Abstract
Purpose:
Serpiginous choroiditis (SC) is primarily an inflammation of choriocapillaris leading to nonperfusion. A quantitative assessment of choriocapillaris perfusion can be done by measuring the flow-density by OCT-Angiography (OCTA). This study measures a change in the flow-density of choriocapillaris with the resolution of inflammation.
Methods:
The OCTA images of a choriocapillaris slab of 30 eyes with active SC were subjected to binarization and vessel density was measured at baseline and final visits and compared.
Results:
Upon comparing the vessel density of the affected areas by OCTA of choriocapillaris-slab at baseline and final visits, there was statistically significant (P < 0.0001) improvement after the resolution of inflammation. The vessel density of a demarcated normal area was significantly higher when compared to that of lesions at baseline (P < 0.0001) and final visit (P < 0.0001).
Conclusion:
OCTA is a useful tool to assess reduction in the inflammatory activity on treatment in SC. This study shows that with treatment the perfusion of choriocapillaris improves; however, it remains lower than normal.
Keywords: OCT angiography, OCT angiography vessel density, OCTA serpiginous choroiditis, serpiginous choroiditis, serpiginous choroiditis vessel density
Serpiginous choroiditis (SC) is a rare form of bilateral, chronic, or recurrent posterior uveitis that is thought to primarily involve the choriocapillaris, with subsequent involvement of the retinal pigment epithelium (RPE) and outer retina.[1,2] Currently, SC is diagnosed based on the clinical appearance and characteristic changes of active lesions using fundus autofluoroscence (FAF), fluorescein angiography (FA) with indocyanine green angiography (ICGA) as an adjunct that can identify subclinical lesions.[1] Optical coherence tomography angiography (OCTA) is a noninvasive imaging technique that generates signals from the movement of intravascular blood cells that are processed to create maps of the vasculature of the choroid and retina. Therefore, its role is being established in SC as it is able to identify areas of reduced choroidal perfusion indicative of active choroiditis and these areas can then be quantified with high repeatability and reproducibility. OCTA can detect ischemia at the level of the choriocapillaris, reperfusion in the form of reemergence of choriocapillaris on the resolution of inflammation, and occurrence of neovascularization in old or scarred lesions.[3,4] The decision to treat, however, is based on the activity on FAF. Although FAF, which is an indirect indicator of RPE function, can monitor disease progression in SC, it cannot provide information regarding the choroidal circulation in this disease which primarily involves the choriocapillaris followed by RPE. The aim of our study was to use OCTA to measure the vessel density of active choroiditis lesions and then comparing with the same areas at the remission stage of inflammation in SC.
Methods
The study was conducted at Medical Research Foundation, Chennai, a tertiary eye care center in Southern India between October 2017 and May 2019. A retrospective chart review of the medical records of consecutive patients diagnosed with serpiginous choroiditis examined between October 1, 2017, and May 31, 2019, was performed. The study was performed in accordance with the tenets of Helsinki declaration and was approved by the Ethics Review Committee at Vision Research Foundation, Sankara Nethralaya, Chennai.
All patients underwent standard examination and the data of demographic details, best-corrected visual acuity, intraocular pressure, slit lamp examination, and dilated funduscopic examination were gathered at baseline and final follow-up. All the patients were evaluated by senior uveitis specialists and the investigations were analyzed in detail by them.
Patients diagnosed with SC involving posterior pole having complete multimodal imaging, consisting of color fundus (CF) photography, FAF, FA, ICGA, Spectral Domain-OCT (SD-OCT), and OCTA at least at baseline visit were included in the analysis. All selected patients underwent an evaluation to rule out common infectious and inflammatory causes, that included laboratory testing with a Mantoux test, an Interferon-G release assay for tuberculosis (QuantiFERON-TB Gold, Qiagen, Hilden, Germany), serum angiotensin converting enzyme (ACE) levels, and a high-resolution computed tomography (HRCT) of chest and systemic evaluation by a general physician. All patients were treated with oral and/or intravenous steroids.
Eyes with active lesions at baseline visit showing complete or partial resolution at final visit were included in the study. Eyes with scarred lesions or inactive lesions at baseline, reactivation of choroiditis at follow-up and those with media opacification, poor fixation precluding OCTA at follow-up were excluded from the study. Eyes with age-related macular degeneration or chorioretinal diseases such as toxoplasma chorioretinitis, multiple evanescent white dot syndrome, birdshot chorioretinopathy, and acute posterior multifocal placoid pigment epitheliopathy were excluded. Patients with an underlying diagnosis suggestive of tuberculosis were excluded from the study.
CF and FAF were performed using an FF450Plus (Carl Zeiss Medica, Zens, Germany). FA and ICGA were performed using Spectralis Heidelberg retinal angiography (Heidelberg Engineering, Heidelberg, Germany). High-resolution SD-OCT (Macular Cube 512 × 128 pattern) examinations were performed using Zeiss Cirrus (Cirrus SD-OCT; Carl Zeiss, Dublin, CA). Based on multimodal imaging, the activity of lesions was determined at baseline and final follow up visit. On FAF, the active lesions were defined as hypoautofluorescent areas with fuzzy margins and inactive areas as hyperautofluorescent areas with sharp borders. On FA, active lesions were defined as early hypoflourescence with late hyperfluorescent fuzzy edges and inactive lesions as those showing late staining with sharp borders. Activity in SC was defined as dark hypocyanescent areas with fuzzy margins in the early phase of ICGA. Healed areas were defined as hypocyanescent areas with sharp borders on ICGA. Partially resolved lesions were defined as those having the presence of features of activity with either reduction in the size of active area or presence of features of both active and healed lesions. This was assessed both clinically and with FAF at every visit.
OCTA was performed using AngioPlex™ CIRRUS™ HD-OCT model 5000 with a scanning area of 8 x 8 mm centered on the foveal center. The minimum signal strength required was 8/10. AngioPlex™ uses optical microangiography (OMAG) algorithm with a central wavelength of 840 nm and an A-scan speed of 68 kHz. The automatic segmentation provided by the OCTA software uses a 30-μm-thick slab starting 19 μm posterior to the automated RPE segmentation which was adjusted manually when necessary by modulating the segmentation lines for correct visualization of the choriocapillaris. Automated removal of projection artifacts was done in all images. Each B-scan was repeated twice at the same position.
For the evaluation of vascular density and change in size of lesion, all OCTA images were analyzed with the ImageJ V.1.48 (National Institutes of Health, Bethesda, Maryland, USA) software. All images were subjected to image thresholding and binarization using the Otzu's thresholding. The images were first standardized to have a normal distribution of pixel intensity in order to make all images have a comparable pixel-intensity distribution by normalizing the brightness intensity of histogram similar to modifications used by Al-Sheikh et al.[5] Choriocapillaris-slab images were overlaid with enface images at superficial and deep capillary plexus to align and eliminate the artefacts from superficial layers.
An area of interest was marked at the level of the choriocapillaris by a single observer fellowship trained in medical retina and uveitis on the OCTA image at baseline corresponding to the area of active inflammation based on multimodal imaging [Fig. 1]. This same area was marked on the OCTA image at final follow up and vessel density was compared in both visits. Vessel density was calculated as the ratio of white pixels over the total number of pixels of the area selected for analysis [Fig. 2]. Another unaffected area of dimensions 300 μm2 was also marked for all scans at baseline visit and vessel density was calculated in the same for comparison with the affected area [Fig. 3].[3]
Figure 1.
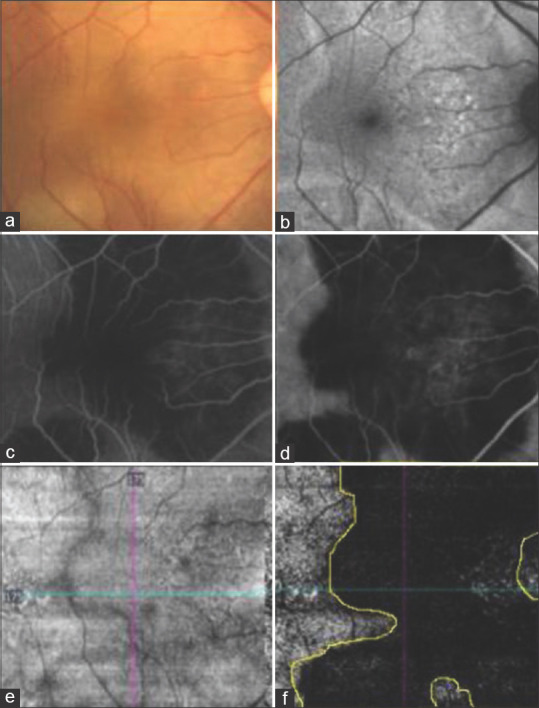
(a) Color fundus photograph of the right eye showing active SC involving posterior pole. (b) Fundus autofluorescence image showing hyperautofluoroscent margins. (c) Fundus fluorescein angiography in the arteriovenous phase showing hypofluorescent lesion with early leakage. (d) Indocyanine green angiography image showing hypocyanescent lesion. (e) Enface OCT angiography image at the CC slab showing the absence of any projection artefacts. (f) OCT angiography showing flow at CC slab with the demarcated area of interest based on activity in CF, FAF, FFA and ICGA
Figure 2.
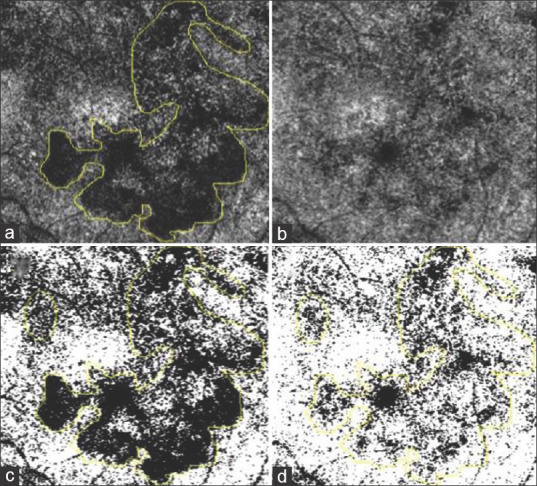
(a) The marked area of interest on choriocapillaris slab in OCT-A at first visit. (b) OCT-A image of choriocapillaris slab at final visit. (c) Image A converted into binary via Otzu thresholding with a marked area of interest. (d) Image B converted into binary via Otzu thresholding with marked area of interest
Figure 3.
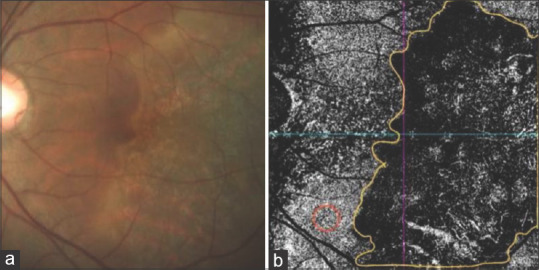
(a and b) Color fundus photograph and OCT-A of choriocapillaris slab at first visit. Area of interest are marked in yellow for measuring flow density. Red marked area is a normal area for measuring normal flow density
Primary outcome was analyzed quantitatively in two aspects. Firstly, to compare the vessel density of choroiditis lesions at baseline and final follow-up. Secondly, to compare it with the vessel density of an unaffected area using OCTA. The secondary outcome was a descriptive change in lesions from baseline to final follow-up as seen on OCT-A.
Statistical analyses were performed using SPSS Statistics V.20 (IBM, Armonk, New York, USA). The results of descriptive analyses are expressed as mean ± SD for quantitative variables, and as counts (percentages) for categorical variables. Fisher exact test was used to compare binary variables. The comparisons of mean BCVA between subgroups were performed using the Student's independent samples t-test. The comparisons of the vessel density between two visits and that with marked unaffected area was performed using Wilcoxon sign rank test. In all analyses, the P values <0.05 were considered statistically significant.
Results
Thirty eyes of 24 patients (16 males) with a mean age of 32 ± 10 years; range 16–53 years) met the inclusion criteria for this study; 18 patients had unilateral and six had bilateral involvement. The median BCVA at presentation was 0.2 logMAR units (range 0-0.57 logMAR units) and at final follow up visit was 0 log MAR units (range 0-0.2 logMAR units). All 30 eyes had active lesions at baseline visit of which at final follow up, eight eyes had healed lesions and 22 eyes had persistent but reduced activity. The mean duration of follow-up was 121.5 ± 66.7 days (range 45 to 283 days).
Qualitative analysis
The active lesions of SC had flow void defined as areas with the absence of flow signals[6] at the level of choriocapillaris suggestive of reduced choriocapillaris flow. As the lesions healed the choriocapillaris became visible, the large choroidal vessels continued to be visible in both active and healed lesions on OCTA. When compared with the normal area in OCTA, the choriocapillaris flow was significantly reduced in the active lesions [Fig. 4]. The first visit in all cases showed active inflammation confirmed by FA, ICG, and FAF. 26 (86.7%) eyes showed flow void lesions at baseline while only 10 (33.3%) lesions showed void lesions at follow-up.
Figure 4.
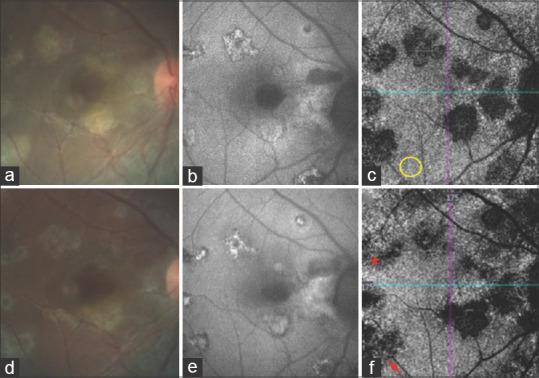
(a) CF at first visit showing active SC. (b) Corresponding FAF image with lesions having hypoautofluorescent margins. (c) Corresponding OCTA of CCslab showing flow voids colocalizing with active areas on CF and FAF. Yellow circle is an area of normal flow. (d) CF at final visit showing the clinical resolution with flattening of lesions. (e) FAF image at final visit with lesions having hyperautofluorescent margins. (f) OCTA of CCslab at final visit showing a reduction in the size of flow void areas with the reemergence of choriocapillaris (asterisk) and the presence of large choroidal vessels at the base of few lesions (arrow)
Quantitative analysis
On quantitative analysis of choroiditis lesions on OCTA, the mean vessel density in the demarcated area at baseline visit was 27.68 ± 6.20 and that at final follow-up in the same demarcated area was 48.52 ± 14.12. This change was statistically significant (P < 0.0001, Z = -4.576). A specific 300 μm area was demarcated in the unaffected zone, the mean vessel density of which was found to be 81.33 ± 10.13, which was significantly higher when compared to the vessel density of lesions at baseline visit (P < 0.0001, Z = -6.653) and also at final followup (P < 0.0001, Z = -6.372).
Discussion
SC is an inflammation of the choriocapillaris and an underlying choriocapillaris nonperfusion is recognized as the most probable pathogenic mechanism.[7] OCTA has been established as a useful tool in SC because of its ability to visualize the choroidal vascular network. It has previously been noted that in diseases with choroidal inflammation, the OCTA of the choriocapillaris shows clear areas of flow void which precisely colocalize with the hypofluorescent areas on ICGA consistently.[2,8] As the lesions heal, these flow void areas reduce and the preserved choriocapillaris and choroidal vessels become prominent. This is hypothesized to be due to the lack of decorrelation signal on OCTA as a result of the choroidal ischaemia. Studies in retinal vasculitis have shown that when there is leakage of plasma from an inflamed vessel, it may lead to a decrease in the flow inside a vessel and OCTA may not detect the retinal capillary flow if it is outside the range of 0.3 mm/s to 2 mm/s.[9] Similarly, it must be noted that in SC, the inflammatory edema could reduce the flow making it difficult to visualize the vessels in active inflammation. With the resolution of inflammation and the associated edema, the flow in the underlying choroidal vessels and the choriocapillaris becomes visible again. However, the absence of shadowing in the enface slabs suggest that these are areas of true choroidal ischemia rather than inflammatory edema.[10] In inactive or healed lesions, there is still some flow visible in the larger choroidal vessels and these areas are presumed to be devoid of choriocapillaris with reduced vessel density. The objective quantification of this flow void or the corresponding flow density has the potential to become a biomarker for these diseases involving the choriocapillaris.[11] The confirmation of inactivity by comparing these demarcated areas with FAF or ICGA establishes that the residual areas of flow void in a healed lesion are the areas of true ischemia.
In this study, an area of interest is compared qualitatively and quantitatively in its active stage and on followup. With the quantification of these areas, this study objectively assessed the areas of inflammation and their subsequent resolution. The study also compares the choroidal vessel density in the inactive areas with the normal areas and establishes the presence of persistent reduced vessel density or ischemia on resolution of inflammation.
In this study, we analyzed and compared the choriocapillaris flow density in the active and remission stages of SC and found a statistically significant difference. Previous studies have either analyzed the areas of nonperfusion qualitatively[1,2] or quantitatively for a single visit.[3] Montorio et al.[3] have analyzed the choroidal vessel density in the different regions of active and inactive lesions at a single visit. We analyzed each OCTA image with standard imaging techniques like FAF, FA, and ICGA at the initial visit to confirm the diagnosis and area of activity of the disease and again at the final visit to confirm a reduction or complete inactivity of the lesion.
The study compared areas demarcated on OCTA corresponding to those on ICGA and FAF both in the active and remission stages. Binarized imaging was able to objectively quantify this in the form of vessel density and compare a change in choroidal ischemia. The observer error was limited by using the same observer and identical area of measurement for both baseline and final visits. This study could identify areas where the choriocapillaris and medium sized choroidal vessels became visible in a healed lesion [Fig. 4]. Mandadi et al.[12] have also shown that in comparison to ICGA, OCTA is better enabled, in some cases, to distinguish between choriocapillaris atrophy and choriocapillaris hypoperfusion.
The limitation of OCTA in this aspect lies in its ability to image only areas within the arcades whereas FAF and ICGA can better assess the peripheral lesions. With the advent of wide-field OCTA imaging, this limitation can be overcome. Our study has its limitations due to its retrospective nature. Also with SD-OCTA, we could only analyze within an area of 8 × 8 mm inside the arcade, where many lesions could only partially be analyzed [Fig. 1]. Further studies are needed to correlate these changes demonstrated by OCTA with the visual outcome after treatment.
Conclusion
OCTA is a useful tool to assess the activity of serpiginous choroiditis. It can ascertain the perfusion or its deficit in an inactive lesion. It can aid as an adjunct to FAF in determining the persistence of activity on followup.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Desai R, Nesper P, Goldstein DA, Fawzi AA, Jampol LM, Gill M. OCT angiography imaging in serpiginous choroidopathy. Ophthalmol Retina. 2018;2:351–9. doi: 10.1016/j.oret.2017.07.023. [DOI] [PubMed] [Google Scholar]
- 2.Herbort CP., Jr Serpiginous choroiditis imaged by optical coherence tomography angiography. Retin Cases Brief Rep. 2018;12:279–85. doi: 10.1097/ICB.0000000000000512. [DOI] [PubMed] [Google Scholar]
- 3.Montorio D, Giuffrè C, Miserocchi E, Modorati G, Sacconi R, Mercuri S, et al. Swept-source optical coherence tomography angiography in serpiginous choroiditis. Br J Ophthalmol. 2018;102:991–5. doi: 10.1136/bjophthalmol-2017-310989. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Q, Zheng F, Motulsky EH, Gregori G, Chu Z, Chen CL, et al. A novel strategy for quantifying choriocapillaris flow voids using swept-source OCT angiography. Invest Ophthalmol Vis Sci. 2018;59:203–11. doi: 10.1167/iovs.17-22953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Sheikh M, Falavarjani KG, Pfau M, Uji A, Le PP, Sadda SR. Quantitative features of the choriocapillaris in healthy individuals using swept-source optical coherence tomography angiography. Ophthalmic Surg Lasers Imag Retina. 2017;48:623–31. doi: 10.3928/23258160-20170802-04. [DOI] [PubMed] [Google Scholar]
- 6.Lauermann JL, Eter N, Alten F. Optical coherence tomography angiography offers new insights into choriocapillaris perfusion. Ophthalmologica. 2018;239:74–84. doi: 10.1159/000485261. [DOI] [PubMed] [Google Scholar]
- 7.Khanamiri HN, Rao NA. Serpiginous choroiditis and infectious multifocal serpiginoid choroiditis. Surv Ophthalmol. 2013;58:203–32. doi: 10.1016/j.survophthal.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pichi F, Aggarwal K, Neri P, Salvetti P, Lembo A, Nucci P, et al. Choroidal biomarkers. Indian J Ophthalmol. 2018;66:1716–26. doi: 10.4103/ijo.IJO_893_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pichi F, Sarraf D, Morara M, Mazumdar S, Neri P, Gupta V. Pearls and pitfalls of optical coherence tomography angiography in the multimodal evaluation of uveitis. J Ophthalmic Inflamm Infect. 2017;7:20. doi: 10.1186/s12348-017-0138-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pakzad-Vaezi K, Khaksari K, Chu Z, Van Gelder RN, Wang RK, Pepple KL. Swept-source OCT angiography of serpiginous choroiditis. Ophthalmol Retina. 2018;2:712–9. doi: 10.1016/j.oret.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao SS, Jia Y, Zhang M, Su JP, Liu G, Hwang TS, et al. Optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2016;57:27–36. doi: 10.1167/iovs.15-19043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mandadi SK, Agarwal A, Aggarwal K, Moharana B, Singh R, Sharma A, et al. OCTA Study Group. Novel findings on optical coherence tomography angiography in patients with tubercular serpiginous-like choroiditis. Retina. 2017;37:1647–59. doi: 10.1097/IAE.0000000000001412. [DOI] [PubMed] [Google Scholar]


