A 26-year-old female with exudative retinal detachment due to Vogt–Koyanagi–Harada (VKH) disease presented to us. She had received five doses of intravenous methylprednisolone (1 g/day) followed by oral steroids (80 mg/day) elsewhere. At the presentation to us, she was on oral corticosteroid 40 mg/day and topical corticosteroid. Her visual acuity was counting fingers close to the face in both eyes. Clinical examination revealed a quiet anterior chamber, plenty of cells in anterior vitreous, and persistent bullous retinal detachment with shifting fluid [Fig. 1a and b] in both eyes. In consultation with a rheumatologist, tofacitinib (Xeljanz®) 10 mg/day was instituted along with an oral corticosteroid. At a 1-month follow-up, her visual acuity was 6/15 in both eyes and fundus examination revealed resolution of exudative retinal detachment with coarse retinal pigment epithelium pigmentations throughout the fundus [Fig. 1c and d].
Figure 1.
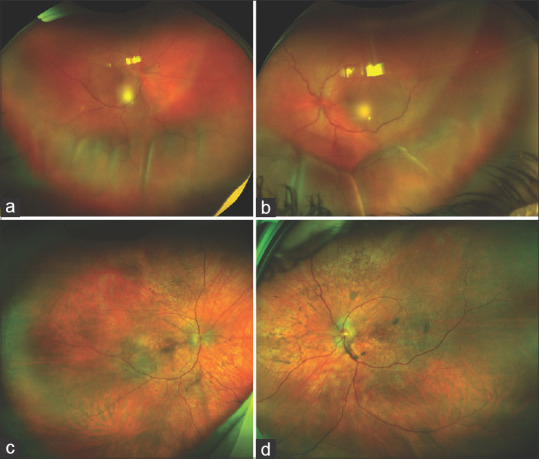
(a and b) fundus photographs of both eyes showing exudative retinal detachment; (c and d) fundus photographs of both eyes showing resolution of exudative retinal detachment in both eyes
Discussion
Tofacitinib, a small-molecule inhibitor of Janus kinase (JAK) 1 and 3, has been increasingly being used for rheumatoid arthritis, psoriatic arthritis, and ulcerative colitis.[1] Being a small molecule, tofacitinib offers the theoretical advantage of crossing the blood-retinal barrier more efficiently and also provide the ease of administration by oral and topical route. Tofacitinib has been reported to be useful in controlling recurrences of human leukocyte antigen (HLA) B27-associated uveitis and in a case of scleritis.[2] There have been no reports on the use of tofacitinib in patients with exudative retinal detachment, refractory to standard immunomodulatory therapy. Role of helper T-cell subsets producing Th1 cytokines including interleukin 2 (IL-2) and interferon-gamma have been implicated in causing pathological changes such as the granulomatous choroid inflammation in the acute phase of VKH.[3,4] Thus, tofacitinib, which can reduce the production of IL-2 may be efficacious in VKH. Thus, tofacitinib can be a useful alternative in treatment-resistant exudative retinal detachment secondary to intraocular inflammation.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Singh JA, Hossain A, Tanjong Ghogomu E, Mudano AS, Maxwell LJ, Buchbinde R, et al. Biologics or tofacitinib for people with rheumatoid arthritis unsuccessfully treated with biologics: A systematic review and network meta-analysis. Cochrane Database Syst Rev. 2017;3:CD012591. doi: 10.1002/14651858.CD012591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paley MA, Karacal H, Rao PK, Margolis TP, Miner JJ. Tofacitinib for refractory uveitis and scleritis. Am J Ophthalmol Case Rep. 2019;13:53–5. doi: 10.1016/j.ajoc.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang J-F, Zhang Y, Hirakawa B. Evaluation of JAK inhibition with topical tofacitinib in an experimental autoimmune uveitis model (EAU) Invest Ophthalmol Vis Sci. 2013;54:2536. [Google Scholar]
- 4.Lavezzo MM, Sakata VM, Morita C, Rodriguez EEC, Abdallah SF, da Silva FTG, et al. Vogt-Koyanagi-Harada disease: Review of a rare autoimmune disease targeting antigens of melanocytes. Orphanet J Rare Dis. 2016;11:29. doi: 10.1186/s13023-016-0412-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


