Abstract
Tubercular granulomas are a common manifestation of intraocular tuberculosis. These are said to be hypoxic granulomas with increased expression of vascular endothelial growth factor (VEGF). Management of these granulomas includes a combination of antitubercular therapy (ATT) and oral corticosteroids. We report a case of tubercular granuloma with exudative retinal detachment which was treated with weekly intravitreal anti-VEGF and antibiotic injections along with ATT and corticosteroids. The VEGF levels measured paralleled with the clinical regression of the granuloma.
Keywords: Anti-VEGF drug, bevacizumab, tubercular granuloma, vascular endothelial growth factor, VEGF
Tubercular granulomas are the most well-recognized sign of intraocular tuberculosis (IOTB).[1] They are said to be hypoxic granulomas associated with high vascularity and angiogenesis due to increased vascular endothelial growth factor (VEGF) production.[1] Pharmacological inhibition of the VEGF pathway has shown to decrease the infection load, reduce angiogenesis, and limit the spread of infection.[2,3] Management of these granulomas includes a combination of antitubercular therapy (ATT) and oral corticosteroids.[1] Case reports by Babu et al. and Bansal et al. have highlighted the use of anti-VEGF drugs as an adjunct to systemic therapy.[4,5] Our patient with multiple bilateral tubercular granulomas was treated with ATT, oral corticosteroids along with weekly intravitreal anti-VGEF bevacizumab and moxifloxacin injections. The intraocular VEGF levels were measured after every intravitreal injection and correlated with the clinical response.
We report this case to highlight the need for weekly anti-VEGF injections to decrease the excessive VEGF load produced by the tubercular granuloma and the quantitative correlation between intraocular VEGF levels and the clinical regression of the granuloma.
Case Report
A 23-year-old female presented with insidious onset of diminution of vision in the left eye for the last 3 months. There was a history of hospitalization 3 months back for severe headache and altered consciousness when she was diagnosed with pulmonary and cerebral tuberculosis. She underwent a cartridge-based nucleic acid amplification test which showed rifampicin sensitivity and was subsequently started on four-drug ATT.
The best-corrected visual acuity (BCVA) in the right eye was 6/6, N6 and hand movements in the left eye. Anterior segment examination was normal in both the eyes and the intraocular pressure was 17 mmHg in the right eye and 19 mmHg in the left eye (Goldmann applanation tonometer). Fundus examination of the right eye showed multiple small yellowish lesions suggestive of choroidal tubercles with one tubercle approximately half-disc diameter in size, superonasal to the disc surrounded with chorioretinal atrophy (CRA) [Fig. 1a]. Left eye fundus showed a subretinal elevated yellowish lesion suggestive of a tubercular granuloma temporal to the macula measuring two-disc diameter in size with exudative retinal detachment superiorly and surrounding subretinal fluid (SRF) extending till the macula. Two smaller granulomas were seen inferotemporal to the macula and in the inferior quadrant. A small choroidal tubercle was noted in the superonasal quadrant [Fig. 1b].
Figure 1.
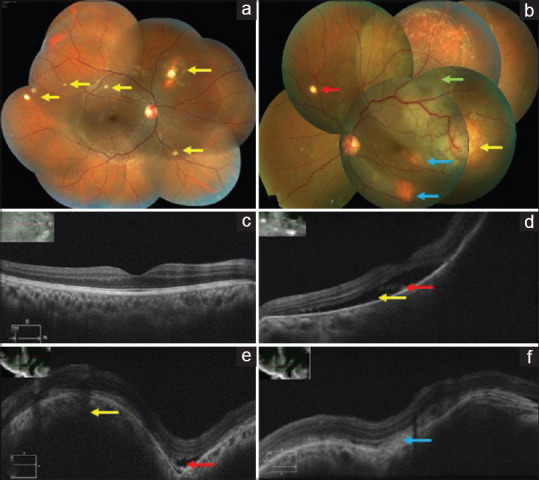
(a) Right eye color fundus montage photo showing multiple choroidal tubercles (yellow arrows). (b) Left eye color fundus montage photo showing a tubercular granuloma temporal to the macula two-disc diameter in size (yellow arrow) with superior exudative retinal detachment (green arrow) and SRF extending till the macula. Two smaller granulomas noted inferotemporal to the macula and in the inferior quadrant (blue arrows). A single choroidal tubercle noted in the superonasal quadrant (red arrow). (c) Right eye OCT scan showing normal foveal contour. (d) Left eye OCT macular scan showing SRF (yellow arrow) with shaggy photoreceptors (red arrow). (e and f) Left eye OCT scan through the granuloma temporal to the macula showing an elevation of the retinal layers with an underlying choroidal bump (yellow arrow) and a small pocket of SRF (red arrow) and subretinal fibrin (blue arrow)
Optical coherence tomography (OCT) of the right eye showed normal foveal contour. Left eye OCT showed SRF at the macula, and the scan passing through the granuloma temporal to the macula showed an elevation of the retinal layers with an underlying choroidal bump and a small pocket of SRF [Fig. 1c-f].
Mantoux test was positive with an induration of 30 × 35 mm. HIV 1 and 2 was nonreactive. The blood counts were normal with a raised erythrocyte sedimentation rate of 23 mm/h. Contrast-enhanced computed tomography (CECT) of the chest showed subpleural centrilobular, nodular, patchy, and ill-defined opacities with ground glass haze in the lateral segment of the left lower lobe, anterior segment of the left upper lobe, and apical segment of the right upper lobe suggestive of tuberculomas. Subcentrimetric lymph nodes were noted in pretracheal, subcarinal, and right hilar regions suggestive of infective etiology. Noncontrast CT of the brain showed multiple ring-like enhancing lesions with surrounding edema suggestive of tubercular granulomas in both the cerebral hemispheres.
After an informed consent, she underwent an intravitreal injection of anti-VEGF drug bevacizumab (1.25 mg/0.05 mL) (off label use) with moxifloxacin (500 μg/0.1 mL) (off label use) in the left eye under topical anesthesia and strict aseptic precautions. An anterior chamber tap was done in order to lower the intraocular pressure and aqueous humor sample (0.1–0.2 mL) thus collected was sent for VEGF level analysis. VEGF-A levels were calculated by sandwich enzyme-linked immunosorbent assay by using human VEGF antibody pair kit {Invitrogen; 10 plate Format; Lot#*: 650073; Catalog # CHG0113}. The linear range of detection was 2–2000 pg/mL (picograms/millilitre). VEGF concentration was recorded as 1180.70 pg/mL. She was continued on ATT and oral prednisolone (1 mg/kg/day) was added after taking clearance by the treating pulmonologist who discussed the risks and benefits of the therapy with the patient.
Post injection on day one, there was no evidence of any reaction in the left eye. Follow-up at 1 week, BCVA in the left eye was counting fingers close to face. Left eye fundus showed a minimal decrease in the size of the temporal granuloma with a marked decrease in the SRF and an increase in the surrounding CRA [Fig. 2]. A second injection of the same drugs was repeated after 1 week. ATT and oral corticosteroids were continued as before. The VEGF level of the aqueous sample after first injection thus collected measured as 867.81 pg/mL.
Figure 2.
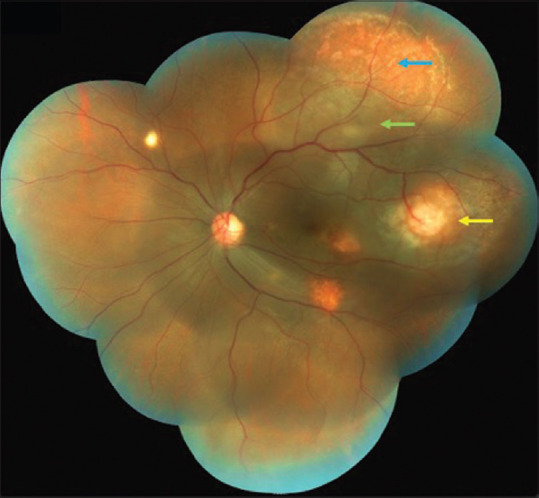
Left eye color fundus montage photo 1 week after the first injection showing a minimal decrease in the size of the temporal granuloma (yellow arrow) with marked decrease in the SRF (green arrow) and an increase in the surrounding CRA (blue arrow)
Post injection on day one, there was no evidence of any reaction in the left eye. Follow-up at 1 week, BCVA in the left eye was 6/60, N36 and the fundus showed further scarring of the temporal granuloma and increasing CRA [Fig. 3a]. Repeat OCT showed a decrease in the height of the choroidal bump [Fig. 3b-d]. Third intravitreal injection was repeated after 1 week. VEGF level of the aqueous sample recorded after two injections was 391.50 pg/mL.
Figure 3.
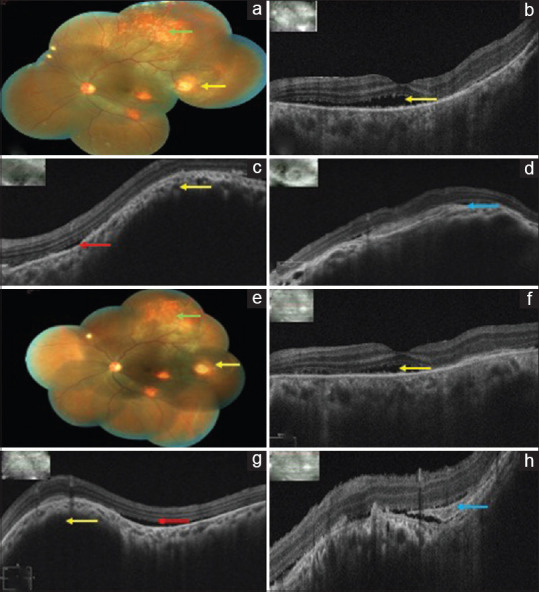
(a) Left eye color fundus montage photo 1 week after the second injection showing scarring of the granuloma (yellow arrow) and increasing CRA (green arrow). (b) Left eye OCT macular scan showing a decrease in the SRF (yellow arrow). (c and d) Left eye OCT scan through the granuloma showing a decrease in the height of the choroidal bump (yellow arrow) with SRF (red arrow) and fibrin (blue arrow). (e) Left eye color fundus montage photo 1 week after the third injection showing further regression of the granuloma (yellow arrow) with increasing pigmentation and scarring (green arrow). (f) Left eye OCT macular scan showing minimum SRF (yellow arrow). (g and h) Left eye OCT scan through the granuloma showing further decrease in the height of the choroidal bump (yellow arrow) with SRF (red arrow) and fibrin (blue arrow)
Post injection on day one, there was no evidence of any reaction in the left eye. Follow-up at 1 week, BCVA in the left eye was 6/24, N24 with further reduction in the size of the granuloma and resolution of the SRF on OCT [Fig. 3e-h].
Follow-up at 1 month, BCVA in the left eye was 6/9p, N6 and the VEGF level was measured as 255.61 pg/mL from the aqueous humor sample. Left eye fundus showed regressed granulomas without any evidence of SRF on OCT [Fig. 4]. She continued to remain stable on regular follow-ups. Follow-up at 6 months, BCVA was 6/6, N6 in both the eyes and the fundus did not show any evidence of active lesions. ATT was stopped on completion of 9 months by the treating pulmonologist after a thorough systemic evaluation. At 1-year follow-up, she continued to maintain a BCVA of 6/6, N6 in both the eyes without any recurrence in either eye.
Figure 4.
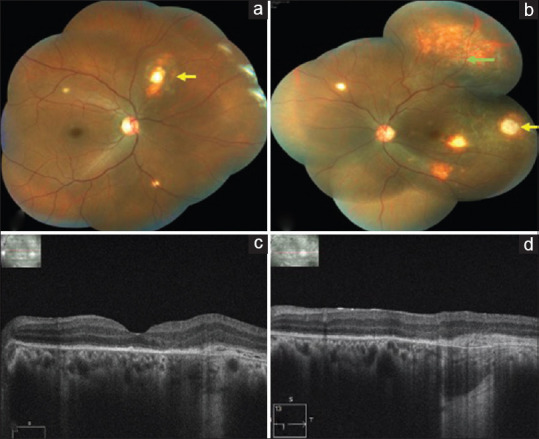
(a) Right eye color fundus montage photo showing regressed choroidal tubercles (yellow arrows). (b) Left eye color fundus montage photo 1 month after the third injection showing complete regression of the granuloma (yellow arrow) with scarring and resolved exudative retinal detachment (green arrow). (c) Left eye OCT macular scan showing complete resorption of SRF. (d) Left eye OCT scan through the granuloma showing flattening of the RPE with complete resolution of SRF
Discussion
Tuberculosis is a chronic bacterial infection caused by Mycobacterium tuberculosis.[1] The classic histopathological finding is epithelioid cell granuloma with central area of caseation necrosis.[1] Ocular involvement has been reported in 6.8% of the patients with pulmonary TB (PTB).[6] IOTB is a form of extrapulmonary TB presenting as uveitis in TB-endemic countries like India.[7]
Localized tissue hypoxia is found in tubercular necrotic granulomas.[8,9] In response to the hypoxia, there is an upregulation of VEGF which is a known biomarker for active TB disease in pulmonary as well as extrapulmonary sites.[10] Studies have found VEGF as a useful biomarker to monitor disease severity, bacterial burden, and therapeutic responses in PTB.[10,11] Thayil et al. showed that the tubercular granulomas have reduced oxygen tension, with increased expression of VEGF in the retinal pigment epithelium (RPE) and photoreceptors.[2] Datta et al. demonstrated that the anti-VEGF drug bevacizumab decreases hypoxia and promotes vascular normalization in TB granulomas.[12] Bansal et al. and Jain et al. have used anti-VEGF therapy to treat tubercular granulomas which were highly vascular or associated with either exudation or massive serous retinal detachments or showing poor response to conventional ATT and corticosteroids.[5,13]
Our patient had multiple nonresolving tubercular granulomas with persisting SRF despite being on ATT for the last 3 months leading to gradual progressive deterioration of vision. She was treated with three weekly intravitreal injections of bevacizumab and moxifloxacin along with corticosteroids as an adjunct to the ongoing ATT. Intravitreal moxifloxacin has been used for the management of bacterial endophthalmitis.[14] Moxifloxacin belongs to the fluoroquinolone group which are second-line ATT drugs.[15] Keeping this in mind, we gave a combination of intravitreal bevacizumab and moxifloxacin for treating the granulomas.
The rationale for repeating the bevacizumab and moxifloxacin injections every week can be explained by the following: first, the level of VEGF in the aqueous sample of a patient with a single choroidal neovascular membrane (CNVM) has been reported between 74.5 and 521.6 pg/mL; however, the load of the VEGF produced by the multiple granulomas is likely to be much higher.[16] In our patient, we recorded the VEGF level as high as 1180.70 pg/mL which is approximately twice as high as in a patient with CNVM. The VEGF level reduced consistently on giving repeat anti-VEGF injections. On complete regression of the granulomas, the value of the VEGF level dropped to 255.61 pg/mL. The second reason being that the half-life of intravitreal bevacizumab in nonvitrectomized eyes varies between 2.5 and 7.3 days with a mean of 4.9 days.[17] However, it may be shortened further due to the breakdown of the blood retinal barrier secondary to inflammation leading to early washout of the drugs.
Conclusion
We report this case to show the quantitative decrease in the intraocular VEGF levels which paralleled with the clinical regression of the granulomas and the resolution of the SRF in response to the intravitreal anti-VEGF injections. We added moxifloxacin which may have had an adjunctive role as a second-line ATT to decrease the bacterial load.
This case also highlights the fact that there may be a need of more frequent administration of intravitreal anti-VEGF injections due to a much higher VEGF load in tubercular granulomas in comparison to treating a CNVM which requires monthly injections.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Gupta V, Gupta A, Rao NA. Intraocular tuberculosis-an update. Surv Ophthalmol. 2007;52:561–87. doi: 10.1016/j.survophthal.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 2.Thayil SM, Albini TA, Nazari H, Moshfeghi AA, Parel JM, Rao NA, et al. Local ischemia and increased expression of vascular endothelial growth factor following ocular dissemination of Mycobacterium tuberculosis. PLoS One. 2011;6:e28383. doi: 10.1371/journal.pone.0028383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oehlers SH, Cronan MR, Scott NR, Thomas MI, Okuda KS, Walton EM, et al. Interception of host angiogenic signalling limits mycobacterial growth. Nature. 2015;517:612–5. doi: 10.1038/nature13967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Babu K, Murthy P, Murthy K. Intravitreal bevacizumab as an adjunct in a patient with presumed vascularised choroidal tubercular granuloma. Eye. 2010;24:397–9. doi: 10.1038/eye.2009.83. [DOI] [PubMed] [Google Scholar]
- 5.Bansal R, Beke N, Sharma A, Gupta A. Intravitreal bevacizumab as an adjunct in the management of a vascular choroidal granuloma. BMJ Case Rep. 2013;2013 doi: 10.1136/bcr-2013-200255. bcr2013200255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lara LP, Ocampo V., Jr Prevalence of presumed ocular tuberculosis among pulmonary tuberculosis patients in a tertiary hospital in the Philippines. J Ophthalmic Inflamm Infect. 2013;3:1. doi: 10.1186/1869-5760-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta A, Sharma A, Bansal R, Sharma K. Classification of intraocular tuberculosis. Ocul Immunol Inflamm. 2015;23:7–13. doi: 10.3109/09273948.2014.967358. [DOI] [PubMed] [Google Scholar]
- 8.Via LE, Lin PL, Ray SM, Carrillo J, Allen SS, Eum SY, et al. Tuberculous granulomas are hypoxic in guinea pigs, rabbits, and nonhuman primates. Infect Immun. 2008;76:2333–40. doi: 10.1128/IAI.01515-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsai MC, Chakravarty S, Zhu G, Xu J, Tanaka K, Koch C, et al. Characterization of the tuberculous granuloma in murine and human lungs: Cellular composition and relative tissue oxygen tension. Cell Microbiol. 2006;8:218–32. doi: 10.1111/j.1462-5822.2005.00612.x. [DOI] [PubMed] [Google Scholar]
- 10.Matsuyama W, Hashiguchi T, Matsumuro K, Iwami F, Hirotsu Y, Kawabata M, et al. Increased serum level of vascular endothelial growth factor in pulmonary tuberculosis. Am J Respir Crit Care Med. 2000;162(3 Pt 1):1120–2. doi: 10.1164/ajrccm.162.3.9911010. [DOI] [PubMed] [Google Scholar]
- 11.Kumar NP, Banurekha VV, Nair D, Babu S. Circulating angiogenic factors as biomarkers of disease severity and bacterial burden in pulmonary tuberculosis. PLoS One. 2016;11:e0146318. doi: 10.1371/journal.pone.0146318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Datta M, Via LE, Kamoun WS, Liu C, Chen W, Seano G, et al. Anti-vascular endothelial growth factor treatment normalizes tuberculosis granuloma vasculature and improves small molecule delivery. Proc Natl Acad Sci U S A. 2015;112:1827–32. doi: 10.1073/pnas.1424563112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jain S, Agarwal A, Gupta V. Resolution of large choroidal tuberculoma following monotherapy with intravitreal ranibizumab. Ocul Immunol Inflamm. 2020;28:494–7. doi: 10.1080/09273948.2019.1582786. [DOI] [PubMed] [Google Scholar]
- 14.Jacobs DJ, Grube TJ, Flynn HW, Jr, Greven CM, Pathengay A, Miller D, et al. Intravitreal moxifloxacin in the management of Ochrobactrum intermedium endophthalmitis due to metallic intraocular foreign body. Clin Ophthalmol. 2013;7:1727–30. doi: 10.2147/OPTH.S44212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agrawal R, Gupta B, Gonzalez-Lopez JJ, Rahman F, Phatak S, Triantafyllopoulou I, et al. The role of anti-tubercular therapy in patients with presumed ocular tuberculosis. Ocul Immunol Inflamm. 2015;23:40–6. doi: 10.3109/09273948.2014.986584. [DOI] [PubMed] [Google Scholar]
- 16.Cabral T, Lima LH, Polido J, Duong J, Okuda É, Oshima A, et al. Aqueous vascular endothelial growth factor and clinical outcomes correlation after single intravitreal injection of bevacizumab in patients with neovascular age-related macular degeneration. Int J Retina Vitreous. 2017;3:6. doi: 10.1186/s40942-017-0066-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moisseiev E, Waisbourd M, Ben-Artsi E, Levinger E, Barak A, Daniels T, et al. Pharmacokinetics of bevacizumab after topical and intravitreal administration in human eyes. Graefes Arch Clin Exp Ophthalmol. 2014;252:331–7. doi: 10.1007/s00417-013-2495-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


