Abstract
Purpose:
To report the clinical profile of a series of anterior nodular scleritis in Indian population.
Methods:
We conducted a retrospective review of medical records of 140 eyes of 123 consecutive patients with nodular scleritis who presented to a tertiary eye care institute between 2007 and 2018.
Results:
The mean age at presentation was 46.8 ± 13.1 years and 70.7% of the patients were female. Bilateral involvement was observed in 14% patients. The most common presenting symptom was redness (92.6%) and ocular pain (69.1%). Twenty-seven patients (22%) had some systemic association and rheumatoid arthritis (5%) was the most common autoimmune disease. Presumed ocular tuberculosis was diagnosed in 13% patients. Methotrexate was the most common immunosuppressive used in these patients and an additional immunosuppressive was required in 6.5% patients. Recurrence of inflammation was observed in 74.8% patients. Deterioration of vision noted in 2.8% eyes.
Conclusion:
Tuberculosis remains an important cause of nodular scleritis in India. Recurrence of scleritis is common in nodular scleritis and cases with non infectious nodular scleritis often require treatment with immune suppressives.
Keywords: Anterior nodular scleritis, cyclophosphamide, methotrexate, scleritis, tuberculosis
Scleritis is a chronic, painful, and potentially blinding inflammatory disease that is characterized by edema and cellular infiltration of the scleral and epi scleral tissues. Nodular scleritis remains the second most common subtype of anterior scleritis after diffuse scleritis.[1,2] Nodular scleritis pose both a diagnostic as well as management challenges for ophthalmologists due to varied differentials as well as its refractory nature to the standard treatment protocol. Most of the available studies in the literature had described the clinical profile of patients where nodular scleritis was analyzed only as a subgroup of scleral inflammation. There is a paucity of literature on isolated case series on nodular scleritis. The largest series published till date is a retrospective case series of 585 patients of scleritis and epi scleritis by Sainz de la Maza et al.[2] which included 71 patients of nodular scleritis. The present study was undertaken to evaluate the clinical profile of nodular scleritis in an Indian population.
Methods
This was a hospital-based retrospective case series that reviewed the files of all consecutive patients with nodular scleritis at a single tertiary center between 2007 and 2018. The study was approved by the institutional review board of the hospital and adhered to the declaration of Helsinki. Nodular anterior scleritis, as per Watson and Hayreh's classification, was diagnosed clinically on the basis of characteristic ophthalmic findings such as congestion of the deeper episcleral plexus of vessels with or without excruciating pain; and nodular swelling of the sclera with scleral edema. Deeper episcleral congestion was confirmed by phenylephrine blanching test. Other sub-types of scleritis, other causes of nodular swelling and patients with insufficient documentation were excluded from the study. The information regarding the presenting complaints of the patient, the best-corrected visual acuity (BCVA) at the time of presentation and the follow up, detailed ocular examination including presence or absence of anterior segment inflammation, intraocular pressure (IOP), and fundus findings were noted. Patients in our series underwent a thorough laboratory evaluation that included routine hemogram, erythrocyte sedimentation rate, tuberculin skin test, antinuclear antibodies, rheumatoid factor (RF), human leukocyte antigen B27 (HLA-B27), and antinuclear cytoplasmic antibody. All the patients were evaluated by an in-house internist before starting systemic medications and a rheumatologist's opinion was sought to find out associated systemic diseases. Consultation from a pulmonologist was sought in relevant cases such as patients with history of contact with tuberculosis patients, positive Mantoux test and interferon - gamma release assay, and radiological evidence of healed or active pulmonary tuberculosis. All patients were initially treated with a combination of topical and oral non-steroidal anti-inflammatory agents and topical corticosteroid. Patients who were already treated with these agents, received oral corticosteroids. Oral corticosteroid was started at 0.5–1 mg per kilogram of body weight per day and was tapered gradually depending upon the clinical response. Immunosuppressives were considered in cases where the scleral inflammation did not respond to the systemic corticosteroid therapy alone, in cases with systemic rheumatic diseases as suggested by the rheumatologists; and in cases with recurrences. Immunosuppressive medications included methotrexate, azathioprine, mycophenolate mofetil, and cyclophosphamide. Oral methotrexate was started at a weekly oral dose of 15 mg and gradually decreased by 2.5 mg/week according to clinical response. Folate was administered concomitantly in patients receiving methotrexate to reduce bone marrow suppression. Oral azathioprine was started at a dose of 50 mg three times a day and tapered gradually. Patients on both methotrexate and azathioprine were monitored closely with liver function test and blood counts at regular intervals. Mycophenolate mofetil was started at 1 gram twice daily. Oral cyclophosphamide was started at a dose of 2 mg per kg per day in addition to oral corticosteroid therapy. Complete blood counts and urine analyses were monitored regularly.
Diminution of vision was defined as a decrease in visual acuity by more than two Snellen lines after the resolution of the scleral inflammation clinically. An IOP more than 21 mm of Hg was defined as ocular hypertension. The resolution was defined as lack of any symptom with resolution of clinically detectable episcleral injection, or scleral nodule and surrounding scleral edema on objective evaluation. Non responsiveness to treatment was defined as persistence of scleral edema with congested and/or engorged deeper episcleral vessels with no change in the size of scleral nodules for more than 8 weeks. BCVA results were converted to logarithm of the minimal angle of resolution (logMAR) for statistical analysis and were presented as logMAR and Snellen equivalent.
Results
The index study included 140 eyes of 123 patients with nodular anterior scleritis who visited the uvea clinic of a tertiary eye care center between 2007 to 2018. Of 123 patients, 87 (70.7%) were women and 36 (29.3%) were men. The mean age of the patients in the study was 46.8 ± 13.1 years (range: 9–80 years). The median duration of follow-up was 363.5 days. A total of 106 patients (86.2%) had a unilateral presentation, while 17 patients (13.8%) had bilateral nodular scleritis. The most common presenting symptom was redness (92.6%) followed by ocular pain (69.1%). Corneal involvement was noted in 3 eyes (2.4%) and 8 eyes (6.5%) showed anterior uveitis. The fundus examination was unremarkable in all the patients. Two patients had internal limiting membrane striae; the possibility of posterior scleritis was ruled out in these two patients by B-scan ultrasonography and fundus fluorescein angiography. The demographic and clinical characteristics of all patients in the current study are summarized in Table 1.
Table 1.
Demographic and clinical characteristics of all patients with nodular scleritis
| Clinical characteristics | Results |
|---|---|
| Age | 46.8±13.2 years |
| Median Duration of follow-up (Range) | 363.5 (90-5110) days |
| Gender | |
| Male | 36 (29.3) |
| Female | 87 (70.7) |
| Laterality | |
| Unilateral | 106 (86.2) |
| Bilateral | 17 (13.8) |
| Symptoms | |
| Ocular Pain | 85 (69.1%) |
| Foreign body sensations | 14 (11.3% ) |
| Redness | 114 (92.6%) |
| Watering | 3 (2.45) |
| Systemic association | |
| Rheumatoid arthritis | 6 (4.8%) |
| HLA-B27 - associated | 1 (0.8%) |
| Polymyositis | 1 (0.8%) |
| Granulomatosis polyangiitis | 1 (0.8%) |
| Behçet’s disease | 1 (0.8%) |
| Syphilis | 1 (0.8%) |
| Tuberculosis | 16 (13%) |
| Complications | |
| Anterior Uveitis | 8 (3.1%) |
| Ocular Hypertension | 11 (8.9%) |
| Cataract | 7 (5.7%) |
| Corneal involvement | 3 (2.4%) |
| Recurrences | 92 (74.8%) |
| Medications | |
| Topical NSAID | 26 (21.1%) |
| Oral NSAID | 16 (13.0%) |
| Oral Steroid | 97 (78.9%) |
| Topical Steroid | 101 (82.1%) |
| Immunosuppressives | |
| Cyclophosphamide | 7 (5.6%) |
| Methotrexate | 22 (17.8%) |
| Azathioprine | 10 (8.1%) |
| Mycophenolate mofetil | 21 (17.1%) |
NSAID=Nonsteroidal anti-inflammatory drug
Twenty-seven (21.9%) patients had a systemic association in our study. The most common systemic association was presumed tuberculosis (13%) [Fig. 1] followed by rheumatoid arthritis (4.8%) [Fig. 2]. Diagnosis of presumed tuberculosis was made based on positive tuberculin skin test, radiological assessment, and consultation with a chest physician. Scleral biopsy was obtained in only two patients. None of them yielded any positive result on microbiological examination. Methotrexate (17.8%) was the most commonly used immunosuppressive in the current study, followed by mycophenolate mofetil (17.1%). An additional immunosuppressive was required in 8 patients (6.5%). Eighty-nine patients (74.8%) had recurrences of scleral inflammation during the follow-up period - frequency of recurrences reduced after the introduction of immunosuppressives in the management. Deterioration of BCVA was noted in 4 (2.8%) of the eyes, whereas BCVA improved in 14 (10%) of eyes; BCVA remained unchanged in 122 eyes. Seven (5.69%) patients developed complicated cataract; two (1.6%) of them required surgical intervention phacoemulsification with foldable intraocular lens implantation under steroid cover. Ocular hypertension was noted in 11 patients (8.9%) and only one of them (0.8%) required a filtering procedure. Two patients (1.6%) required a scleral patch graft due to the gradual thinning of sclera; one of them had undergone scleral biopsy for the nodule. On comparing the mean log MAR visual acuity values, the study observed improvement in vision following treatment which was statistically significant (P = 0.0149).
Figure 1.
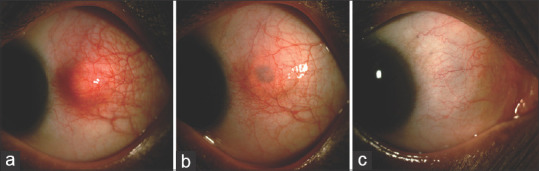
(a) Slit-lamp photograph of a 43-year-old man who presented with painful nodular swelling of sclera in his left eye. His Mantoux test was positive (22 mm induration) and erythrocyte sedimentation rate was raised. His interferon gamma release assay was also positive and high-resolution chest tomography revealed multiple nodes on the posterior segment of right upper lobe. He was started on ATT and oral corticosteroid 1 mg/kg/bodyweight. (b) Slit-lamp photograph of the left eye after 2 months. (c) Slit-lamp photograph of the left eye after 6 months
Figure 2.
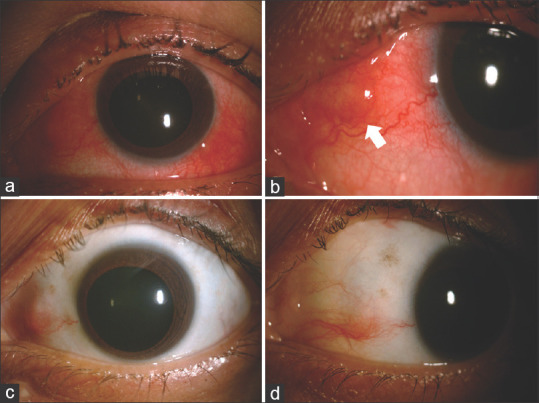
(a and b): Slit-lamp photograph of left eye of a 68-year-old female. She had recurrent attacks of redness, ocular pain which did not resolve with topical corticosteroid. Her laboratory investigations revealed a positive rheumatoid factor and on subsequent consultation with a rheumatologist, a diagnosis of rheumatoid arthritis was made. (c and d): Slit-lamp photograph of left eye after 2 months of therapy with oral methotrexate (15 mg/week) and oral steroid 40 mg/day in tapering schedule
Discussion
There is a paucity of literature describing the clinical profile of patients with nodular scleritis alone, and the majority of the existing literature has described nodular scleritis as a subset of scleritis. To the best of our knowledge, ours is the largest series entirely on anterior nodular scleritis, which included 123 patients.
Diffuse anterior and necrotizing scleritis remain the most common cause of bilateral scleritis.[2,3] In the current study, four patients (13.8%) had bilateral nodular scleritis. Compared to the other subtypes of scleritis, nodular scleritis has been reported to have relatively lower rates of bilateral involvement, ranging from 0 to 33%.[2,4,5,6] Also, in accordance with the previous studies, the index study observed similar age group involvement and female preponderance.[6] The majority of the patients presented with redness in the current study and ocular pain was complained by 69% of patients in the study at the time of presentation to us. Compared to necrotizing scleritis, ocular pain in nodular scleritis is mild to moderate, as reported by Sainz de la Maza et al.[2]
Association of nodular scleritis with various systemic rheumatic diseases remains variable, which ranges from nil to as high as 45%.[7,8] In a retrospective analysis, Raiji et al.[9] observed that patients with systemic disease are more likely to have diffuse or necrotizing scleritis, while those with isolated scleritis are more likely to have nodular scleritis. In our case series, 27 (21.9%) patients had a systemic association, and only 10 patients had an association with systemic autoimmune disorders. Keino et al.[7] could not establish an association with systemic autoimmune disease in any of the nine patients in their cohort of nodular scleritis patients. Rheumatoid arthritis is by far the most common systemic autoimmune condition reported to be associated with nodular scleritis. In our study, 4.8% of the cases of nodular scleritis were associated with rheumatoid arthritis. Relative lower incidence of systemic rheumatic or autoimmune disorders was observed in other case series on scleritis published from India. Kumar et al.[10] could not detect any systemic association in their patients with posterior scleritis. In another case series by the same authors on necrotizing scleritis, a subset of scleritis, which is reported to have a high association with the systemic rheumatic disease often up to 95% in the literature, had a systemic association is only in 37% patients.[11] In the index study, the most common systemic association was presumed ocular tuberculosis (12.1%). Bin Ismail et al.[12] reported higher rates of infectious etiology in their cohort with nodular scleritis patients. They found that 24% of patients to have an association with infectious etiology, whereas an association with systemic autoimmune disorders could be established only in 8% of patients. In a recently published case series on scleritis from Japan, Tanake et al.[5], an equal number of patients were associated with systemic rheumatic diseases and infectious etiology (11.8%). In another cohort of scleritis patients from Japan, 55.5% of patients with positive tuberculin test had nodular scleritis, and two had tuberculosis; the same study could not detect systemic autoimmune disorders in any patients with nodular scleritis. In a retrospective analysis of patients with tuberculous sclerokeratitis, the nodular involvement of the sclera was observed in 75% of patients.[13] Mycobacterium tuberculosis has been implicated in nodular scleral inflammation by various authors.[14,15,16,17]
The most common complication in our series was cataract (5.7%), which can be attributed to the frequent use of topical corticosteroid. Nodular scleritis usually responds slowly to topical corticosteroids and but usually prescribed often by the clinicians in increased frequency and for a longer period. Also, the anterior uveitis associated with nodular scleritis warrants topical corticosteroid. In the current study, anterior uveitis was detected in 6.5% of patients. In two separate studies by Tanaka et al.[5] and Yang et al.[4] the incidences of anterior uveitis in patients with nodular scleritis were 41.2% and 32%. Both the studies included a relatively smaller number of patients, which can be attributed to the higher number of anterior uveitis cases. Anterior uveitis was lowest in patient with nodular scleritis (14%) and most frequently observed in patients with necrotizing scleritis (45%) in a retrospective analysis of 500 patients with scleritis by Sainz de la Maza et al.[2] Thus, our results support the finding by various other studies that reported the incidence of anterior uveitis from 5%–14%.[2,6,7,8] However, the majority of the patients were on topical corticosteroid when they presented to us, making it difficult for us to calculate the exact incidence of anterior uveitis associated with nodular scleritis in our study. The current study did not observe vitritis, cystoid macular edema in patients with nodular scleritis, as reported by a case series from Japan.[5]
The index study showed a relatively higher use of immunosuppressive agents when compared with the existing literature. An immunosuppressive agent was used in 41.5% (51) patients. Being a referral tertiary care institute, there is a possibility of treating a greater number of difficult-to-treat cases or treatment-resistant cases; which may explain the higher use of immunosuppressive in our study. The most common immunosuppressive used was methotrexate (17.8%) and mycophenolate mofetil (17.1%) followed by azathioprine (8.1%). Eight patients (6.5%) required a combination of immunosuppressives. Methotrexate, because of the relatively low side-effects and cheaper, was the preferred agent of choice. However, methotrexate was found to be a weaker immunosuppressive in control of nodular scleral inflammation in our study, as 22.7% of the cases, initially treated with the drug, required additional or alternate immunosuppressives. Cyclophosphamide, however, was proven to be an effective immunosuppressive in control of nodular scleritis. A similar observation was reported by Sainz de la Maza et al.[18] The use of immunosuppressive helped in reducing the number of recurrent attacks of scleritis. Only two patients underwent scleral biopsy and one of them required a scleral patch graft later. Microbiological example from the sample obtained by scleral biopsy did not show the presence of any microorganism.
Our study has several limitations. As India is a developing nation with a vast geography, it is impractical to ascertain that our study population is a representative population of India.
Conclusion
A clear pattern of nodular scleritis has emerged out from the current study, which may help the clinicians to understand this subtype of scleral inflammation better and manage them accordingly. Anterior nodular scleritis remains largely idiopathic in India, followed by presumed tuberculosis and rheumatoid arthritis as the commonest infective and non-infective associations. Recurrences are frequent despite treatment with immunosuppressive agents. All patients of nodular scleritis should be evaluated for tuberculosis, especially in the tuberculosis-endemic region.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Okhravi N, Odufuwa B, McCluskey P, Lightman S. Scleritis. Surv Ophthalmol. 2005;50:351–63. doi: 10.1016/j.survophthal.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Sainz de la Maza M, Molina N, Gonzalez-Gonzalez LA, Doctor PP, Tauber J, Foster CS. Clinical characteristics of a large cohort of patients with scleritis and episcleritis. Ophthalmology. 2012;119:43–50. doi: 10.1016/j.ophtha.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 3.Wieringa WG, Wieringa JE, ten Dam-van Loon NH, Los LI. Visual outcome, treatment results, and prognostic factors in patients with scleritis. Ophthalmology. 2013;120:379–86. doi: 10.1016/j.ophtha.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Yang P, Ye Z, Tang J, Du L, Zhou Q, Qi J, et al. Clinical features and complications of scleritis in chinese patients. Ocul Immunol Inflamm. 2018;26:387–96. doi: 10.1080/09273948.2016.1241282. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka R, Kaburaki T, Ohtomo K, Takamoto M, Komae K, Numaga J, et al. Clinical characteristics and ocular complications of patients with scleritis in Japanese. Jpn J Ophthalmol. 2018;62:517–24. doi: 10.1007/s10384-018-0600-y. [DOI] [PubMed] [Google Scholar]
- 6.Erkanli L, Akova YA, Guney-Tefekli E, Tugal-Tutkun I. Clinical features, prognosis, and treatment results of patients with scleritis from 2 tertiary eye care centers in Turkey. Cornea. 2010;29:26–33. doi: 10.1097/ICO.0b013e3181ac9fad. [DOI] [PubMed] [Google Scholar]
- 7.Keino H, Watanabe T, Taki W, Nakashima C, Okada AA. Clinical features and visual outcomes of Japanese patients with scleritis. Br J Ophthalmol. 2010;94:1459–63. doi: 10.1136/bjo.2009.171744. [DOI] [PubMed] [Google Scholar]
- 8.Jabs DA, Mudun A, Dunn JP, Marsh MJ. Episcleritis and scleritis: Clinical features and treatment results. Am J Ophthalmol. 2000;130:469–76. doi: 10.1016/s0002-9394(00)00710-8. [DOI] [PubMed] [Google Scholar]
- 9.Raiji VR, Palestine AG, Parver DL. Scleritis and systemic disease association in a community-based referral practice. Am J Ophthalmol. 2009;148:946–50. doi: 10.1016/j.ajo.2009.07.021. [DOI] [PubMed] [Google Scholar]
- 10.Kumar A, Ghose A, Biswas J, Majumder PD. Clinical profile of patients with posterior scleritis: A report from Eastern India. Indian J Ophthalmol. 2018;66:1109–12. doi: 10.4103/ijo.IJO_121_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dutta Majumder P, Ghose A, Chidambaram M, Ganesh SK, Biswas J. Clinical profile of patients with necrotizing scleritis in a tertiary eye care center in southern. India Ocul Immunol Inflamm. 2016:1–5. doi: 10.1080/09273948.2016.1223857. [DOI] [PubMed] [Google Scholar]
- 12.Bin Ismail MA, Lim RHF, Fang HM, Wong EPY, Ling HS, Lim WK, et al. Ocular autoimmune systemic inflammatory infectious study (OASIS)-report 4: Analysis and outcome of scleritis in an East Asian population. J Ophthalmic Inflamm Infect. 2017;7:6. doi: 10.1186/s12348-017-0124-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shoughy SS, Jaroudi MO, Tabbara KF. Clinical manifestations and outcome of tuberculous sclerokeratitis. Br J Ophthalmol. 2016;100:1301–3. doi: 10.1136/bjophthalmol-2015-307599. [DOI] [PubMed] [Google Scholar]
- 14.Chansangpetch S, Manassakorn A, Laksanaphuk P, Reinprayoon U. Case report: Atypical presentation of mycobacterium tuberculosis uveitis preceding nodular scleritis. BMC Infect Dis [Internet] 2015;15:476. doi: 10.1186/s12879-015-1221-4. Available from: https://wwwncbinlmnihgov/pmc/articles/PMC4625575/ Last cited on 2019 Oct 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vargas-Kelsh JG, Fonollosa A, Artaraz J, del Rio R, Silva-Ribeiro AR, Ruiz-Arruza I. Anterior tuberculous scleritis: A diagnostic challenge. Arch Soc Espanola Oftalmol. 2015;90:585–7. doi: 10.1016/j.oftal.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 16.Bathula BP, Pappu S, Epari SR, Palaparti JB, Jose J, Ponnamalla PK. Tubercular nodular episcleritis. Indian J Chest Dis Allied Sci. 2012;54:135–6. [PubMed] [Google Scholar]
- 17.Biswas J, Aparna AC, Annamalai R, Vaijayanthi K, Bagyalakshmi R. Tuberculous scleritis in a patient with rheumatoid arthritis. Ocul Immunol Inflamm. 2012;20:49–52. doi: 10.3109/09273948.2011.628195. [DOI] [PubMed] [Google Scholar]
- 18.Sainz de la Maza M, Molina N, Gonzalez-Gonzalez LA, Doctor PP, Tauber J, Foster CS. Scleritis therapy. Ophthalmology. 2012;119:51–8. doi: 10.1016/j.ophtha.2011.07.043. [DOI] [PubMed] [Google Scholar]


