Abstract
Purpose:
The objective of this study was to correlate seasonal variation of epidemic retinitis (ER) with concurrent community outbreaks.
Methods:
This is a retrospective, observational, comparative study conducted in a tertiary care eye hospital in south India. Monthly variation in number of ER cases in comparison with reported community outbreaks by Integrated Disease Surveillance Program (IDSP) from 2009 to 2020 in the same region were studied. Month-wise graphs against number of patients were plotted for ER and for each community outbreak.
Results:
ER was diagnosed in 163 patients. Diagnosis of presumed rickettsial ER was made in 48 cases (29.44%), chikungunya in 5, dengue in 3 and typhoid in 6 cases, while in other cases the etiological diagnosis remained uncertain (n = 101). Multiple positive serological tests were seen in 6 patients (Weil Felix Test (WFT) with WIDAL in 4 and chikungunya IgM with WFT in 2 patients). Relevant reported outbreaks by IDSP were: Pyrexia of unknown origin (PUO) (n = 5148), Chikungunya (n = 6577), Dengue (n = 7350), Measles (n = 1422), Mumps (n = 881), Rubella (n = 288), Malaria (n = 2262), Chicken Pox (n = 2385), Typhoid (n = 597), Kyasanur Forest Disease (n = 381), Scrub Typhus (n = 13), Typhus fever (n = 4), Japanese Encephalitis (n = 15). None of the outbreak graphs pattern was identical or similar to the graph of ER. Inverse relation of the graph of dengue outbreak with ER was observed.
Conclusion:
Inverse correlation between dengue and ER should be further studied for causation, which we believe may prove dengue as least common cause. Reporting of rickettsial outbreaks should be enhanced by undertaking statewide awareness and procurement of gold standard tests.
Keywords: Community outbreaks, epidemic retinitis, post fever retinitis, rickettsia, seasonal variation
Uveitis with posterior segment involvement has been described in many infectious epidemics: Leptospirosis,[1] Chikungunya,[2] Dengue,[3] West Nile fever,[4] Rickettsiosis,[5] and even in Toxoplasmosis.[6] Many of these conditions repeat seasonally and some of them are active throughout the year in tropical countries like India. Retinitis has been reported in many of these epidemics.[7] Causative organisms could be different, but manifestation could be same in the form of focal or multifocal retinitis. Club of those entities has been termed “epidemic retinitis” (ER) due to their seasonal variation.[8,9] A clear pattern of variation with peaks in colder months and troughs in warmer season has been reported previously.[8] Taking into consideration relation of ER with community outbreaks, one may expect similar pattern of variation of the contemporary infectious community outbreaks which can cause ER.
On this background we planned our study to analyze pattern of seasonal variation of ER in our set up and correlate it with contemporary systemic community outbreaks in our state.
Methods
This is a retrospective, observational, cross-sectional, comparative study. Case records of patients diagnosed as ER were reviewed from June 2009 to January 2020 in uveitis department of tertiary eye care hospital. Patient presented from our state (Karnataka, India) were included in the study. Patients hailing from outside Karnataka or having history of inter-state travel just prior to their fever were excluded. The study was approved by the ethics committee and adhered to the declaration of Helsinki. Diagnosis of ER was made based on previously described criteria: recent history of fever followed by ocular symptoms, presence of focal or multifocal cotton wool spots (CWS)-like retinitis lesions around the disc or on the posterior pole with or without macular edema.[8,9] Investigations and likely etiological diagnosis were noted for each patients. In case of multiple positive investigations or all negative investigations the etiological diagnosis was labeled “unconfirmed.” Diagnosis made by patient's physicians during their febrile illness based on the records provided by the patients was also noted where available.
Epidemiological data for weekly reported community outbreaks in our state (Karnataka, India) was collected from 'integrated disease surveillance program's (IDSP) website (https://idsp.nic.in/index4.php?lang=1&level=0&linkid=406&lid=3689) from June 2009 to January 2020. Disease outbreaks known to cause ER such as rickettsia, dengue, chikungunya, West Nile Virus (WNV) fever, typhoid, as well as other outbreaks which cannot be ruled out causing ER, like Measles, Mumps, Rubella, Chicken pox, Malaria, Kyasanur Forest disease (KFD), Japanese Encephalitis (JE) and pyrexia of unknown origin (PUO) were included in the study. Outbreaks not known to cause ER such as food poisoning, cholera, dysentery, influenza were excluded from the data collection.
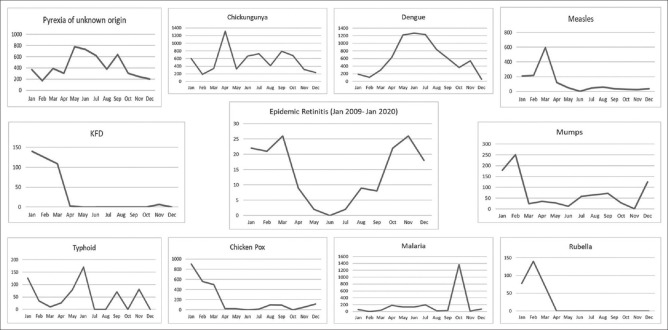
A chart of months against number of patients of ER presented from June 2009 to January 2020 was plotted by adding number of patients for that particular month [Fig. 1]. Similarly, months and number of patients reported weekly for our state were noted down from the IDSP website. The weekly reported number of patients were added according to the outbreaks from June 2009 to January 2020. And a month-wise graph was produced for each outbreak [Fig. 1]. ER graph was then compared with community outbreak graphs to compare the pattern.
Figure 1.
A comparative month-wise graphical representation of individual systemic disease outbreaks as reported by IDSP from June 2009 to January 2020 against ER from June 2009 – January 2020. X-axis represents months and Y-axis total number of patients seen in that particular month for the entire study duration
Results
A total of 163 patients of ER were studied. Mean age of presentation was 38 years (range: 5-73 years). Hundred and one were male and 62 were females. Maximum numbers of ER patients were seen between October and March, whereas least number of patients presented in May, June and July in 11 years [Fig. 1]. Following diagnosis was made for the fever by their physicians: typhoid (n = 25), viral fever (n = 22), malaria (n = 5), dengue (n = 11), chikungunya (n = 3), measles (n = 1), viral meningoencephalitis (n = 2), chickenpox (n = 1), and rickettsial disease (Typhus fever) (n = 1). In rest of the cases diagnosis remained PUO or was not available.
In patients with ER who presented to us, investigations revealed positive Weil-Felix test (WFT) in 54/116 (46.55%), Dengue (IgG) 23/101 (22.77%), Dengue (IgM) 4/101 (3.96%), Chikungunya (IgM) 7/102 (6.8%), WIDAL 13/87 (15%), West Nile Virus (IgM ELISA, Sandwich ELISA, RT-LAMP) 0/9 (0%) and blood films for malaria parasites 0/49 (0%). Rubella IgM was positive in (1/13), Measles (0/5) and Mumps (0/5). Anterior Chamber Tap (AC Tap) PCR for HSV/VZV/CMV (n = 11), chikungunya (n = 2), dengue (n = 1), WNV (n = 9), and scrub typhus (n = 4) was negative in all. Thrombocytopenia was seen in 20 cases, of which 3 were diagnosed as rickettsial disease and 1 each as chikungunya, dengue and typhoid. One patient tested positive for enterovirus on PCR-AC Tap done elsewhere but the same patient also tested positive for chikungunya on PCR-blood. Multiple positive serological tests were seen in 6 patients: positive WFT with WIDAL was seen in 4 patients, and positive chikungunya IgM with WFT was seen in 2 patients. We made presumed diagnosis of rickettsial ER in 48 cases, chikungunya in 5, dengue in 3 and typhoid in 6 cases, while in other cases, the etiological diagnosis remained unconfirmed (n = 101). There was mismatch between our diagnosis and physician's diagnosis in many cases [Table 1].
Table 1.
Presumed etiological diagnosis of ER and fever
| Our diagnosis | Physician’s diagnosis |
|---|---|
| Rickettsia (n=16) | Viral fever (n=6), Typhoid (n=5), Chicken pox (n=1), Dengue (n=2), Malaria (n=1), Chikungunya (n=1) |
| Typhoid (n=2) | Viral fever (n=1), Dengue (n=1) |
| Dengue (n=1) | Malaria (n=1) |
| Chikungunya (n=4) | Malaria (n=1), Typhoid (n=1), Viral fever (n=2) |
| Unconfirmed (n=43) | Malaria (n=2), Chikungunya (n=1), Dengue (n=7), Viral fever (n=14), Typhoid (n=17), Measles (n=1), Typhus (n=1) |
The following community outbreaks and total number of patients were studied: PUO (n = 5148), chikungunya (n = 6577), dengue (n = 7350), measles (n = 1422), mumps (n = 881), rubella (n = 288), malaria (n = 2262), chicken pox (n = 2385), typhoid (n = 597), KFD (n = 381), JE (n = 15) and rickettsial disease [scrub typhus (n = 13), typhus fever (n = 4)] [Fig. 2]. Month-wise graphs were plotted for all outbreaks except for JE and rickettsial diseases as they were poorly reported [Fig. 1]. In 11 years, only 8 cases of scrub typhus were reported in September and 5 cases in December, whereas 4 cases of typhus fever were reported in month of June. Cases of JE were reported in June (n = 2), July (n = 10) and December (n = 3).
Figure 2.
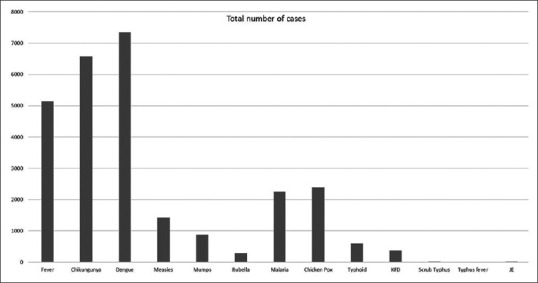
A graphical representation of relevant systemic outbreaks reported by IDSP for state of Karnataka, India between June 2009 to January 2020. X-axis denotes name of the epidemic and Y-axis total number of patients reported for the study period
None of the outbreak graphs was found identical or similar with the graph of ER. PUO, and Chikungunya were reported throughout the year with few peak incidences between April and October. Peak of dengue graph matched with trough of ER graph. First half of the ER graph showed more or less similarities with measles, mumps, rubella, chicken pox and KFD graphs. Malaria was slightly prevalent between April and July, but there was disproportionate peak in October due to a single large outbreak. Typhoid graph had several intermittent peaks throughout the year. Unfortunately (or fortunately), no outbreak reports were found for WNV in our state.
Discussion
Numerous causes like chikungunya, dengue, WNV and rickettsia have been reported as possible aetiology for ER.[4,5,6,7,8,9,10,11] A battery of investigations is needed to pinpoint the causative agent. Many times, it is not possible to do all serological investigations due to financial constraints and unavailability of that particular test. Contemporary epidemic in that particular region may give a clue towards the etiology of ER. We have noted obvious seasonal variation in ER with high incidence in colder months and least in warmer months. Each year the epidemic (ER) began between July and August, gradually picked up and showed maximum incidence between October and March, after which there was a sharp decline [Fig. 1]. It was interesting for us to look for similar pattern of contemporary outbreaks ongoing in the community. Taking into consideration the latent period of ER (duration between fever and onset of ocular symptoms, around 18 days[9]) which may vary from few days to few weeks, we expected a similar graph pattern in few of the outbreaks in the community with a delay of 1 or 2 months (to explain the latent period).
IDSP reported weekly outbreaks of several diseases from our state which are known to cause ER. But to our surprise, we did not find similar seasonal variation pattern of ER in any of the reported outbreaks. Rather, inverse correlation of dengue outbreak with ER was evident. Furthermore, we also noted that most of our patients tested positive for WFT but rickettsial outbreaks were poorly reported in the community.
Comparing the graph pattern of dengue outbreak with ER, it's evident that the epidemic continues with peak incidence in May, June and July and then gradual decline in December, January and February, whereas ER begins from July onwards with peak incidence in colder months, followed by steep decline from March onwards. Similarly, for chikungunya and typhoid outbreaks were reported in warmer months in contrast to ER. Ignoring the single spike in malaria-graph which was due to a single large outbreak, incidence for malaria was also slightly high in warmer months. First half pattern of the Mumps/Measles/Rubella (MMR), Chicken pox and KFD graphs were somewhat similar to the first half of ER graph. Hence based on the patterns of these community outbreaks one can speculate MMR, Chicken pox and KFD to be the relatively common causes for ER.
Chicken pox and mumps causing retinitis and neuro-retinitis has been described in the literature.[12,13,14] None of our patients presented with history of mumps, which is otherwise an obvious clinical diagnosis. Only one patient gave history of chicken pox, but upon investigation (by us), the patient was tested positive for WFT. Neppert and Bonamour have reported retinitis in measles.[15,16] One of our patients was diagnosed as measles by her physician, but WFT was not done in this case. IDSP has reported Measles outbreaks mainly in children whereas our ER population was mainly adults. We had only 10 patients below 18 years of age in the ER series. Rubella retinopathy is known in children, but little is known about its presentation in adults. Damasceno et al. have reported a case of retinitis and vasculitis after a febrile illness, proven to be 'rubella in adult'.[17] Rubella IgM was detected positive only in 1 out of 13 cases in our ER series. Whereas IgM were negative for all cases tested for Measles and Mumps. It is possible that MMR cases when presented to ophthalmologist weeks after the fever, the titers of IgM antibodies might have receded from their serum, precluding the diagnosis. KFD is a tick-borne viral hemorrhagic fever seen in forest inhabitants or visitors and is endemic in south-western parts of India. Ocular manifestations of KFD is well described in an old report from India which described iritis and retinal hard exudates as the manifestations but no comments on vitritis or retinitis or soft exudates were made.[18] Their study included patients seen only in first 2 weeks of the disease, this could have missed cases of uveitis (with retinitis) presented after 2 to 4 weeks of latent period. None of our patients were forest dweller and none of them gave history of a recent forest visit. Unfortunately, diagnostic tests for KFD was not done in our ER patients. WNV another possible cause for ER remained unaddressed as IDSP has no data on this epidemic during our study period for our state. Only 9 of our ER patients were investigated for same and tested negative. Scrub typhus, Typhus fever and JE were reported in negligible numbers by IDSP from 2009 to 2020 in our state, and hence could not be compared with the graph of ER.
Taking into consideration, high WFT positivity (29.44%) in our series along with growing literature support on rickettsial diseases in the country,[10,11,19,20] we feel that community outbreak of rickettsial diseases were under-reported in IDSP registry from our state. Seasonal trend of rickettsial diseases has been reported soon after the rainy season (August–November).[20] This trend was reflected in our graph of ER. Many of the diseases can be confused with rickettsiosis including typhoid as reported by Gerhard.[21] Taking into consideration typhoid is a water-borne disease, one would expect the outbreak in warmer season when the chances of water consumption and hence the contamination is more. This was partially expressed in the typhoid-graph, as there were outbreaks in colder months as well. We speculate those colder month outbreaks in typhoid-graph could have been due to confusing rickettsial diseases with typhoid. Many cases where diagnosis of typhoid was made by physicians, tested positive for WFT in our series. The test was also positive in several cases of so called “viral fever.” The WFT test, although not a gold standard and having low sensitivity and specificity (43% and 98%, respectively), may confirm the presence of infection 2 weeks after the onset of disease.[20] And this is the time when patients develop signs of ER and rush to the ophthalmologist. Unfortunately, demonstration of 4-fold increase in titers of WFT was not possible in our series as the test was not done at the onset of the fever in most of the cases. Thrombocytopenia was seen in few of our patients in setting of negative serology for dengue. Thrombocytopenia (which frequently confused with dengue fever) can be seen during early course of rickettsial disease as well.[22] We presume a good number of cases were diagnosed as PUO, dengue and typhoid due to lack of testing for rickettsial diseases. Similar to the possible failure of consideration of rickettsial diseases by the physician, the likelihood of measles, mumps, rubella, chickenpox and KFD causing ER was also not paid attention to and investigations were poorly done in that direction in our study. But knowing that fact that the patients come to the ophthalmologist after few weeks of the fever, investigations done by physician may yield positive results compared to investigations done by ophthalmologists.
Limitations of our study were retrospective nature, lack of standard investigation pattern, lack of communication between physician and ophthalmologists. Another drawback was consideration of geographic region for study rather than meteorological zone. The merits of the study were a novel idea and a different angle of correlating infectious or para-infectious uveitides with systemic diseases where the diagnosis is precluded by unavailability of gold standard diagnostic tests, and less clues from the systemic examination as the systemic disease has already resolved by the time the patient presents to the ophthalmologist. In attempts to correlate ER with systemic outbreaks, our study also found lacunae in reporting/screening of emerging epidemics to IDSP.
Conclusion
In conclusion, our study is the largest cohort of ER which confirms the seasonal variation with least incidence during May, June and July. No identical or similar pattern of seasonal variation was recognized between ER and community outbreaks reported by IDSP. Inverse correlation of dengue outbreak with ER as noted in our study should be further studied for causation which we believe may result into dengue being least common cause of ER. Reporting of rickettsial diseases to IDSP should be enhanced in the state of Karnataka. There is need of statewide training, awareness and procurement of better laboratory instruments that can perform testing at global gold standard levels.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Rathinam SR, Rathnam S, Selvaraj S, Dean D, Nozik RA, Namperumalsamy P. Uveitis associated with an epidemic outbreak of leptospirosis. Am J Ophthalmol. 1997;124:71–9. doi: 10.1016/s0002-9394(14)71646-0. [DOI] [PubMed] [Google Scholar]
- 2.Mahendradas P, Avadhani K, Shetty R. Chikungunya and the eye: A review. J Ophthalmic Inflamm Infect. 2013;3:35. doi: 10.1186/1869-5760-3-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta A, Srinivasan R, Setia S, Soundravally R, Pandian DG. Uveitis following dengue fever. Eye (Lond) 2009;23:873–6. doi: 10.1038/eye.2008.124. [DOI] [PubMed] [Google Scholar]
- 4.Sivakumar RR, Prajna L, Arya LK, Muraly P, Shukla J, Saxena D, et al. Molecular diagnosis and ocular imaging of West Nile virus retinitis and neuroretinitis. Ophthalmology. 2013;120:1820–6. doi: 10.1016/j.ophtha.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Agahan A, Torres J, Fuentes-Páez G, Martínez-Osorio H, Orduña A, Calonge M. Intraocular inflammation as the main manifestation of Rickettsia conorii infection. Clin Ophthalmol. 2011;5:1401–7. doi: 10.2147/OPTH.S21257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palanisamy M, Madhavan B, Balasundaram M, Andavar R, Venkatapathy N. Outbreak of ocular toxoplasmosis in Coimbatore. Indian J Ophthalmol. 2006;54:129–31. doi: 10.4103/0301-4738.25839. [DOI] [PubMed] [Google Scholar]
- 7.Khairallah M, Chee SP, Rathinam SR, Attia S, Nadella V. Novel infectious agents causing uveitis. Int Ophthalmol. 2010;30:465–83. doi: 10.1007/s10792-009-9319-6. [DOI] [PubMed] [Google Scholar]
- 8.Kawali A, Mahendradas P, Mohan A, Mallavarapu M, Shetty B. Epidemic retinitis. Ocul Immunol Inflamm. 2019;27:571–7. doi: 10.1080/09273948.2017.1421670. [DOI] [PubMed] [Google Scholar]
- 9.Kawali A, Srinivasan S, Mohan A, Bavaharan B, Mahendradas P, Shetty B. Epidemic retinitis with macular edema -Treatment outcome with and without steroids? Ocul Immunol Inflamm. 2020:1–5. doi: 10.1080/09273948.2019.1704792. doi: 10.1080/09273948.2019.1704792. [DOI] [PubMed] [Google Scholar]
- 10.Kawali A, Mahendradas P, Srinivasan P, Priya S, Yadav NK, Avadhani K, et al. Rickettsial retinitis-an Indian perspective. J Ophthalmic Inflamm Infect. 2015;5:37. doi: 10.1186/s12348-015-0066-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balasundaram MB, Manjunath M, Baliga G, Kapadi F. Ocular manifestations of Rickettsia conorii in South India. Indian J Ophthalmol. 2018;66:1840–4. doi: 10.4103/ijo.IJO_420_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biswas J, Nagpal A, Chopra S, Karna S. Resolution of chicken pox neuroretinitis with oral acyclovir: A case report. Ocul Immunol Inflamm. 2003;11:315–8. doi: 10.1076/ocii.11.4.315.18267. [DOI] [PubMed] [Google Scholar]
- 13.Khubchandani R, Rane T, Agarwal P, Nabi F, Patel P, Shetty AK. Bilateral neuroretinitis associated with mumps. Arch Neurol. 2002;59:1633–6. doi: 10.1001/archneur.59.10.1633. [DOI] [PubMed] [Google Scholar]
- 14.Foster RE, Lowder CY, Meisler DM, Kosmorsky GS, Baetz-Greenwalt B. Mumps neuroretinitis in an adolescent. Am J Ophthalmol. 1990;110:91–3. doi: 10.1016/s0002-9394(14)76948-x. [DOI] [PubMed] [Google Scholar]
- 15.Neppert B. Measles retinitis in an immunocompetent child. Klin Monbl Augenheilkd. 1994;205:156–60. doi: 10.1055/s-2008-1045509. [DOI] [PubMed] [Google Scholar]
- 16.Bonamour G, Bonnet M. Post-measles severe retinitis. Bull Soc Ophtalmol Fr. 1965;65:517–21. [PubMed] [Google Scholar]
- 17.Damasceno N, Damasceno E, Souza E. Acquired unilateral rubella retinopathy in adult. Clin Ophthalmol. 2011;5:3–4. doi: 10.2147/OPTH.S15273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ocular manifestations of Kyasanur forest disease (a clinical study) Indian J Ophthalmol. 1983;31:700–2. [PubMed] [Google Scholar]
- 19.Mittal V, Gupta N, Bhattacharya D, Kumar K, Ichhpujani RL, Singh S, et al. Serological evidence of rickettsial infections in Delhi. Indian J Med Res. 2012;135:538–41. [PMC free article] [PubMed] [Google Scholar]
- 20.Sharma A, Mishra B. Rickettsial disease existence in India: Resurgence in outbreaks with the advent of 20th century. Indian J Health Sci Biomed Res. 2020;13:5–10. [Google Scholar]
- 21.Gerhard WW. TYPHOID vs. TYPHUS FEVER? JAMA. 1962;181:154–5. doi: 10.1001/jama.1962.03050280084012. [Google Scholar]
- 22.Rahi M, Gupte MD, Bhargava A, Varghese GM, Arora R. DHR-ICMR Guidelines for diagnosis and management of Rickettsial diseases in India. Indian J Med Res. 2015;141:417–22. doi: 10.4103/0971-5916.159279. [DOI] [PMC free article] [PubMed] [Google Scholar]