Abstract
BACKGROUND AND IMPORTANCE
Apraxia of speech is a disorder of articulatory coordination and planning in speech sound production. Its diagnosis is based on deficits in articulation, prosody, and fluency. It is often described concurrent with aphasia or dysarthria, while pure apraxia of speech is a rare entity.
CLINICAL PRESENTATION
A right-handed man underwent focal surgical resection of a recurrent grade III astrocytoma in the left hemisphere dorsal premotor cortex located in the posterior middle frontal gyrus. After the procedure, he experienced significant long-term speech production difficulties. A battery of standard and custom language and articulatory assessments were administered, revealing intact comprehension and naming abilities, and preserved strength in orofacial articulators, but considerable deficits in articulatory coordination, fluency, and prosody—consistent with diagnosis of pure apraxia of speech. Tractography and resection volumes compared with publicly available imaging data from the Human Connectome Project suggest possible overlap with area 55b, an under-recognized language area in the dorsal premotor cortex and has white matter connectivity with the superior longitudinal fasciculus.
CONCLUSION
The case reported here details a rare clinical entity, pure apraxia of speech resulting from resection of posterior middle frontal gyrus. While not a classical language area, emerging literature supports the role of this area in the production of fluent speech, and has implications for surgical planning and the general neurobiology of language.
Keywords: Apraxia of speech, AOS, Premotor cortex, Area 55b, Language deficit
ABBREVIATIONS
- 3D
three-dimensional
- AAPS-3
Arizona Articulation Proficiency Scale, Third Revision
- AOS
Apraxia of speech
- BNT
Boston Naming Test
- DTI
diffusion tensor imaging
- FAT
frontal aslant tract
- MRI
magnetic resonance imaging
- QAB
Quick Aphasia Battery
- SLF
superior longitudinal fasciculus
- WAB
Western Aphasia Battery
Apraxia of speech (AOS) is a disorder of articulatory coordination and planning in speech sound production1,2 and is typically reported secondary to ischemic infarcts. Diagnosis is based on deficits in articulation, prosody, and fluency.3 Almost always, AOS is described coincident with other disorders of speech and language.2,4-6 Pure AOS following a brain injury is rare.4,7-11
Literature around AOS dates back to Broca's original description of what he termed aphemia in the 1860s,12,13 which he localized to left premotor frontal cortex. Most contemporary etiological literature on AOS focuses on vascular infarct or neurodegeneration. Early studies using maximal overlap of AOS-associated lesions from strokes suggested involvement of the insula.2,5 However, some have suggested anatomic areas more susceptible to ischemia create a bias in lesion-deficit association.6,14 Neuroanatomic studies of neurodegenerative AOS point to volume loss in supplementary motor, premotor, and precentral gyri.4,7-11 While rare, cases of pure AOS demonstrate lesions near the precentral gyrus and premotor cortex in the left hemisphere.4,7-11
One cortical area that has re-emerged as a focus of language function is area 55b. The posterior aspect of 55b occupies the medial-lateral midpoint of the precentral gyrus. The anterior aspect of 55b generally occupies the posterior portion of the middle frontal gyrus where it joins the precentral gyrus. That said, 55b is one of the more variable cortical areas, as described in Glasser et al15 and may be found adjacent to the middle frontal gyrus and precentral sulcus. The boundaries of area 55b stem from early 20th century studies segmenting the cortex into ∼200 fields, based on high gradients of myelin content.16 These data have been re-examined more recently in combination with techniques such as magnetic resonance imaging (MRI)-based myelin mapping.16-19 A multimodal neuro-anatomic parcellation study from the human connectome project demonstrated that area 55b is selectively activated in language production tasks, and shows distinct functional connectivity from surrounding cortex.15
Neurosurgical etiology is a critical piece missing from AOS literature. Here, we report the first detailed characterization of pure AOS due to focal surgical resection of left dorsal premotor cortex. This may also represent the first overt documentation of language deficits from a lesion to area 55b.
CLINICAL PRESENTATION
Patient Information and Timeline
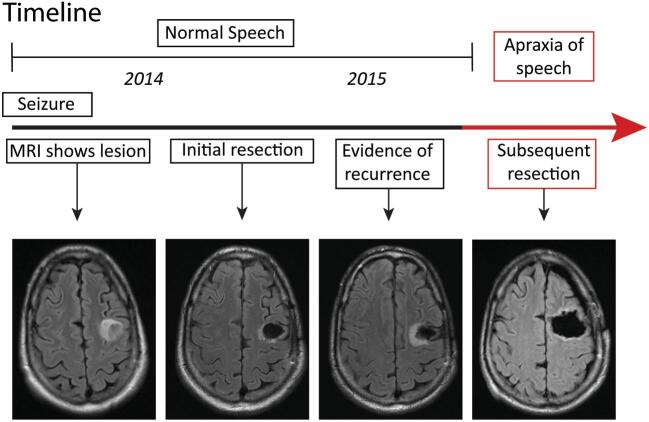
The patient is a 49-yr-old, right-handed male accountant, without significant medical or family history, who initially presented with an unprovoked seizure (Figure 1). MRI demonstrated a nonenhancing mass lesion in posterior middle frontal gyrus. He initially underwent a craniotomy for resection of tumor without incident or residual deficits. Pathology showed WHO grade III astrocytoma. A year later, the tumor showed signs of early recurrence around the rim of the prior resection cavity. The patient underwent an awake craniotomy with speech mapping for resection of tumor. Intraoperative motor and speech mapping were performed and neither demonstrate interruption of speech on counting, naming pictures, or reading tasks nor was there motor arrest during these tasks.
FIGURE 1.
Timeline.
Clinical Findings and Follow-up
Immediately after the procedure, the patient was found to have significant speech production deficits that have persisted for over 3 yr. His comprehension and naming abilities were intact and he has been able to communicate by typing difficult-to-produce words on his phone/tablet.
His communication difficulties suggest a motor speech disorder, but his articulators have preserved muscle strength as demonstrated in formal testing of articulatory musculature, including moving tongue side to side, opening and closing jaw, puffing cheeks, pursing lips, and holding vowel or consonant sounds. This leads to a diagnosis of pure AOS, a disorder involving difficulty of articulation despite having intact language skills and muscular function.1
Diagnostic Assessments
Speech, Language, and Motor Assessments
Testing was administered in March 2018. The tests were (1) Arizona Articulation Proficiency Scale, Third Revision (AAPS-3),20 (2) Western Aphasia Battery (WAB),21 (3) The Quick Aphasia Battery (QAB),22 (4) Boston Naming Test (BNT),23 and (5) customized, nonstandard batteries. The first nonstandard battery was a word/pseudoword repetition task24; the second was a supplementary motor battery.25,26 The patient provided informed consent for data analysis and presentation. Table 1 shows the summary scores from each language test administered. For detailed documentation, see Supplemental Digital Content.
TABLE 1.
Tests Administered and Linguistic/Motor Domains Tested
| Test | Domains tested | Summary score |
|---|---|---|
| AAPS-3 | Naming | 81.5/100 |
| WAB | Fluency, comprehension, repetition, naming | 66.3/100 |
| QAB | Fluency, comprehension, repetition, naming, motor speech | 73.3/100 |
| Boston Naming Task, 15-item (BNT) | Naming | 86.6/100 |
| 50-item word/pseudoword repetition task | Repetition | 62/100 |
| Supplementary motor assessment battery | Motor speech | N/A |
All scores are reported as normalized out of 100.
AAPS-3
The patient scored “severely impaired” or below the second percentile of speakers. The prose description on this score bracket is “speech is intelligible with careful listening.” Aggregate score was 81.5/100.
WAB
Scores were low in fluency (20/100), within normal limits on comprehension (92.5/100), and poor on Repetition (72/100). While the patient's naming ability was near normal, many of the items were scored as partial credit due to apraxic errors (77/100). See Supplemental Digital Content.
QAB
The patient scored 16.6/100 for absence of AOS, indicating severe apraxia. On absence of dysarthria, he scored 83.3/100 (smile slightly asymmetric). As a motor speech assessment, the patient was asked to move his tongue from side to side, say “aah” and sustain, and repeat strings of DDK tokens (eg, /p∧p∧p∧/,/p∧t∧k∧/). The patient had no difficulty with any of these tasks except the complex DDK token (/p∧t∧k∧/), which suggests his articulators are still strong and the difficulty is one of motor coordination as opposed to dysarthria. See Supplemental Digital Content.
BNT
Scores were within normal limits (86.6/100).
Word/Pseudoword Repetition
The patient had increasing difficulty with differential diadochokinetic rate (repeating different syllables, /p∧//t∧//k∧/) than sequential diadochokinetic rate (repeating the same syllable, /p∧//p∧//p∧/). He also had increasing difficulty repeating longer words (for “thick, thicken, thickening”). For oral and manual movements, he scored mostly within normal limits, suggesting normal strength in articulators.
Linguistic Analysis
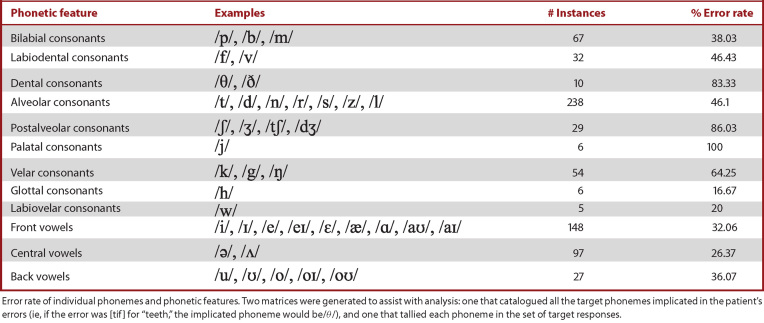
Table 2 shows the error rate of individual phonemes and phonetic features. The highest error rates were associated with palatal (100%), postalveolar (86%), and dental (83%) consonants. For error codes (Table 3), the most common error was incorrect place of articulation (77), followed by incorrect manner of articulation (66) and deletion (58). See tables and Supplemental Digital Content for details on linguistic analyses.
TABLE 2.
Phonetic Features With Corresponding Error Percentages
|
TABLE 3.
Error Codes and Examples
| Error code | # Instances | Example error | Example target |
|---|---|---|---|
| Incorrect place | 77 | pɹeɪn | train |
| Incorrect manner | 66 | ka.bi k∧p | coffee cup |
| Incorrect voicing | 23 | pɪk | pig |
| Incorrect vowel | 43 | bol | ball |
| Deletion | 58 | kol | cold |
| Addition | 30 | hoʊ.pɛ.f∂l | hopeful |
| Metathesis | 6 | nɛts | nest |
Each error was coded using 7 possible error codes. (1) Incorrect place of articulation, (2) manner, (3) voicing, and (4) vowel. (5) Addition was defined as the insertion of extra phonemes or syllables into the target structure, while (6) deletion was the omission of phonemes or syllables from the target structure. (7) Metathesis was coded when the patient transposed phonemes or syllables in the target word.
Neuroanatomy
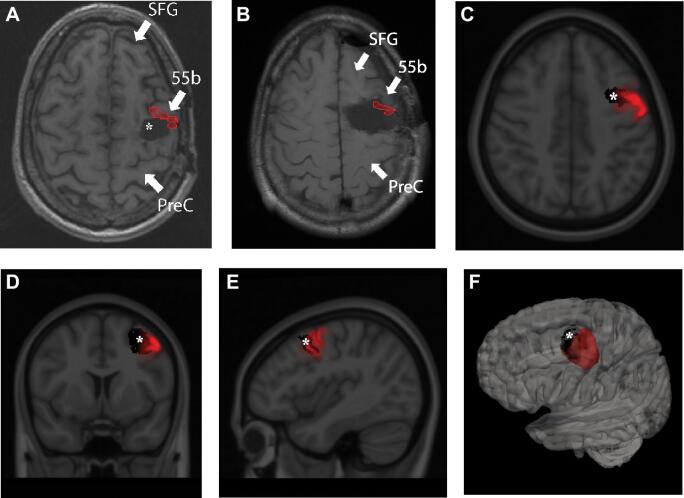
Publicly available imaging data from the Human Connectome Project were obtained for this study (http://humanconnectome.org, release Q3).27-35 See Supplemental Digital Content for location determinates of area 55b.15,36 Reconstructions demonstrating approximations of the shape and location of area 55b are shown in Figure 2. Area 55b is subject to individual anatomic variation37 and our localizing techniques are limited by the patient's nonuniform anatomy status post-resection, the resolution of postoperative imaging, and the neoplasm disrupting native anatomy.
FIGURE 2.
A, Area 55b projected onto axial MRI of the patient prior to the resection causing his AOS. Asterix marks the first resection cavity sparing Area 55b (corresponding to the panel of Figure 1 labelled “initial resection”), which did not cause language deficits. B, Area 55b projected onto the cavity after the resection, which led to AOS deficits. C, Axial, D, coronal, and E, sagittal cuts of the MNI brain co-registered with probability maps of Area 55b (Red) and the defect prior to AOS-causing resection (black). F, 3D reconstruction with the probability map of Area 55b depicted in red and the defect prior to AOS-causing resection (black). SFG = Superior frontal gyrus; 55b = Area 55b; PreC = Pre-central gyrus; * = original resection cavity prior to AOS-causing resection.
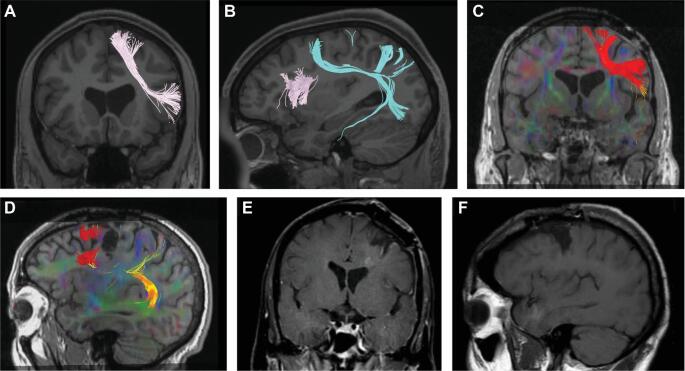
Fiber tractography reveals that area 55b predominantly contributes fibers to the superior longitudinal fasciculus (SLF), as shown in Figure 3. The relationship between the intact frontal aslant tract (FAT) and fibers from area 55b can be seen in Figure 3A and 3B.
FIGURE 3.
A and B, Diffusion tensor imaging (DTI) of a healthy control, showing Frontal aslant tracts (FAT) in pink and tracts arising from area 55b in coronal A and saggital B cuts. C and D, DTI of the patient prior to onset of AOS, showing FAT tracts in red and area 55b tracts in yellow/orange, in coronal C and saggital D cuts. E and F, T1-weighted MRI of the patient after the resection leading to AOS in coronal E and saggital F cuts.
Preoperative diffusion tensor images are shown in Figure 3C and 3D. In Figure 3D, the FAT can be identified within the frontal lobe, arising anterior to the patient's initial resection cavity. Post-resection images in Figure 3E and 3F show that the area of resection essentially spares the cortex with terminating FAT fibers. See Supplemental Digital Content.
DISCUSSION
We report a case of surgical resection at the posterior middle frontal gyrus, the dorsal premotor cortex, that resulted in pure AOS. Tractography was integrated with human connectome project data to suggest potential localization to area 55b, with functional deficits documented by comprehensive language testing.
The patient had severely non-fluent speech but scores inconsistent with anomia or expressive aphasia. He scored within normal limits on QAB and WAB sections for comprehension, and on BNT, weighing against a diagnosis of expressive or receptive aphasias. The motor segment of the QAB demonstrated intact gross motor function, weighing against unilateral upper motor neuron dysarthric deficits. He exhibited word groping, increasing difficulty with increasing word length,38,39 and differential diadochokinetic rate,3,40 as well as slow speech rate, sound distortions and substitutions, and prosodic abnormalities.41 These results suggest an isolated, pure AOS.
As shown in Figure 3, area 55b contributes to the SLF, a complex white matter tract with connections including higher-order language areas of cortex.42-44 The areas that are referred to here as SLF may in certain categorizations be associated with arcuate fasciculus (AF), while in others may be referred to as SLF-IV.45 It should be noted that the tractography presented here is preoperative and can inform, but not confirm, hypotheses on integrity of traversing white matter tracts. Tracts from centers of speech praxis not identified in this report could be interrupted by this focal resection, leading to the observed deficit, rather than cortical dissociation of area 55b.44,46
The localization of area 55b comes with a degree of uncertainty in a brain that has anatomic variations induced by an underlying neoplastic process as well as prior neurological surgery. While the association of this lesion with area 55b is not proven by this single case, better candidates for this patient's neuroanatomic etiology have yet to be described.
CONCLUSION
Through unequivocal documentation of pure AOS with extensive language testing, paired to structural analysis of resection imaging and associated tractography, this case offers a clinically significant example of language deficits from surgical excision of the posterior middle frontal gyrus.
Disclosures
This work was funded in part by NIH 5R01DC012379-08. The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Supplementary Material
Acknowledgments
The authors would like to acknowledge Benjamin Speidel for his contributions to the complex imaging data depicted here. Gratitude is also expressed to the patient at the center of this work. Data were provided (in part) by the Human Connectome Project, WU-Minn Consortium (principal investigators: David Van Essen and Kamil Ugurbil; 1U54MH091657) funded by the 16 NIH Institutes and Centers that support the NIH Blueprint for Neuroscience Research; and by the McDonnell Center for Systems Neuroscience at Washington University.
Contributor Information
Edward F Chang, Department of Neurological Surgery, University of California, San Francisco, San Francisco, California.
Garret Kurteff, Department of Neurological Surgery, University of California, San Francisco, San Francisco, California.
John P Andrews, Department of Neurological Surgery, University of California, San Francisco, San Francisco, California.
Robert G Briggs, Department of Neurosurgery, University of Oklahoma Health Sciences Center, Oklahoma City, Oklahoma.
Andrew K Conner, Department of Neurological Surgery, University of California, San Francisco, San Francisco, California.
James D Battiste, Department of Neurology, University of Oklahoma Health Sciences Center, Oklahoma City, Oklahoma.
Michael E Sughrue, Prince of Wales Private Hospital, Randwick, Australia.
Supplemental Digital Content. Text. Supplemental Information.
COMMENT
The authors describe a persistent pure speech apraxia following re-resection of a tumor in the posterior aspect of the dominant middle frontal gyrus (MFG). The dominant MFG has important speech and language connections via the middle longitudinal fasciculus, the superior longitudinal fasciculus, and the frontal aslant tract (FAT). While the exact connections of the dominant FAT remain conjectural, with a number of putative subcomponents,1-4 connections to the supplementary motor area have been clearly demonstrated.
In the case presented, the prior resection was extended both medially and anteriorly. The authors’ hypothesis, that resection of area 55b is the culprit for the deficits, is interesting. It is also interesting that the posterior MFG is often resected with impunity (as in this patient's first resection). It remains unclear whether the patient's speech apraxia was due to cortical resection, white matter resection, or a combination of the 2.
Daniel L. Silbergeld
Seattle, Washington
REFERENCES
- 1. Broce I, Bernal B, Altman N, Tremblay P, Dick AS. Fiber tracking of the frontal aslant tract and subcomponents of the arcuate fasciculus in 5-8-year-olds: relation to speech and language function. Brain Lang. 2015;149:66-76. [DOI] [PubMed] [Google Scholar]
- 2. Fujii M, Maesawa S, Motomura K et al. Intraoperative subcortical mapping of a language-associated deep frontal tract connecting the superior frontal gyrus to Broca's area in the dominant hemisphere of patients with glioma. J Neurosurg. 2015;122(6):1390-1396. [DOI] [PubMed] [Google Scholar]
- 3. Kinoshita M, de Champfleur NM, Deverdun J, Moritz-Gasser S, Herbet G, Duffau H. Role of fronto-striatal tract and frontal aslant tract in movement and speech: an axonal mapping study. Brain Struct Funct. 2014;220(6):3399-3412. [DOI] [PubMed] [Google Scholar]
- 4. Vassal F, Boutet C, Lemaire J-J, Nuti C. New insights into the functional significance of the frontal aslant tract – an anatomo-functional study using intraoperative electrical stimulations combined with diffusion tensor imaging-based fiber tracking. Br J Neurosurg. 2014;28:685-687. [DOI] [PubMed] [Google Scholar]
REFERENCES
- 1. Ogar J, Slama H, Dronkers N, Amici S, Luisa Gorno-Tempini M. Apraxia of speech: an overview. Neurocase. 2005;11(6):427-432. [DOI] [PubMed] [Google Scholar]
- 2. Ogar J, Willock S, Baldo J, Wilkins D, Ludy C, Dronkers N. Clinical and anatomical correlates of apraxia of speech. Brain Lang. 2006;97(3):343-350. [DOI] [PubMed] [Google Scholar]
- 3. Haley KL, Jacks A, de Riesthal M, Abou-Khalil R, Roth HL. Toward a quantitative basis for assessment and diagnosis of apraxia of speech. J Speech Lang Hear Res. 2012;55(5):S1502-S1517. [DOI] [PubMed] [Google Scholar]
- 4. Graff-Radford J, Jones DT, Strand EA, Rabinstein AA, Duffy JR, Josephs KA. The neuroanatomy of pure apraxia of speech in stroke. Brain Lang. 2014;129:43-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dronkers NF. A new brain region for coordinating speech articulation. Nature. 1996;384(6605):159-161. [DOI] [PubMed] [Google Scholar]
- 6. Hillis AE, Work M, Barker PB, Jacobs MA, Breese EL, Maurer K. Re-examining the brain regions crucial for orchestrating speech articulation. Brain. 2004;127(7):1479-1487. [DOI] [PubMed] [Google Scholar]
- 7. Ramos-Estebanez C, Gokhale S, Goddeau R, Kumar S. Apraxia of speech in healthy 36-year-old man. J Clin Neurosci. 2013;20(8):1176-1177. [DOI] [PubMed] [Google Scholar]
- 8. Fox RJ, Kasner SE, Chatterjee A, Chalela JA. Aphemia: an isolated disorder of articulation. Clin Neurol Neurosurg. 2001;103(2):123-126. [DOI] [PubMed] [Google Scholar]
- 9. Polanowska KE, Pietrzyk-Krawczyk I. Post-stroke pure apraxia of speech – a rare experience. Neurol Neurochir Pol. 2016;50(6):497-503. [DOI] [PubMed] [Google Scholar]
- 10. Patira R, Ciniglia L, Calvert T, Altschuler EL. Pure apraxia of speech due to infarct in premotor cortex. Neurol Neurochir Pol. 2017;51(6):519-524. [DOI] [PubMed] [Google Scholar]
- 11. Itabashi R, Nishio Y, Kataoka Y et al. Damage to the left precentral gyrus is associated with apraxia of speech in acute stroke. Stroke. 2016;47(1):31-36. [DOI] [PubMed] [Google Scholar]
- 12. Broca P. Remarques sur le siège de la faculté du langage articulé, suivies d’une observation d’aphémie (perte de la parole). Bulletin et Memoires de la Societe anatomique de Paris. 1861;6:330-357. [Google Scholar]
- 13. de Oliveira-Souza R, Moll J, Tovar-Moll F. Broca's aphemia: the tortuous story of a nonaphasic nonparalytic disorder of speech. J Hist Neurosci. 2016;25(2):142-168. [DOI] [PubMed] [Google Scholar]
- 14. Basilakos A, Rorden C, Bonilha L, Moser D, Fridriksson J. Patterns of poststroke brain damage that predict speech production errors in apraxia of speech and aphasia dissociate. Stroke. 2015;46(6):1561-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Glasser MF, Coalson TS, Robinson EC et al. A multi-modal parcellation of human cerebral cortex. Nature. 2016;536(7615):171-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hopf A. Distribution of myeloarchitectonic marks in the frontal cerebral cortex in man. J Hirnforsch. 1956;2(4):311-333. [PubMed] [Google Scholar]
- 17. Glasser MF, Van Essen DC. Mapping Human cortical areas in vivo based on myelin content as revealed by T1- and T2-weighted MRI. J Neurosci. 2011;31(32):11597-11616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nieuwenhuys R, Broere CA. A map of the human neocortex showing the estimated overall myelin content of the individual architectonic areas based on the studies of Adolf Hopf. Brain Struct Funct. 2017;222(1):465-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Geyer S, Weiss M, Reimann K, Lohmann G, Turner R. Microstructural parcellation of the human cerebral cortex –from Brodmann's post-mortem map to in vivo mapping with high-field magnetic resonance imaging. Front Hum Neurosci. 2011;5:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fudala JB. Arizona 3: Arizona Articulation Proficiency Scale. 3rd Rev. Torrance, CA: Western Psychological Services; 2000. [Google Scholar]
- 21. Kertesz A. The Western Aphasia Battery. New York: Grune and Stratton; 1982. [Google Scholar]
- 22. Wilson SM, Eriksson DK, Schneck SM, Lucanie JM. A quick aphasia battery for efficient, reliable, and multidimensional assessment of language function. PLoS One. 2018;13(2):e0192773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test. 2nd ed. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- 24. Cibelli ES, Leonard MK, Johnson K, Chang EF. The influence of lexical statistics on temporal lobe cortical dynamics during spoken word listening. Brain Lang. 2015;147:66-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Robertson SJ, Thomson FE. Working With Dysarthric Clients: Practical Guide to Therapy for Dysarthria. Austin, Texas: Communication Skill Builders; 1987. [Google Scholar]
- 26. Dabul BL. Apraxia Battery for Adults: Examiner's Manual and Picture Plates. Austin, Texas: Pro-Ed; 1979. [Google Scholar]
- 27. Van Essen DC, Smith SM, Barch DM, Behrens TE, Yacoub E, Ugurbil K. The WU-Minn human connectome project: an overview. Neuroimage. 2013;80:62-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Glasser MF, Sotiropoulos SN, Wilson JA et al. The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage. 2013;80:105-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. FSL. Neuroimage. 2012;62(2):782-790. [DOI] [PubMed] [Google Scholar]
- 30. Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825-841. [DOI] [PubMed] [Google Scholar]
- 31. Fischl B. FreeSurfer. Neuroimage. 2012;62(2):774-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sotiropoulos SN, Moeller S, Jbabdi S et al. Effects of image reconstruction on fiber orientation mapping from multichannel diffusion MRI: reducing the noise floor using SENSE. Magn Reson Med. 2013;70(6):1682-1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Andersson JLR, Skare S, Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage. 2003;20(2):870-888. [DOI] [PubMed] [Google Scholar]
- 34. Andersson JL, Sotiropoulos SN. Non-parametric representation and prediction of single- and multi-shell diffusion-weighted MRI data using Gaussian processes. Neuroimage. 2015;122:166-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Andersson JLR, Sotiropoulos SN. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage. 2016;125:1063-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Van Essen DC, Smith J, Glasser MF et al. The brain analysis library of spatial maps and atlases (BALSA) database. Neuroimage. 2017;144:270-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Coalson TS, Van Essen DC, Glasser MF. The impact of traditional neuroimaging methods on the spatial localization of cortical areas. Proc Natl Acad Sci USA. 2018;115(27):E6356-E6365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Haley KL, Overton HB. Word length and vowel duration in apraxia of speech: the use of relative measures. Brain Lang. 2001;79(3):397-406. [DOI] [PubMed] [Google Scholar]
- 39. Ziegler W. A nonlinear model of word length effects in apraxia of speech. Cogn Neuropsychol. 2005;22(5):603-623. [DOI] [PubMed] [Google Scholar]
- 40. Jonkers R, Feiken J, Stuive I. Diagnosing apraxia of speech on the basis of eight distinctive signs. Canad J Speech Lang Pathol Audiol. 2017;41(3):303-319. [Google Scholar]
- 41. Wambaugh JL, Duffy JR, McNeil MR, Robin DA, Rogers MA. Treatment guidelines for acquired apraxia of speech: a synthesis and evaluation of the evidence. J Med Speech Lang Pathol. 2006;14(2):15-34. [Google Scholar]
- 42. Makris N, Kennedy DN, McInerney S et al. Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cereb Cortex. 2004;15(6):854-869. [DOI] [PubMed] [Google Scholar]
- 43. Fujii M, Maesawa S, Motomura K et al. Intraoperative subcortical mapping of a language-associated deep frontal tract connecting the superior frontal gyrus to Broca's area in the dominant hemisphere of patients with glioma. J Neurosurg. 2015;122(6):1390-1396. [DOI] [PubMed] [Google Scholar]
- 44. Basilakos A, Fillmore PT, Rorden C, Guo D, Bonilha L, Fridriksson J. Regional white matter damage predicts speech fluency in chronic post-stroke aphasia. Front Hum Neurosci. 2014;8:845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Madhavan KM, McQueeny T, Howe SR, Shear P, Szaflarski J. Superior longitudinal fasciculus and language functioning in healthy aging. Brain Res. 2014;1562:11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vergani F, Lacerda L, Martino J et al. White matter connections of the supplementary motor area in humans. J Neurol Neurosurg Psychiatr. 2014;85(12):1377-1385. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.