Abstract
Breast involvement of Adult T-cell leukemia-lymphoma (ATLL) is extremely rare, and the data on the characteristics are limited. We herein describe a 49-year-old woman who presented with skin involvement of ATLL. Positron emission tomography/computed tomography showed bilateral breast lesions. Although the patient once achieved a complete metabolic response, a relapse of her ATLL occurred. The patient received subsequent allogeneic hematopoietic stem cell transplantation (HSCT). To our knowledge, only four cases of ATLL with breast involvement have previously been reported, and the prognoses have generally been poor. Breast lesions of ATLL have aggressive features, and intensive systemic chemotherapy and HSCT are required to improve survival.
Keywords: adult T-cell leukemia-lymphoma, breast involvement, positron emission tomography/computed tomography
Introduction
Adult T-cell leukemia-lymphoma (ATLL) is a lymphoproliferative neoplasm of T lymphocytes caused by human T-cell leukemia virus type 1 (HTLV-1). ATLL shows various clinical symptoms, including hypercalcemia, lymphadenopathy, hepatosplenomegaly, and skin lesions. The diversity of the disease has led to the classification of clinical subtypes, referred to as acute, lymphoma, chronic, and smoldering types, according to the percentage of abnormal T cells, called “flower cells,” in the blood and the involvement of extranodal organs such as the spleen, liver, and central nervous system (1, 2). The acute and lymphoma subtypes are defined as “aggressive” subtypes that require immediate treatment by systemic chemotherapy. ATLL involvement in the mammary gland is very rare, and its significance has not yet been defined. This case report describes a patient with ATLL involving the breast and also reviews the literature of ATLL cases with breast lesions.
Case Report
A 49-year-old woman presented to a clinic with gradually developing erythematous lesions on her upper chest. She was referred to our hospital because she was a carrier of HTLV-1. She had no history of fever, weight loss, or night sweats. Physical examination revealed erythematous lesions measuring 0.5-2 cm in diameter and nodules on the chest without any peripheral lymphadenopathy. Complete blood counts revealed the presence of abnormal lymphocytes (leukocyte count: 10,360 /μL and abnormal lymphocyte proportion: 7%) that were a cluster of differentiation (CD)3+, CD4+, CD7-, CD25+, and C-C chemokine receptor type 4 (CCR4)+ on a flow cytometry analysis. Biochemistry tests showed elevated lactate dehydrogenase (267 IU/L, normal range 124-222 IU/L) and soluble interleukin-2 receptor (3,228 U/mL; normal range, 122-496 U/mL) levels, but other data, including albumin, calcium, and blood urea nitrogen, were unremarkable (Table 1). A biopsy obtained from the erythematous skin lesion revealed that large lymphoid cells with pleomorphic nuclei and prominent nucleoli had infiltrated from the dermis to the adipose tissue, and they were CD3+, CD4+, CD5+, CD7-, CD20-, and CD25+ by immunohistochemistry, compatible with the diagnosis of ATLL. The monoclonal integration of HTLV-1 proviral DNA in the blood was confirmed by southern blotting.
Table 1.
Patient’s Laboratory Data on Admission.
| <Complete Blood Count> | <Coagulation> | |||||||||||||
| WBC | 10,360 | /μL | PT | 10.3 | s | BUN | 10.1 | mg/dL | ||||||
| RBC | 425 | ×104/μL | APTT | 28.9 | s | Cre | 0.72 | mg/dL | ||||||
| Hb | 13.3 | g/dL | Fib | 386 | mg/dL | UA | 4.3 | mg/dL | ||||||
| Het | 40.2 | % | FDP | <2.5 | μg/mL | Na | 141 | mmol/L | ||||||
| MCV | 94.4 | fL | D-dimer | <0.5 | μg/mL | Cl | 109 | mmol/L | ||||||
| MCH | 31.3 | pg | <Biochemistry> | K | 3.9 | mmol/L | ||||||||
| MCHC | 33.1 | g/dL | TP | 6.8 | g/dL | Ca | 8.7 | mg/dL | ||||||
| PLT | 286 | ×103/μL | Alb | 4.0 | g/dL | IP | 3.7 | mg/dL | ||||||
| Seg | 64.0 | % | T-Bil | 0.38 | mg/dL | CRP | 0.04 | mg/dL | ||||||
| St | 0.5 | % | AST | 14 | IU/L | IgG | 1,081.2 | ng/mL | ||||||
| Ly | 19.5 | % | ALT | 14 | IU/L | IgA | 156.4 | ng/mL | ||||||
| Mon | 6.5 | % | ALP | 195 | IU/L | IgM | 85.2 | pg/mL | ||||||
| Eos | 2.5 | % | γ-GTP | 17 | IU/L | sIL-2R | 3,228 | U/mL | ||||||
| Bas | 0 | % | ChE | 291 | IU/L | |||||||||
| Abnormal-Ly | 7.0 | % | LDH | 267 | IU/L | |||||||||
WBC: white blood cells, RBC: red blood cells, Hb: hemoglobin, Het: hematocrit, MCV: mean corpuscular volume, MCH: mean corpuscular hemoglobin, MCHC: mean corpuscular hemoglobin concentration, PLT: platelets, Seg: segmented cell, St: stab cell, Ly: lymphocyte, Mon: monocyte, Eos: eosinophils, Bas: basophils, Abnormal-Ly: abnormal lymphocytes, PT: prothrombin time, APTT: activated partial thromboplastin time, Fib: fibrinogen, FDP: fibrin/fibrinogen degradation products, TP: total protein, Alb: albumin, T-Bil: total bilirubin, AST: aspartate aminotransaminase, ALT: alanine aminotransaminase, ALP: alkaline phosphatase, γ-GTP: gamma glutamyltranspeptidase, ChE: cholinesterase, LDH: lactate dehydrogenase, BUN: blood urea nitrogen, Cre: creatinine, UA: uric acid, Na: natrium, Cl: chloride, K: potassium, Ca: calcium, IP: inorganic phosphorus, CRP: C-reactive protein, IgG: immunoglobulin G, IgA: immunoglobulin A, IgM: immunoglobulin M, sIL-2R: soluble interleukin-2 receptor
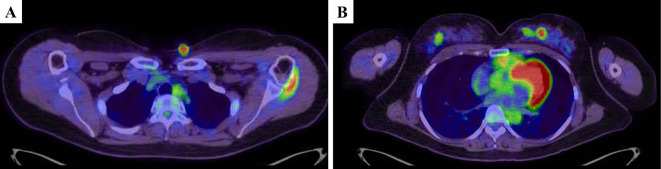
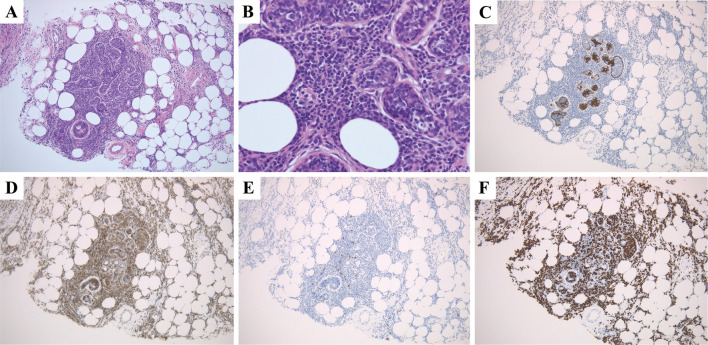
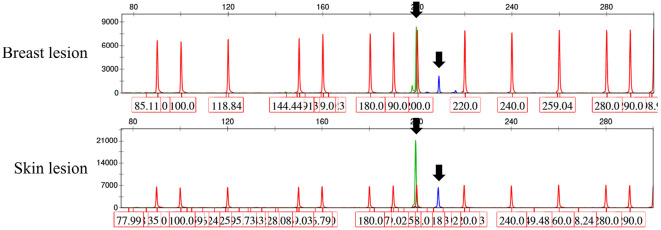
18F-fluorodeoxyglucose (FDG) positron emission tomography/computed tomography (PET/CT) demonstrated an increased FDG uptake, not only in the skin nodules, but also in the bilateral breasts with standardized uptake values of 10.6 and 12.9, respectively (Fig. 1). The mammary masses detected by PET/CT were palpable but undetectable by either ultrasonography or mammography. A core needle biopsy of the right breast mass revealed ductal infiltration of abnormal lymphoid cells that were CD3+, CD4+, and CD25+ with a high proliferative index as defined by Ki-67 staining (Fig. 2). Polymerase chain reaction of the skin and breast lesions for T-cell receptor gene rearrangements showed the same clonal gene rearrangement (Fig. 3). Magnetic resonance imaging of the head and an analysis of the cerebrospinal fluid profile showed no evidence of central nervous system invasion. She was diagnosed with an acute type of ATLL because according to the diagnostic criteria, patients with smoldering type ATLL should have no involvement of extranodal organs except for skin and pulmonary lesions (1). Her ATL prognostic index and Japan Clinical Oncology Group prognostic index were classified as low- and moderate-risk, respectively (3,4).
Figure 1.
Pretreatment positron emission tomography/computed tomography shows a skin lesion on the chest (A) and bilateral breast lesions (B).
Figure 2.
Histological findings of the right breast lesion reveal large lymphoid cells infiltrating the mammary ducts (Hematoxylin and Eosin staining; A, magnification ×100; B, magnification ×400). Immunohistochemical staining of cytokeratin highlighted ductal cells (C, magnification ×100). Immunohistochemical staining reveals that the tumor cells are positive for CD3 (D, magnification ×100) and negative for CD20 (E, magnification ×100). The Ki-67 labeling index is high (F, magnification ×100).
Figure 3.
Clonality for the T-cell receptor (TCR) assessed by polymerase chain reaction for TCR gamma chain gene rearrangements using formalin-fixed paraffin-embedded sections according to the established BIOMED-2 protocol (24). Identical peaks obtained from skin and breast lesions, suggesting the presence of the same TCR gene rearrangement (black arrow).
The patient received two cycles of the modified Lymphoma Study Group (mLSG15) regimen (VCAP-AMP-VECP) (5). Although the patient achieved a complete metabolic response on PET/CT after two cycles of chemotherapy, skin lesions recurred after three cycles of mLSG15, suggesting the aggressive features of this disease. The patient was treated with salvage chemotherapy. However, the disease was refractory for salvage chemotherapy. She received allogeneic hematopoietic stem cell transplantation (HSCT) from a haploidentical sibling with a reduced-intensity conditioning regimen composed of fludarabine (30 mg/m2 on day -7 to -2), melphalan (40 mg/m2 on day -3 and -2), and total body irradiation (2 Gy). Three months after HSCT, she complained of a headache and abnormal sensation of her right jaw, and magnetic resonance imaging of the head revealed an abnormal enhancement of the right trigeminal nerve and multiple skull lesions with bone lysis. A biopsy specimen obtained from the skull lesion revealed the infiltration of large lymphoid cells, which was compatible with the relapse of ATLL. The immunosuppressant was discontinued, and mogamulizumab administration was commenced in anticipation of a direct anti-tumor effect and an alloreactive immune response (6).
Discussion
Most breast lymphomas are B-cell lymphomas, and breast T-cell lymphomas are rare (7,8). The common subtypes of breast T-cell lymphoma are breast implant-associated anaplastic large cell lymphoma and peripheral T-cell lymphoma, unspecified (9,10). ATLL cases with breast lesions at diagnosis are very rare and have only been sporadically reported as isolated cases. We found only four cases reported between 1990 and 2018 (Table 2) (11-14). According to these previous reports, the first clinical presentation of breast ATLL lesions is a painless palpable mass. The mammography in these cases showed a well-circumscribed, round mass of high density without calcification. The main ultrasonographic feature was a heterogeneous hypoechoic mass with an irregular border. These findings are similar to the radiological features of other types of breast lymphoma (15). Our patient, however, did not present with these radiological findings, and only PET/CT could detect the breast lesions. A previous study reported that no abnormalities were found mammographically in about 10% of all patients with breast lymphoma (15). PET/CT is an essential modality for the initial evaluation of all types of lymphoma (16), and the revised adult T-cell leukemia-lymphoma International Consensus Meeting Report also recommends PET/CT at diagnosis because ATLL is frequently associated with extranodal disease (17). PET/CT, therefore, plays an essential role in clinical staging in patients with ATLL. Additionally, PET/CT helps predict the prognosis of ATLL. Nakachi et al. demonstrated that the intensity of FDG uptake was significantly higher in the acute and lymphoma subtypes than in the smoldering and chronic subtypes (18).
Table 2.
Clinical Characteristics of Adult T-cell Leukemia/lymphoma with Breast Lesions.
| No | Cases | Age (year) | Sex | Site | Extramammary disease | Treatment | Follow-up (months) |
|---|---|---|---|---|---|---|---|
| 1 | Our case | 49 | F | Bilateral | Skin | VCAP-AMP-VECP Allogenic HSCT | Alive (11) |
| 2 | 11 | 69 | F | Left | No | Surgery+CHOP | Alive (18) |
| 3 | 12 | 84 | F | Left | No | Surgery | Dead (19) |
| 4 | 13 | 45 | F | Left | Axial lymph node | Surgery+CHOP, bleomycin | Dead (19) |
| 5 | 14 | 45 | F | Left | No | Surgery+CHOP, etoposide | Dead (39) |
F: female,VCAP: vincristine, cyclophosphamide, doxorubicin, and prednisone, AMP: doxorubicin, ranimustine, and prednisone, VECP: vindesine, etoposide, carboplatin, and prednisone, HSCT: hematopoietic stem cell transplantation, CHOP: cyclophosphamide, doxorubicin, vincristine, and prednisone
The prognosis of ATLL patients with breast involvement as an extranodal site has not been assessed because of the rarity of the disease. As shown in Table 2, previous studies showed that three of four patients died within approximately 3 years after diagnosis despite receiving systemic chemotherapy, indicating that the prognosis of patients with breast lesions seems to be comparable to that of patients with acute and lymphoma ATLL (1). A histopathological analysis of the four cases revealed that the tumor cells in the breast lesion were medium to large cells with mitotic figures. In our case, the breast lesion also showed a high Ki-67 index, suggesting its aggressive nature. Since the clinical features of ATLL with breast lesions are similar to those of acute or lymphoma ATLL, the prognosis of breast ATLL is estimated to be poor. Similar to previous reports, our patient showed progression after three cycles of intensive chemotherapy. According to previous reports, allogenic HSCT for ATLL demonstrated favorable outcomes (19-21). Thus, the revised adult T-cell leukemia-lymphoma International Consensus Meeting Report recommends that allogeneic HSCT should be considered for all suitable patients with an aggressive type of ATLL (17). Therefore, our patient received allogeneic HSCT. However, she experienced a relapse 3 months after allogeneic HSCT. We thought her clinical course reflected the aggressive features of ATLL with breast lesions. In contrast, despite the disease involvement of a visceral organ, patients diagnosed to have ATLL with pulmonary lesions are classified as smoldering type. Yoshioka et al. surveyed ATLL patients with pulmonary lesions and reported that pulmonary ATLL cell infiltration occurred in the early stage, which had been considered as a chronic lung disease for 2-6 years before the diagnosis of ATLL (22). Itai et al. showed that patients with pulmonary lesions had a long survival period and did not die during observation (23). Accordingly, it took several years before patients with pulmonary lesions experienced transformation, with aggressive features. It remains unclear why a different organ involvement of ATLL leads to a distinct prognosis.
In conclusion, we experienced an ATLL patient with breast involvement detected only by PET/CT. Taken together with previous reports, patients with breast ATLL lesions generally have a worse prognosis in comparison to patients with indolent type ATLL. Although the optimal treatment for patients with breast ATLL lesions remains unknown, intensive systemic chemotherapy or further allogeneic HSCT is considered to be required to improve the prognosis.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
The authors thank Misa Sakamoto for performing PCR.
References
- 1. Shimoyama M. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma. A report from the Lymphoma Study Group (1984-87). Br J Haematol 79: 428-437, 1991. [DOI] [PubMed] [Google Scholar]
- 2. Tsukasaki K. Adult T-cell leukemia-lymphoma. Hematology 17 (Suppl): S32-S35, 2012. [DOI] [PubMed] [Google Scholar]
- 3. Katsuya H, Yamanaka T, Ishitsuka K, et al. Prognostic index for acute- and lymphoma-type adult T-cell leukemia/lymphoma. J Clin Oncol 30: 1635-1640, 2012. [DOI] [PubMed] [Google Scholar]
- 4. Fukushima T, Nomura S, Shimoyama M, et al. Japan Clinical Oncology Group (JCOG) prognostic index and characterization of long-term survivors of aggressive adult T-cell leukaemia-lymphoma (JCOG0902A). Br J Haematol 166: 739-748, 2014. [DOI] [PubMed] [Google Scholar]
- 5. Tsukasaki K, Utsunomiya A, Fukuda H, et al. VCAP-AMP-VECP compared with biweekly CHOP for adult T-cell leukemia-lymphoma: Japan Clinical Oncology Group Study JCOG9801. J Clin Oncol 25: 5458-5464, 2007. [DOI] [PubMed] [Google Scholar]
- 6. Inoue Y, Endo S, Matsuno N, et al. Safety of mogamulizumab for relapsed ATL after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant 54: 338-342, 2019. [DOI] [PubMed] [Google Scholar]
- 7. Guo HY, Zhao XM, Li J, Hu XC. Primary non-Hodgkin's lymphoma of the breast: eight-year follow-up experience. Int J Hematol 87: 491-497, 2008. [DOI] [PubMed] [Google Scholar]
- 8. Cheah CY, Campbell BA, Seymour JF. Primary breast lymphoma. Cancer Treat Rev 40: 900-908, 2014. [DOI] [PubMed] [Google Scholar]
- 9. Gualco G, Chioato L, Harrington WJ Jr, Weiss LM, Bacchi CE. Primary and secondary T-cell lymphomas of the breast: clinico-pathologic features of 11 cases. Appl Immunohistochem Mol Morphol 17: 301-306, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Aguilera NS, Tavassoli FA, Chu WS, Abbondanzo SL. T-cell lymphoma presenting in the breast: a histologic, immunophenotypic and molecular genetic study of four cases. Mod Pathol 13: 599-605, 2000. [DOI] [PubMed] [Google Scholar]
- 11. Kosaka M, Tsuchihashi N, Takishita M, et al. Primary adult T-cell lymphoma of the breast. Acta Haematol 87: 202-205, 1992. [DOI] [PubMed] [Google Scholar]
- 12. Sakoda Y, Kohno N, Nakata S, Ishikawa Y, Sashikata T. A case of T cell malignant lymphoma of the breast. J Jpn Surg Assoc 58: 85-89, 1997(in Japanese, Abstarct in English). [Google Scholar]
- 13. Morozumi K, Miyazaki H, Narai S, Kodaira K, Sugiura J. Malignant T-cell lymphoma of the breast -report of a case-. J Jpn Surg Assoc 60: 2320-2325, 1999(in Japanese, Abstarct in English). [Google Scholar]
- 14. Fujita Y, Kageyama N. A case of adult T-cell lymphoma of the breast. J Jpn Surg 65: 328-332, 2004(in Japanese, in Abstarct English). [Google Scholar]
- 15. Surov A, Holzhausen HJ, Wienke A, et al. Primary and secondary breast lymphoma: prevalence, clinical signs and radiological features. Br J Radiol 85: e195-e205, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol 32: 3059-3068, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cook LB, Fuji S, Hermine O, et al. Revised Adult T-Cell Leukemia-Lymphoma International Consensus Meeting Report. J Clin Oncol 37: 677-687, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nakachi S, Okada M, Morishima S, et al. Clinical usefulness of FDG-PET/CT for the evaluation of various types of adult T-cell leukemia. Hematology 22: 536-543, 2017. [DOI] [PubMed] [Google Scholar]
- 19. Hishizawa M, Kanda J, Utsunomiya A, et al. Transplantation of allogeneic hematopoietic stem cells for adult T-cell leukemia: a nationwide retrospective study. Blood 116: 1369-1376, 2010. [DOI] [PubMed] [Google Scholar]
- 20. Ishida T, Hishizawa M, Kato K, et al. Allogeneic hematopoietic stem cell transplantation for adult T-cell leukemia-lymphoma with special emphasis on preconditioning regimen: a nationwide retrospective study. Blood 120: 1734-1741, 2012. [DOI] [PubMed] [Google Scholar]
- 21. Bazarbachi A, Cwynarski K, Boumendil A, et al. Outcome of patients with HTLV-1-associated adult T-cell leukemia/lymphoma after SCT: a retrospective study by the EBMT LWP. Bone Marrow Transplant 49: 1266-1268, 2014. [DOI] [PubMed] [Google Scholar]
- 22. Yoshioka R, Yamaguchi K, Yoshinaga T, Takatsuki K. Pulmonary complications in patients with adult T-cell leukemia. Cancer 55: 2491-2494, 1985. [DOI] [PubMed] [Google Scholar]
- 23. Itai Y, Kamizikkoku S, Yul LS, Tomino S, Yamaguchi K, Takatsuki K. Interstitial pneumonitis and smoldering adult T-cell leukemia. Study of anti ATLA antibody in patients with chronic pulmonary diseases in ATL endemic area. Rinsho Ketsueki (Jpn J Clin Hematol) 26: 499-503, 1985(in Japanese, Abstarct in English). [PubMed] [Google Scholar]
- 24. Miyata-Takata T, Takata T, Yamanouchi S, Sato Y, Harada M, Oka T. Detection of T-cell receptor gamma gene rearrangement in paraffin-embedded T or natural killer/T-cell lymphoma samples using the BIOMED-2 protocol. Leuk Lymphoma 55: 2161-2164, 2014. [DOI] [PubMed] [Google Scholar]