PubMed] [Google Scholar]
- 77.Cawthorn W.P., Scheller E.L., Learman B.S., Parlee S.D., Simon B.R., Mori H., Ning X., Bree A.J., Schell B., Broome T., et al. Bone marrow adipose tissue is an endocrine organ that contributes to increased circulating adiponectin during caloric restriction. Cell Metab. 2015;20:368–375. doi: 10.1016/j.cmet.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Strong A.L., Burow M.E., Gimble J.M., Bunnell B.A. Concise review: The obesity cancer paradigm: Exploration of the interactions and crosstalk with adipose stem cells. Stem Cells. 2015;33:318–326. doi: 10.1002/stem.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ibrahim M.M. Subcutaneous and visceral adipose tissue: Structural and functional differences. Obes. Rev. 2010;11:11–18. doi: 10.1111/j.1467-789X.2009.00623.x. [DOI] [PubMed] [Google Scholar]
- 80.Rittig K., Hieronimus A., Thamer C., Machann J., Peter A., Stock J., Schick F., Fritsche A., Stefan N., Häring H.U., et al. Reducing visceral adipose tissue mass is essential for improving endothelial function in type 2 diabetes prone individuals. Atherosclerosis. 2010;212:575–579. doi: 10.1016/j.atherosclerosis.2010.06.042. [DOI] [PubMed] [Google Scholar]
- 81.Tam C.S., Heilbronn L.K., Henegar C., Wong M., Cowell C.T., Cowley M.J., Kaplan W., Clément K., Baur L.A. An early inflammatory gene profile in visceral adipose tissue in children. Int. J. Pediatrics Obes. 2011;6:e360–e363. doi: 10.3109/17477166.2011.575152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wajchenberg B.L., Giannella-Neto D., Da Silva M.E.R., Santos R.F. Depot-specific hormonal characteristics of subcutaneous and visceral adipose tissue and their relation to the metabolic syndrome. Horm. Metab. Res. 2002;34:616–621. doi: 10.1055/s-2002-38256. [DOI] [PubMed] [Google Scholar]
- 83.Lopes H.F., Corrêa-Giannella M.L., Consolim-Colombo F.M., Egan B.M. Visceral adiposity syndrome. Diabetol. Metab. Syndr. 2016;8:1–8. doi: 10.1186/s13098-016-0156-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.West D.B., Prinz W.A., Francendese A.A., Greenwood M.R.C. Adipocyte blood flow is decreased in obse Zucker rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1987;253:228–233. doi: 10.1152/ajpregu.1987.253.2.R228. [DOI] [PubMed] [Google Scholar]
- 85.Trayhurn P. Hypoxia and adipose tissue function and dysfunction in obesity. Physiol. Rev. 2013;93:1–21. doi: 10.1152/physrev.00017.2012. [DOI] [PubMed] [Google Scholar]
- 86.Pecht T., Gutman-Tirosh A., Bashan N., Rudich A. Peripheral blood leucocyte subclasses as potential biomarkers of adipose tissue inflammation and obesity subphenotypes in humans. Obes. Rev. 2014;15:322–337. doi: 10.1111/obr.12133. [DOI] [PubMed] [Google Scholar]
- 87.Evans J., Goedecke J.H., Söderström I., Burén J., Alvehus M., Blomquist C., Jonsson F., Hayes P.M., Adams K., Dave J.A., et al. Depot- and ethnic-specific differences in the relationship between adipose tissue inflammation and insulin sensitivity. Clin. Endocrinol. (Oxf.) 2011;74:51–59. doi: 10.1111/j.1365-2265.2010.03883.x. [DOI] [PubMed] [Google Scholar]
- 88.Kitade H., Sawamoto K., Nagashimada M., Inoue H., Yamamoto Y., Sai Y., Takamura T., Yamamoto H., Miyamoto K.I., Ginsberg H.N., et al. CCR5 plays a critical role in obesity-induced adipose tissue inflammation and insulin resistance by regulating both macrophage recruitment and M1/M2 status. Diabetes. 2012;61:1680–1690. doi: 10.2337/db11-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Suganami T., Tanaka M., Ogawa Y. Adipose tissue inflammation and ectopic lipid accumulation. Endocr. J. 2012;59:849–857. doi: 10.1507/endocrj.EJ12-0271. [DOI] [PubMed] [Google Scholar]
- 90.Samaan M.C. The macrophage at the intersection of immunity and metabolism in obesity. Diabetol. Metab. Syndr. 2011;3:29. doi: 10.1186/1758-5996-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Behan J.W., Yun J.P., Proektor M.P., Ehsanipour E.A., Arutyunyan A., Moses A.S., Avramis V.I., Louie S.G., Butturini A., Heisterkamp N., et al. Adipocytes impair leukemia treatment in mice. Cancer Res. 2009;69:7867–7874. doi: 10.1158/0008-5472.CAN-09-0800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sheng X., Mittelman S.D. The role of adipose tissue and obesity in causing treatment resistance of acute lymphoblastic leukemia. Front. Pediatrics. 2014;2:1–8. doi: 10.3389/fped.2014.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zuccaro P., Guandalini S., Pacifici R., Simona P., Di L.M., Guiducci M., Giuliano M., Di Tullio M.T., Mantovani M.P. Fat body mass and pharmacokinetics of oral 6-mercaptopurine in children with acute lymphoblastic leukemia. Ther. Drug Monit. 1991;13:37–41. doi: 10.1097/00007691-199101000-00004. [DOI] [PubMed] [Google Scholar]
- 94.Sheng X., Parmentier J.H., Tucci J., Pei H., Cortez-Toledo O., Dieli-Conwright C.M., Oberley M.J., Neely M., Orgel E., Louie S.G., et al. Adipocytes sequester and metabolize the chemotherapeutic daunorubicin. Mol. Cancer Res. 2017;15:1704–1713. doi: 10.1158/1541-7786.MCR-17-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.De Lourdes Perim A., Amarante M.K., Guembarovski R.L., De Oliveira C.E.C., Watanabe M.A.E. CXCL12/CXCR4 axis in the pathogenesis of acute lymphoblastic leukemia (ALL): A possible therapeutic target. Cell. Mol. Life Sci. 2015;72:1715–1723. doi: 10.1007/s00018-014-1830-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Orgel E., Sea J.L., Mittelman S.D. Mechanisms by which obesity impacts survival from acute lymphoblastic leukemia. JNCI Monogr. 2019;2019:152–156. doi: 10.1093/jncimonographs/lgz020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen J. Multiple signal pathways in obesity-associated cancer. Obes. Rev. 2011;12:1063–1070. doi: 10.1111/j.1467-789X.2011.00917.x. [DOI] [PubMed] [Google Scholar]
- 98.Beksaç M., Ertürk S., Akan H., Koç H., Ilhan O. The clinical correlations of serum tumor necrosis factor-alpha in acute leukemias: A predictor of response and relapse? Leukemia. 1993;7:1773–1776. [PubMed] [Google Scholar]
- 99.Eisenkraft A., Keidan I., Bielorai B., Keller N., Toren A., Paret G. MCP-1 in the cerebrospinal fluid of children with acute lymphoblastic leukemia. Leuk. Res. 2006;30:1259–1261. doi: 10.1016/j.leukres.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 100.Protas P.T., Holownia A., Muszynska-Roslan K., Wielgat P., Krawczuk-Rybak M., Braszko J.J. Cerebrospinal fluid IL-6, TNF-α and MCP-1 in children with acute lymphoblastic leukaemia during chemotherapy. Neuropediatrics. 2011;42:254–256. doi: 10.1055/s-0031-1295477. [DOI] [PubMed] [Google Scholar]
- 101.Jaime-Pérez J.C., Gamboa-Alonso C.M., Jiménez-Castillo R.A., López-Silva L.J., Pinzón-Uresti M.A., Gómez-De León A., Gómez-Almaguer D. TNF-α increases in the CSF of children with acute lymphoblastic leukemia before CNS relapse. Blood Cells Mol. Dis. 2017;63:27–31. doi: 10.1016/j.bcmd.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 102.Zenatti P.P., Ribeiro D., Li W., Zuurbier L., Silva M.C., Paganin M., Tritapoe J., Hixon J.A., Silveira A.B., Cardoso B.A., et al. Oncogenic IL7R gain-of-function mutations in childhood T-cell acute lymphoblastic leukemia. Nat. Genet. 2011;43:932–941. doi: 10.1038/ng.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ribeiro D., Melão A., Barata J.T. IL-7R-mediated signaling in T-cell acute lymphoblastic leukemia. Adv. Biol. Regul. 2013;53:211–222. doi: 10.1016/j.jbior.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 104.Peschon J.J., Morrissey P.J., Grabstein K.H., Ramsdell F.J., Maraskovsky E., Gliniak B.C., Park L.S., Ziegler S.F., Williams D.E., Ware C.B., et al. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J. Exp. Med. 1994;180:1955–1960. doi: 10.1084/jem.180.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Puel A., Ziegler S.F., Buckley R.H., Leonard W.J. Defective IL7R expression in T-B+NK+ severe combined immunodeficiency. Nat. Genet. 1998;20:394–397. doi: 10.1038/3877. [DOI] [PubMed] [Google Scholar]
- 106.Ramanathan S., Gagnon J., Leblanc C., Rottapel R., Ilangumaran S. Suppressor of Cytokine Signaling 1 Stringently Regulates Distinct Functions of IL-7 and IL-15 In Vivo during T Lymphocyte Development and Homeostasis. J. Immunol. 2006;176:4029–4041. doi: 10.4049/jimmunol.176.7.4029. [DOI] [PubMed] [Google Scholar]
- 107.Juarez J., Baraz R., Gaundar S., Bradstock K., Bendall L. Interaction of interleukin-7 and interleukin-3 with the CXCL12-induced proliferation of B-cell progenitor acute lymphoblastic leukemia. Haematologica. 2007;92:450–459. doi: 10.3324/haematol.10621. [DOI] [PubMed] [Google Scholar]
- 108.Chiarini F., Lonetti A., Evangelisti C., Buontempo F., Orsini E., Evangelisti C., Cappellini A., Neri L.M., McCubrey J.A., Martelli A.M. Advances in understanding the acute lymphoblastic leukemia bone marrow microenvironment: From biology to therapeutic targeting. Biochim. Biophys. Acta Mol. Cell Res. 2016;1863:449–463. doi: 10.1016/j.bbamcr.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 109.Juarez J., Bradstock K.F., Gottlieb D.J., Bendall L.J. Effects of inhibitors of the chemokine receptor CXCR4 on acute lymphoblastic leukemia cells in vitro. Leukemia. 2003;17:1294–1300. doi: 10.1038/sj.leu.2402998. [DOI] [PubMed] [Google Scholar]
- 110.Housa D., Housová J., Vernerová Z., Haluzík M. Adipocytokines and cancer. Physiol. Res. 2006;55:233–244. doi: 10.33549/physiolres.930848. [DOI] [PubMed] [Google Scholar]
- 111.Aref S., Ibrahim L., Azmy E., Al Ashary R. Impact of serum adiponectin and leptin levels in acute leukemia. Hematology. 2013;18:198–203. doi: 10.1179/1607845412Y.0000000059. [DOI] [PubMed] [Google Scholar]
- 112.Wang Z.V., Scherer P.E. Adiponectin, the past two decades. J. Mol. Cell Biol. 2016;8:93–100. doi: 10.1093/jmcb/mjw011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.El-Baz H.A., Mosa T.E., Elabd E.M., Ramadan A., Elharoun A.S., Elmorsy E.A., Fouda M.I. Serum adiponectin and resistin levels in de novo and relapsed acute lymphoblastic leukemia children patients. Iran. J. Public Health. 2013;42:504–510. [PMC free article] [PubMed] [Google Scholar]
- 114.Leclerc G.M., Leclerc G.J., Kuznetsov J.N., DeSalvo J., Barredo J.C. Metformin Induces Apoptosis through AMPK-Dependent Inhibition of UPR Signaling in ALL Lymphoblasts. PLoS ONE. 2013;8:1–10. doi: 10.1371/journal.pone.0074420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dalamaga M., Diakopoulos K.N., Mantzoros C.S. The role of adiponectin in cancer: A review of current evidence. Endocr. Rev. 2012;33:547–594. doi: 10.1210/er.2011-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dalamaga M., Christodoulatos G.S. Adiponectin as a biomarker linking obesity and adiposopathy to hematologic malignancies. Horm. Mol. Biol. Clin. Investig. 2015;23:5–20. doi: 10.1515/hmbci-2015-0016. [DOI] [PubMed] [Google Scholar]
- 117.Zhang Y., Proenca R., Maffei M., Barone M., Leopold L., Friedman J.M. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 118.Park J., Scherer P.E. Leptin and cancer: From cancer stem cells to metastasis. Endocr. Relat. Cancer. 2011;18:25–29. doi: 10.1530/ERC-11-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Considine R.V., Sinha M.K., Heiman M.L., Kriauciunas A., Stephens T.W., Nyce M.R., Ohannesian J.P., Marco C.C., McKee L.J., Bauer T.L., et al. Serum Immunoreactive-Leptin Concentrations in Normal-Weight and Obese Humans. N. Engl. J. Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 120.Lu Z., Xie J., Wu G., Shen J., Collins R., Chen W., Kang X., Luo M., Zou Y., Huang L.J.-S., et al. Fasting selectively blocks development of acute lymphoblastic leukemia via leptin-receptor upregulation. Nat. Med. 2017;23:79–90. doi: 10.1038/nm.4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Myers M.G., Leibel R.L., Seeley R.J., Schwartz M.W. Obesity and leptin resistance: Distinguishing cause from effect. Trends Endocrinol. Metab. 2010;21:643–651. doi: 10.1016/j.tem.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Carbone F., La Rocca C., Matarese G. Immunological functions of leptin and adiponectin. Biochimie. 2012;94:2082–2088. doi: 10.1016/j.biochi.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 123.Perez-Atayde A.R., Sallan S.E., Tedrow U., Connors S., Allred E., Folkman J. Spectrum of tumor angiogenesis in the bone marrow of children with acute lymphoblastic leukemia. Am. J. Pathol. 1997;150:815–821. [PMC free article] [PubMed] [Google Scholar]
- 124.Iversen P.O., Drevon C.A., Reseland J.E. Prevention of leptin binding to its receptor suppresses rat leukemic cell growth by inhibiting angiogenesis. Blood. 2002;100:4123–4128. doi: 10.1182/blood-2001-11-0134. [DOI] [PubMed] [Google Scholar]
- 125.Ho M., Garnett S.P., Baur L.A. Childhood Obesity and Insulin Resistance: How Should It Be Managed? Curr. Treat. Options Cardiovasc Med. 2014;16 doi: 10.1007/s11936-014-0351-0. [DOI] [PubMed] [Google Scholar]
- 126.Frohnert B.I., Jacobs D.R., Steinberger J., Moran A., Steffen L.M., Sinaiko A.R. Relation between serum free fatty acids and adiposity, insulin resistance, and cardiovascular risk factors from adolescence to adulthood. Diabetes. 2013;62:3163–3169. doi: 10.2337/db12-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sciacca L., Cassarino M.F., Genua M., Vigneri P., Giovanna Pennisi M., Malandrino P., Squatrito S., Pezzino V., Vigneri R. Biological Effects of Insulin and Its Analogs on Cancer Cells With Different Insulin Family Receptor Expression. J. Cell. Physiol. 2014;229:1817–1821. doi: 10.1002/jcp.24635. [DOI] [PubMed] [Google Scholar]
- 128.Steelman L.S., Abrams S.L., Whelan J., Bertrand F.E., Ludwig D.E., Bäsecke J., Libra M., Stivala F., Milella M., Tafuri A., et al. Contributions of the Raf/MEK/ERK, PI3K/PTEN/Akt/mTOR and Jak/STAT pathways to leukemia. Leukemia. 2008;22:686–707. doi: 10.1038/leu.2008.26. [DOI] [PubMed] [Google Scholar]
- 129.Pan J., Chen C., Jin Y., Fuentes-Mattei E., Velazquez-Tores G., Benito J.M., Konopleva M., Andreeff M., Lee M.H., Yeung S.C.J. Differential impact of structurally different anti-diabetic drugs on proliferation and chemosensitivity of acute lymphoblastic leukemia cells. Cell Cycle. 2012;11:2314–2326. doi: 10.4161/cc.20770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang J., Xue H.M., Chen Y.R., Xu H.G., Lin S.F., Tang X.K., Chen C. Evaluation of Insulin-mediated Regulation of AKT Signaling in Childhood Acute Lymphoblastic Leukemia. J. Pediatric Hematol. Oncol. 2019;41:96–104. doi: 10.1097/MPH.0000000000001425. [DOI] [PubMed] [Google Scholar]
- 131.Adamaki M., Tsotra M., Vlahopoulos S., Zampogiannis A., Papavassiliou A.G., Moschovi M. STAT transcript levels in childhood acute lymphoblastic leukemia: STAT1 and STAT3 transcript correlations. Leuk. Res. 2015;39:1285–1291. doi: 10.1016/j.leukres.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 132.Morishita N., Tsukahara H., Chayama K., Ishida T., Washio K., Miyamura T., Yamashita N., Oda M., Morishima T. Activation of Akt is associated with poor prognosis and chemotherapeutic resistance in pediatric B-precursor acute lymphoblastic leukemia. Pediatric Blood Cancer. 2012;59:83–89. doi: 10.1002/pbc.24034. [DOI] [PubMed] [Google Scholar]
- 133.Weroha S.J., Haluska P. The Insulin-Like Growth Factor System in Cancer. Endocrinol. Metab. Clin. North. Am. 2012;41:335–350. doi: 10.1016/j.ecl.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Orgel E., Mittelman S.D. The links between insulin resistance, diabetes, and cancer. Curr. Diab. Rep. 2013;13:213–222. doi: 10.1007/s11892-012-0356-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Medyouf H., Gusscott S., Wang H., Tseng J.C., Wai C., Nemirovsky O., Trumpp A., Pflumio F., Carboni J., Gottardis M., et al. High-level IGF1R expression is required for leukemia-initiating cell activity in T-ALL and is supported by Notch signaling. J. Exp. Med. 2011;208:1809–1822. doi: 10.1084/jem.20110121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gusscott S., Jenkins C.E., Lam S.H., Giambra V., Pollak M., Weng A.P. IGF1R derived PI3K/AKT signaling maintains growth in a subset of human T-cell acute lymphoblastic leukemias. PLoS ONE. 2016;11:1–23. doi: 10.1371/journal.pone.0161158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.O’Shea D., Hogan A.E. Dysregulation of natural killer cells in obesity. Cancers. 2019;11:573. doi: 10.3390/cancers11040573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lynch L.A., O’Connell J.M., Kwasnik A.K., Cawood T.J., O’Farrelly C., O’Shea D.B. Are natural killer cells protecting the metabolically healthy obese patient? Obesity. 2009;17:601–605. doi: 10.1038/oby.2008.565. [DOI] [PubMed] [Google Scholar]
- 139.Michelet X., Dyck L., Hogan A., Loftus R.M., Duquette D., Wei K., Beyaz S., Tavakkoli A., Foley C., Donnelly R., et al. Metabolic reprogramming of natural killer cells in obesity limits antitumor responses. Nat. Immunol. 2018;19:1330–1340. doi: 10.1038/s41590-018-0251-7. [DOI] [PubMed] [Google Scholar]
- 140.O’Shea D., Cawood T.J., O’Farrelly C., Lynch L. Natural killer cells in obesity: Impaired function and increased susceptibility to the effects of cigarette smoke. PLoS ONE. 2010;5:1–8. doi: 10.1371/journal.pone.0008660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tobin L.M., Mavinkurve M., Carolan E., Kinlen D., O’Brien E.C., Little M.A., Finlay D.K., Cody D., Hogan A.E., O’Shea D. NK cells in childhood obesity are activated, metabolically stressed, and functionally deficient. JCI Insight. 2017;2:e94939. doi: 10.1172/jci.insight.94939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Viel S., Besson L., Charrier E., Marçais A., Disse E., Bienvenu J., Walzer T., Dumontet C. Alteration of Natural Killer cell phenotype and function in obese individuals. Clin. Immunol. 2017;177:12–17. doi: 10.1016/j.clim.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 143.Wensveen F.M., Jelenčić V., Valentić S., Šestan M., Wensveen T.T., Theurich S., Glasner A., Mendrila D., Štimac D., Wunderlich F.T., et al. NK cells link obesity-induced adipose stress to inflammation and insulin resistance. Nat. Immunol. 2015;16:376–385. doi: 10.1038/ni.3120. [DOI] [PubMed] [Google Scholar]
- 144.Zuk P.A., Zhu M., Mizuno H., Huang J., Futrell J.W., Katz A.J., Benhaim P., Lorenz H.P., Hedrick M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 145.Gimble J.M., Guilak F. Adipose-derived adult stem cells: Isolation, characterization, and differentiation potential. Cytotherapy. 2003;5:362–369. doi: 10.1080/14653240310003026. [DOI] [PubMed] [Google Scholar]
- 146.Zuk P.A., Zhu M., Ashjian P., De Ugarte D.A., Huang J.I., Mizuno H., Alfonso Z.C., Fraser J.K., Benhaim P., Hedrick M.H. Human Adipose Tissue Is a Source of Multipotent Stem Cells. Mol. Biol. Cell. 2002;13:4279–4295. doi: 10.1091/mbc.e02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Trayhurn P., Beattie J.H. Physiological role of adipose tissue: White adipose tissue as an endocrine and secretory organ. Proc. Nutr. Soc. 2001;60:329–339. doi: 10.1079/PNS200194. [DOI] [PubMed] [Google Scholar]
- 148.Rehman J., Traktuev D., Li J., Merfeld-Clauss S., Temm-Grove C.J., Bovenkerk J.E., Pell C.L., Johnstone B.H., Considine R.V., March K.L. Secretion of Angiogenic and Antiapoptotic Factors by Human Adipose Stromal Cells. Circulation. 2004;109:1292–1298. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 149.Nakagami H., Morishita R., Maeda K., Kikuchi Y., Ogihara T., Kaneda Y. Adipose tissue-derived stromal cells as a novel option for regenerative cell therapy. J. Atheroscler. Thromb. 2006;13:77–81. doi: 10.5551/jat.13.77. [DOI] [PubMed] [Google Scholar]
- 150.Wang M., Crisostomo P.R., Herring C., Meldrum K.K., Meldrum D.R. Human progenitor cells from bone marrow or adipose tissue produce VEGF, HGF, and IGF-I in response to TNF by a p38 MAPK-dependent mechanism. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;291:880–884. doi: 10.1152/ajpregu.00280.2006. [DOI] [PubMed] [Google Scholar]
- 151.Kilroy G.E., Foster S.J., Wu X., Ruiz J., Sherwood S., Heifetz A., Ludlow J.W., Stricker D.M., Potiny S., Green P., et al. Cytokine profile of human adipose-derived stem cells: Expression of angiogenic, hematopoietic, and pro-inflammatory factors. J. Cell. Physiol. 2007;212:702–709. doi: 10.1002/jcp.21068. [DOI] [PubMed] [Google Scholar]
- 152.Lee M.W., Park Y.J., Kim D.S., Park H.J., Jung H.L., Lee J.W., Sung K.W., Koo H.H., Yoo K.H. Human Adipose Tissue Stem Cells Promote the Growth of Acute Lymphoblastic Leukemia Cells in NOD/SCID Mice. Stem Cell Rev. Rep. 2018;14:451–460. doi: 10.1007/s12015-018-9806-0. [DOI] [PubMed] [Google Scholar]
- 153.Lee M.W., Ryu S., Kim D.S., Lee J.W., Sung K.W., Koo H.H., Yoo K.H. Mesenchymal stem cells in suppression or progression of hematologic malignancy: Current status and challenges. Leukemia. 2019;33:597–611. doi: 10.1038/s41375-018-0373-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Joe A.W.B., Lin Y., Even Y., Vogl A.W., Rossi F.M.V. Depot-specific differences in adipogenic progenitor abundance and proliferative response to high-fat diet. Stem Cells. 2009;27:2563–2570. doi: 10.1002/stem.190. [DOI] [PubMed] [Google Scholar]
- 155.Maumus M., Sengenès C., Decaunes P., Zakaroff-Girard A., Bourlier V., Lafontan M., Galitzky J., Bouloumie A. Evidence of in situ proliferation of adult adipose tissue-derived progenitor cells: Influence of fat mass microenvironment and growth. J. Clin. Endocrinol. Metab. 2008;93:4098–4106. doi: 10.1210/jc.2008-0044. [DOI] [PubMed] [Google Scholar]
- 156.Zhang Y., Daquinag A.C., Amaya-Manzanares F., Sirin O., Tseng C., Kolonin M.G. Stromal progenitor cells from endogenous adipose tissue contribute to pericytes and adipocytes that populate the tumor microenvironment. Cancer Res. 2012;72:5198–5208. doi: 10.1158/0008-5472.CAN-12-0294. [DOI] [PubMed] [Google Scholar]
- 157.Strong A.L., Semon J.A., Strong T.A., Santoke T.T., Zhang S., McFerrin H.E., Gimble J.M., Bunnell B.A. Obesity-associated dysregulation of calpastatin and MMP-15 in adipose-derived stromal cells results in their enhanced invasion. Stem Cells. 2012;30:2774–2783. doi: 10.1002/stem.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.DelaRosa O., Sánchez-Correa B., Morgado S., Ramírez C., Del Río B., Menta R., Lombardo E., Tarazona R., Casado J.G. Human adipose-derived stem cells impair natural killer cell function and exhibit low susceptibility to natural killer-mediated lysis. Stem Cells Dev. 2012;21:1333–1343. doi: 10.1089/scd.2011.0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bochev I., Elmadjian G., Kyurkchiev D., Tzvetanov L., Altankova I., Tivchev P., Kyurkchiev S. Mesenchymal stem cells from human bone marrow or adipose tissue differently modulate mitogen-stimulated B-cell immunoglobulin production in vitro. Cell Biol. Int. 2008;32:384–393. doi: 10.1016/j.cellbi.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 160.Wang Y.C., Chen R.F., Brandacher G., Lee W.P.A., Kuo Y.R. The suppression effect of dendritic cells maturation by adipose-derived stem cells through TGF-β1 related pathway. Exp. Cell Res. 2018;370:708–717. doi: 10.1016/j.yexcr.2018.07.037. [DOI] [PubMed] [Google Scholar]
- 161.Vicente López Á., Vázquez García M.N., Melen G.J., Entrena Martínez A., Cubillo Moreno I., García-Castro J., Ramírez Orellana M., Zapata González A.G. Mesenchymal stromal cells derived from the bone marrow of acute lymphoblastic leukemia patients show altered BMP4 production: Correlations with the course of disease. PLoS ONE. 2014;9:1–11. doi: 10.1371/journal.pone.0084496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Luo G., He Y., Yu X. Bone Marrow Adipocyte: An intimate partner with tumor cells in bone metastasis. Front. Endocrinol. (Lausanne) 2018;9:1–14. doi: 10.3389/fendo.2018.00339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Boag J.M., Beesley A.H., Firth M.J., Freitas J.R., Ford J., Hoffmann K., Cummings A.J., de Klerk N.H., Kees U.R. Altered glucose metabolism in childhood pre-B acute lymphoblastic leukaemia. Leukemia. 2006;20:1731–1737. doi: 10.1038/sj.leu.2404365. [DOI] [PubMed] [Google Scholar]
- 164.Liu T., Kishton R.J., Macintyre A.N., Gerriets V.A., Xiang H., Liu X., Abel E.D., Rizzieri D., Locasale J.W., Rathmell J.C. Glucose transporter 1-mediated glucose uptake is limiting for B-cell acute lymphoblastic leukemia anabolic metabolism and resistance to apoptosis. Cell Death Dis. 2014;5:1–13. doi: 10.1038/cddis.2014.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ye H., Adane B., Khan N., Alexeev E., Nusbacher N., Minhajuddin M., Stevens B.M., Winters A.C., Lin X., Ashton J.M., et al. Subversion of Systemic Glucose Metabolism as a Mechanism to Support the Growth of Leukemia Cells. Cancer Cell. 2018;34:659–673.e6. doi: 10.1016/j.ccell.2018.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Roberson J.R., Spraker H.L., Shelso J., Zhou Y., Inaba H., Metzger M.L., Rubnitz J.E., Ribeiro R.C., Sandlund J.T., Jeha S., et al. Clinical consequences of hyperglycemia during remission induction therapy for pediatric acute lymphoblastic leukemia. Leukemia. 2009;23:245–250. doi: 10.1038/leu.2008.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Shafat M.S., Oellerich T., Mohr S., Robinson S.D., Edwards D.R., Marlein C.R., Piddock R.E., Fenech M., Zaitseva L., Abdul-Aziz A., et al. Leukemic blasts program bone marrow adipocytes to generate a protumoral microenvironment. Blood. 2017;129:1320–1332. doi: 10.1182/blood-2016-08-734798. [DOI] [PubMed] [Google Scholar]
- 168.Van’t Veer M.B., Brooijmans A.M., Langerak A.W., Verhaaf B., Goudswaard C.S., Graveland W.J., Van Lom K., Valk P.J.M. The predictive value of lipoprotein lipase for survival in chronic lymphocytic leukemia. Haematologica. 2006;91:56–63. [PubMed] [Google Scholar]
- 169.Tabe Y., Yamamoto S., Saitoh K., Sekihara K., Monma N., Ikeo K., Mogushi K., Shikami M., Ruvolo V., Ishizawa J., et al. Bone marrow adipocytes facilitate fatty acid oxidation activating AMPK and a transcriptional network supporting survival of acute monocytic leukemia cells. Cancer Res. 2017;77:1453–1464. doi: 10.1158/0008-5472.CAN-16-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Tucci J., Sheng X., Mittelman S.D. Acute lymphoblastic leukemia cells stimulate adipocyte lipolysis and utilize adipocyte-derived free-fatty acids for proliferation; Proceedings of the Molecular and Cellular Biology; San Diego, CA, USA. 5–9 April 2014; Philadelphia, PA, USA: American Association for Cancer Research; p. 4339. [Google Scholar]
- 171.Oettgen H.F., Old L.J., Boyse E.A., Campbell H.A., Philips F.S., Clarkson B.D., Tallal L., Leeper R.D., Schwartz M.K., Kim J.H. Inhibition of leukemias in man by L-asparaginase. Cancer Res. 1967;27:2619–2631. [PubMed] [Google Scholar]
- 172.Ehsanipour E.A., Sheng X., Behan J.W., Wang X., Butturini A., Avramis V.I., Mittelman S.D. Adipocytes cause leukemia cell resistance to l-asparaginase via release of glutamine. Cancer Res. 2013;73:2998–3006. doi: 10.1158/0008-5472.CAN-12-4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Tucci J., Alhushki W., Chen T., Sheng X., Kim Y.-M., Mittelman S.D. Switch to low-fat diet improves outcome of acute lymphoblastic leukemia in obese mice. Cancer Metab. 2018;6:6–13. doi: 10.1186/s40170-018-0189-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wang K.W., Ladhani S., Empringham B., Portwine C., Fleming A., Banfield L., Balakumaran J., Sarpong L., Sims E.D., Popa A.N., et al. Bariatric interventions in obesity treatment and prevention in pediatric acute lymphoblastic leukemia: A systematic review and meta-analysis. Cancer Metastasis Rev. 2020;39:79–90. doi: 10.1007/s10555-020-09849-y. [DOI] [PubMed] [Google Scholar]
- 175.Zhou G., Goodyear L.J., Moller D.E., Zhou G., Myers R., Li Y., Chen Y., Shen X., Fenyk-melody J., Wu M., et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Grimaldi C., Chiarini F., Tabellini G., Ricci F., Tazzari P.L., Battistelli M., Falcieri E., Bortul R., Melchionda F., Iacobucci I., et al. AMP-dependent kinase/mammalian target of rapamycin complex 1 signaling in T-cell acute lymphoblastic leukemia: Therapeutic implications. Leukemia. 2012;26:91–100. doi: 10.1038/leu.2011.269. [DOI] [PubMed] [Google Scholar]
- 177.Trucco M., Barredo J.C., Goldberg J., Leclerc G.M., Hale G.A., Gill J., Setty B., Smith T., Lush R., Lee J.K., et al. A phase I window, dose escalating and safety trial of metformin in combination with induction chemotherapy in relapsed refractory acute lymphoblastic leukemia: Metformin with induction chemotherapy of vincristine, dexamethasone, PEG-asparaginase, and doxo. Pediatric Blood Cancer. 2018;65:e27224. doi: 10.1002/pbc.27224. [DOI] [PubMed] [Google Scholar]