Abstract
Introduction
The safety of upper gastrointestinal cancer patients in the SARS-CoV-2 outbreak is extremely important and most surgeons need to establish a contingency management.
Aim
In this study, we present the surgical outlines of patients suffering from upper gastrointestinal cancers.
Materials and Methods
Data were obtained from PubMed, Cochrane Database of Controlled Trials, and SCOPUS of reports up to September 2020.
Results
The COVID-19 outbreak makes surgical procedures extremely difficult to be performed. The most common criteria to prioritize patients for surgical treatment are stage, tumor biology, presence of tumor-related symptoms, the risk of tumor to become non-resectable, and time interval from neoadjuvant therapy. The multidisciplinary teams can help assigning a priority level to each clinical case.
Conclusion
We have to continue providing treatment to oncologic patients in the face of COVID-19 uncertainty, with higher caution and responsibility in order to develop a safer and more effective personalized treatment plan.
Keywords: Upper gastrointestinal cancers, Surgery, COVID-19, Pandemic
Introduction
Coronavirus disease 2019 (COVID-19) pandemic is the major healthcare issue of 2020. It is caused by a novel beta-coronavirus, known as severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). This new respiratory illness is characterized by rapid human-to-human transmission, which resulted in its quick global spread. Since its outbreak at the end of 2019 and until the 15th of November 2020, COVID-19 has affected more than 53.5 million of individuals and is responsible for 1,305,164 deaths globally [1].
Scientific community has been focused on identifying predisposing factors for increased morbidity and complications. Patients with comorbidities, like cancer, seem to develop complications after COVID-19 infection [2]. More specifically, Chinese cancer patients with COVID-19 demonstrated a higher risk for developing severe events (admission to intensive care unit, need for mechanical ventilation, or death), compared to patients without cancer [2, 3]. Moreover, several factors that are present in the majority of cancer patients have been demonstrated to significantly increase the risk of infection, including frequent hospital visits and immunosuppression caused by an underlying malignancy or anticancer therapy [4]. Due to this and by taking into consideration the high pressure of health systems after the initial outbreak of the pandemic, a re-evaluation of cancer patients’ management seems to be necessary.
Upper gastrointestinal (GI) tract (esophageal and gastric) malignancies rank between the ten most common malignancies worldwide, while gastric cancer still remains one of the leading causes of cancer-associated mortality. The incidence of upper GI malignancies varies widely, based on geographic location, race, and socioeconomic status. Interestingly, regions with high COVID-19 incidence, such as China, Japan, Central, and South America, also represent areas with the highest occurrence of esophageal and non-cardiac gastric cancer [5].
The purpose of the present study is to present the risk factors for developing a COVID-19 infection and to summarize all standardized medical practice modifications, during the pandemic, in terms of diagnosis and management of upper GI tract cancer patients.
Materials and Methods
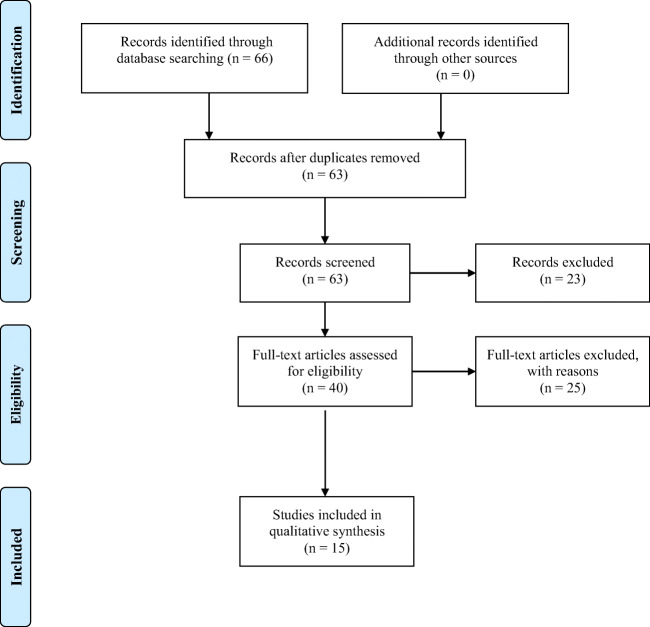
We included papers offering data concerning surgical aspects of upper gastrointestinal cancer patients, during the COVID-19 outbreak. Electronic databases were searched, by using the following terms: “Covid-19 upper gastrointestinal cancer,” “Covid-19 gastric cancer,” “Covid-19 esophageal cancer.” PubMed, Cochrane Database of Controlled Trials, and SCOPUS were searched until September 2020. Cross references from the included studies were hand-searched. In order to identify studies not captured by our search method, we examined the reference lists of similar articles. The studies that clearly did not meet the inclusion criteria were excluded. We used papers only in the English language (Fig. 1).
Fig. 1.
The studies that clearly did not meet the inclusion criteria were excluded. We used papers only in the English language
Results
COVID-19 Infection and Associated Outcomes in Upper GI Tract Cancer Patients
Most of the oncologic patients are immunosuppressed, in poor nutritional status, and, combined with other systemic primary diseases such as chronic lung disease, diabetes, cardiovascular, and chronic kidney disease, they are at increased risk of COVID-19 infection. Immunosuppression constitutes a sequela (a) of the malignancy itself and (b) of the anticancer treatments (e.g., chemotherapy, immunotherapy, radiotherapy, or surgery) [6]. Considering their altered immunologic status, it has been assumed that cancer patients might present an increased risk for COVID-19 infection resulting in poor prognosis [4] (Table 1).
Table 1.
A comprehensive table of the included studies with the most prominent outcomes
| Author | Year | Type of study | Country of origin | Number of patients (n) | Type of cancer | Morbidity/mortality | Conclusion |
|---|---|---|---|---|---|---|---|
| Wu Z et al. | 2020 | Retrospective | China | NR | Esophageal or gastric |
Morbidity: 4.7% Mortality: 2.3% |
More common adverse events in COVID-19 cancer patients |
| Perioperative neoadjuvant therapy significant predictor of severe clinical events | |||||||
| Torzilli et al. | 2020 | Retrospective | Italy | NR | Esophageal or gastric | NR | 60% reduction of hospital beds |
| 55% reduction of surgical activity | |||||||
| Fligor et al. | 2020 | Review | NR | 3961 | Gastric | NR | No impact of treatment delay on gastric patients’ survival rate |
| Shipe et al. | 2020 | Decision analysis model | USA | NR | Esophageal | NR | A delayed approach of early esophageal cancer is associated with an improved 5-year survival rate, because of COVID-19 perioperative complications and adverse events |
The first study that investigated the clinical characteristics of cancer patients infected by COVID-19 was published in China in January 2020, with lung cancer being the most frequently encountered malignancy (28%), whereas no upper GI tract cancer cases were included. The findings of this study demonstrated that cancer incidence was 1% among COVID-19-infected patients (18 out of 1590 confirmed COVID-19 cases), which is much higher than the general Chinese cancer population (0.29% according to 2015 cancer epidemiology statistics) [7]. Additionally, cancer patients presented an increased risk for severe adverse events (admission to intensive care unit requiring invasive ventilation or death), compared to non-cancer patients, and the highest risk was observed in patients who underwent chemotherapy, radiotherapy, or surgical operation within the last 30 days [6].
Oncologic patients’ increased risk for adverse outcomes due to COVID-19 was also confirmed by another retrospective study conducted in Wuhan, China, which revealed that 2.2% (28 out of 1276) of patients admitted for COVID-19 had a cancer history, with 53.6% of them developing a severe event and 28.6% finally succumbing [3].
However, the aforementioned high morbidity and mortality rates are in contrast to the findings of another study, which demonstrated morbidity and mortality rates of 4.7% and 2.3% in COVID-19 confirmed cancer cases, respectively [8]. In more detail, 17.8% of the participants suffered from esophageal or gastric cancer, with a mean age of 65.4 years. Severe events (admission to intensive care unit, life-threatening complications, or death) were presented in 60% of the upper GI cancer patients, and anti-tumor therapies (chemotherapy, radiotherapy, targeted therapy, and immunotherapy), offered within 14 days before COVID-19 diagnosis, seemed to be significant predictors of severe clinical events.
Regarding the clinical characteristics of COVID-19 upper GI tract cancer patients, the previously mentioned study revealed that these patients suffer from the same symptoms as the general population, except for anemia and hypoproteinemia, which were observed more frequently in them [8]. Published data suggest that approximately 70% of all esophageal cancer patients suffer from weight loss, caused by gastrointestinal obstruction, anorexia, and/or cancer cachexia that may adversely affect their immune status increasing their susceptibility to respiratory pathogens [9]. Considering these findings, the nutritional deterioration of upper GI tract cancer patients might be a plausible explanation for the increased rates of anemia and hypoproteinemia, which have been observed in such patients diagnosed with COVID-19.
Considering the aforementioned results, cancer patients seem to be at an increased risk for COVID-19 infection, with the infected cancer patients demonstrating a higher complication rate, compared to their non-infected counterparts. Anti-tumor therapies have been demonstrated as an important factor predisposing COVID-19 cancer patients to adverse events.
Upper GI Tract Endoscopy During COVID-19 Pandemic
Endoscopy and biopsy still remain the gold standard for the diagnosis of GI tract lesions. However, endoscopy is an aerosol-generating procedure and may cause cross infection of COVID-19 to doctors and patients [10].
The high prevalence of asymptomatic carriers in the community, the limited availability of COVID-19 testing in many GI units, the conversion of endoscopy suites to COVID-19 care units, and the re-deployment of healthcare workers to COVID-19 units led to an update of GI endoscopy guidelines during the pandemic [11]. Many organizations, medical associations, and societies have recommended that only emergent endoscopic procedures should be performed during the pandemic outburst. More specifically, the New York Society for Gastrointestinal Endoscopy issued guidelines that minimize endoscopy utilization and conserve resources. According to them, physicians should consider non-invasive testing as a first option, maximize medical therapies before the intervention, consider interventional radiology procedures, prioritize procedures that reduce the length of hospital stay, and encourage endoscopic procedures aiming to avoid surgery [10, 11]. At the same context, the European Society of Gastrointestinal Endoscopy (ESGE) issued guidelines that minimize endoscopy utilization during the pandemic. In more detail, endoscopic screening of high-risk patients for esophageal or gastric cancer should not be performed during the pandemic outburst but should be postponed for a period of at least 12 weeks, as these cases were classified as low priority procedures [12].
Following these updated guidelines, a retrospective cohort study was conducted in China between February 20 and March 6, 2020, which analyzed all clinical data of elective endoscopy as well as the detection rate of GI malignancy and compared them with the data of the same period of the previous year. The number of elective endoscopic procedures was greatly decreased compared to 2019 (911 vs 5746). The detection rate of GI malignancies during the epidemic was significantly increased for both esophageal and gastric cancer. Upper GI endoscopy identified 39 cancer cases (7.2%) during the epidemic, compared to 77 cancer cases (2.2%) during the same period of the previous year [13].
Apparently, endoscopy remained the procedure of choice for the diagnosis of GI tract lesions during the pandemic, maintaining its advantages over other diagnostic procedures in terms of diagnostic accuracy. However, the diagnostic management of upper GI tract lesions during the pandemic had to be adapted to the updated guidelines; thus, non-invasive diagnostic techniques, including contrast-enhanced computed tomography (CT) and positron emission tomography-computed tomography (PET-CT), had been widely implemented [14].
Considering the aforementioned results, endoscopy and biopsy still remain the gold standard for the diagnosis of GI tract lesions. However, its aerosol-generating potential may be responsible for COVID-19 cross infection to doctors and patients. As a result, the role of endoscopy has to be adapted during the COVID-19 pandemic, by prioritizing only emergent endoscopic procedures, whereas elective endoscopy as an initial diagnostic modality should be either substituted by non-invasive diagnostic techniques or postponed for period of at least 12 weeks.
The Impact of COVID-19 Pandemic on Esophagogastric Oncological Surgery and the Need for Implementation of Hub-and-Spoke Frameworks
COVID-19 pandemic outburst shocked national health systems, resulting in massive subtraction of resources that were planned to be used for the management of other diseases. Due to the SARS-CoV-2 virus pandemic, the anesthetic unavailability may represent a capacity problem. All these led to the cancelation of elective surgical operations, including surgeries for the treatment of upper GI tract cancer. More specifically, the results of a study published in Italy, which had been heavily affected by the COVID-19 pandemic, were highly representative of this phenomenon. Following the pandemic outbreak, 70% of the oncological units had a reduction of hospital beds (median: 50%) and a 76% reduction of their surgical activity. Additionally, the number of oncological surgical procedures was significantly decreased, from 3.8 to 2.6 procedures per week (p = 0.036). The most common criteria to prioritize patients for surgical treatment were tumor biology (80%), time interval from neoadjuvant therapy (61%), risk of tumor becoming non-resectable (57%), and presence of tumor-related symptoms (52%). Despite all these efforts, the time interval between multidisciplinary discussion and surgical treatment was disproportionally increased, from 3 weeks prior to the pandemic outbreak to 7 weeks after it (p < 0.001) [15].
Considering the delay in the surgical management of oncological cases, a recently published review aimed to evaluate its impact on patients’ oncological outcomes. Six studies reporting on the impact of gastric cancer treatment delay on oncological outcomes were included. The authors concluded that there was no deterioration of gastric cancer patients’ survival rate because of increased time to surgery [16].
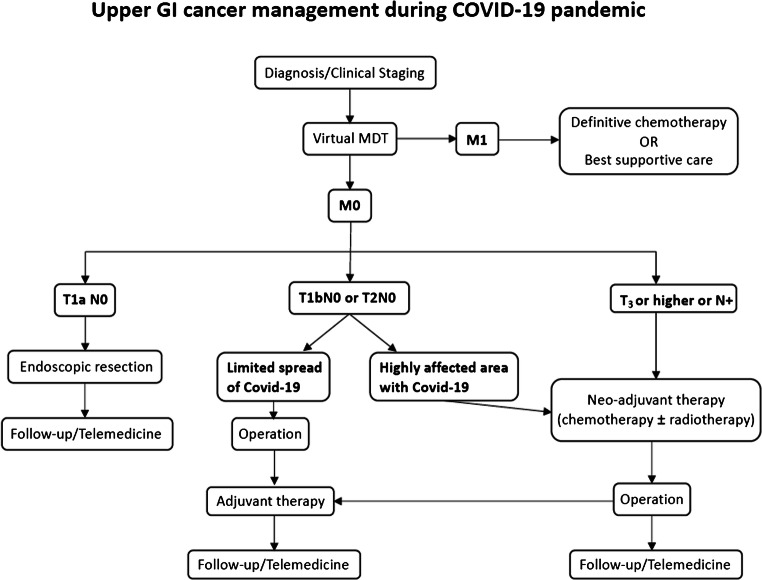
According to the Society of Surgical Oncology (SSO) updated guidelines for the surgical management of esophageal or gastric cancer cases during the pandemic, all cT1a lesions amenable to endoscopic resection should be preferentially managed by endoscopic procedures, cT1b cancers should be surgically resected, while cT2 or higher T status tumors and lymph node-positive tumors (N positivity) should be first treated with neoadjuvant therapy, followed by chemotherapy in patients that responded to neoadjuvant treatment [17]. The aforementioned guidelines are in agreement with the European Society for Diseases of the Esophagus (ESDE) statement, except for the management of cT1b esophageal cancers, which should also be treated by endoscopic procedures [18].
Considering the alterations in the management of upper GI cancer cases stated in the updated guidelines, the aforementioned lack of association between increased time to surgery and gastric cancer survival rate should be cautiously interpreted [16]. The increased utilization of neoadjuvant therapy in the updated guidelines (in an effort to delay invasive procedures due to their associated risk for the viral spread) might be responsible for the unaffected survival rate of gastric cancer patients after an increased time to surgery, which needs further investigation.
As previously stated, the experience from highly affected regions has demonstrated that lack of adherence to a national hub-and-spoke program inevitably leads to healthcare system overload, resulting in a dramatic reduction of oncological hospital beds and surgical activity [15]. Therefore, every effort should be made to establish clinical frameworks that would allow a time critical oncological surgery during the COVID-19 pandemic. The principles and strategy during the pandemic of a center in the UK designated as “cancer hub” have been recently published [19]. According to their protocol, all elective surgical cancer patients were preoperatively requested to self-isolate for 14 days, complete a COVID-19-associated symptoms questionnaire and a COVID-19-associated risk consent form, and undergo a COVID-19 swab testing and a thoracic CT prior to surgery. Furthermore, full personal protective equipment usage was compulsory for all members of the theater team. All cases were reviewed in multidisciplinary meetings and therapeutic plans were individualized. In addition, their priority had been assessed with “grade one” for emergent/urgent surgery or “grade two” for operations that could be deferred up to 4 weeks. Regarding upper GI tract cancer cases, patients presenting tumors causing either hemorrhage or gastric outlet obstruction not amenable to endoscopic/interventional radiological management were considered as surgical candidates. Given their poor prognosis and the subsequent need for a prolonged postoperative level three care, all esophageal cancer emergencies (bleeding/perforation) were considered for endoscopic/radiological intervention. Additionally, cT1a and cT1b esophageal/gastric tumors were initially evaluated by a gastroenterologist specialized in upper GI endoscopy and were considered for endoscopic resection. Esophageal or gastric cancer patients on an intention-to-cure pathway, who had already completed their neoadjuvant chemotherapy and had undergone a post-chemotherapy assessment of resectability/treatment response, were also considered for surgical resection. Following the aforementioned framework, authors successfully managed to triage upper GI cancer patients in a resource-limited environment and safely deliver the maximum number of potentially curative operations during the COVID-19 pandemic [19].
The COVID-19 pandemic has definitely led to a reduction in healthcare resources, which resulted in a decreased surgical oncologic activity and an increased time interval between cancer diagnosis and its surgical treatment. Despite this treatment delay, the unaltered survival rate of gastric cancer patients during the pandemic should be cautiously interpreted, considering the increased implementation of neoadjuvant therapy in these patients. Apparently, the hub-and-spoke program implementation on a national basis has demonstrated the most efficient utilization of healthcare resources, which has led to the most effective and timely management of oncologic patients during the COVID-19 outburst.
Could COVID-19 Pandemic Reform the Treatment Algorithm of Upper GI Cancer Patients in the Post-COVID Era?
Regular healthcare services have already started to resume at a population—and health—service level in Europe. However, a backlog of patients with cancer symptoms needing urgent assessment should be awaited. Moreover, accurate pandemic recovery planning and strategies for the management of a possible second surge of COVID-19 should also be designed [11].
Establishing new forms of physician-patient relationship seems mandatory, addressing ongoing healthcare needs. Furthermore, the management of procedure backlogs definitely requires the implementation of three primary strategies/components: telehealth (to continue social distancing and maintain disease’s mitigation), risk stratification to maximize the benefit of scheduled procedures and minimize adverse events (which include risk of infection for the patients and/or medical staff and procedure-related complications), and methods for healthcare capacity and safety optimization, aiming to provide healthcare services to most possible people [20].
The appropriate therapeutic approach for each patient should be individualized, considering primarily the healthcare needs and the local health care system’s capability to meet existing and projected needs [21]. In areas with a limited spread of the pandemic, surgeons and physicians may propose more conventional indications [21]. On the other hand, in highly affected areas, healthcare workers should be prepared to compromise, following strategies which have low short-term complication rates and require less organizational efforts.
Considering upper GI cancer patients’ management, the increased implementation of neoadjuvant chemotherapy proposed by the updated guidelines during the pandemic has already demonstrated its advantages over the conventional treatment algorithm, in terms of successful management of these patients in a resource-limited environment. Before the implantation of these treatment alterations in post-COVID-19 everyday clinical practice, it would be interesting to see if these alterations could lead to better or at least equal upper GI cancer-associated survival rates, compared to the conventional (pre-COVID-19) treatment algorithms.
The COVID-19 pandemic could be seen as a great opportunity for the healthcare system reframing. Following the current pandemic successful management, it would be useful for health systems to adopt all the improvements of telemedicine and subsequently form the basis for an alternative form of patient-physician relationship [22]. Remote follow-up and screening programs might be established, leading to a more careful and quick evaluation of oncologic patients’ needs and complications. This approach might also favor a reduced length of hospital stay for all patients, especially upper GI tract oncologic patients. The multidisciplinary teams (MDT) should determine therapeutic options case-by-case. The MDT attendance can be virtual (e.g., video consultations or by telephone [23].
The COVID-19 pandemic may be a great opportunity for healthcare systems’ reframing. It would be useful to see all the improvements in telemedicine, patients’ risk stratification, and triage as well as the adoption of hub-and-spoke programs to be implemented in contemporary healthcare systems (Fig. 2).
Fig. 2.
It would be useful to see all the improvements in telemedicine, patients’ risk stratification, and triage as well as the adoption of hub-and-spoke programs to be implemented in contemporary healthcare systems
Conclusion
The entire landscape of cancer management, from case identification to management of people living with and beyond cancer, is rapidly evolving during the COVID-19 pandemic. This unprecedented healthcare crisis has resulted in enormous disruption of routine medical procedures, challenging the existing approaches for optimal oncologic planning, and care. In the setting of ongoing rates of COVID-19, reconsidering deferred medical and surgical procedures essential for upper GI tract cancer management requires a balanced and data-driven framework that prioritizes procedures scheduling based on medical urgency (likelihood of patient benefiting), the risk for COVID-19 infection, inherent procedural risk and availability of personal protective equipment, and preoperative SARS-CoV-2 testing. Adoption of this framework may help minimize the potential impact of deferred care and diminish additional waves of adverse outcomes among those affected by the COVID-19 pandemic until the threat of recurrent epidemic surges subsides. The uncertainty of this crisis demands healthcare systems to prepare for sustained mitigation/suppression policies, along with physicians’ and patients’ commitment to discuss critical therapeutic decisions as well as alterations to the conventional therapeutic plans.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Organization WH (2020) WHO Coronavirus Disease (COVID-19) Dashboard. https://covid19.who.int. Accessed 15 Nov 2020.
- 2.Moujaess E, Kourie HR, Ghosn M. Cancer patients and research during COVID-19 pandemic: a systematic review of current evidence. Crit Rev Oncol Hematol. 2020;150:102972. doi: 10.1016/j.critrevonc.2020.102972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang L, Zhu F, Xie L, Wang C, Wang J, Chen R, Jia P, Guan HQ, Peng L, Chen Y, Peng P, Zhang P, Chu Q, Shen Q, Wang Y, Xu SY, Zhao JP, Zhou M. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol. 2020;31(7):894–901. doi: 10.1016/j.annonc.2020.03.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Shamsi HO, Alhazzani W, Alhuraiji A, Coomes EA, Chemaly RF, Almuhanna M, Wolff RA, Ibrahim NK, Chua MLK, Hotte SJ, Meyers BM, Elfiki T, Curigliano G, Eng C, Grothey A, Xie C. A practical approach to the management of cancer patients during the novel coronavirus disease 2019 (COVID-19) pandemic: an international collaborative group. Oncologist. 2020;25(6):e936–e945. doi: 10.1634/theoncologist.2020-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crew KD, Neugut AI. Epidemiology of upper gastrointestinal malignancies. Semin Oncol. 2004;31(4):450–464. doi: 10.1053/j.seminoncol.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 6.Xia Y, Jin R, Zhao J, Li W, Shen H. Risk of COVID-19 for patients with cancer. Lancet Oncol. 2020;21(4):e180. doi: 10.1016/S1470-2045(20)30150-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng RS, Sun KX, Zhang SW, Zeng HM, Zou XN, Chen R, Gu XY, Wei WW, He J. Report of cancer epidemiology in China, 2015. Zhonghua Zhong Liu Za Zhi. 2019;41(1):19–28. doi: 10.3760/cma.j.issn.0253-3766.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Qin JJ, Wang Z, Yu Y, Wen YY, Chen XK, Liu WX, Li Y. Surgical treatment for esophageal cancer during the outbreak of COVID-19. Zhonghua Zhong Liu Za Zhi. 2020;42(4):296–300. doi: 10.3760/cma.j.cn112152-20200226-00128. [DOI] [PubMed] [Google Scholar]
- 10.Philip M, Lakhtakia S, Aggarwal R, Madan K, Saraswat V, Makharia G. Joint guidance from SGEI, ISG and INASL for gastroenterologists and gastrointestinal endoscopists on the prevention, care, and management of patients with COVID-19. J Clin Exp Hepatol. 2020;10(3):266–270. doi: 10.1016/j.jceh.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sethi A, Swaminath A, Latorre M, Behin DS, Jodorkovsky D, Calo D, Aroniadis O, Mone A, Mendelsohn RB, Sharaiha RZ, Gonda TA, Khanna LG, Bucobo JC, Nagula S, Ho S, Carr-Locke DL, Robbins DH, New York Society for Gastrointestinal E Donning a new approach to the practice of gastroenterology: perspectives from the COVID-19 pandemic epicenter. Clin Gastroenterol Hepatol. 2020;18(8):1673–1681. doi: 10.1016/j.cgh.2020.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gralnek IM, Hassan C, Beilenhoff U, Antonelli G, Ebigbo A, Pellise M, Arvanitakis M, Bhandari P, Bisschops R, Van Hooft JE, Kaminski MF, Triantafyllou K, Webster G, Pohl H, Dunkley I, Fehrke B, Gazic M, Gjergek T, Maasen S, Waagenes W, de Pater M, Ponchon T, Siersema PD, Messmann H, Dinis-Ribeiro M. ESGE and ESGENA position statement on gastrointestinal endoscopy and the COVID-19 pandemic. Endoscopy. 2020;52(6):483–490. doi: 10.1055/a-1155-6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu L, Cai MY, Shi Q, Wang P, Li QL, Zhong YS, Yao LQ, Zhou PH. Analysis of selective endoscopy results during the epidemic of coronavirus disease 2019 (COVID-19) Zhonghua Wei Chang Wai Ke Za Zhi. 2020;23(4):327–331. doi: 10.3760/cma.j.issn.1671-0274.2020-0316-00147. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Xu JM. Medical diagnosis and treatment strategies for malignant tumors of the digestive system during the outbreak of COVID-19. Zhonghua Zhong Liu Za Zhi. 2020;42(3):184–186. doi: 10.3760/cma.j.cn112152-20200227-00141. [DOI] [PubMed] [Google Scholar]
- 15.Torzilli G, Vigano L, Galvanin J, Castoro C, Quagliuolo V, Spinelli A, Zerbi A, Donadon M, Montorsi M, group C-S-I A snapshot of elective oncological surgery in Italy during COVID-19 emergency: pearls, pitfalls, and perspectives. Ann Surg. 2020;272(2):e112–e117. doi: 10.1097/SLA.0000000000004081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fligor SC, Wang S, Allar BG, Tsikis ST, Ore AS, Whitlock AE, Calvillo-Ortiz R, Arndt KR, Gangadharan SP, Callery MP. Gastrointestinal malignancies and the COVID-19 pandemic: evidence-based triage to surgery. J Gastrointest Surg. 2020;24:2357–2373. doi: 10.1007/s11605-020-04712-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bartlett DL, Howe JR, Chang G, Crago A, Hogg M, Karakousis G, Levine E, Maker A, Mamounas E, McGuire K, Merchant N, Shibata D, Sohn V, Solorzano C, Turaga K, White R, Yang A, Yoon S, Society of Surgical O Management of cancer surgery cases during the COVID-19 pandemic: considerations. Ann Surg Oncol. 2020;27(6):1717–1720. doi: 10.1245/s10434-020-08461-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barbieri L, Talavera Urquijo E, Parise P, Nilsson M, Reynolds JV, Rosati R. Esophageal oncologic surgery in SARS-CoV-2 (COVID-19) emergency. Dis Esophagus. 2020;33(5). 10.1093/dote/doaa028. [DOI] [PMC free article] [PubMed]
- 19.Bhogal RH, Patel PH, Doran SLF, Zar S, Pollok JM, Jiao LR, Allum WH, Chaudry MA, Kumar S. Approach to upper gastroIntestinal cancer surgery during the COVID-19 pandemic - experience from a UK cancer centre. Eur J Surg Oncol. 2020;46:2156–2157. doi: 10.1016/j.ejso.2020.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rouillard S, Liu VX, Corley DA. COVID-19: long-term planning for procedure-based specialties during extended mitigation and suppression strategies. Gastroenterology. 2020. 10.1053/j.gastro.2020.05.047. [DOI] [PMC free article] [PubMed]
- 21.Mauri D, Tzachanis D, Valachis A, Kamposioras K, Tolia M, Dambrosio M, Zarkavelis G, Gkoura S, Gazouli I, De Lorenzo F, Apostolidis K. Behind the numbers and the panic of a viral pandemic: fixed restrictive oncology guidance may jeopardize patients’ survival. J BUON. 2020;25(3):1277–1280. [PubMed] [Google Scholar]
- 22.Mauri D, Kamposioras K, Tolia M, Alongi F, Tzachanis D, International Oncology P, European Cancer Patient Coalition c Summary of international recommendations in 23 languages for patients with cancer during the COVID-19 pandemic. Lancet Oncol. 2020;21(6):759–760. doi: 10.1016/S1470-2045(20)30278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.(FSSA) FoSSA (2020) Clinical guide to surgical prioritisation during the coronavirus pandemic.