Abstract
Antimicrobial resistance (AMR) is a global threat. A better understanding of how antibiotic use and between-ward patient transfers (or connectivity) impact population-level AMR in hospital networks can help optimize antibiotic stewardship and infection control strategies. Here, we used a metapopulation framework to explain variations in the incidence of infections caused by seven major bacterial species and their drug-resistant variants in a network of 357 hospital wards. We found that ward-level antibiotic consumption volume had a stronger influence on the incidence of the more resistant pathogens, while connectivity had the most influence on hospital-endemic species and carbapenem-resistant pathogens. Piperacillin-tazobactam consumption was the strongest predictor of the cumulative incidence of infections resistant to empirical sepsis therapy. Our data provide evidence that both antibiotic use and connectivity measurably influence hospital AMR. Finally, we provide a ranking of key antibiotics by their estimated population-level impact on AMR that might help inform antimicrobial stewardship strategies.
Research organism: E. coli, Other
Introduction
Antimicrobial resistance (AMR) of pathogenic bacteria progresses worldwide, imposing a considerable burden of morbidity, mortality and healthcare costs (Laxminarayan et al., 2013; Cassini et al., 2019). AMR is increasingly recognized to emerge in various settings including agriculture (Johnson et al., 2016) or polluted environments (Lübbert et al., 2017; Venter et al., 2017). However, hospitals continue to be important hotspots for AMR in clinically-relevant pathogens (Chatterjee et al., 2018; David et al., 2019) due to the confluence of strong antibiotic selection pressure, fragile patients, and highly resistant pathogens that can disseminate between wards and facilities through patient transfers (Safdar and Maki, 2002; Snitkin et al., 2012). These conditions result in hospitals becoming reservoirs of resistant bacteria (Clarivet et al., 2016; Cohen, 1992; Pogue et al., 2013), which can later enter the community (Huttner et al., 2013).
The primary hospital-based strategies against AMR are antimicrobial stewardship, which aims to lower the antibiotic pressure, and infection control whose goal is to reduce the transmission of pathogens (Manning et al., 2018). The need for such strategies is widely accepted but because they are often implemented together, the relative importance of each is unclear (Chatterjee et al., 2018; Goff et al., 2017). The details of how to best implement these strategies are hotly debated (Lemmen and Lewalter, 2018), especially regarding which antibiotics should be restricted first as a part of antibiotic stewardship strategies (Chastain et al., 2018) or the risk-benefit balance of screening-based patient isolation procedures to reduce transmission (Kardaś-Słoma et al., 2017; Robotham et al., 2016). Designing effective antibiotic stewardship strategies has been hindered by the paucity of evidence concerning which antibiotics exert the strongest selection pressure. Current rankings of antibiotics for de-escalation and sparing strategies rely on expert consensus with partial agreement (Weiss et al., 2015), themselves based on conflicting evidence (Acar, 1997; Huttner et al., 2016). This is further complicated by the fact that the association between antibiotic use and resistance is not uniform across pathogen species (Bell et al., 2014) or classes of antibiotics (Niehus et al., 2020). Moreover, antibiotic consumption can also have long-term effects on the carriage of resistant bacteria within patients (Niehus et al., 2020).
Understanding the drivers of AMR at the hospital level requires consideration not only of the selection pressure from antibiotics on individual patients, but also of the transmission and dissemination of drug-resistant pathogens in the patient population (Lipsitch, 2001; Lipsitch and Samore, 2002). However, we lack a quantitative understanding of the respective impacts of selection and transmission on the incidence of AMR infections in hospitals. Observational studies of AMR usually report on the proportion of resistant variants in a limited set of species, which conceals the overall AMR burden and can make interpretation difficult when resistant variants apparently increase in proportion while decreasing in incidence (Burton, 2009). Moreover, linking antibiotic use and AMR prevalence at the population level is difficult due to the confounding effects of bacterial transmission and the complexity of the ecological processes underlying AMR (review in Schechner et al., 2013). Thus, studies of AMR in hospitals could benefit from ecological frameworks able to simultaneously model the impact of antibiotic use and patient transfers on the incidence of infections with the most relevant pathogens. Metapopulation ecology is such a framework. It was introduced by Levins, 1969 to explain the persistence of agricultural pests across a set of habitat patches, such as crop fields, and refined by Hanski to account for the characteristics of patches, such as their size, and the connectivity between them (Hanski, 1998; Hanski, 1994). The metapopulation concept, in which populations of organisms are spread across inter-connected patches with varying characteristics, is useful to describe bacterial pathogens in a hospital network containing wards with different sizes and levels of antibiotic pressure, connected by the transfers of infected patients. Models using the metapopulation framework, beyond their frequent use in wildlife and conservation biology (Dolrenry et al., 2014; Heard et al., 2015; MacPherson and Bright, 2011), have recently provided theoretical grounds for pathogen persistence in the healthcare setting (Spagnolo et al., 2018). So far, however, metapopulation models of hospital AMR have been applied to simulated rather than empirical data (Spagnolo et al., 2018; Vilches et al., 2019).
Here, we used a metapopulation framework to model and isolate the population-level effects of antibiotic use and inter-ward connectivity on the incidence of infections with major pathogen species and their drug-resistant variants within a 357-ward hospital network, using detailed data collected over the course of one year. Our objectives were: (1), to determine the respective impacts of antibiotic use and connectivity on the incidence of infections with resistant pathogens at the population level; and (2), to compare the impacts of the use of specific antibiotics on the ward-level incidence of AMR infections, after controlling for the effect of connectivity. Based on the association patterns between the incidence of 17 pathogen variants and the use of 11 antibiotic classes, our findings highlight both common patterns and species-specific behaviors of pathogens and provide a ranking of key antibiotics by their estimated population-level impact on AMR.
Results
Distribution of bacterial pathogens and antibiotic use in a hospital network
We analyzed pathogen isolation incidence in clinical samples, antibiotic use, and patient transfers in 357 hospital wards from the region of Lyon, France. Data for all three measures were collected during the same period from October 2016 to September 2017. The hospital network contained a total of 4,685 beds. The median ward size was 12 beds (interquartile range, 5 to 20).
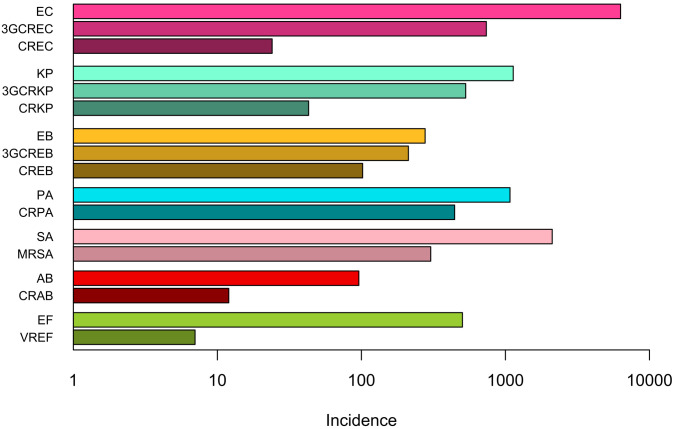
Ward-level data were aggregated from 13,915 infection episodes, defined as ward admissions with ≥1 clinical sample positive for E. coli or one of the so-called ESKAPE pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa and Enterobacter cloacae complex), collectively termed ESKAPE2 (Table 1). Pathogens were grouped into species-resistance pattern combinations, namely 3rd-generation cephalosporin (3GC)-resistant E. coli, E. cloacae complex and K. pneumoniae, carbapenem-resistant E. coli, E. cloacae complex, K. pneumoniae, P. aeruginosa and A. baumannii, vancomycin-resistant E. faecium and methicillin-resistant S. aureus (MRSA). Pathogen variants not falling into these resistance groups were collectively referred to as the less-resistant variants (Table 1). The median yearly incidence of infection episodes per ward was 24 (interquartile range, 7 to 55).
Table 1. Distribution of ESKAPE2 pathogen infection episodes in 357 hospital wards.
| Species | Resistance profile | Acronym | No. of episodes (%), n = 13,915 | No. of wards (%), n = 357 | Concentration indexa (%) (95% CI) |
|---|---|---|---|---|---|
| E. coli | Susceptible to 3GC and carbapenems | EC | 6,303 (45.3) | 328 (91.9) | 0.6 (0.6, 0.7) |
| 3GC-resistant | 3GCREC | 737 (5.3) | 207 (58.0) | 0.7 (0.6, 0.8) | |
| Carbapenem-resistant | CREC | 24 (0.2) | 24 (5.6) | 1.4 (0.0, 3.9) | |
| K. pneumoniae | Susceptible to 3GC and carbapenems | KP | 1,133 (8.1) | 249 (69.7) | 0.7 (0.6, 0.8) |
| 3GC-resistant | 3GCRKP | 530 (3.8) | 175 (49.0) | 0.9 (0.7, 1.0) | |
| Carbapenem-resistant | CRKP | 43 (0.3) | 32 (9.0) | 1.7 (0.0, 3.5) | |
| E. cloacae complex | Susceptible to 3GC and carbapenems | EB | 277 (2.0) | 140 (39.2) | 1.0 (0.7, 1.3) |
| 3GC-resistant | 3GCREB | 212 (1.5) | 116 (32.5) | 0.8 (0.5, 1.0) | |
| Carbapenem-resistant | CREB | 102 (0.7) | 74 (20.7) | 0.7 (0.3, 1.1) | |
| P. aeruginosa | Carbapenem-susceptible | PA | 1,076 (7.7) | 231 (64.7) | 0.8 (0.7, 0.9) |
| Carbapenem-resistant | CRPA | 444 (3.2) | 148 (41.5) | 1.5 (1.2, 1.7) | |
| A. baumannii | Carbapenem-susceptible | AB | 96 (0.7) | 61 (17.1) | 1.3 (0.5, 2.1) |
| Carbapenem-resistant | CRAB | 12 (0.1) | 10 (2.8) | 3.0 (0.0, 9.8) | |
| E. faecium | Vancomycin-susceptible | EF | 503 (3.6) | 133 (27.3) | 1.4 (1.2, 1.6) |
| Vancomycin-resistant | VREF | 7 (<0.1) | 7 (2.0) | 0.0 (0.0, 9.6) | |
| S. aureus | Methicillin-susceptible | SA | 2,113 (15.2) | 273 (76.5) | 1.1 (1.0, 1.1) |
| Methicillin-resistant | MRSA | 303 (2.2) | 151 (42.3) | 0.7 (0.5, 0.9) |
NOTE. aThe concentration index estimates the probability that two episodes taken at random occurred in the same ward. Here we report the concentration index as a percent (0–100%). 3GC, 3rd-generation cephalosporins.
Infection episodes most frequently involved the less-resistant variants, especially E. coli, which were also found in the largest number of wards (Table 1 and Figure 1—figure supplement 1). Resistant variants were consistently less frequent than their less-resistant counterparts in all species (Figure 1—figure supplement 1). In enterobacteria (E. coli, K. pneumoniae and E. cloacae), carbapenem-resistant variants were consistently less frequent than 3GC-resistant variants. Infections with vancomycin-resistant E. faecium and carbapenem-resistant A. baumannii were exceptional, with seven and twelve episodes respectively (Table 1).
To estimate the degree of concentration of each variant in the network, we calculated concentration indices defined as the probability that two random occurrences of the same variant originated from the same ward, analogous to the asymptotic Simpson index (see Materials and methods). The concentration index varies from 0% for a uniformly random distribution (each occurrence is in a different ward) to 100% for a maximally concentrated distribution (all occurrences are in the same ward). The concentration of infection episodes was weak (<5%) for all variants, indicating a global lack of clustering (Table 1). Concentration increased with resistance (~2-fold increase from the least to the most resistant variant) in E. coli, K. pneumoniae, P. aeruginosa and A. baumannii, suggesting an adaptation of resistant variants to more specific wards within the hospital network compared to their less-resistant, less-concentrated counterparts. This pattern was not found in E. cloacae complex, E. faecium or S. aureus, in which the concentration index remained comparable across resistance categories.
Over the same period (October 2016 to September 2017), antibiotics were prescribed in 86.3% of wards (Table 2), with a total consumption of 125.7 defined daily doses per year per bed (ddd/y/b). Antibiotics usually suspected to select for AMR in the selected variants were grouped into 11 classes (Table 2). Antibiotics with comparatively rare use (e.g., rifampicin) were excluded. The distribution of antibiotic use in the network was analyzed using the concentration index described above, here representing the probability that two random drug doses were delivered in the same ward. Antibiotic use was diffuse, with concentration indices < 4%, ranging from 0.8% for cefotaxime-ceftriaxone and fluoroquinolones to 3.6% for oxacillin.
Table 2. Distribution of the use of 11 antibiotics in 357 hospital wards.
| Antibiotics | Acronym | Prescription volume in ddd/y (%) | No. of wards (%), n = 357 | Concentration indexa (%), (95% CI) |
|---|---|---|---|---|
| Amoxicillin | AMX | 141,293 (24.0) | 252 (70.6) | 1.8 (1.7, 1.8) |
| Coamoxiclav | AMC | 78,072 (13.3) | 247 (69.3) | 1.0 (0.9, 1.0) |
| First- and second- generation cephalosporins | 1GC/2GC | 12,915 (2.2) | 191 (53.5) | 1.2 (1.1, 1.3) |
| Non-antipseudomonal 3GCs, cefotaxime and ceftriaxone | CTX/CRO | 53,406 (9.1) | 259 (72.5) | 0.8 (0.8, 0.9) |
| Antipseudomonal 3GCs, ceftazidime and cefepime | CTZ/FEP | 29,204 (5.0) | 184 (51.5) | 1.9 (1.8, 1.9) |
| Piperacillin-tazobactam | TZP | 27,593 (4.7) | 198 (55.5) | 1.9 (1.7, 1.9) |
| Carbapenems | IPM/MEM | 25,093 (4.3) | 204 (57.1) | 1.5 (1.4, 1.6) |
| Oxacillin | OXA | 12,374 (2.1) | 143 (40.1) | 3.6 (3.1, 3.7) |
| Vancomycin and teicoplanin | VAN/TEC | 25,376 (4.3) | 206 (57.7) | 1.5 (1.4, 1.5) |
| Fluoroquinolones | FQ | 52,549 (8.9) | 249 (69.7) | 0.8 (0.8, 0.8) |
| Aminoglycosides | AMIN | 12,745 (2.2) | 207 (58.0) | 1.9 (1.5, 1.9) |
| All antibioticsb | - | 589,014 (100) | 308 (86.3) | 0.8 (0.8, 0.8) |
NOTE. aThe concentration index estimates the probability that two antibiotic ddds taken at random were prescribed in the same ward. Here we report the concentration index as a percent (0–100%). bTotal consumption of systemic-use antibiotics (ATC class J01) including those not considered in the 11 specific drug groups. 3GC, 3rd-generation cephalosporin; ddd, defined daily dose.
Antibiotic use and connectivity predict the incidence of drug-resistant infections
We used multivariable generalized linear models (GLMs) within the metapopulation framework to disentangle the influences of antibiotic pressure, connectivity, ward size, and ward type on the incidence of infections with the selected pathogens and their resistant variants.
Connectivity quantifies the incoming flux of each pathogen variant in a downstream ward via the transfer of infected patients from upstream wards. Practically, we estimated connectivity for each variant and downstream ward as the sum of the direct transfers from each upstream ward multiplied by the variant’s prevalence in that ward (see Materials and methods). Mean connectivity ranged from 168.2 estimated introductions per year for less-resistant E. coli to 0.09 for vancomycin-resistant E. faecium. Connectivity was always higher for the less-resistant variants compared to the resistant variants, consistent with the higher prevalence of the former (Supplementary file 1 Table 1a).
Wards were characterized by their size (no. of beds) and type, representing patient fragility. Ward type was coded as one of the following categorical variables: intensive care and blood cancer units, progressive care units, and other wards. ‘Other wards’ was considered the reference category in all models. In several pathogen variants, namely carbapenem-resistant E. coli, carbapenem-resistant A. baumannii, and vancomycin-resistant E. faecium, the small sample size in one or several ward categories prevented the inclusion of ward type as a model covariate.
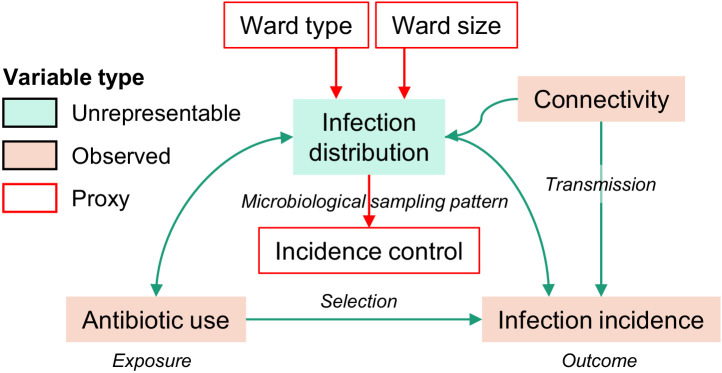
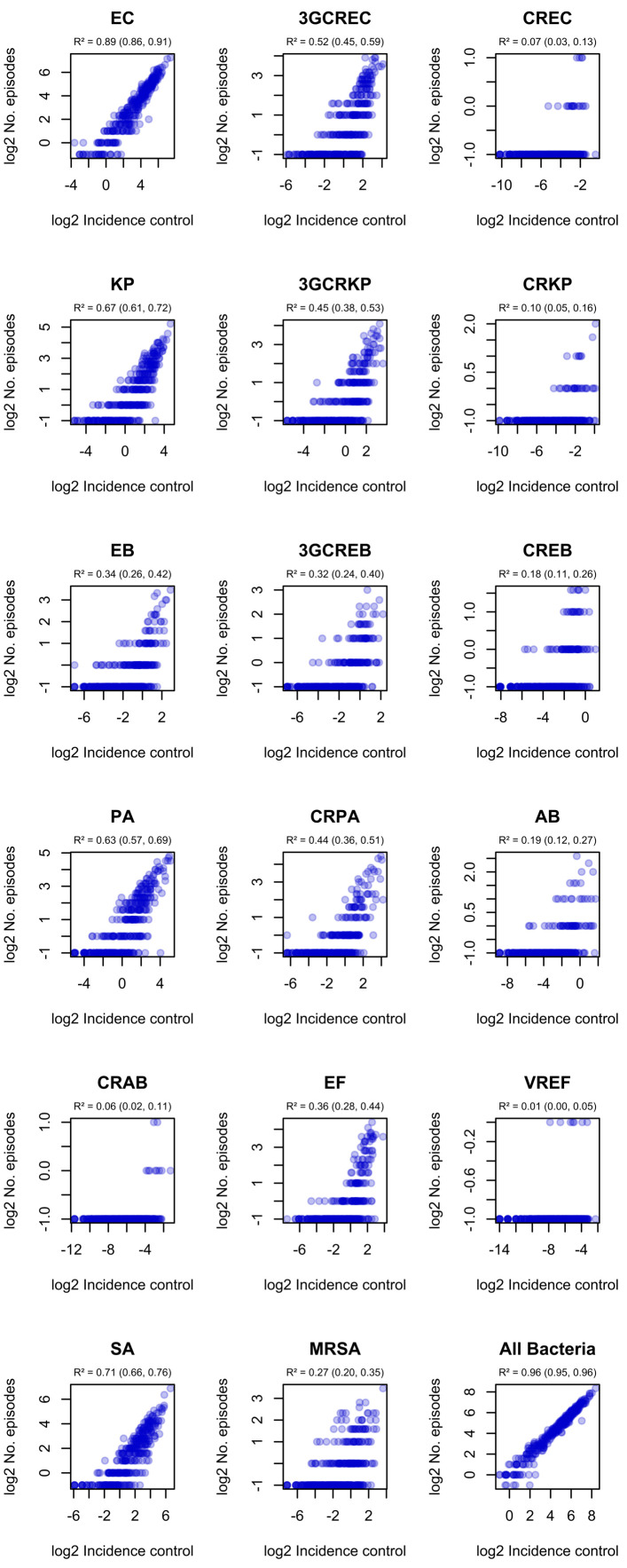
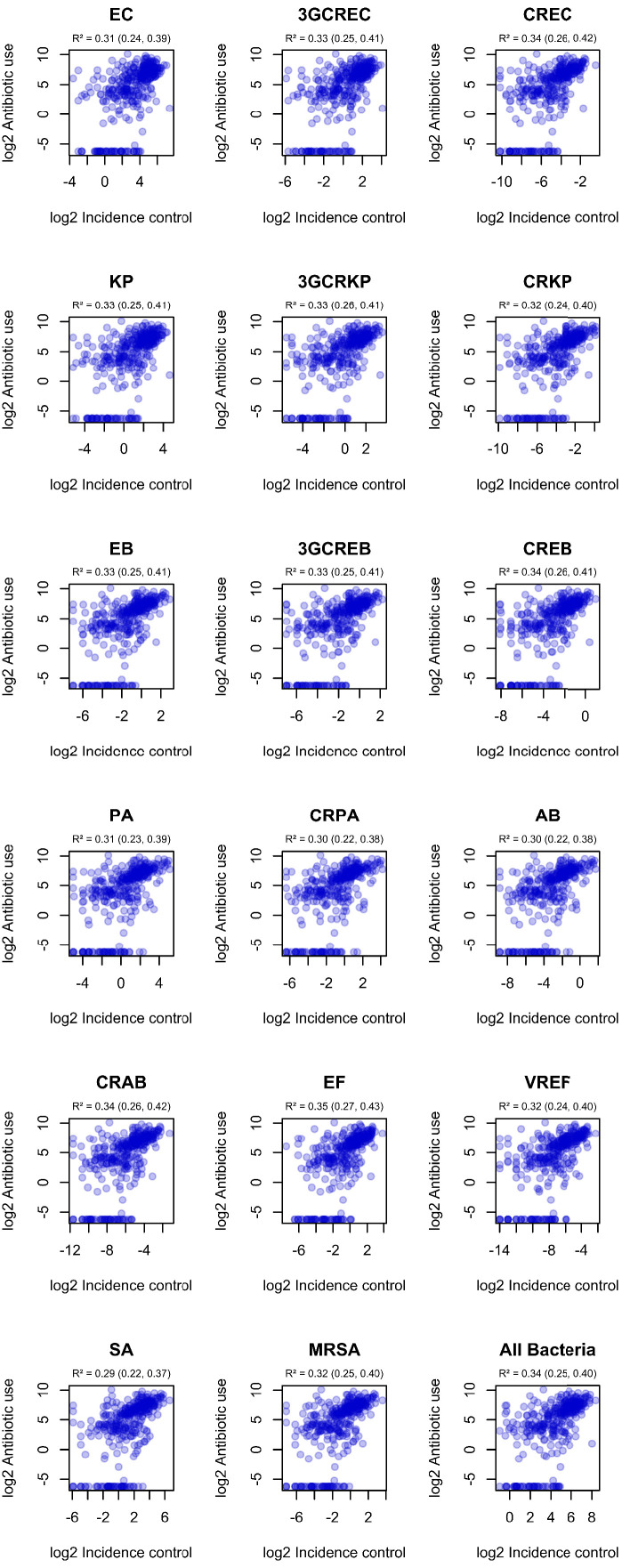
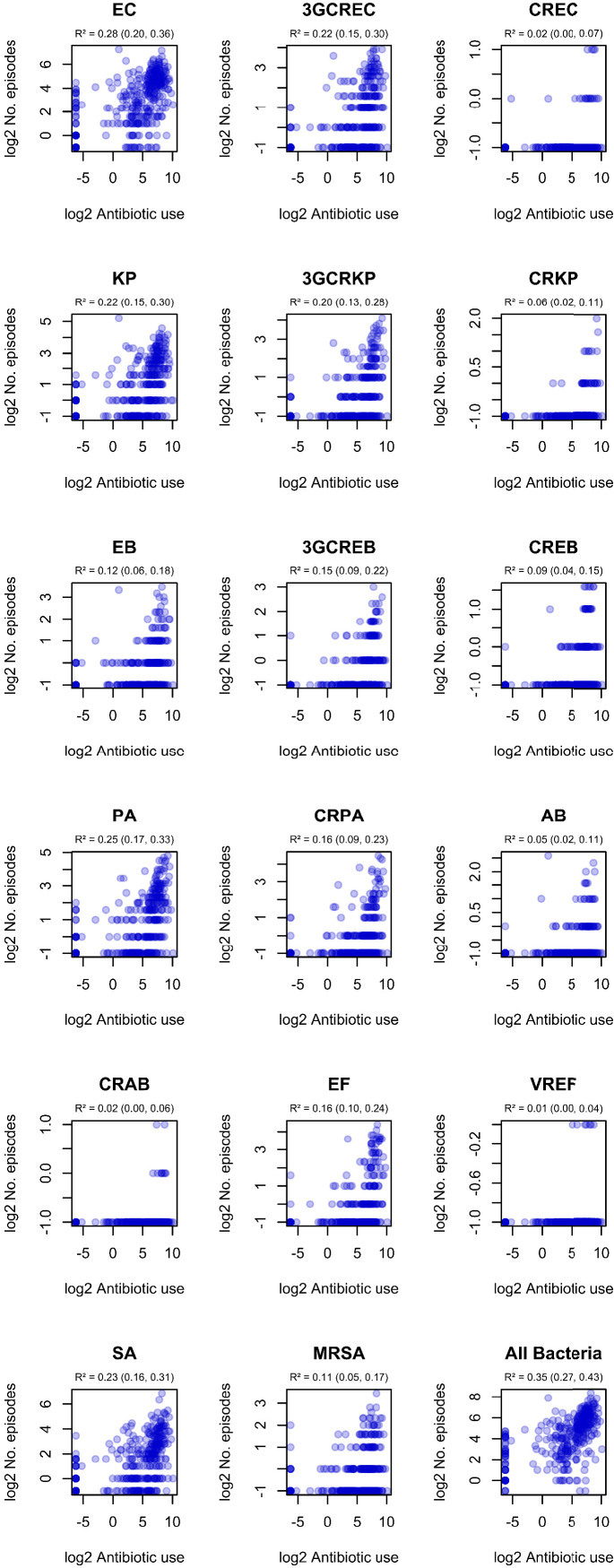
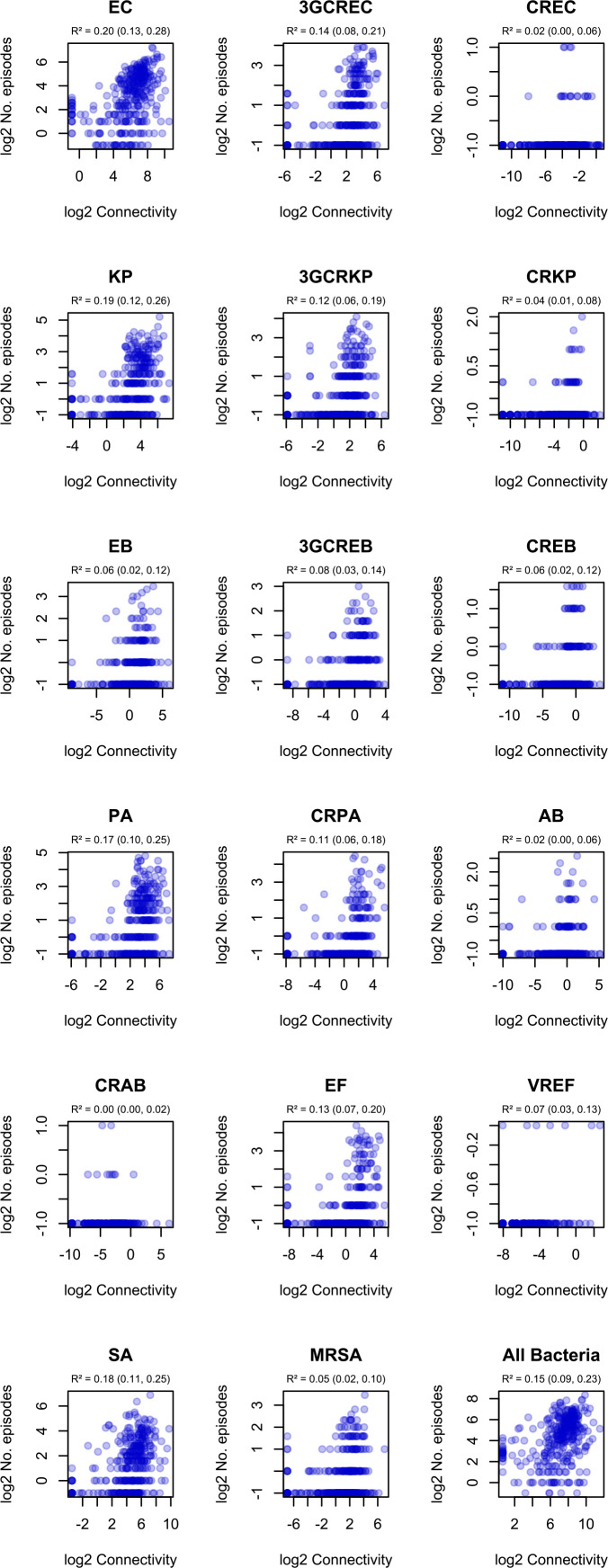
We also considered that the distribution of infections across wards was a source of bias that required a specific adjustment procedure (see Materials and methods). The local prevalence of specific infections (e.g., respiratory tract infections) in a ward influences both the antibiotic use and the observed incidence of infections with a given pathogen, which might confound the relationship between antibiotic use and incidence (Figure 1—figure supplement 2). However, the distribution of infections would be difficult to represent as an adjustment covariate with a sufficiently small number of categories. We used a proxy method to circumvent this issue. We assumed that the distribution of infections directly influences the frequency and specimen types (e.g., respiratory vs. urinary tract specimens) of microbiological samples in each ward. Under this assumption, we replaced the unrepresentable distribution of infections with a proxy variable summarizing the distribution of microbiological samples. This proxy variable, which we refer to as the incidence control, was defined as the ward-level incidence of a pathogen variant predicted by patterns of microbiological sampling alone. As expected, the incidence control correlated with both antibiotic use and the incidence of infections in all prevalent variants (Figure 1—figure supplements 3 and 4). Of note, the incidence of each variant also correlated with both antibiotic use (Figure 1—figure supplement 5) and connectivity (Figure 1—figure supplement 6) in bivariate analyses, except for the very rare A. baumannii and E. faecium resistant variants. Hereafter, all models included the incidence control covariate to adjust for the confounding effect of the distribution of infections.
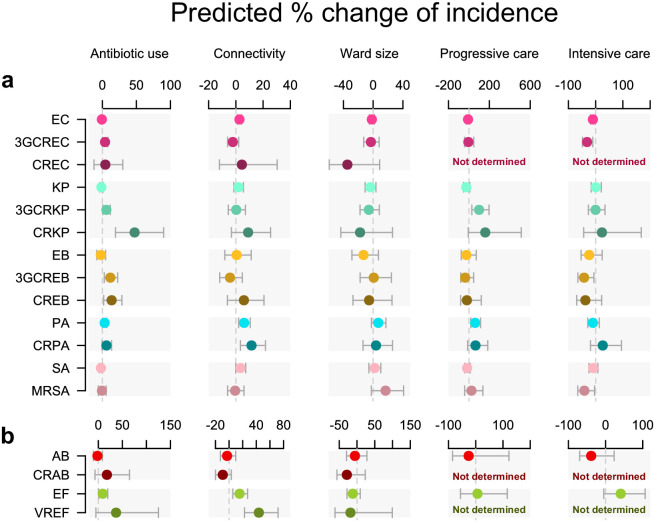
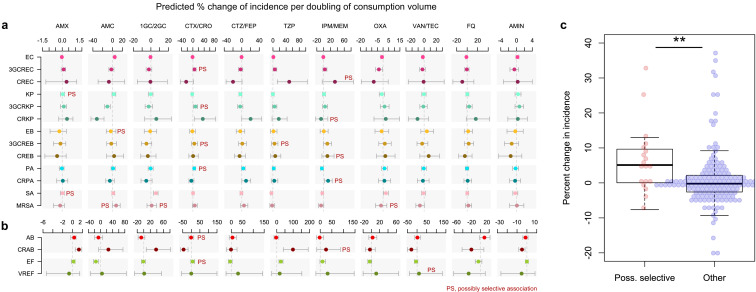
The incidence of each pathogen variant was modeled in a separate multivariable quasi-Poisson GLM (Figure 1). In these GLMs, global antibiotic use was associated with infection incidence in seven pathogen variants independent of connectivity, ward size, and ward type (Figure 1), including five resistant variants (3GC-resistant E. coli, 3GC-resistant K. pneumoniae, carbapenem-resistant K. pneumoniae, 3GC-resistant E. cloacae, and carbapenem-resistant E. cloacae) and two less-resistant variants (P. aeruginosa and E. faecium). The largest effect size was found in carbapenem-resistant K. pneumoniae, in which every doubling of antibiotic use predicted a 47% increase in incidence (95% confidence interval, 19% to 90%).
Figure 1. Antibiotic use and connectivity predict the incidence of infection with ESKAPE2 pathogen variants.
Shown are the predicted percent changes in incidence (points) with 95% confidence interval (bars) for each variant in each ward (n = 357) for every doubling of antibiotic use, connectivity (estimated no. of patients infected with the same variant entering the ward), ward size (no. of beds), and ward type. All models were multivariable quasi-Poisson regressions that included the incidence control covariate (see Materials and methods). Models involving A. baumannii and E. faecium, which exhibited larger 95% confidence intervals due to smaller incidence of the resistant variants, are shown with separate scales (panel b) for readability. In models of CREC, CRAB, and VREF incidence, small sample size in at least one ward category prevented the inclusion of ward type as a covariate and the estimation of the coefficient, marked as ‘not determined’. Variant acronyms are listed in Table 1.
Figure 1—figure supplement 2. Directed acyclic graph representation of causal assumptions.
Figure 1—figure supplement 3. Correlation of ward-level incidence control values with infection incidence in ESKAPE2 pathogen variants.
Figure 1—figure supplement 4. Correlation of incidence control values with observed ward-level antibiotic consumption.
Figure 1—figure supplement 5. Correlation of ward-level antibiotic consumption and infection incidence in ESKAPE2 variants.
Figure 1—figure supplement 6. Correlation of ward-level connectivity and infection incidence in ESKAPE2 variants.
Connectivity predicted a higher incidence in all variants of P. aeruginosa and E. faecium, with a stronger effect size in the resistant variants (11.5 and 43.7%, respectively) compared to their less-resistant counterparts (6.1 and 15.3%). A significant association with connectivity was also found for the less-resistant E. coli, although with a much smaller effect size (2.6%).
Ward characteristics only weakly predicted infection incidence compared to antibiotic use and connectivity. Ward type, or patient fragility, predicted incidence in several variants, although with large uncertainty margins. Interestingly, associations of incidence with intensive care and blood cancer units were negative (in E. coli, 3GC-resistant E. coli, 3GC-resistant E. cloacae, and MRSA) while associations with progressive care units were positive (in 3GC-resistant K. pneumoniae and P. aeruginosa). Ward size did not predict incidence in any variant.
Do associations between antibiotic use and resistance represent AMR selection?
The metapopulation models illustrated in Figure 1 identified positive associations between total antibiotic use in hospital wards and increased incidences of infections with the more resistant variants of several species. Yet, a correlation with AMR does not necessarily establish a selective role of antibiotics. For instance, a high incidence of resistant infections in a ward can increase antibiotic use through prolonged or combined therapies (Schechner et al., 2013). Conversely, the prescription of antibiotics always inactive against a variant is unlikely to be motivated by this variant’s incidence and such antibiotics are more likely to provide a direct benefit to the resistant variant. Based on this rationale, we propose stringent criteria to identify whether an association between the use of an antibiotic and the incidence of a variant possibly reflects AMR selection (hereafter, possibly selective associations; see Materials and methods). Under the hypothesis that antibiotic use is either a consequence of AMR or spuriously correlated with AMR, the strength of an association between the use of an antibiotic and the incidence of a variant should not depend on whether the association fulfills the criteria for possible selection.
To test this hypothesis, we identified possibly selective associations in our data and we examined whether they were equally likely to be positive compared to other associations. We constructed quasi-Poisson multivariable regression models where the total antibiotic use was replaced with the use of specific antibiotics, along with the incidence control and connectivity covariates (Figure 2a and b). Ward type and size, which were weak predictors of incidence in Figure 1 models, were excluded to avoid introducing additional noise. The 17 variant-specific models each included all antibiotic groups as predictors, including antibiotic classes not expected to exert any direct selection pressure on the variant, such as aminoglycosides on E. coli. The models yielded 187 coefficients of which 19 (10.2%) represented possibly selective associations (see Materials and methods). The mean percent change in incidence for every doubling of consumption volume in possibly selective associations was 6.5%, compared to 0.9% in other associations (95% CI of the difference of the means, 0.6 to 10.6%). Three of the four strongest possibly selective associations involved cefotaxime-ceftriaxone, which selected for 3GC-resistant E. coli, K. pneumoniae and P. aeruginosa; the other involved carbapenems selecting for carbapenem-resistant P. aeruginosa. Overall, the larger magnitude of the coefficients of possibly selective associations suggests that the local, ward-level selection of drug-resistant variants by antibiotics is measurably pervasive throughout our hospital network.
Figure 2. Possibly selective associations between the use of specific antibiotics and the incidence of infection with ESKAPE2 pathogen variants.
Shown are the predicted percent changes in incidence (points) with 95% confidence interval (bars) for each variant in each ward (n = 357) for every doubling in the consumption volume of 11 antibiotic groups, based on multivariable quasi-Poisson regression models of the incidence of each variant in each ward (n = 357) that included the connectivity and incidence control covariates (see Materials and methods). Associations classified as possibly selective (n = 19) are indicated by a ‘PS’ mark. Models involving A. baumannii and E. faecium, which exhibited larger 95% confidence intervals due to smaller incidence of the resistant variants, are shown with separate scales (panel b) for readability. (c), possibly selective associations had higher coefficients compared to other associations. The center line indicates the median; box limits indicate the upper and lower quartiles; whiskers indicate the 1.5x interquartile range; points indicate the individual coefficients. **p<0.01, two-sided Mann–Whitney U-test. Acronyms of pathogen variants and antibiotics are listed in Tables 1 and 2, respectively.
Quantifying the drivers of resistance to first-line sepsis therapy
From a clinical standpoint, the most immediate consequence of AMR is the failure to control sepsis with empirical antibiotics, mainly carbapenems and the non-antipseudomonal 3GCs cefotaxime and ceftriaxone. Because such failure can equally result from acquired or intrinsic resistance, the incidence of intrinsically resistant pathogens such as E. faecium is of equal clinical importance as that of pathogen variants with acquired resistance mechanisms. To examine the impact of antibiotics on both intrinsic and acquired resistance, we modeled the cumulative incidence of infections with 3GC- and/or carbapenem-resistant variants of the selected pathogens (see Materials and methods).
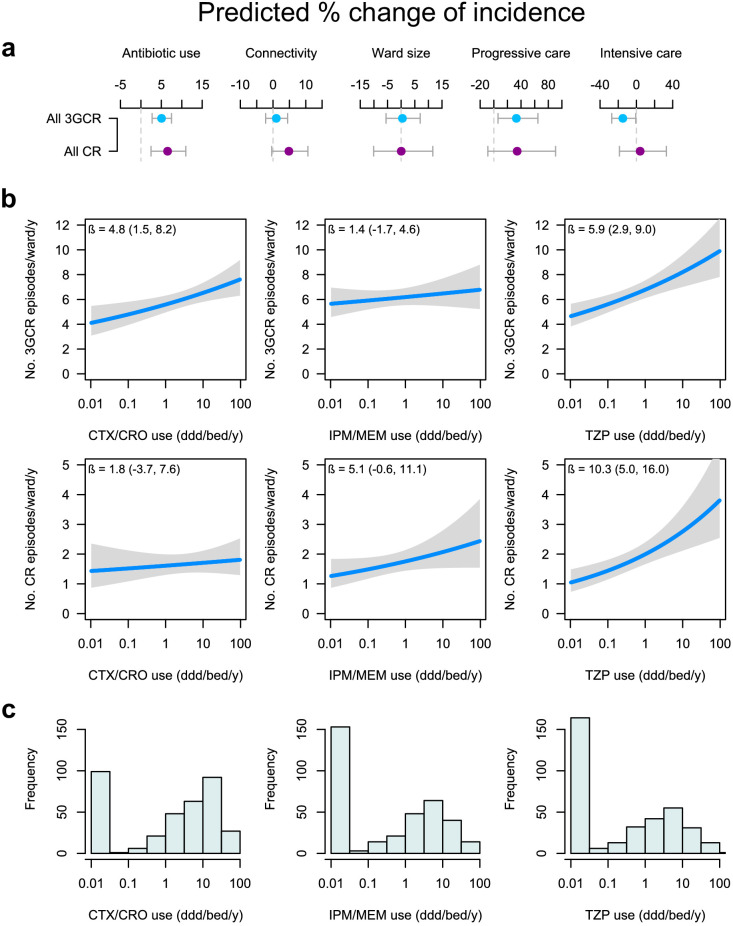
In these models, antibiotic use was the strongest predictor of the incidence of both carbapenem-resistant (6.5% increase in incidence for every doubling of consumption volume, 95% CI, 2.5 to 11.0%) and 3GC-resistant infections (5.1% increase, 95% CI, 2.7 to 7.5%; Figure 3a). Connectivity better predicted the incidence of carbapenem-resistant infections (4.8%, 95% CI, −0.4 to 10.7%) compared with 3GC-resistant infections (1.0%, 95% CI, −2.3 to 4.5%), in line with the comparatively stronger association of connectivity with the incidence of individual carbapenem-resistant variants (Figure 1). Ward size had no measurable effect in either model. Carbapenem-resistant infections were not associated with ward type, while 3GC-resistant infections were positively associated with progressive care units and negatively associated with intensive care and blood cancer units (Figure 3a). These findings provide an unambiguous link between population-level antibiotic use and global resistance to empirical sepsis therapy that was robust to confounding by connectivity, microbiological sampling patterns, and other ward characteristics.
Figure 3. Global and specific antibiotics consumption predict the incidence of infection with 3rd-generation cepalosporin- or carbapenem-resistant ESKAPE2 pathogen variants.
(a) Predicted percent change in incidence (points) with the 95% confidence interval (bars) of all 3GCR and CR infections for every doubling of antibiotic use, connectivity (estimated no. of patients infected with the same variant entering the ward), ward size (no. of beds); and ward type, based on quasi-Poisson regression models of the pooled incidence of 3GCR and CR infections in each ward (n = 357) that included the incidence control covariate (see Materials and methods). (b) Predicted incidence and 95% confidence bands of infections with 3GCR and CR pathogen variants depending on the consumption of CTX/CRO, IPM/MEM, and TZP, in models that included connectivity, the incidence control, and the consumption of 8 other antibiotic groups as covariates. (c) Consumption patterns of CTX/CRO, IPM/MEM, and TZP per ward in the hospital network. Variants classified as 3GCR were 3GCREC, 3GCRKP, CRKP, 3GCREB, CREB, PA, CRPA, AB, CRAB, EF, VREF, and MRSA; the CR category included CREC, CRKP, CREB, CRPA, CRAB, EF, VREF, and MRSA. Acronyms of pathogen variants and antibiotics are listed in Tables 1 and 2, respectively.
To identify the antibiotics whose use was most strongly associated with global carbapenem and cefotaxime-ceftriaxone resistance, we examined the effect of replacing the total antibiotic use in our models with individual antibiotic classes, similar to Figure 2 models. Besides the consumption of cefotaxime-ceftriaxone and carbapenems, the consumption of piperacillin-tazobactam was a strong predictor of the incidence of both 3GC- or carbapenem-resistant infections (Table 3). To visualize the independent associations of incidence with the use of cefotaxime-ceftriaxone, carbapenems and piperacillin-tazobactam, we plotted the average ward-level infection incidence predicted by variations in the consumption volumes in models including the connectivity and incidence control covariates (Figure 3b). The incidence of 3GC-resistant infections was predicted by the consumption of cefotaxime-ceftriaxone (4.8% increase, 95% CI, 1.5 to 8.2%) but not carbapenems (1.4% increase, 95% CI, −1.7 to 4.6%). In the same vein, the use of carbapenems, but not 3GCs, predicted the incidence of carbapenem-resistant infections, although with a wide uncertainty margin (5.1% increase, 95% CI, −0.6 to 11.1%). Strikingly, the use of piperacillin-tazobactam predicted the incidence of both 3GC-resistant (5.9% increase, 95% CI, 2.9 to 9.0%) and carbapenem-resistant infections (10.3% increase, 95% CI, 5.0 to 16.0%). In both models, the amplitude of the piperacillin-tazobactam coefficient outweighed those of all other antibiotics (Table 3). Overall, these results indicate a specific association of cefotaxime-ceftriaxone and, to a slightly lesser extent, carbapenem use with resistance to the same antibiotic group, but not other groups, and identify a major role of piperacillin-tazobactam consumption in predicting the incidence of both 3GC- and carbapenem-resistant infections. To propose a unified ranking of the population-level impact of antibiotics on 3GC and carbapenem resistance, a final model was constructed by pooling all 3GC- and carbapenem-resistant variants together (Table 3). In this model, piperacillin-tazobactam and cefotaxime-ceftriaxone had the largest positive coefficients while 1GC/2GC and coamoxiclav had the largest negative coefficients (Table 3).
Table 3. Associations between the consumption volume of 11 antibiotics and the cumulative incidence of 3GC- and/or carbapenem-resistant infections in 357 wards.
| Predicted percent change in incidence (95% CI) per doubling of consumption volume | |||
|---|---|---|---|
| Antibiotics | 3GCR incidence model | CR incidence model | 3GCR or CR incidence model |
| TZP | 5.9 (2.9, 9.0) | 10.3 (5.0, 16.0) | 6.1 (3.1, 9.2) |
| CTX/CRO | 4.8 (1.5, 8.2) | 1.8 (-3.7, 7.6) | 4.6 (1.4, 8.0) |
| AMX | 2.7 (-1.5, 7.0) | 3.6 (-3.4, 11.2) | 2.7 (-1.4, 7.0) |
| CTZ/FEP | 1.7 (-1.0, 4.5) | 3.3 (-1.5, 8.3) | 1.6 (-1.1, 4.4) |
| IPM/MEM | 1.4 (-1.8, 4.7) | 5.1 (-0.6, 11.1) | 1.5 (-1.6, 4.8) |
| FQ | 0.5 (-1.9, 2.9) | −1.2 (-5.2, 3.0) | 0.4 (-1.9, 2.8) |
| OXA | −0.6 (-2.2, 1.1) | −2.0 (-4.6, 0.7) | −0.6 (-2.2, 1.0) |
| AMIN | −1.7 (-4.3, 0.9) | −1.1 (-5.4, 3.6) | −1.7 (-4.3, 0.9) |
| VAN/TEC | −2.3 (-4.9, 0.4) | −3.8 (-8.3, 0.9) | −2.3 (-4.9, 0.5) |
| 1GC/2GC | −2.1 (-4.0,–0.2) | −2.3 (-5.4, 1.0) | −2.1 (-4.0,–0.3) |
| AMC | −4.1 (-7.0,–1.1) | −5.8 (-10.6,–0.7) | −4.1 (-7.0,–1.1) |
NOTE. a Percent change was estimated from multivariable quasi-Poisson regression models that included the connectivity and the incidence control covariates (see Materials and methods). 3GCR, 3rd-generation cephalosporins-resistant infections; CR, carbapenem-resistant infections. Acronyms of antibiotics are listed in Table 2.
Discussion
Understanding the respective impacts of antibiotic use and connectivity on the incidence of drug-resistant infections is essential for optimizing interventions against AMR. Pathogens whose incidence is strongly predicted by antibiotic use might be most effectively targeted by antibiotic restrictions. On the other hand, infection control interventions might be most relevant against pathogens whose incidence is predicted by connectivity. By applying a metapopulation framework to explain variations in infection incidences across a large network of hospital wards, we found that both antibiotic use and connectivity independently contribute to ward-level AMR in several pathogen species. Our study also provides the first quantitative ranking of the predicted impact of several key antibiotics on the global burden of drug-resistant infections in a hospital network.
While previous theoretical work based on modeling and simulation has predicted how patient transfers contribute to AMR prevalence through pathogen dissemination, they have typically considered only a single resistant variant or used a generic simulated pathogen (Donker et al., 2017; Donker et al., 2010; Vilches et al., 2019). Our study provides empirical evidence to support these predictions in general, while identifying substantial variation in the response to connectivity between different species and variants (Figure 1). In our closed network model of the hospital, the influence of connectivity on the incidence of a pathogen variant is expected to be higher if the variant is endemic to the hospital, its prevalence varies across wards, and changes from one variant category to another are rare. If a variant is frequently introduced from outside of the network, the contribution of within-network movements, hence of connectivity, to the prediction of the local incidence will be diminished. The measured influence of connectivity should also be reduced if a variant frequently undergoes transitions between resistance categories (e.g., by evolving or losing resistance) because local emergence will affect incidence more strongly than inter-ward introduction.
Consistent with this theoretical interpretation of connectivity, we found that its influence was strongest in the hospital-endemic pathogens P. aeruginosa and E. faecium (Blanc et al., 2007; Wurster et al., 2016; Zhou et al., 2020). Intriguingly, MRSA incidence was not predicted by connectivity. This is at odds with the classical perception of MRSA as typically nosocomial and, by extension, hospital-endemic. However, available evidence does not strongly support the qualification of MRSA as a hospital-endemic pathogen in our setting. In France, the proportions of MRSA among S. aureus are comparable in community and hospital settings, at about 10% (ECDC, 2019; ONERBA France, 2018; Santé Publique France, 2019). MRSA population structures in hospitals and the community are comparably dominated by the so-called ST8 Lyon clone which is equally found in in- and outpatients (Dauwalder et al., 2008). Finally, MRSA infections were especially diffuse in our network, with a concentration index even lower than that of the less-resistant S. aureus infections (Table 1). Collectively, this does not support the conclusion that MRSA concentrates in French hospitals compared to the community, which might explain why connectivity did not predict MRSA incidence in our study.
The incidence of global carbapenem-resistant infections was strongly predicted by connectivity (Figure 3), with an effect size comparable to that of antibiotic use, while connectivity did not predict global 3GC-resistant infections. This suggests that reducing connectivity with infection control interventions could be more effective at preventing the spread of carbapenem-resistant pathogens between wards compared to 3GC-resistant pathogens. Contrasting with hospital-endemic pathogens, connectivity was a comparatively weaker predictor for community-associated variants (3GC-resistant E. coli and K. pneumoniae) that enter from outside the hospital network, and in variants whose resistance is selected locally because they can frequently shift between susceptible and resistant categories. Indeed, the weak association of connectivity with the incidence of resistant E. cloacae complex variants, for instance, might be explained by the plasticity of their resistance profile, facilitating their local selection. While resistance in E. coli and K. pneumoniae typically requires gene acquisition (Manges et al., 2019; Wyres and Holt, 2016), E. cloacae complex can resist cephalosporins and carbapenems through increased AmpC beta-lactamase and decreased porin expression (Pavez et al., 2016; Babouee Flury et al., 2016; Lee et al., 2017). Such resistance emerges through adaptation and de novo mutations that are rapidly selected from the local reservoir of susceptible progenitors under antibiotic selection pressure (Hawken et al., 2018; Moradigaravand et al., 2016).
Ecological studies have repeatedly identified associations between the use of antibiotics and AMR prevalence, but such associations do not necessarily reflect AMR selection (Schechner et al., 2013). Specific associations at the level of antibiotic and variant pairs are not all equally likely to result from selection by antibiotics or from increased antibiotic use in response to AMR. Based on medical and biological reasoning, we identified associations more likely to represent possible selection and showed that the average strength of these associations outweighed the others (Figure 2). Noteworthy, several associations with a sizeable strength could not be formally classified as possibly selective but might reflect co-selection. The association of cefotaxime-ceftriaxone with carbapenem-resistant variants in K. pneumoniae, but not in E. coli, possibly reflected selection for the highly frequent 3GC-resistance in carbapenemase-producing K. pneumoniae. This contrasts with E. coli Oxa48 producers that frequently remain susceptible to 3GCs but resistant to piperacillin-tazobactam (Huang et al., 2014), consistent with the strong association between the use of piperacillin-tazobactam and the incidence of carbapenem-resistant E. coli infections (Figure 2a). These findings suggest that the associations between antibiotic use and resistance preferentially reflected AMR selection by antibiotics rather than adaptation of antibiotic use in response to AMR. However, this observation does not eliminate temporal ambiguity between antibiotic use and resistance in our models. Further research using, for instance, multi-state models or time-series analyses, may better clarify this ambiguity.
Because intrinsic and acquired resistances to an antibiotic equally lead to treatment failure, we modeled the pooled incidences of infections with 3GC- or carbapenem-resistant variants of the ESKAPE2 pathogens, including those with intrinsic resistance. This approach allowed us to rank antibiotics by their potential association strength with global AMR. The use of piperacillin-tazobactam and cefotaxime-ceftriaxone predicted 3GC resistance and the use of piperacillin-tazobactam predicted carbapenem resistance (Figure 3). The positive association of the use of piperacillin-tazobactam with both 3GC- and carbapenem-resistant infections deserves further attention. Based on its in vitro efficacy against extended-spectrum beta-lactamase (ESBL)-producing enterobacteria, piperacillin-tazobactam has been repeatedly considered as an alternative drug of choice in carbapenem-sparing strategies (Harris et al., 2015; Peterson, 2008). The strategy of replacing carbapenems with piperacillin-tazobactam assumes: (1), that piperacillin-tazobactam is clinically as effective as carbapenems on piperacillin-tazobactam-susceptible pathogens; and (2), that AMR selection under piperacillin-tazobactam pressure is weaker than under carbapenem pressure, as reflected by a recent consensus-based ranking of beta-lactams for de-escalation therapy (Weiss et al., 2015). Yet, in several recent reports including a multicenter randomized clinical trial, piperacillin-tazobactam treatment of sepsis with ESBL-producing enterobacteria was associated with poorer outcomes compared to carbapenem treatment (Harris et al., 2018; Ofer-Friedman et al., 2015; Tamma et al., 2015). From an epidemiological standpoint, studies of the respective associations of piperacillin-tazobactam and carbapenem use with AMR yielded conflicting results. At the population level, the incidence of carbapenem-resistant enterobacteria was negatively associated with the use of piperacillin-tazobactam in a 5 year, single-hospital trend analysis study (McLaughlin et al., 2013). At the patient level, however, the exposure to piperacillin-tazobactam was associated with the acquisition of carbapenem-resistant P. aeruginosa in a meta-analysis (Raman et al., 2018) and carbapenem-resistant Gram-negative bacilli in a single-hospital prospective cohort study (Marchenay et al., 2015). If confirmed in other settings, our finding that piperacillin-tazobactam use correlates with both 3GC- and carbapenem-resistant infections might call for a reevaluation of the rationale of recommending piperacillin-tazobactam over other drugs for ecological reasons. As a note of caution, our observation that piperacillin-tazobactam use predicted a higher incidence of 3GC- and carbapenem-resistant variants does not imply selection for acquired resistance through any specific mechanism such as carbapenemase production. We also note that the link between piperacillin-tazobactam use and global resistance resulted from the accumulation of small, positive associations with most 3GC- and carbapenem-resistant variants, including those with intrinsic resistance (Figure 2). Because of this, links between piperacillin-tazobactam use and 3GC- or carbapenem-resistant infections might go undetected in studies focusing on individual pathogen variants.
Our study has several limitations. First, we did not consider the role of healthcare workers in pathogen transmission, nor the role of direct patient admissions from the community. Second, although our 3-level categorical coding of ward types captures variations in patient fragility within our study system, such a coarse-grained classification could potentially leave residual confounding. Third, the small sample sizes of resistant variants of E. faecium and A. baumannii limited our ability to draw robust inferences regarding these variants and further studies are required to confirm these results (Arias and Murray, 2012; Hsu et al., 2017). Finally, our findings reflect the AMR ecology of a Western European area, with generally lower prevalences of carbapenemase-producing pathogens, vancomycin-resistant E. faecium, and MRSA than in other regions of the world.
To conclude, the modeling of the incidence of infections with seven major bacterial species and their drug-resistant variants in hospital wards using a metapopulation framework indicates that both antibiotic use and inter-ward connectivity may predict the burden of AMR in a variant-specific fashion. This supports the need to tailor strategies against AMR to the targeted pathogen. Along with novel hospital-level insights into the drivers of AMR, our work illustrates the application of the methodological framework of metapopulation ecology to the problem of hospital AMR. Applying this framework to other healthcare settings could help inform the local and regional antibiotic stewardship and infection control strategies.
Materials and methods
Data collection and compilation
We obtained data on infection incidence from the information system of the Institut des Agents Infectieux, the clinical microbiology laboratory of the Hospices Civils de Lyon, a group of university hospitals serving the Greater Lyon urban area (~1.4 million inhabitants) of France. For each ward from October 1st, 2016 to September 30th, 2017, we extracted the number of clinical samples, after exclusion of screening samples, positive for at least one of the ESKAPE2 species (as determined using Vitek MS MALDI-ToF identification, bioMérieux), falling into one of the resistance variant categories defined in Table 1. Resistance was based on available results for susceptibility to, where applicable, ceftriaxone, cefotaxime, ceftazidime, cefepime, imipenem, meropenem, oxacillin, and vancomycin. Samples were deduplicated per patient, ward, and pathogen variant, so that only the first sample positive for the same variant in the same ward was considered for each patient. Patients positive for multiple variants and/or sampled from multiple wards were considered as multiple, distinct infection episodes. Antibiotic use in defined daily doses (ddd) of all systemic antibacterial drugs (ATC classification term J01), as well as of specific (groups of) molecules defined in Table 2, were extracted from the pharmacy department information system. For each pair of wards, the number of patient transfers was extracted from the hospital information system along with, for each ward, the number of beds, the type of medical activity and the number of patient admissions. Because of the aggregated nature of the data, informed consent was not sought, in accordance with French regulations. Our main response variable was the number of patients per ward infected with each pathogen variant, expressed as incidence over 1y.
Controlling for microbiological sampling patterns
The estimation of a causal effect between antibiotic use and the incidence of infections with a given pathogen variant is biased by variations in the distribution of infections across wards, because this distribution influences both antibiotic prescriptions and microbiological sampling efforts. A causal network representation of this situation is shown in Figure 1—figure supplement 2. The distribution of infections is difficult to determine and to represent as a model covariate, preventing its direct inclusion in our models. To circumvent this issue, the unrepresentable distribution of infections was replaced with a proxy variable that we called the incidence control, designed to capture variations of microbiological sampling frequencies and of the sampled anatomic sites (e.g. urinary vs. respiratory tract) across wards. Of note, ward size and type were also assumed to correlate with the infection distribution and to contribute to the proxy adjustment. However, the inclusion of ward size and type had a negligible impact on model fit compared to the incidence control value, suggesting that this latter variable captures most of the signal.
The incidence control was defined as the expected incidence of a pathogen variant explained by microbiological sampling alone, under the assumption that the pathogen incidence is conditionally independent of the ward given the sampled anatomic site. Anatomic sites were assigned to seven site groups, namely, skin and soft tissues, respiratory tract, urinary tract, digestive tract, vascular access devices, sterile sites (such as cerebrospinal fluid and peripheral blood cultures) and other sites.
The incidence control was computed as follows for each pathogen variant in each ward. In this section, wards are indexed by , groups of anatomical sites are indexed by , and pathogen variants are indexed by . For conciseness, we introduce the special case to denote a sample not positive for any of the considered pathogen variants. Contrary to the incidence calculation described in the previous section, all samples were considered without deduplication to account for how repeated sampling increases the likelihood of pathogen detection.
First, for each pathogen variant and group of anatomic sites , we computed the average probability of variant detection aggregated for all wards in the network as
where is the number of samples in ward taken from site positive for the variant (or, if , negative for all variants) and the denominator denotes all samples taken irrespective of their result. For simplicity, we assumed independence between the pathogen variants isolated from a same sample and between samples collected from a same patient. Of note, most patients (n = 22,646 / 26,064; 86.9%) were sampled only once.
To account for repeated sampling, we considered the probability that samples from a site in a patient remained negative for pathogen variant ,
Sampling from multiple sites was represented using the vector notation to denote the respective numbers of samples taken from site groups 1 to 7. For instance, denotes 1 skin or soft tissue sample and 2 urinary samples. Using this notation, the probability that all samples from all sites remained negative is,
and the probability that at least one sample was positive for variant is,
This relationship was used to calculate the expected incidence of variant , that is the number of patients with at least one sample positive for , by considering in each ward the number of patients with the same number of samples from each site and the probability of being tested positive given . The expected incidence of variant in ward was then defined as,
where denotes the set of possible sample combinations.
Clearly, variations in the incidence control value between wards depend only on the number and sites of microbiological samples taken, thus reflecting the incidence and types of bacterial infections at ward-level independent of between-ward variations of pathogen community structure. Under our assumption that the incidence control is a valid proxy to the unrepresentable distribution of infections in each ward, the incidence control should correlate both with antibiotic use and the incidence of infections. Bivariate analyses confirmed that the incidence control correlated with the observed cumulative incidence of all bacteria (R²=0.96, 95% CI, 0.95 to 0.96, Figure 1—figure supplement 3) and, to a lesser extent, with the total antibiotic use (R²=0.34, 95% CI, 0.25 to 0.40, Figure 1—figure supplement 4). These correlations remained substantial for most pathogen variants and specific antibiotics. The incidence control was added as an adjustment covariate in all models predicting infection incidence. The adjusted models, thus, predicted the incidence of infections in excess of what would be expected based on variations in sampling intensity alone.
Connectivity and other ward characteristics
In ecology, habitat quality refers to the resources and conditions that allow individuals and populations to persist in a location (Hall et al., 1997), such as food or cover from predators (Johnson, 2005). We described the habitat quality of hospital wards for bacterial pathogens using explanatory variables adapted from Hanski’s metapopulation models (Hanski, 1998; Hanski, 1994), namely patch size and connectivity, along with additional variables capturing patient fragility and antibiotic selection pressure. We considered each ward within the hospital system as a distinct habitat patch, i. We used the number of beds both as a measure of patch size (capturing the number of patients available for colonization) and as a proxy for contact opportunities between patients within the same ward.
The connectivity estimate was implemented as a proxy to the unobservable number of introductions of each variant in each ward during the study period (Donker et al., 2017). To estimate this quantity, we measured directional, partial connectivity Sj,i from ward j to ward i as the number of patients transferred directly from j to i, times the inferred probability that each patient tested positive for the variant. Partial connectivity, thus, was the expected number of positive patients transferred from j to i. Finally, connectivity for ward i was the sum of all directional connectivities, Si = ∑jSj,i. This estimation procedure relies on simplifying assumptions, namely that all positive cases are detected and remain positive upon transfer to the downstream ward; and that the probability and destination ward of a patient transfer does not depend on infection.
Along with size and connectivity, wards were characterized by ward type based on patient fragility, and antibiotic consumption. Ward type was coded as a categorical variable with the following three levels: general wards, intermediate (progressive) care units, and intensive care and blood cancer units. Antibiotic use was normalized by dividing by the number of beds in each ward and expressed in ddd/bed/y.
Statistical analysis
The statistical unit was the individual ward (n = 357) in all analyses. We used the asymptotic Simpson index (Simpson, 1949), also known as the Hunter-Gaston index (Hunter and Gaston, 1988), to determine the probability that two random isolates of a given variant were isolated in the same ward or that two random doses of a given antibiotic were delivered in the same ward. The index is defined as [∑ni (ni-1)]/[N (N-1)] where ni is the number of infection episodes (or antibiotic doses) detected (or delivered) in ward i and Ni = ∑ni is the total number of infection episodes (or antibiotic doses). In ecology, the Simpson index is typically used to measure biodiversity by estimating the probability that two individuals from a sample belong to the same species (Simpson, 1949). Here, we use this index to examine the distribution of sampling locations relative to the taxa, measuring the probability that two infection episodes occur, or two antibiotic doses are consumed, in the same ward. Hence, we used the term ‘concentration index’ to avoid confusion with a diversity measure. The iNext R package was used to determine bootstrap-based 95% confidence intervals of the concentration index (Hsieh et al., 2016).
Models of infection incidence were constructed using multivariable quasi-Poisson regression of the form
where denotes expectation, is the vector of predictors, such as antibiotic consumptions, the ’s are model coefficients, denotes variance and is the overdispersion parameter of the quasi-Poisson model. The overdispersion parameter is used to relax the Poisson assumption of equality of the expectation and variance of the response variable. We used the quasi-Poisson distribution because we found evidence of both under- and overdispersion, as evidenced by fitted quasi-Poisson dispersion parameters ranging from 0.24 (strong underdispersion, found for carbapenem-resistant A. baumannii in Figure 1 model) to 2.5 (moderate overdispersion, found for less-resistant E. faecium) (Supplementary file 1 Table 1b). We favored the quasi-Poisson model over the alternative, negative binomial model because the latter gives greater weight to smaller sites, whereas the former gives greater weight to the larger sites (Ver Hoef and Boveng, 2007). In our application, greater weight should be given to wards with greater incidence rather than to those with less.
We constructed a model for each variant. The response variable was the deduplicated patient counts. All non-categorical explanatory variables including the incidence control, ward size, connectivity and antibiotic use were log2-transformed before further analyses. To avoid negative infinity values from this transformation, all zeroes were first converted to half the minimum non-zero value. This transformation was associated with better model fit (using the model structure of Figure 1), in terms of Akaike information criterion (with Poisson distribution), compared with: (1) replacing zeroes with the minimum non-zero value before taking logs; (2) adding one to zero values before taking logs; or (3) avoiding log transformation. The log2 transformed data were used for all subsequent analyses.
To ease interpretation of model coefficients, we converted the raw coefficients from all models to percent changes in infection incidence, equal to . All analyses used R software version 3.6.0.
Possibly selective associations between antibiotic use and resistance
We examined criteria to identify a priori possibly selective associations between antibiotic use and resistance. The criteria were based on medical and biological considerations, namely, that antibiotics inactive against a variant are unlikely to be prescribed in response to this variant’s prevalence; and that antibiotics are most likely to select for a variant when resistance provides a specific advantage, hence, when the variant is not resistant to more potent antibiotics (e.g., CTX/CRO is more likely to select for PA than for CRPA in which carbapenem resistance provides no additional benefit under CTX/CRO pressure). This rationale led to the following criteria: (1) the variant is always resistant to the antibiotics of interest; (2) the variant is not resistant to antibiotics more potent (in terms of spectrum or efficacy) than the antibiotics of interest; and (3) the antibiotics of interest can be plausibly used against the variant in empirical therapy. A total of 19 associations fulfilled the criteria for possibly selective associations: 3GCREC with CTX/CRO; CREC with IPM/MEM; KP with AMX; 3GCRKP with CTX/CRO; CRKP with IPM/MEM; EB with AMC; C3GREB with CTX/CRO and TZP; CREB with IPM/MEM; PA with CTX/CRO; CRPA with IPM/MEM; SA with AMX; MRSA with C1G/C2G, OXA and AMC; AB with CTX/CRO; CRAB with IPM/MEM; EF with CTX/CRO; and VREF with VAN/TEC. Of note, the associations not fulfilling the criteria for possible selection can be interpreted as negative controls in our models, and their coefficients are expected to be distributed around zero (null distribution) in the absence of residual confounding. In line with this interpretation, the coefficients of most of the negative control associations followed a near-zero-centered distribution (Figure 2c), suggesting that residual confounding was negligible in the adjusted models.
Pooled analysis of CTX/CRO- and IPM/MEM-resistant variants
To model the cumulative incidences of 3GC- and carbapenem-resistant infections, pathogen variants were pooled into resistance categories. When resistance to CTX/CRO or IPM/MEM was not determined by design (such as 3GC resistance in 3GCREC) or by intrinsic resistance (such as 3GC resistance in E. faecium), variants were classified as resistant when the proportion of resistance in our setting was above 80%. This less-stringent resistance criterion, compared to the criterion used to determine possibly selective associations, was chosen to avoid the exclusion of variants that are mostly resistant to an antibiotic group, which would bias pooled analyses. Applying the 80% threshold for the proportion of resistance led to classifying CRKP and CREB as 3GC-resistant (91% and 93% 3GC resistance, respectively) but not CREC (61% 3GC resistance); and EF and VREF as carbapenem-resistant (84 and 100% carbapenem resistance, respectively, inferred from ampicillin resistance [Weinstein, 2001]). Overall, the 3GCR category included 3GCREC, 3GCRKP, CRKP, 3GCREB, CREB, PA, CRPA, AB, CRAB, EF, VREF, and MRSA; and the CR category included CREC, CRKP, CREB, CRPA, CRAB, EF, VREF, and MRSA.
Data and software code availability
All data and software code that support the findings of this study are available at: Shapiro, 2020, https://github.com/rasigadelab/metapop (copy archived at https://github.com/elifesciences-publications/metapop).
Acknowledgements
The authors thank Arnaud Friggeri, Alain Lepape, and Florent Wallet for fruitful discussion. Julie Teresa Shapiro, Gérard Lina, François Vandenesch, and Jean-Philippe Rasigade are part of the French Laboratory of Excellence project ECOFECT (ANR-11-LABX-0048).
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Contributor Information
Jean-Philippe Rasigade, Email: jean-philippe.rasigade@univ-lyon1.fr.
Ben S Cooper, Mahidol University, Thailand.
Eduardo Franco, McGill University, Canada.
Funding Information
This paper was supported by the following grants:
Fondation Innovations en Infectiologie R18037CC to Jean-Philippe Rasigade.
French Laboratory of Excellence project ECOFECT ANR-11-LABX-0048 to Julie Teresa Shapiro, Gilles Leboucher, François Vandenesch, Jean-Philippe Rasigade.
Additional information
Competing interests
No competing interests declared.
Author contributions
Conceptualization, Software, Formal analysis, Visualization, Methodology, Writing - original draft, Writing - review and editing.
Data curation, Investigation, Writing - review and editing.
Data curation, Investigation, Writing - review and editing.
Data curation, Investigation, Writing - review and editing.
Data curation, Investigation, Writing - review and editing.
Data curation, Formal analysis, Investigation, Writing - review and editing.
Data curation, Formal analysis, Investigation, Writing - review and editing.
Validation, Writing - review and editing.
Investigation, Writing - review and editing.
Writing - review and editing.
Conceptualization, Writing - review and editing.
Writing - review and editing.
Data curation, Investigation, Writing - review and editing.
Writing - review and editing.
Conceptualization, Investigation, Writing - review and editing.
Conceptualization, Software, Formal analysis, Supervision, Funding acquisition, Investigation, Methodology, Writing - original draft, Project administration, Writing - review and editing.
Additional files
(a) Summary statistics (mean, interquartile range) of connectivity for each variant. (b) Comparison of log-likelihood values from multivariable Poisson and negative binomial regressions and overdispersion parameters from quasi-Poisson multivariable regression of the incidence of infections with 17 pathogen variants. All models included total antibiotic consumption, connectivity, ward size, ward type, and the incidence control value as variables.
Data availability
All data and software code that support the findings of this study are available at: https://github.com/rasigadelab/metapop (copy archived at https://github.com/elifesciences-publications/metapop).
References
- Acar J. Broad- and narrow-spectrum antibiotics: an unhelpful categorization. Clinical Microbiology and Infection. 1997;3:395–396. doi: 10.1111/j.1469-0691.1997.tb00274.x. [DOI] [PubMed] [Google Scholar]
- Arias CA, Murray BE. The rise of the Enterococcus: beyond vancomycin resistance. Nature Reviews Microbiology. 2012;10:266–278. doi: 10.1038/nrmicro2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babouee Flury B, Ellington MJ, Hopkins KL, Turton JF, Doumith M, Woodford N. The differential importance of mutations within AmpD in cephalosporin resistance of Enterobacter aerogenes and Enterobacter cloacae. International Journal of Antimicrobial Agents. 2016;48:555–558. doi: 10.1016/j.ijantimicag.2016.07.021. [DOI] [PubMed] [Google Scholar]
- Bell BG, Schellevis F, Stobberingh E, Goossens H, Pringle M. A systematic review and meta-analysis of the effects of antibiotic consumption on antibiotic resistance. BMC Infectious Diseases. 2014;14:13. doi: 10.1186/1471-2334-14-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanc DS, Francioli P, Zanetti G. Molecular epidemiology of Pseudomonas aeruginosa in the intensive care units - A review. The Open Microbiology Journal. 2007;1:8–11. doi: 10.2174/1874285800701010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton DC. Methicillin-Resistant Staphylococcus aureus Central Line–Associated Bloodstream Infections in US Intensive Care Units, 1997-2007. Jama. 2009;301:727–736. doi: 10.1001/jama.2009.153. [DOI] [PubMed] [Google Scholar]
- Cassini A, Högberg LD, Plachouras D, Quattrocchi A, Hoxha A, Simonsen GS, Colomb-Cotinat M, Kretzschmar ME, Devleesschauwer B, Cecchini M, Ouakrim DA, Oliveira TC, Struelens MJ, Suetens C, Monnet DL, Burden of AMR Collaborative Group Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant Bacteria in the EU and the european economic area in 2015: a population-level modelling analysis. The Lancet Infectious Diseases. 2019;19:56–66. doi: 10.1016/S1473-3099(18)30605-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chastain DB, White BP, Cretella DA, Bland CM. Is it time to rethink the notion of Carbapenem-Sparing therapy against Extended-Spectrum β-Lactamase-Producing Enterobacteriaceae bloodstream infections? A critical review. Annals of Pharmacotherapy. 2018;52:484–492. doi: 10.1177/1060028017748943. [DOI] [PubMed] [Google Scholar]
- Chatterjee A, Modarai M, Naylor NR, Boyd SE, Atun R, Barlow J, Holmes AH, Johnson A, Robotham JV. Quantifying drivers of antibiotic resistance in humans: a systematic review. The Lancet Infectious Diseases. 2018;18:e368–e378. doi: 10.1016/S1473-3099(18)30296-2. [DOI] [PubMed] [Google Scholar]
- Clarivet B, Grau D, Jumas-Bilak E, Jean-Pierre H, Pantel A, Parer S, Lotthé A. Persisting transmission of carbapenemase-producing Klebsiella pneumoniae due to an environmental reservoir in a university hospital, France, 2012 to 2014. Eurosurveillance. 2016;21:30213. doi: 10.2807/1560-7917.ES.2016.21.17.30213. [DOI] [PubMed] [Google Scholar]
- Cohen ML. Epidemiology of drug resistance: implications for a post-antimicrobial era. Science. 1992;257:1050–1055. doi: 10.1126/science.257.5073.1050. [DOI] [PubMed] [Google Scholar]
- Dauwalder O, Lina G, Durand G, Bes M, Meugnier H, Jarlier V, Coignard B, Vandenesch F, Etienne J, Laurent F. Epidemiology of invasive methicillin-resistant Staphylococcus aureus clones collected in France in 2006 and 2007. Journal of Clinical Microbiology. 2008;46:3454–3458. doi: 10.1128/JCM.01050-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David S, Reuter S, Harris SR, Glasner C, Feltwell T, Argimon S, Abudahab K, Goater R, Giani T, Errico G, Aspbury M, Sjunnebo S, Feil EJ, Rossolini GM, Aanensen DM, Grundmann H. Epidemic of carbapenem-resistant Klebsiella pneumoniae in Europe is driven by nosocomial spread. Nature Microbiology. 2019;4:1919–1929. doi: 10.1038/s41564-019-0492-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolrenry S, Stenglein J, Hazzah L, Lutz RS, Frank L. A metapopulation approach to african lion (Panthera leo) conservation. PLOS ONE. 2014;9:e88081. doi: 10.1371/journal.pone.0088081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donker T, Wallinga J, Grundmann H. Patient referral patterns and the spread of hospital-acquired infections through national health care networks. PLOS Computational Biology. 2010;6:e1000715. doi: 10.1371/journal.pcbi.1000715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donker T, Smieszek T, Henderson KL, Johnson AP, Walker AS, Robotham JV. Measuring distance through dense weighted networks: the case of hospital-associated pathogens. PLOS Computational Biology. 2017;13:e1005622. doi: 10.1371/journal.pcbi.1005622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECDC . European Centre for Disease Prevention and Control Surveillance of Antimicrobial Resistance in Europe. Annual report of the European Antimicrobial Resistance Surveillance Network (EARS-Net); 2019. https://www.ecdc.europa.eu/sites/default/files/documents/surveillance-antimicrobial-resistance-Europe-2018.pdf [Google Scholar]
- Goff DA, Kullar R, Goldstein EJC, Gilchrist M, Nathwani D, Cheng AC, Cairns KA, Escandón-Vargas K, Villegas MV, Brink A, van den Bergh D, Mendelson M. A global call from five countries to collaborate in antibiotic stewardship: united we succeed, divided we might fail. The Lancet Infectious Diseases. 2017;17:e56–e63. doi: 10.1016/S1473-3099(16)30386-3. [DOI] [PubMed] [Google Scholar]
- Hall LS, Krausman PR, Morrison ML. The habitat concept and a Plea for standard terminology. Wildlife Society Bulletin. 1997;25:173–182. [Google Scholar]
- Hanski I. A practical model of metapopulation dynamics. The Journal of Animal Ecology. 1994;63:151–162. doi: 10.2307/5591. [DOI] [Google Scholar]
- Hanski I. Metapopulation dynamics. Nature. 1998;396:41–49. doi: 10.1038/23876. [DOI] [Google Scholar]
- Harris PN, Tambyah PA, Paterson DL. β-lactam and β-lactamase inhibitor combinations in the treatment of extended-spectrum β-lactamase producing Enterobacteriaceae: time for a reappraisal in the era of few antibiotic options? The Lancet Infectious Diseases. 2015;15:475–485. doi: 10.1016/S1473-3099(14)70950-8. [DOI] [PubMed] [Google Scholar]
- Harris PNA, Tambyah PA, Lye DC, Mo Y, Lee TH, Yilmaz M, Alenazi TH, Arabi Y, Falcone M, Bassetti M, Righi E, Rogers BA, Kanj S, Bhally H, Iredell J, Mendelson M, Boyles TH, Looke D, Miyakis S, Walls G, Al Khamis M, Zikri A, Crowe A, Ingram P, Daneman N, Griffin P, Athan E, Lorenc P, Baker P, Roberts L, Beatson SA, Peleg AY, Harris-Brown T, Paterson DL, MERINO Trial Investigators and the Australasian Society for Infectious Disease Clinical Research Network (ASID-CRN) Effect of Piperacillin-Tazobactam vs meropenem on 30-Day mortality for patients with E coli or Klebsiella pneumoniae bloodstream infection and ceftriaxone resistance: a randomized clinical trial. Jama. 2018;320:984–994. doi: 10.1001/jama.2018.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawken SE, Washer LL, Williams CL, Newton DW, Snitkin ES. Genomic investigation of a putative Endoscope-Associated Carbapenem-Resistant Enterobacter cloacae Outbreak Reveals a Wide Diversity of Circulating Strains and Resistance Mutations. Clinical Infectious Diseases. 2018;66:460–463. doi: 10.1093/cid/cix934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heard GW, Thomas CD, Hodgson JA, Scroggie MP, Ramsey DSL, Clemann N. Refugia and connectivity sustain amphibian metapopulations afflicted by disease. Ecology Letters. 2015;18:853–863. doi: 10.1111/ele.12463. [DOI] [PubMed] [Google Scholar]
- Hsieh TC, Ma KH, Chao A. iNEXT: an R package for rarefaction and extrapolation of species diversity ( H ill numbers) Methods in Ecology and Evolution. 2016;7:1451–1456. doi: 10.1111/2041-210X.12613. [DOI] [Google Scholar]
- Hsu LY, Apisarnthanarak A, Khan E, Suwantarat N, Ghafur A, Tambyah PA. Carbapenem-Resistant Acinetobacter baumannii and Enterobacteriaceae in south and southeast asia. Clinical Microbiology Reviews. 2017;30:1–22. doi: 10.1128/CMR.00042-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang TD, Poirel L, Bogaerts P, Berhin C, Nordmann P, Glupczynski Y. Temocillin and piperacillin/tazobactam resistance by disc diffusion as antimicrobial surrogate markers for the detection of carbapenemase-producing Enterobacteriaceae in geographical Areas with a high prevalence of OXA-48 producers. Journal of Antimicrobial Chemotherapy. 2014;69:445–450. doi: 10.1093/jac/dkt367. [DOI] [PubMed] [Google Scholar]
- Hunter PR, Gaston MA. Numerical index of the discriminatory ability of typing systems: an application of Simpson's index of diversity. Journal of Clinical Microbiology. 1988;26:2465–2466. doi: 10.1128/JCM.26.11.2465-2466.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttner A, Harbarth S, Carlet J, Cosgrove S, Goossens H, Holmes A, Jarlier V, Voss A, Pittet D. Antimicrobial resistance: a global view from the 2013 world Healthcare-Associated infections forum. Antimicrobial Resistance and Infection Control. 2013;2:31. doi: 10.1186/2047-2994-2-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttner B, Pulcini C, Schouten J. De-constructing de-escalation. Clinical Microbiology and Infection. 2016;22:958–959. doi: 10.1016/j.cmi.2016.09.024. [DOI] [PubMed] [Google Scholar]
- Johnson MD. Habitat quality: a brief review for wildlife biologists. Transactions of the Western Section of the Wildlife Society. 2005;41:31–41. [Google Scholar]
- Johnson TA, Stedtfeld RD, Wang Q, Cole JR, Hashsham SA, Looft T, Zhu YG, Tiedje JM. Clusters of antibiotic resistance genes enriched together stay together in swine agriculture. mBio. 2016;7:e02214. doi: 10.1128/mBio.02214-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardaś-Słoma L, Lucet JC, Perozziello A, Pelat C, Birgand G, Ruppé E, Boëlle PY, Andremont A, Yazdanpanah Y. Universal or targeted approach to prevent the transmission of extended-spectrum beta-lactamase-producing Enterobacteriaceae in intensive care units: a cost-effectiveness analysis. BMJ Open. 2017;7:e017402. doi: 10.1136/bmjopen-2017-017402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laxminarayan R, Duse A, Wattal C, Zaidi AK, Wertheim HF, Sumpradit N, Vlieghe E, Hara GL, Gould IM, Goossens H, Greko C, So AD, Bigdeli M, Tomson G, Woodhouse W, Ombaka E, Peralta AQ, Qamar FN, Mir F, Kariuki S, Bhutta ZA, Coates A, Bergstrom R, Wright GD, Brown ED, Cars O. Antibiotic resistance-the need for global solutions. The Lancet Infectious Diseases. 2013;13:1057–1098. doi: 10.1016/S1473-3099(13)70318-9. [DOI] [PubMed] [Google Scholar]
- Lee JY, Hong YK, Lee H, Ko KS. High prevalence of non-clonal imipenem-nonsusceptible Enterobacter spp. isolates in Korea and their association with porin down-regulation. Diagnostic Microbiology and Infectious Disease. 2017;87:53–59. doi: 10.1016/j.diagmicrobio.2016.10.004. [DOI] [PubMed] [Google Scholar]
- Lemmen SW, Lewalter K. Antibiotic stewardship and horizontal infection control are more effective than screening, isolation and eradication. Infection. 2018;46:581–590. doi: 10.1007/s15010-018-1137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levins R. Some Demographic and Genetic Consequences of Environmental Heterogeneity for Biological Control. Bulletin of the Entomological Society of America. 1969;15:237–240. doi: 10.1093/besa/15.3.237. [DOI] [Google Scholar]
- Lipsitch M. Measuring and Interpreting Associations between Antibiotic Use and Penicillin Resistance in Streptococcus pneumoniae. Clinical Infectious Diseases. 2001;32:1044–1054. doi: 10.1086/319604. [DOI] [PubMed] [Google Scholar]
- Lipsitch M, Samore MH. Antimicrobial use and antimicrobial resistance: a population perspective. Emerging Infectious Diseases. 2002;8:347–354. doi: 10.3201/eid0804.010312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lübbert C, Baars C, Dayakar A, Lippmann N, Rodloff AC, Kinzig M, Sörgel F. Environmental pollution with antimicrobial agents from bulk drug manufacturing industries in Hyderabad, South India, is associated with dissemination of extended-spectrum beta-lactamase and carbapenemase-producing pathogens. Infection. 2017;45:479–491. doi: 10.1007/s15010-017-1007-2. [DOI] [PubMed] [Google Scholar]
- MacPherson JL, Bright PW. Metapopulation dynamics and a landscape approach to conservation of lowland water voles (Arvicola amphibius) Landscape Ecology. 2011;26:1395–1404. doi: 10.1007/s10980-011-9669-0. [DOI] [Google Scholar]
- Manges AR, Geum HM, Guo A, Edens TJ, Fibke CD, Pitout JDD. Global extraintestinal pathogenic Escherichia coli (ExPEC) Lineages. Clinical Microbiology Reviews. 2019;32:e00135. doi: 10.1128/CMR.00135-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning ML, Septimus EJ, Ashley ESD, Cosgrove SE, Fakih MG, Schweon SJ, Myers FE, Moody JA. Antimicrobial stewardship and infection prevention-leveraging the synergy: a position paper update. American Journal of Infection Control. 2018;46:364–368. doi: 10.1016/j.ajic.2018.01.001. [DOI] [PubMed] [Google Scholar]
- Marchenay P, Blasco G, Navellou J-C, Leroy J, Cholley P, Talon D, Bertrand X, Gbaguidi-Haore H. Acquisition of carbapenem-resistant Gram-negative bacilli in intensive care unit: predictors and molecular epidemiology. Médecine Et Maladies Infectieuses. 2015;45:34–40. doi: 10.1016/j.medmal.2014.12.003. [DOI] [PubMed] [Google Scholar]
- McLaughlin M, Advincula MR, Malczynski M, Qi C, Bolon M, Scheetz MH. Correlations of antibiotic use and carbapenem resistance in Enterobacteriaceae. Antimicrobial Agents and Chemotherapy. 2013;57:5131–5133. doi: 10.1128/AAC.00607-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moradigaravand D, Reuter S, Martin V, Peacock SJ, Parkhill J. The dissemination of multidrug-resistant Enterobacter cloacae throughout the UK and Ireland. Nature Microbiology. 2016;1:16173. doi: 10.1038/nmicrobiol.2016.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niehus R, van Kleef E, Mo Y, Turlej-Rogacka A, Lammens C, Carmeli Y, Goossens H, Tacconelli E, Carevic B, Preotescu L, Malhotra-Kumar S, Cooper BS. Quantifying antibiotic impact on within-patient dynamics of extended-spectrum beta-lactamase resistance. eLife. 2020;9:e49206. doi: 10.7554/eLife.49206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofer-Friedman H, Shefler C, Sharma S, Tirosh A, Tal-Jasper R, Kandipalli D, Sharma S, Bathina P, Kaplansky T, Maskit M, Azouri T, Lazarovitch T, Zaidenstein R, Kaye KS, Marchaim D. Carbapenems versus Piperacillin-Tazobactam for bloodstream infections of nonurinary source caused by Extended-Spectrum Beta-Lactamase-Producing Enterobacteriaceae. Infection Control & Hospital Epidemiology. 2015;36:981–985. doi: 10.1017/ice.2015.101. [DOI] [PubMed] [Google Scholar]
- ONERBA France . Observatoire National d’Epidémiologie De La Résistance Bactérienne Aux Antibiotiques: Rapport D’activité. Annual report 2017; 2018. http://onerba-doc.onerba.org/Rapports/Rapport-ONERBA-2017/ONERBA_rapport_2017.pdf [Google Scholar]
- Pavez M, Vieira C, de Araujo MR, Cerda A, de Almeida LM, Lincopan N, Mamizuka EM. Molecular mechanisms of membrane impermeability in clinical isolates of Enterobacteriaceae exposed to imipenem selective pressure. International Journal of Antimicrobial Agents. 2016;48:78–85. doi: 10.1016/j.ijantimicag.2016.04.016. [DOI] [PubMed] [Google Scholar]
- Peterson LR. Antibiotic policy and prescribing strategies for therapy of extended-spectrum beta-lactamase-producing Enterobacteriaceae: the role of piperacillin-tazobactam. Clinical Microbiology and Infection. 2008;14 Suppl 1:181–184. doi: 10.1111/j.1469-0691.2007.01864.x. [DOI] [PubMed] [Google Scholar]
- Pogue JM, Mann T, Barber KE, Kaye KS. Carbapenem-resistant Acinetobacter baumannii: epidemiology, surveillance and management. Expert Review of Anti-Infective Therapy. 2013;11:383–393. doi: 10.1586/eri.13.14. [DOI] [PubMed] [Google Scholar]
- Raman G, Avendano EE, Chan J, Merchant S, Puzniak L. Risk factors for hospitalized patients with resistant or multidrug-resistant Pseudomonas aeruginosa infections: a systematic review and meta-analysis. Antimicrobial Resistance & Infection Control. 2018;7:79. doi: 10.1186/s13756-018-0370-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robotham JV, Deeny SR, Fuller C, Hopkins S, Cookson B, Stone S. Cost-effectiveness of national mandatory screening of all admissions to english national health service hospitals for meticillin-resistant Staphylococcus aureus: a mathematical modelling study. The Lancet Infectious Diseases. 2016;16:348–356. doi: 10.1016/S1473-3099(15)00417-X. [DOI] [PubMed] [Google Scholar]
- Safdar N, Maki DG. The commonality of risk factors for nosocomial colonization and infection with antimicrobial-resistant Staphylococcus aureus, Enterococcus, gram-negative bacilli, Clostridium difficile, and candida. Annals of Internal Medicine. 2002;136:834–844. doi: 10.7326/0003-4819-136-11-200206040-00013. [DOI] [PubMed] [Google Scholar]
- Santé Publique France . Données De Surveillance: Surveillance De La Résistance Bactérienne Aux Antibiotiques en Soins De Ville Et en Établissements Pour Personnes Âgées Dépendentes. Année; 2019. https://www.santepubliquefrance.fr/content/download/263473/2657301 [Google Scholar]
- Schechner V, Temkin E, Harbarth S, Carmeli Y, Schwaber MJ. Epidemiological interpretation of studies examining the effect of antibiotic usage on resistance. Clinical Microbiology Reviews. 2013;26:289–307. doi: 10.1128/CMR.00001-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro JT. metapop. bf3f075GitHub. 2020 https://github.com/rasigadelab/metapop
- Simpson EH. Measurement of Diversity. Nature. 1949;163:688. doi: 10.1038/163688a0. [DOI] [Google Scholar]
- Snitkin ES, Zelazny AM, Thomas PJ, Stock F, Henderson DK, Palmore TN, Segre JA, NISC Comparative Sequencing Program Group Tracking a hospital outbreak of carbapenem-resistant Klebsiella pneumoniae with whole-genome sequencing. Science Translational Medicine. 2012;4:148ra116. doi: 10.1126/scitranslmed.3004129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spagnolo F, Cristofari P, Tatonetti NP, Ginzburg LR, Dykhuizen DE. Pathogen population structure can explain hospital outbreaks. The ISME Journal. 2018;12:2835–2843. doi: 10.1038/s41396-018-0235-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamma PD, Han JH, Rock C, Harris AD, Lautenbach E, Hsu AJ, Avdic E, Cosgrove SE, Antibacterial Resistance Leadership Group Carbapenem therapy is associated with improved survival compared with piperacillin-tazobactam for patients with extended-spectrum β-lactamase bacteremia. Clinical Infectious Diseases. 2015;60:1319–1325. doi: 10.1093/cid/civ003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venter H, Henningsen ML, Begg SL. Antimicrobial resistance in healthcare, agriculture and the environment: the biochemistry behind the headlines. Essays in Biochemistry. 2017;61:1–10. doi: 10.1042/EBC20160053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ver Hoef JM, Boveng PL. Quasi-Poisson vs. negative binomial regression: how should we model overdispersed count data? Ecology. 2007;88:2766–2772. doi: 10.1890/07-0043.1. [DOI] [PubMed] [Google Scholar]
- Vilches TN, Bonesso MF, Guerra HM, Fortaleza C, Park AW, Ferreira CP. The role of intra and inter-hospital patient transfer in the dissemination of heathcare-associated multidrug-resistant pathogens. Epidemics. 2019;26:104–115. doi: 10.1016/j.epidem.2018.11.001. [DOI] [PubMed] [Google Scholar]
- Weinstein MP. Comparative evaluation of penicillin, ampicillin, and imipenem MICs and susceptibility breakpoints for vancomycin-susceptible and vancomycin-resistant Enterococcus faecalis and Enterococcus faecium. Journal of Clinical Microbiology. 2001;39:2729–2731. doi: 10.1128/JCM.39.7.2729-2731.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E, Zahar JR, Lesprit P, Ruppe E, Leone M, Chastre J, Lucet JC, Paugam-Burtz C, Brun-Buisson C, Timsit JF, De-escalation Study Group Elaboration of a consensual definition of de-escalation allowing a ranking of β-lactams. Clinical Microbiology and Infection. 2015;21:649.e1–64649. doi: 10.1016/j.cmi.2015.03.013. [DOI] [PubMed] [Google Scholar]
- Wurster JI, Saavedra JT, Gilmore MS. Impact of antibiotic use on the evolution of Enterococcus faecium. The Journal of Infectious Diseases. 2016;213:1862–1865. doi: 10.1093/infdis/jiv598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyres KL, Holt KE. Klebsiella pneumoniae population genomics and Antimicrobial-Resistant clones. Trends in Microbiology. 2016;24:944–956. doi: 10.1016/j.tim.2016.09.007. [DOI] [PubMed] [Google Scholar]
- Zhou X, Willems RJL, Friedrich AW, Rossen JWA, Bathoorn E. Enterococcus faecium: from microbiological insights to practical recommendations for infection control and diagnostics. Antimicrobial Resistance & Infection Control. 2020;9:130. doi: 10.1186/s13756-020-00770-1. [DOI] [PMC free article] [PubMed] [Google Scholar]