Abstract
During an investigation of the chemistry of the Red Sea Verongiid sponge Pseudoceratina arabica, we discovered a small molecule, pseudoceratonic acid (1), along with the new moloka’iamine derivatives, ceratinines N (2), O (3), and the previously reported compounds moloka’iamine (4), hydroxymoloka’iamine (5) and ceratinamine (6). The structural assignments of 1–6 were accomplished by interpretation of their NMR and HRESIMS spectral data. Pseudoceratonic acid possesses a dibrominated hydrazine-derived functional group not found in any reported chemical compound. Pseudoceratonic acid selectively inhibited the growth of E. coli and S. aureus, while ceratinine N selectively inhibited C. albicans. Further, ceratinine N showed potent cytotoxic effects against the triple-negative breast cancer, colorectal carcinoma, and human cervical carcinoma cell lines down to 2.1 µM.
Keywords: Red Sea Verongiid sponge, Pseudoceratina arabica, pseudoceratonic acid, dibromotyrosine alkaloids, ceratinines N and O, structure determinations, antibiotics and cytotoxic activities
1. Introduction
As a result of the global and expanding resistance against today’s available antibiotics and antitumor therapies, novel chemical entities with antibiotic and anticancer potential are in high demand to support the clinical pipelines with enough drug leads. Infections with Staphylococcus aureus, malaria, and Tuberculosis represent a few examples of diseases that have become difficult to cure with current antibiotics [1]. Therefore, there is an urgent need for new drugs/drug leads to combat expanding drug resistance. Further, the declining number of FDA-approved candidates has warranted a renewed interest in the role of secondary metabolites with the goal of characterizing new and bioactive structural scaffolds. Marine organisms represent an unlimited foundation of secondary metabolites with antibiotic and antitumor potential.
Sponges of the Verongida order are well-known for producing bromotyrosine-derived secondary metabolites with diverse bioactivities [2,3,4,5,6]. Bromotyrosines include several classes of brominated marine alkaloids such as spirocyclohexadienylisoxazolines, spirooxepinisoxazolines (psammaplysins), oximes, bastadins, and many other compounds [5]. Members of these classes have been associated with diverse bioactivities including antiviral [7], antimicrobial [8,9,10], antifungal [11], anticancer [12,13,14,15], antimigratory [14], antimalarial [16,17,18], parasympatholytic [8], enzyme inhibition [19] and antifouling properties [20,21].
As a continuation of our interest in bioactive chemical entities from Red Sea Verongiid sponges [8,22], a new sample of the sponge Pseudoceratina arabica was collected and investigated. The current study aimed to identify the cytotoxic and antimicrobial compounds from the methanolic extract of the sponge. Fractionation and purification of the active fractions gave pseudoceratonic acid (1), ceratnines N (2) and O (3) along with moloka’iamine (4) [2], hydroxymoloka’iamine (5) [8] and ceratinamine (6) [6]. Herein, we report on the purification, structural determinations, and the biological effects of compounds 1–6.
2. Results and Discussion
2.1. Purification of Compounds 1–6
Extraction of the freeze-dried sponge P. arabica with MeOH, followed by partition of the resulted extract between H2O-MeOH and n-hexanes, CH2Cl2 and EtOAc gave three organic fractions. The EtOAc-soluble fraction was subjected to chromatographic fractionation on reversed phase (C18) Sep-Pak cartridge and Sephadex LH-20. Purification of the active fractions on C18 HPLC afforded compounds 1–6.
2.2. Structural Determination of 1–6
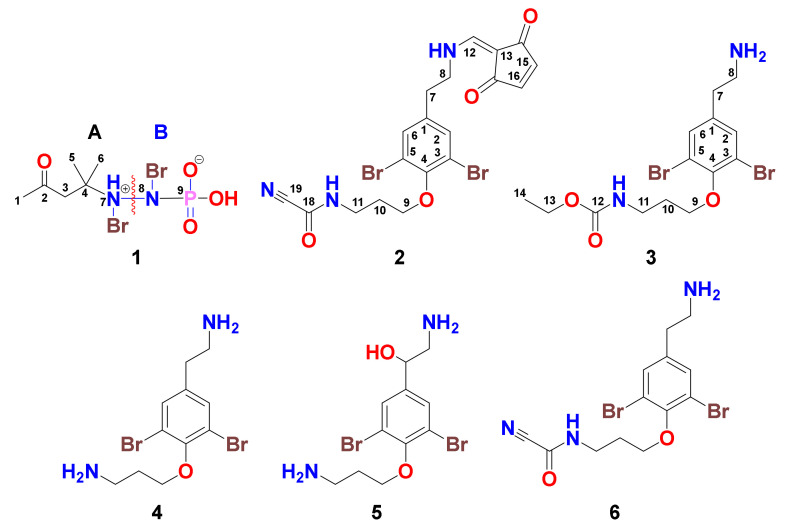
The positive ESIMS of 1 showed three ion peaks at m/z 366.9, 368.9 and 370.9 (1:2:1), supporting the existence of 2 bromine atoms in 1. The molecular formula C6H1379Br2N2O4P was obtained from the ion peak at 366.9064 [M + H]+ in the positive HRESIMS (Figure S1). Also, the negative HRESIMS (Figure S2) of 1 confirmed the molecular formula. Compound 1 (Figure 1) was assigned by interpretation of its 1D (1H and 13C) (Figures S3 and S4) and 2D NMR spectral data including multiplicity-edited HSQC (Figure S5), and 1H-13C (Figure S6) and 1H-15N HMBC (Figure S7) experiments. These combined data, along with the HRESIMS and MS fragmentation of 1, supported the structural determination of two subunits in 1 including 4-(bromoamino)-4-methylpentan-2-one (A) and N-bromo-phosphoramidic acid (B) connected together through N-7 and N-8 (Figure 1).
Figure 1.
Structures of compounds 1–6.
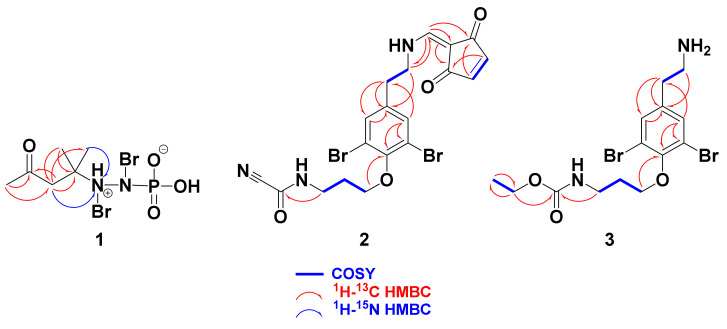
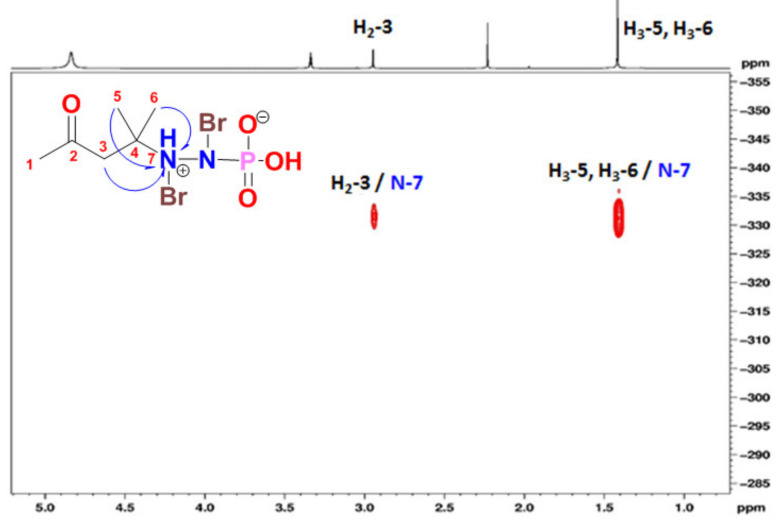
The 1H NMR spectrum of 1 showed three singlets in the ratio of 1.5:1.0:3.0 (3.0:2.0:6.0) at δH 2.23 (3H, s), 2.91 (2H, s) and 1.41 (6H, s) corresponding to H3-1, H2-3 and H3-5/6 (Table 1). In the HSQC, these signals are correlated to the 13C signals at δC 31.0 (CH3, C-1), 50.7 (CH2, C-3) and 26.0 (2 × CH3, C-5 and C-6), respectively. The quaternary signals at δC 209.3 and 53.4 (Table 1) were assigned to a ketone (C-2) and a quaternary carbon (C-4) attached to a nitrogen atom (N-7) as supported by HMBC correlations from H2-3 to C-2, from H3-1 to C-2 (δC 209.3), from H2-3 to C-4 (δC 53.4) and from H3-5/H3-6 to C-4. Furthermore, the 1H-15N HMBC cross-peaks from H2-3 to N-7 and from H3-5/H3-6 to N-7 supported the connection of N-7 to C-4 (Figure 2 and Figure 3). The fragmentation ion peaks of 1 (Figure 4) supported the N-bromination of part A. Thus, fragment A was assigned as 4-(bromoamino)-4-methylpentan-2-one.
Table 1.
NMR data of pseudoceratonic acid (1) (CD3OD) a.
| No. | δC (mult.) | δN b | δH (mult.) | 1H-13C HMBC | 1H-15N HMBC |
|---|---|---|---|---|---|
| 1 | 31.0, CH3 | 2.23 (3H, s) | C-2, C-3 | ||
| 2 | 209.3, qC | ||||
| 3 | 50.7, CH2 | 2.91 (2H, s) | C-2, C-4, C-6 | N-7 | |
| 4 | 53.4, qC | ||||
| 5 | 26.0, CH3 | 1.42 (3H, s) | C-3, C-4 | N-7 | |
| 6 | 26.0, CH3 | 1.42 (3H, s) | C-3, C-4 | N-7 | |
| N-7 | −331.6 |
a 1H and 13C NMR were acquired at 400 and 100 MHz, respectively. b 15N Signal was traced from 1H-15N HMBC and was referenced to CH3NO2 at 0.0 ppm.
Figure 2.
COSY, 1H-13C HMBC of 1–3 and 1H-15N HMBC of 1.
Figure 3.
1H-15N HMBC spectrum of 1.
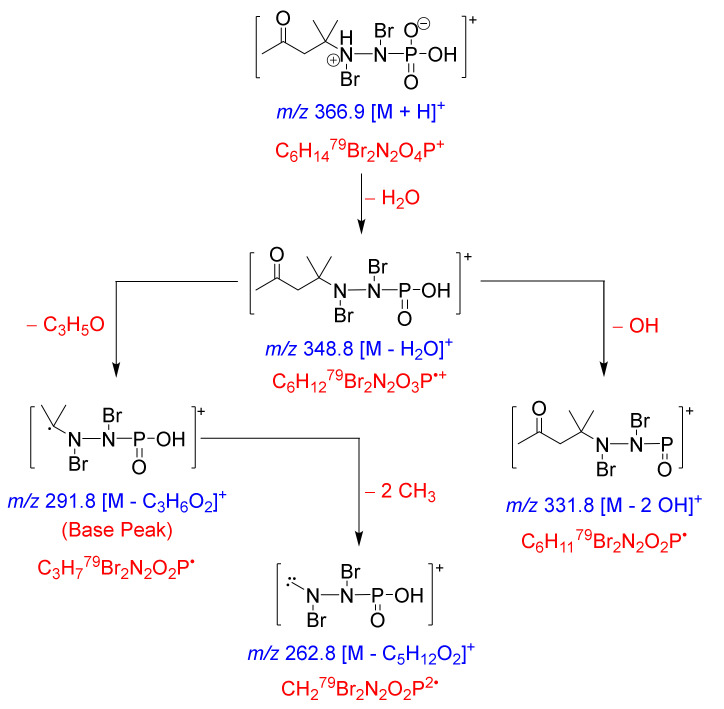
Figure 4.
Important MS fragmentation ion peaks of 1.
The molecular formula of fragment A is counted for “C6H11BrNO”. Thus, the remaining elements of 1 “H2BrNO3P” are counted for an N-bromophosphoramidic acid moiety (Fragment B), as confirmed from the fragmentation ion peaks of 1 (Figure 4 and Figure S8). The fragment ion peaks at m/z 348.8, 350.8 and 352.8 (1:2:1) resulted from the loss of OH from the phosphoramidic acid [M + H − H2O]+ (Figure 4). Additional loss of C3H5O fragment from the other terminus of 1 gave a base peak at m/z 291.8/293.8/295.8 (1:2:1) [M + H − H2O − C3H5O]+. The loss of another OH from the ion peak at m/z 348.8 resulted in an ion peak at m/z 331.8/333.8/335.8 [M + H – H2O − OH]+. Finally, the loss of two methyls from the base peak at m/z 291.8/293.8/295.8 gave a minor ion peak at m/z 262.8/264.8/266.8 [M + H − C5H12O2]+ (Figure 4).
The existence of the fragmentation ion peaks at m/z 348.8, 331.8 and 291.8 and 262.8 supported the existence of both subunits (A and B), the dibromination of the nitrogen atoms in 1 and the linkage between N-7 and N-8. Accordingly, compound 1 was assigned as shown in Figure 1 and named pseudoceratonic acid.
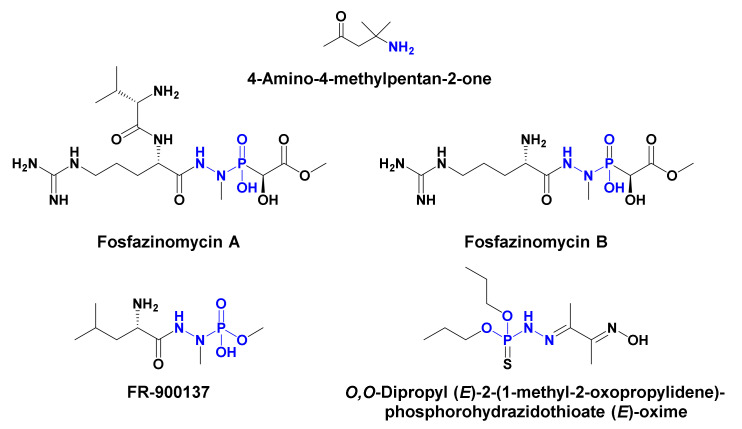
Hydrazine derivatives and other dinitrogen-containing features are commonly found in natural compounds of terrestrial, microbial, and marine origin [23,24]. Similarly, derivatives of phosphoramidic acid are represented in different chemical entities of natural origin [25,26]. A literature search revealed only four compounds with N-N-linked-phosphorous moiety including fosfazinomycins A and B from Streptomyces lavendofoliae [27], FR-900137 from Streptomyces unzenensis [28,29] and O,O-dipropyl (E)-2-(1-methyl-2-oxopropylidene)phosphorohydrazidothioate (E)-oxime) from the dinoflagellate Gymnodinium breve [30]. Similarly, 4-amino-4-methylpentan-2-one (part A in 1) was previously reported from Streptomyces pleomorphus [31] (Figure 5). The existence of such microbial-derived compounds supports the hypothesis of the microbial origin of pseudoceratonic acid.
Figure 5.
Microbial-derived compounds with similar subunits in 1 [27,28,29,30,31].
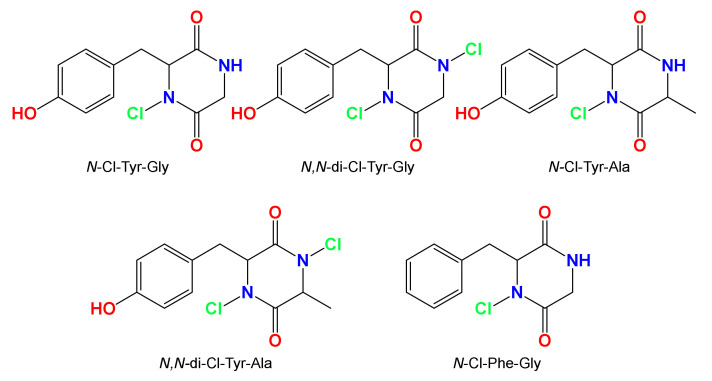
Compound 1 has several features that are not found in previously reported natural products. Perhaps most spectacularly, the dibromination of the hydrazine part, a feature that has only been reported in a few synthetic compounds that were not NMR-characterized. N-Halogenated compounds are extremely rare. Recently, several N-chlorinated dipeptides (Figure 6), which again were not NMR-characterized, were discovered as disinfection byproducts resulted from chlorination of drinking water [32]. Finally, since compound 1 shares most of its functionalities with other microbial-derived compounds [27,28,29,30,31], we speculate that 1 could be a microbial product that is released as a part of a defense mechanism of the sponge after its cutting underwater.
Figure 6.
Chemical structures of N-chlorinated dipeptides [32].
The existence of three pseudomolecular ion peaks at 509.9, 511.9, and 513.9 (1:2:1) [M + H]+ in the ESIMS of 2 confirmed two bromine atoms in 2 (Figure 1). Its molecular formula C19H1779Br2N3O4 was confirmed from the ion peak at m/z 509.9669 [M + H]+ in the positive HRESIMS (Figure S9), confirming 12 degrees of unsaturation. Interpretation of its 1H (Figure S10) and 13C NMR (Figure S11) spectra along with the DEPT (Figure S12), COSY (Figure S13), HSQC (Figure S14) and HMBC (Figure S15) confirmed the assignment of three substructural units (A–C). The 1H and 13C NMR signal at δH/C 138.6 (qC, C-1), 7.54 (2H, s)/134.8 (2 × CH, C-2,6), 119.3 (2 × CH, C-3,5), 153.2 (qC, C-4), 2.86 (2H, t)/36.7 (CH2, C-7), 3.61 (2H, t)/51.9 (CH2, C-8), 4.02 (2H, t)/71.0 (CH2, C-9), 2.08 (2H, quin.)/30.1 (CH2, C-10) and 3.56 (2H, t)/38.4 (CH2, C-11) confirmed the presence of the moloka’iamine moiety (subunit A) [2] (Table 2). In addition, the resonances at δH/C 7.21 (1H, H-12)/150.8 (CH, C-12), 99.5 (qC, C-13), 198.7 (qC, C-14), 6.67 (1H, H-15)/142.7 (CH, C-15), 6.73 (1H, H-16)/142.9 (CH, C-16) and 196.8 (qC, C-17) confirmed the presence of 2-(methylene)cyclopent-4-ene-1,3-dione part (subunit B) [7,14]. Finally, the signals at δC 145.0 (qC, C-18) and 113.1 (qC, C-19) confirmed the existence of a cyanoformamide moiety (subunit C) [6]. The linkage of subunits B and C to the terminal amines of the moloka’iamine at C-8 and C-11, respectively, was confirmed from HMBC of H2-8 (δH 3.61)/C-12 (δC 150.8) and from H-12 (δH 7.21)/C-8 (δC 51.8) as well as HMBC from H2-11 (δH 3.56) to C-18 (δC 145.0) (Figure 2 and Table 2), completing the molecular formula of 2 and its assignment. Accordingly, 2 was assigned as shown in Figure 1 and named ceratinine N.
Table 2.
NMR data of ceratinines N (2) and O (3) (CD3OD) a.
| No. | 2 | 3 | ||||
|---|---|---|---|---|---|---|
| δC (Mult.) a | δH (Mult., J (Hz)) | HMBC (C#) | δC (Mult.) a | δH (Mult., J (Hz)) | HMBC (C#) | |
| 1 | 138.6 (qC) | 139.6 (qC) | ||||
| 2 | 134.8 (CH) | 7.45 (s) | C-1, C-3/5, C-4, C-7 | 134.3 (CH) | 7.46 (s) | C-1, C-3/5, C-4, C-7 |
| 3 | 119.3 (qC) | 119.1 (qC) | ||||
| 4 | 153.2 (qC) | 152.9 (qC) | ||||
| 5 | 119.3 (qC) | 119.1 (qC) | ||||
| 6 | 134.8 (CH) | 7.45 (s) | C-1, C-3/5, C-4, C-7 | 134.3 (CH) | 7.46 (s) | C-1, C-3/5, C-4, C-7 |
| 7 | 36.7 (CH2) | 2.86 (t, 6.6) | C-1, C-2/6, C-8 | 33.0 (CH2) | 2.92 (t, 6.6) | C-1, C-2/6, C-8 |
| 8 | 51.9 (CH2) | 3.61 (t, 6.6) | C-1, C-7, C-12 | 41.0 (CH2) | 3.13 (t, 6.6) | C-1, C-7 |
| 9 | 71.0 (CH2) | 4.02 (t, 6.6) | C-4, C-10, C-11 | 72.0 (CH2) | 4.01 (t, 6.6) | C-4, C-10, C-11 |
| 10 | 30.1 (CH2) | 2.08 (quin, 6.6) | C-9, C-11 | 31.3 (CH2) | 2.01 (quin, 6.6) | C-9, C-11 |
| 11 | 38.4 (CH2) | 3.56 (t, 6.6) | C-9, C-10, C-18 | 39.1 (CH2) | 3.42 (t, 6.6) | C-9, C-10, C-12 |
| 12 | 150.8 (CH) | 7.21 (s) | C-8, C-13, C-14, C-17 | 159.2 (qC) | ||
| 13 | 99.5 (qC) | 61.7 (CH2) | 4.05 (q, 6.6) | C-12, C-14 | ||
| 14 | 198.7 (qC) | 14.5 (CH3) | 1.22 (t, 6.6) | C-13 | ||
| 15 | 142.7 (CH) | 6.67 (d, 6.6) | C-13, C-14, C-16, C-17 | |||
| 16 | 142.9 (CH) | 6.73 (d, 6.6) | C-13, C-14, C-15, C-17 | |||
| 17 | 196.8 (qC) | |||||
| 18 | 145.0 (qC) | |||||
| 19 | 113.1 (qC) | |||||
a Data acquired at 600 and 150 MHz for 1H and 13C, respectively; # means No.; C# = (Carbon No.).
The ESIMS spectrum of 3 with three ion peaks at 422.9, 424.9 and 426.9 (1:2:1) [M + H]+ supported 2 bromine atoms in the molecule. Compound 3 (Figure 1) showed molecular formula C14H2079Br2N2O3 as confirmed by the positive HRESIMS (422.9923, C14H2179Br2N2O3, [M + H]+) (Figure S16), supporting five degrees of unsaturation. Interpretation of the 1H (Figure S17), 13C (Figure S18), DEPT (Figure S19) spectra and the 2D (COSY, HSQC and HMBC) (Figures S20–S22) experiments of 3 allowed its assignment. The 1H and 13C spectra of 3 showed, beside the regular signals of the moloka’iamine moiety [2] (Table 2), additional signals at δH/C 159.2 (QC, C-12), 4.05 (2H, q, H2-13)/61.7 (CH2, C-13) and 1.22 (3H, t, H3-13)/14.5 (CH3, C-13). These signals were assigned for a terminal ethyl carbamate moiety [22,33]. The attachment of the carbamate moiety to the terminal amine at C-11 was confirmed by HMBC cross-peaks from H2-11 (δH 3.42) to C-12 (δC 159.2), H2-13 (δH 4.05) to C-12 (δC 159.2) and from H3-14 (δH 1.22) to C-13 (δC 61.7) (Figure 2 and Table 2), completing the structural assignment of 2. Thus, compound 2 was assigned as shown in Figure 1 and named ceratinine O.
The antimicrobial evaluation of the compounds against E. coli, S. aureus and C. albicans at 50 µg/disc was carried out using disc diffusion assay. Pseudoceratonic acid (1) was the most active against E. coli and S. aureus with inhibition zones of 15 and 17 mm, respectively, while it displayed weak effect towards C. albicans with inhibition zone of 6 mm, suggesting selective antibacterial activity. On the other hand, ceratinine N (2) was more active against C. albicans (inhibition zone = 16 mm) and less active against the other pathogens, suggesting selectivity against C. albicans. The other compounds were weakly active against the three pathogens with inhibition zones of 6–9 mm (Table 3).
Table 3.
Antimicrobial effects of 1–6.
| Compound | E. coli | S. aureus | C. albicans | |||
|---|---|---|---|---|---|---|
| Inhibition Zone (mm) | MIC (µg/mL) | Inhibition Zone (mm) | MIC (µg/mL) | Inhibition Zone (mm) | MIC (µg/mL) | |
| 1 | 15 | 16 | 17 | 16 | 6 | 125 |
| 2 | 6 | 125 | 7 | 125 | 16 | 16 |
| 3 | 6 | 125 | 8 | 64 | 9 | 64 |
| 4 | 6 | 125 | 7 | 125 | 6 | 125 |
| 5 | NT | NT | NT | NT | NT | NT |
| 6 | 7 | 125 | 9 | 64 | 12 | 32 |
| Ciprofloxacin a | 30 | 0.25 | 22 | 0.5 | NT | NT |
| Ketoconazole b | NT | NT | NT | NT | 30 | 0.5 |
a positive antibacterial drug; b positive antifungal drug; NT = Not Tested.
The minimal inhibitory concentrations (MICs) of the compounds were evaluated using a microdilution method (Table 3). Pseudoceratonic acid exhibited the highest activity against E. coli and S. aureus with MIC values of 16 and 16 µg/mL, respectively. Ceratinine N, on the other hand, displayed the highest antifungal activity against C. albicans with an MIC value of 16 µg/mL.
The cytotoxic and antiproliferative effects of the compounds against MDA-MB-231, HeLa, and HCT 116 cell lines were evaluated using MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay (Table 4). Ceratinine N (2) demonstrated potent activities against the cell lines with IC50 of 3.1, 2.3 and 2.9 µM, respectively. Conversely, ceratinine O (3) displayed moderate activity towards HCT 116 with IC50 of 9.5 µM. The remaining compounds displayed IC50 values of ≥10 µM. Furthermore, 2 showed cytotoxicity towards NHDF (normal human dermal fibroblasts) cells with IC50 of 3.5 µM, suggesting marginal selectivity towards the tested cancer cell lines.
Table 4.
Cytotoxic effects of 1–6. a
| Compound | IC50 (µM) | |||
|---|---|---|---|---|
| MDA-MB-231 | HeLa | HCT 116 | NHDF | |
| 1 | ≥10 | ≥10 | ≥10 | |
| 2 | 3.1 ± 0.29 | 2.3 ± 0.16 | 2.9 ± 0.18 | 3.5 ± 0.30 |
| 3 | ≥10 | ≥10 | 9.5 ± 0.85 | |
| 4 | ≥10 | ≥10 | ≥10 | |
| 5 | ≥10 | ≥10 | ≥10 | |
| 6 | ≥10 | ≥10 | ≥10 | |
| 5-FU b | 13.0 ± 0.30 | 12.3 ± 0.25 | 4.6 ± 0.23 | |
a Cells were treated with the compounds for 72 h; b Positive cytotoxic drug; NHDF: normal human dermal fibroblasts.
These results suggest that pseudoceratonic acid (1) possesses selectivity against S. aureus and E. coli, while ceratinine N (2) is selectively active against C. albicans. The potent cytotoxic and antiproliferative activities of ceratinine N (2) suggest the importance of 2-(methylene)cyclopent-4-ene-1,3-dione moiety for maximum cytotoxic activity.
3. Materials and Methods
3.1. General Experimental Procedures
HRESIMS spectra were acquired on Micromass Q-ToF spectrometer (Waters Corporation, Milford, MA, USA). NMR spectra were obtained on Bruker Avance DRX 400 and 600 MHz (Bruker, Rheinstetten, Germany). The UV spectra were measured on a Hitachi 300 spectrometer (Hitachi High-Technologies Corporation, Kyoto, Japan) and as reported earlier [34]. Liquid–Liquid partitions, C18 Sep-Pak and Sephadex LH-20 were used for fractionation of the extract and successive fractions. A Gemini® C18 column (5 µm, Phenomenex, 250 × 0.64 mm) (Torrance, CA, USA) was used for HPLC purification of the compounds. The HCT 116, MDA-MB-231 cell lines were obtained from American Type Culture Collection (ATCC) (Manassas, VA, USA), while NHDF cell line was obtained from PromoCell GmbH (Heidelberg, Germany).
3.2. The Sponge Pseudoceratina arabica
Pseudoceratina arabica was collected via scuba diving (−13–17 m) from the Anas Reef (N 021°39′17.5″, E 038°52′26.3″) in the Saudi Red Sea. The sponge consists of an encrusting mass of 1–2 cm with a conulose surface and bright greenish-yellowish color underwater and greenish-yellow interior. A voucher specimen was stored at University of Amsterdam (code No. RMNHPOR 9161) and another specimen was kept at King Abdulaziz University (code No. KSA-58). A full description of the sponge is reported earlier [14].
3.3. Purification of 1–6
The lyophilized sponge materials (50 g) were macerated in MeOH overnight (3 × 500 mL). The dried methanolic extract was treated with 200 mL of 50% aqueous MeOH followed by partition against n-hexanes, CH2Cl2 and EtOAc. The cytotoxic EtOAc extract (IC50 = 5 µg/mL against HCT 116) was dried and the residue was subjected to partition on C18 cartridge (Sep-Pak, Waters, 1 g) using H2O-CH3CN-MeOH gradients. The fraction eluted with H2O-MeCN (8:2) (175 mg) was partitioned on Sephadex LH-20 affording five fractions (F1–F5). The antimicrobial fraction (F3, 24 mg) (inhibition zone = 9 mm against E. coli) was purified on C18 HPLC column (Gemini® 5 µm, Phenomenex, 250 × 0.64 mm) using H2O-MeCN (1:1) giving compound 1 (1.7 mg), 3 (3.6 mg) and 6 (2.4 mg). Similarly, the cytotoxic fraction (F5, 17 mg) (IC50 = 3.5 µg/mL against HCT 116) was purified on C18 HPLC column (Gemini® 5 µm, Phenomenex, 250 × 0.64 mm) using H2O-MeCN (6:4) to give 2 (2.7 mg), 4 (2.3 mg) and 5 (1.9 mg).
Spectroscopic Data of the Compounds
Pseudoceratonic acid (1)
Colorless oil; positive HRESIMS m/z 366.9064 (calcd for C6H1479Br2N2O4P, 366.9057, [M + H]+); negative HRESIMS m/z 364.8897 (calcd. for C6H1279Br2N2O4P, 364.8901, [M − H]−); NMR: Table 1.
Ceratinine N (2)
Colorless solid; UV (MeOH) λmax (log ε): 2.15 (3.74), 227 (3.81), 276 (2.75), 284 (2.70) nm; HRESIMS m/z 509.9669 (calcd. for C19H1879Br2N3O4, [M + H]+, 509.9664); NMR: Table 2.
Ceratinine O (3)
Colorless solid; UV (MeOH) λmax (log ε): 2.16 (3.69), 230 (3.91), 276 (2.99), 284 (2.96) nm; HRESIMS m/z 422.9923 (calcd. for C14H2179Br2N2O3, [M + H]+, 422.9918), NMR: Table 2.
3.4. Biological Evaluation of the Compounds
Antimicrobial Evaluation of the Compounds
Disc Diffusion Assay
Using S. aureus (ATCC 25923), E. coli (ATCC 25922), and C. albicans (ATCC 14053), a disc diffusion assay was carried out to evaluate the antimicrobial effects of 1–6 at 50 µg/disc as reported before [35,36,37]. Ketoconazole (50 µg/disc) and ciprofloxacin (5.0 µg/disc) served as antibiotics controls.
Determination of the MIC of the Compounds
The MIC values of the compounds were determined of using a macrodilution method [38]. Briefly, the compounds were dissolved in MeOH at a final concentration of 2000 µg/mL. Ciprofloxacin and ketoconazole were dissolved in H2O at final concentrations of 100 µg/mL. Syringe filters (0.2 µm) were used for sterilization of all stock solutions. Two-fold serial dilutions of the solutions were used in Mueller Hinton Broth (MHB) to give concentrations of 1.0–1000 µg/mL (for the compounds) and 0.125–64 µg/mL (for ciprofloxacin and ketoconazole). 500 µL from the 106 CFU/mL microbial suspensions were added in sterile tubes to give inoculua of 5 × 105 CFU/mL. Further, 100 µL of the stock solutions of the compounds and antibiotics were added into the tubes. Control tubes with test microorganisms only and MeOH were prepared. The MeOH did not exhibit any antimicrobial activity. The tubes were kept for 48 h at 37 °C. MIC values were calculated as the lowest concentrations of the compounds/antibiotics, which did not exhibit any microbial growth.
3.5. Determination of the Cytotoxicity of the Compounds
3.5.1. Cell Lines and Cultures
The cell lines MDA-MB-231 (triple-negative breast cancer, ATCC HTB-26), HeLa (human cervical carcinoma, ATCC CCL-2), HCT 116 (colorectal carcinoma, ATCC CCL-247) were used for the evaluation of the cytotoxicity of the compounds. In addition, NHDF (normal human dermal fibroblasts) cells were used to evaluate the selectivity of compound 2 against the cancerous cell lines. DMEM, with Pen–Strep. (1%) and FBS (10%), was used for culturing MDA-MB-231 cells, while RPMI 1640, with Pen–Strep. (1%) and FBS (10%), was used for culturing HCT 116 and HeLa cells. Finally, NHDF cells were cultured in fibroblast basal medium complemented with Pen–Strep. (1% v/v), heat-inactivated fetal bovine serum (FBS) (2% v/v), insulin (5 µg/mL) and basic fibroblast growth factor (1 ng/mL). Culture of all cells was completed at 37 °C with 5% CO2 and 95% humidity [15,39].
3.5.2. MTT Assay
The cytotoxic effects of the compounds were evaluated using MTT assay as reported earlier [15,39]. Briefly, all cells kept overnight in 5% CO2/air and at 37 °C. Tested compounds were added to the upper row of a 96-well plate followed by 1:4 (v/v) serial dilutions. After 72 h incubation of the compounds with the cells (10,000 cells/well for HCT 116 and NHDF, and 12,000 cells/well for HeLa and MDA-MB-231), the cells’ viability was calculated at 490 nm as reported before [15,39]. 5-Fluorouracil (5-FU) and DMSO were used as positive and negative controls. The IC50 values reported in Table 3 represent the mean of three separate experiments. A concentration of 10 µM was set as a cutoff value in this assay.
4. Conclusions
Purification of the cytotoxic and antibacterial fractions of the extract of the sponge Pseudoceratina arabica gave six compounds including pseudoceratonic acid (1), ceratinines N (2) and O (3) along with molka’iamine (4), hydroxymolka’iamine (5) and ceratinamine (6). Further, the structures of 1–6 were assigned by NMR and HRESIMS spectral interpretations. Pseudoceratonic acid possesses an unprecedented N,N-dibromo-phosphorohydrazidic acid that was not encountered previously. The existence of pseudoceratonic acid provides insight into unprecedented biochemical transformations within the sponge P. arabica. The stability of this previously unknown functional group also suggests a new functional group that might be used as an isostere for application in synthetic and medicinal chemistry or as a linking group for other design purposes. Pseudoceratonic acid displayed selective antibiotic activity against S. aureus and E. coli, while ceratinine N was selective against C. albicans. Further, creatinine N was potently active, with a marginal selectivity, against three cancerous human cell lines down to 2.3 µM. This potent effect is attributed to the existence of 2-(methylene)cyclopent-4-ene-1,3-dione moiety in the compound. Thus, creatinine N represents a potential scaffold for the development of novel and more effective anticancer drug leads.
Acknowledgments
This project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, Kingdom of Saudi Arabia, under grant number (D-353-141-1441). The authors therefore acknowledge and thank DSR for technical and financial support. We are grateful for Eric W. Schmidt for the constructive discussion and for editing the manuscript.
Supplementary Materials
The following are available online at https://www.mdpi.com/1660-3397/18/11/525/s1, 1H NMR, 13C NMR, DEPT, COSY, HSQC, 1H-13C HMBC, and HRESIMS data of compounds 1–3 and 1H-15N HMBC of compound 1.
Author Contributions
L.A.S. and D.T.A.Y. conceptualization, conceived and designed the experiments; L.A.S. and D.T.A.Y. performed the experiments and purified the compounds; L.A.S. and D.T.A.Y. interpreted the NMR and MS data, L.A.S. and D.T.A.Y. wrote and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, under grant No. (D-353-141-1441).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sakata T., Winzeler E.A. Genomics, systems biology and drug development for infectious diseases. Mol. Biosyst. 2007;3:841–848. doi: 10.1039/b703924g. [DOI] [PubMed] [Google Scholar]
- 2.Hamann M.T., Scheuer P.J., Kelly-Borges M. Biogenetically diverse, bioactive constituents of a sponge, order Verongida: Bromotyramines and sesquiterpene-shikimate derived metabolites. J. Org. Chem. 1993;58:6565–6569. doi: 10.1021/jo00076a012. [DOI] [Google Scholar]
- 3.Kornprobst J.-M. Encyclopedia of Marine Natural Products. Volume 2. Wiley-Blackwell; Weinheim, Germany: 2010. pp. 796–805. Chapter 19. [Google Scholar]
- 4.Carroll A.R., Copp B.R., Davis R.A., Keyzers R.A., Prinsep M.R. Marine natural products. Nat. Prod. Rep. 2020;37:37–175. doi: 10.1039/C9NP00069K. [DOI] [PubMed] [Google Scholar]
- 5.Peng J., Li J., Hamann M.T. The marine bromotyrosine derivatives. In: Knölker H.-J., editor. The Alkaloids: Chemistry and Biology. Volume 61. Academic Press; San Diego, CA, USA: 2005. pp. 59–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsukamoto S., Kato H., Hirota H., Fusetani N. Ceratinamine: An unprecedented antifouling cyanoformamide from the marine sponge Pseudoceratina purpurea. J. Org. Chem. 1996;61:2936–2937. doi: 10.1021/jo9602884. [DOI] [PubMed] [Google Scholar]
- 7.Ichiba T., Scheuer P.J., Kelly-Borges M. Three bromotyrosine derivatives, one terminating in an unprecedented diketocyclopentenylidene enamine. J. Org. Chem. 1993;58:4149–4150. doi: 10.1021/jo00067a062. [DOI] [Google Scholar]
- 8.Badr J.M., Shaala L.A., Abou-Shoer M.I., Tawfik M.A., Habib A.M. Bioactive brominated metabolites from the Red Sea sponge Pseudoceratina arabica. J. Nat. Prod. 2008;71:1472–1474. doi: 10.1021/np8002113. [DOI] [PubMed] [Google Scholar]
- 9.Takada N., Watanabe R., Suenaga K., Yamada K., Ueda K., Kita M., Uemura D. Zamamistatin, a significant antibacterial bromotyrosine derivative, from the Okinawan sponge Pseudoceratina purpurea. Tetrahedron Lett. 2001;42:5265–5267. doi: 10.1016/S0040-4039(01)00993-5. [DOI] [Google Scholar]
- 10.Jang J., van Soest R.W.M., Fusetani N., Matsunaga S. Pseudoceratins A and B, antifungal bicyclic bromotyrosine-derived metabolites from the marine sponge Pseudoceratina purpurea. J. Org. Chem. 2007;72:1211–1217. doi: 10.1021/jo062010+. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi J., Tsuda M., Agemi K., Shigemori H., Ishibashi M., Sasaki T., Mikami Y. Purealidins B and C, new bromotyrosine alkaloids from the Okinawan marine sponge Psammaplysilla purea. Tetrahedron. 1991;47:6617–6622. doi: 10.1016/S0040-4020(01)82314-0. [DOI] [PubMed] [Google Scholar]
- 12.Dai J., Parrish S., Yoshida W., Yip M., Turkson J., Kelly M., Williams P. Bromotyrosine-derived metabolites from an Indonesian marine sponge in the family Aplysinellidae (Order Verongiida) Bioorg. Med. Chem. Lett. 2016;26:499–504. doi: 10.1016/j.bmcl.2015.11.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tarazona G., Santamaría G., Cruz P.G., Fernández R., Pérez M., Martínez-Leal J.F., Rodríguez J., Jiménez C., Cuevas C. Cytotoxic anomoian B and aplyzanzine B, new bromotyrosine alkaloids from Indonesian sponges. ACS Omega. 2017;2:3494–3501. doi: 10.1021/acsomega.7b00417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaala L.A., Youssef D.T.A., Badr J.M., Sulaiman M., Khedr A., El Sayed K.A. Bioactive alkaloids from the Red Sea marine Verongid sponge Pseudoceratina arabica. Tetrahedron. 2015;71:7837–7841. doi: 10.1016/j.tet.2015.08.024. [DOI] [Google Scholar]
- 15.Shaala L.A., Youssef D.T.A. Cytotoxic psammaplysin analogues from the Verongid Red Sea sponge Aplysinella species. Biomolecules. 2019;9:841. doi: 10.3390/biom9120841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mudianta I.W., Skinner-Adams T., Andrews K.T., Davis R.A., Hadi T.A., Hayes P.Y., Garson M.J. Psammaplysin derivatives from the Balinese marine sponge Aplysinella strongylata. J. Nat. Prod. 2012;75:2132. doi: 10.1021/np300560b. [DOI] [PubMed] [Google Scholar]
- 17.Yang X., Davis R.A., Buchanan M.S., Duffy S., Avery V.M., Camp D., Quinn R.J. Antimalarial bromotyrosine derivatives from the Australian marine sponge Hyattella sp. J. Nat. Prod. 2010;73:985–987. doi: 10.1021/np900834g. [DOI] [PubMed] [Google Scholar]
- 18.Kurimoto S.I., Ohno T., Hokari R., Ishiyama A., Iwatsuki M., Ōmura S., Kobayashi J., Kubota T. Ceratinadins E and F, new bromotyrosine alkaloids from an Okinawan marine sponge Pseudoceratina sp. Mar. Drugs. 2018;16:463. doi: 10.3390/md16120463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pina I.C., Gautschi J.T., Wang G., Sanders M.L., Schmitz F.J., France D., Cornell-Kennon S., Sambucetti L.C., Remiszewski S.W., Perez L.B., et al. Psammaplins from the sponge Pseudoceratina purpurea: Inhibition of both histone deacetylase and DNA methyltransferase. J. Org. Chem. 2003;68:3866–3873. doi: 10.1021/jo034248t. [DOI] [PubMed] [Google Scholar]
- 20.Tsukamoto S., Kato H., Hirota H., Fusetani N. Pseudoceratidine: A new antifouling spermidine derivative from the marine sponge Pseudoceratina purpurea. Tetrahedron Lett. 1996;37:1439–1440. doi: 10.1016/0040-4039(96)00025-1. [DOI] [Google Scholar]
- 21.Tsukamoto S., Kato H., Hirota H., Fusetani N. Ceratinamides A and B: New antifouling dibromotyrosine derivatives from the marine sponge Pseudoceratina purpurea. Tetrahedron. 1996;52:8181–8186. doi: 10.1016/0040-4020(96)00387-0. [DOI] [PubMed] [Google Scholar]
- 22.Shaala L.A., Youssef D.T.A., Sulaiman M., Behery F.A., Foudah A.I., El Sayed K.A. Subereamolline A as a potent breast cancer migration, invasion and proliferation inhibitor and bioactive dibrominated alkaloids from the Red Sea sponge Pseudoceratina arabica. Mar. Drugs. 2012;10:2492–2508. doi: 10.3390/md10112492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Le Goff G., Ouazzani J. Natural hydrazine-containing compounds: Biosynthesis, isolation, biological activities and synthesis. Bioorg. Med. Chem. 2014;22:6529–6544. doi: 10.1016/j.bmc.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 24.Blair L.M., Sperry J. Natural products containing a nitrogen–nitrogen bond. J. Nat. Prod. 2013;76:794–812. doi: 10.1021/np400124n. [DOI] [PubMed] [Google Scholar]
- 25.Kafarski P. Biological Role of Phosphorus. IntechOpen; London, UK: 2019. Phosphonates: Their natural occurrence and physiological role. Open access peer-reviewed chapter. [DOI] [Google Scholar]
- 26.Petkowski J.J., Bains W., Seager S. Natural products containing ‘rare’ organophosphorus functional groups. Molecules. 2019;24:866. doi: 10.3390/molecules24050866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ogita T., Gunji S., Fukazawa Y., Terahara A., Kinoshita T., Nagaki H., Beppu T. The structures of fosfazinomycins A and B. Tetrahedron Lett. 1983;24:2283–2286. doi: 10.1016/S0040-4039(00)81904-8. [DOI] [Google Scholar]
- 28.Kuroda Y., Goto T., Okamoto M., Yamashita M., Iguchi E., Kohsaka M., Aoki H., Imanaka H. FR-900137, a new antibiotic. I. Taxonomy and fermentation of the organism, and isolation and characterization of the antibiotic. J. Antibiot. 1980;33:272–279. doi: 10.7164/antibiotics.33.272. [DOI] [PubMed] [Google Scholar]
- 29.Kuroda Y., Tanaka H., Okamoto M., Goto T., Kohsaka M., Aoki H., Imanaka H. FR-900137, a new antibiotic. II. Structure determination of FR-900137. J. Antibiot. 1980;33:280–283. doi: 10.7164/antibiotics.33.280. [DOI] [PubMed] [Google Scholar]
- 30.Alam M., Sanduja R., Hossain M.B., van der Helm D. Gymnodinium breve toxins. 1. Isolation and x-ray structure of O,O-dipropyl (E)-2-(1-methyl-2-oxopropylidene)phosphorohydrazidothioate (E)-oxime from the red tide dinoflagellate Gymnodinium breve. J. Am. Chem. Soc. 1982;104:5232–5234. doi: 10.1021/ja00383a043. [DOI] [Google Scholar]
- 31.Zheng D., Jiang Y., Han L., Lou K., Chen Y., Xu L., Huang X. Chemical constituents from mycelium of a new streptomycete. Zhongguo Yaowu Huaxue Zazhi. 2010;20:201–205. [Google Scholar]
- 32.Huang G., Jiang P., Li X.-F. Mass spectrometry identification of N-chlorinated dipeptides in drinking water. Anal. Chem. 2017;89:4204–4209. doi: 10.1021/acs.analchem.7b00228. [DOI] [PubMed] [Google Scholar]
- 33.Abou-Shoer M.I., Shaala L.A., Youssef D.T.A., Badr J.M., Habib A.M. Bioactive brominated metabolites from the Red Sea sponge Suberea mollis. J. Nat. Prod. 2008;71:1464–1467. doi: 10.1021/np800142n. [DOI] [PubMed] [Google Scholar]
- 34.Shaala L.A., Youssef D.T.A., Alzughaibi T.A., Elhady S.S. Antimicrobial chlorinated 3-phenylpropanoic acid derivatives from the Red Sea marine actinomycete Streptomyces coelicolor LY001. Mar. Drugs. 2020;18:450. doi: 10.3390/md18090450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Acar J.F. The disc susceptibility test. In: Lorian V., editor. Antibiotics in Laboratory Medicine, Williams and Wilkins, Baltimore. Williams & Wilkins; Philadelphia, PA, USA: 1980. pp. 24–54. [Google Scholar]
- 36.Kiehlbauch J.A., Hannett G.E., Salfinger M., Archinal W., Monserrat C., Carlyn C. Use of the National Committee for Clinical Laboratory Standards Guidelines for Disk Diffusion Susceptibility Testing in New York State Laboratories. J. Clin. Microbiol. 2000;38:3341–3348. doi: 10.1128/JCM.38.9.3341-3348.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shaala L.A., Khalifa S.I., Mesbah M.K., van Soest R.W.M., Youssef D.T.A. Subereaphenol A, a new cytotoxic and antimicrobial dibrominated phenol from the Red Sea sponge Suberea mollis. Nat. Prod. Commun. 2008;3:219–222. doi: 10.1177/1934578X0800300222. [DOI] [Google Scholar]
- 38.CLSI . Performance Standards for Antimicrobial Disk Susceptibility Tests. approved standard 9th ed. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2007. CLSI Documents M07-A9. West Valley Road, Suite 2500. [Google Scholar]
- 39.Youssef D.T.A., Mooberry S.L. Hurghadolide A and swinholide I, potent actin-microfilament disrupters from the Red Sea sponge Theonella swinhoei. J. Nat. Prod. 2006;69:154–157. doi: 10.1021/np050404a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.