Abstract
We herein report the case of a 79-year-old patient with unresectable stage III non-small cell lung cancer who developed immune-related hepatitis caused by durvalumab administration. Durvalumab was administered at 10 mg/kg every two weeks after the treatment with carboplatin (AUC2), paclitaxel (35 mg/m2), and 60 Gy radiation. At the day 208 in which the 14th durvalumab administration was scheduled, the patient was urgently hospitalized due to CTCAE Grade 4 hepatic dysfunction detected during the an outpatient blood sampling test. He was diagnosed with immune-related hepatitis and started on methylprednisolone 60 mg/day. After 51 days, his liver dysfunction improved and he was discharged.
Keywords: immune-related hepatitis, durvalumab, non-small cell lung cancer
Introduction
Durvalumab is an immune checkpoint inhibitor and an anti-programmed death ligand-1 (PD-L1) human IgG1 monoclonal antibody. It has a high affinity to PD-L1, thereby blocking interaction with PD-1 to enhance cancer antigen-specific T-cell cytotoxicity and suppress tumor growth.
Clinically, durvalumab is an immune checkpoint inhibitor for unresectable non-small cell lung cancer (NSCLC), which has very limited treatment options. The Japan Guidelines for the treatment of lung cancer 2018 edition states the “proposal of maintenance therapy with durvalumab after concurrent chemoradiation (2B)”. In the 2019 version, it has been changed to “Recommend maintenance therapy with durvalumab after concurrent chemoradiotherapy (1B)” (1). This modification is based on the results of the phase III PACIFIC trial comparing the maintenance with durvalumab and placebo in patients with unresectable stage III NSCLC who had not progressed following curative concurrent chemoradiotherapy (2,3). Durvalumab maintenance significantly prolonged two primary endpoints as compared with a placebo, i.e., the progression-free survival (PFS) (16.8 months vs. 5.6 months) and the overall survival (OS) (unachieved vs. 28.7 months). Based on these results, durvalumab was approved regardless of PD-L1 expression because the trend was significant (25% or more, less than 25%, unknown) in a subgroup analysis.
However, despite its superior antitumor efficacy, durvalumab may induce immune-related adverse events (irAEs) that are not observed in patients treated with cytotoxic anticancer drugs. This case report presents the first known case of durvalumab-induced irAE liver dysfunction.
Case Report
A 79-year-old man had a height of 160 cm, a weight of 65 kg, and a body mass index (BMI) of 25.3. The patient's alcohol intake was 360 mL/day of Japanese sake. Previous medical conditions included prostate cancer, benign prostatic hyperplasia, hyperlipidemia, chronic gastritis, chronic constipation and insomnia. The patient took silodosin 4 mg/2 tablets, omega-3-acid ethyl esters 2 g/1 packet, lansoprazole OD15 mg/1 tablet, lubiprostone 24 μg/2 capsules, magnesium oxide 250 mg/2 tablets and brotizolam 0.25 mg/1 tablet. While under observation for prostate cancer, he was found to have an enlarged right upper lobe pulmonary nodule. He was finally diagnosed with stage IIIA lung adenocarcinoma. The patient received carboplatin (AUC2) + paclitaxel (35 mg/m2)+60 Gy radiation as concurrent radiation chemotherapy, followed by maintenance therapy with durvalumab (10 mg/kg) at 2-week intervals.
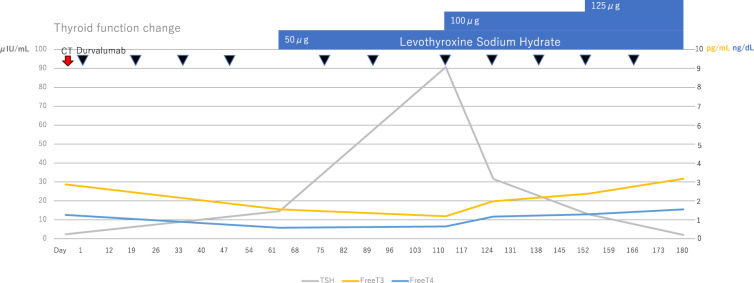
Radiation pneumonitis was observed on a chest X-ray on the day 33, but durvalumab was continuously administered due to Common Terminology Criteria for Adverse Events (CTCAE) Grade 1 with no symptomatic imaging findings. At the day 61, the free T3 and free T4 levels decreased (1.55 pg/mL and 0.58 ng/dL, respectively) while the thyroid-stimulating hormone (TSH) level increased (14.56 μIU/m). The patient was diagnosed with CTCAE Grade 2 hypothyroidism, and an oral administration of 50 μg levothyroxine sodium hydrate was started. Antithyroid antibodies were measured at the day 110; the antithyroid peroxidase antibody (TPOAb) level was 10 IU/mL (<16 IU/mL: normal value), but the antithyroglobulin antibody (TgAb) level was 757 IU/mL (<28 IU/mL: normal value).
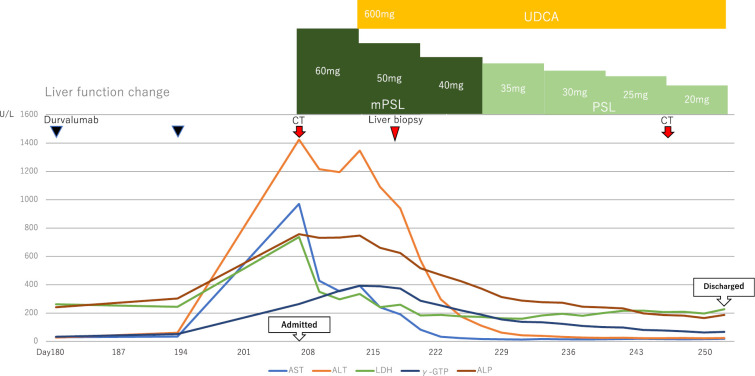
Thereafter, the dose was increased to 125 μg levothyroxine sodium hydrate at the day 152, and the thyroid function eventually stabilized (Fig. 1). CTCAE Grade 1 hepatic dysfunction [aspartate aminotransferase (AST) 34 U/L, alanine aminotransferase (ALT) 61 U/L) was observed by the day 194. His general condition was stable; hence durvalumab was continued. A chest CT scan at the day 208 showed that radiation pneumonitis had spread, but the primary lung lesions had been obscured and the volume of mediastinal lymph node metastasis had shrunk (Fig. 2C, G). In contrast, a blood test showed Grade 4 liver dysfunction [AST 971 U/L, ALT 1425 U/L, lactate dehydrogenase (LDH) 737 U/L, alkaline phosphatase (ALP) 757 U/L, γ-glutamyl transpeptidase (GTP) 264 U/L); hence he was urgently admitted to the Department of Respiratory Surgery (Fig. 3).
Figure 1.
The thyroid function change before day 180.
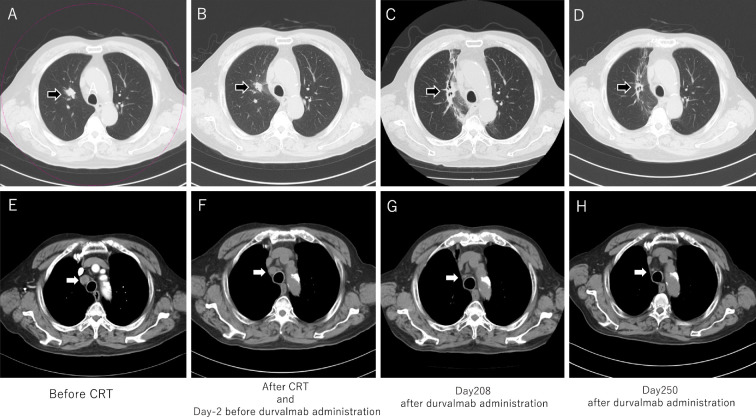
Figure 2.
Chest CT: Lung cancer and lymph node metastasis. A to D: Black arrows indicate lung adenocarcinoma in the upper right lobe. Lung adenocarcinoma was unclear at day 250 after durvalumab administration. Radiation pneumonitis appeared after CRT, but it showed an improvement at day 250. E to H: The white arrow indicates mediastinal lymph node metastasis. The shrinking of the volume of the metastasis was observed after durvalumab administration at day 250.
Figure 3.
The liver function change after day 180. UDCA: ursodeoxycholic acid, mPSL: methylprednisolone, PSL: prednisolone
Abdominal CT showed no apparent liver abnormalities, but an edematous thickening of the gallbladder wall associated with hepatitis was observed (Fig. 4). The biliary system was evaluated by abdominal ultrasound, but no obvious sclerosing cholangitis was found. Additional blood sampling showed the antimitochondrial M2 antibody level to be slightly higher than normal at 10.8 U/mL (Table), but the IgM level was normal at 67 mg/dL. These results showed that the possibility of primary biliary cholangitis (PBC) was low. The patient was diagnosed with immune-related hepatitis and was started with the intravenous administration of methylprednisolone 60 mg/day.
Figure 4.
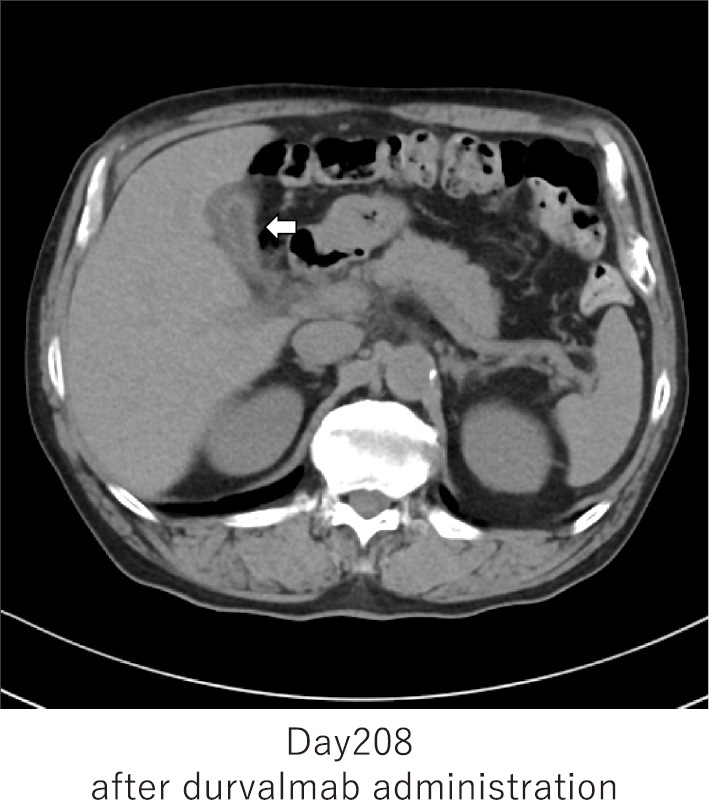
Abdomen CT: gallbladder wall hyperplasia due to edema. The white arrow indicates edematous gallbladder wall thickening associated with liver injury.
Table.
Laboratory Findings on Admission.
| Hematology | Blood Chemistry | Endocrine | |||||||||||
| WBC | 3,550 | /μL | T-Bil | 1.0 | mg/dL | TSH | 1.190 | μIU/mL | |||||
| Neu | 62.1 | % | LDH | 737 | IU/L | F-T3 | 2.68 | pg/mL | |||||
| Lym | 21.0 | % | AST | 971 | IU/L | F-T4 | 1.52 | ng/dL | |||||
| Eos | 1.0 | % | ALT | 1,425 | IU/L | ||||||||
| RBC | 370×104 | /μL | ALP | 757 | IU/L | Serological tests | |||||||
| Hb | 12.8 | g/dL | γ-GTP | 264 | IU/L | HBs-Ag | (-) | ||||||
| Ht | 37.1 | % | Na | 140 | mEq/L | HBs-Ab | (-) | ||||||
| PLT | 11.6×104 | /μL | K | 4.4 | mEq/L | HBc-Ab | (-) | ||||||
| Cl | 105 | mEq/L | HCV-Ab | (-) | |||||||||
| Coagulation | BUN | 17 | mg/dL | IgM-HA | (-) | ||||||||
| PT | 95.0 | % | Cr | 0.66 | mg/dL | IgA-HEV Ab | (-) | ||||||
| APTT | 31.3 | sec | TP | 6.8 | g/dL | CMV IgM | 0.24 | (normal range: <0.8) | |||||
| Fib | 356 | mg/dL | Alb | 3.9 | g/dL | CMV C7-HRP | (-) | ||||||
| CRP | 0.52 | mg/dL | EB VCA IgG | ×10 | |||||||||
| IgG | 1,174 | mg/dL | EB VCA IgM | <×10 | |||||||||
| IgA | 305 | mg/dL | EB EA-DR IgG | <×10 | |||||||||
| IgM | 67 | mg/dL | EBNA | ×20 | |||||||||
| ANA | (-) | ||||||||||||
| AMA-M2 | 10.8 | (normal range: <10.0U/mL) | |||||||||||
CMV: Cytomegalovirus, EB (V): Epstein-Barr (virus), ANA: antinuclear antibody, AMA: anti-mitochondrial antibody
On the 8th hospitalization day (the day 215), the methylprednisolone dosage was reduced to 50 mg/day and the oral administration of ursodeoxycholic acid 600 mg/day was started. However, on the 11th day of admission (the day 218), the patient's recovery showed a tendency to stagnate with AST 241 U/L and ALT 1,091 U/L. Therefore, he was transferred to the Department of Gastroenterology for liver biopsy on the 12th hospitalization day (the day 219).
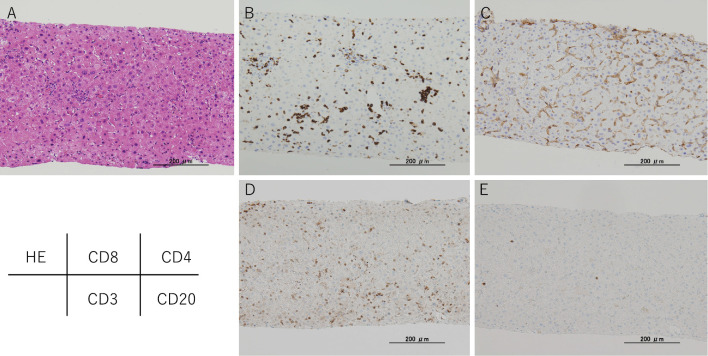
Liver biopsy performed on the 14th hospitalization day (the day 221) indicated that PBC was negative and consistent with immune-related hepatitis (Fig. 5). From the 22nd hospitalization day (the day 229), the prednisolone (PSL) dosage was changed to 35 mg/day. Since then, his liver function improved steadily; hence the dosage was gradually reduced. He was discharged on the 51st hospitalization day (the day 258) when PSL dosage could be reduced to 15 mg.
Figure 5.
Immunohistochemical results of checkpoint inhibitor-induced liver injury. A: Hematoxylin and Eosin staining shows an image of chronic hepatitis mainly due to inflammation of the liver parenchyma. B to E: CD8+and CD3+T cells demonstrated very high levels, while the CD4+and CD20+cells show low levels.
Discussion
The anti-PD-L1 antibodies currently available in Japan include durvalumab, atezolizumab, and avelumab. Under the Japanese Health Insurance System, atezolizumab can be used for NSCLC (combination with first-line chemotherapy or second-line treatment), advanced small-cell lung cancer, and triple-negative breast cancer and avelumab for Merkel cell carcinoma.
Both durvalumab and atezolizumab can be used for lung cancer, but durvalumab has very limited use as a maintenance therapy for unresectable stage III NSCLC, as in this case. However, it is the first immune checkpoint inhibitor available in Japan for unresectable stage III NSCLC and it has had a significant impact on the treatment of the disease, which has not progressed for nearly 20 years.
Immune checkpoint inhibitors other than anti-PD-L1 antibody include anti-cytotoxic T-lymphocyte associated protein (CTLA)-4 antibody and anti-PD-1 antibody. International meta-analyses showed the appearance of irAE by comparing anti-CTLA-4, anti-PD-1, and anti-PD-L1 antibodies in all grades (53.8%, 26.5%, and 17.1%, respectively). For Grade 3 or higher, similar results were obtained (the anti-CTLA-4 antibody was the highest at 21.5%, the anti-PD-1 antibody was 7.1%, and the anti-PD-L1 antibody was the lowest at 6.3%) (4). These results may be due to the difference in the target cell and the point of action of each drug (5). The anti-CTLA-4 antibody targets the priming function of immune cells in the body, whereas the anti-PD-L1 antibody targets the effector function of non-immune tumor cells. Although the target cells are different, the anti-PD-1 and anti-PD-L1 antibodies, which have the same effector function, have the same irAE frequency in Grade 3 and higher, but the anti-PD-L1 antibody in all grades has a lower frequency. The difference in the irAE frequency may be due to the diversity of the mechanism of action, such as PD-1 binding to PD-L1 and PD-L2 and PD-L1 binding to PD-1 and CD80 (6).
In all grades of thyroid dysfunction, the anti-CTLA-4, anti-PD-1, and anti-PD-L1 antibodies were 2.9%, 5.5%, and 4.3%, respectively. The appearance of the anti-PD-1 and anti-PD-L1 antibodies tended to be relatively higher than that of anti-CTLA4 antibody, and this may have led to the onset of irAE in this case. In addition, it has been reported that patients with thyroid dysfunction (destructive thyroiditis) who received anti-PD-1 antibody nivolumab had significantly higher thyroid autoantibodies (TPOAb or TgAb) levels than those who did not (7). It is therefore considered that thyroid autoantibodies can serve as a biomarker to identify patients who are more likely to develop thyroid side effects. Although thyroid autoantibody measurements were carried out post-onset in this case, in the future it may be possible to determine the risk of thyroid side effects by measuring the autoantibodies before durvalumab administration.
In contrast, in all grades of liver dysfunction (ALT elevation), the anti-CTLA-4, anti-PD-1, and anti-PD-L1 antibodies were 4.5%, 2.1%, and 2.7%, respectively. The incidence with anti-PD-1 and anti-PD-L1 antibodies tended to be low. At Grade 3 or a higher level of liver dysfunction, the incidence with the anti-PD-L1 antibody (2.6%) tended to be higher than that of the anti-PD-1 antibody (0.90%). However, it should be noted that once it appears, there is a risk of developing severe irAE of Grade 3 or higher as was observed in this case. Furthermore, in this case, Grade 3 or higher liver damage appeared 6 months (29 weeks, 208 days) after durvalumab administration. In contrast, liver damage after the administration of anti-CTLA-4 antibody ipilimumab and anti-PD-1 antibody nivolumab appeared at 10.0 weeks (3.0-15.1) and 14.1 weeks (1.9-145.1), respectively (8). Even in comparison with these results, our case had a late onset.
A survey of durvalumab immediately after marketing in Japan (9) estimated that it was used in about 1,900 patients between July 2018 and February 2019, of which 19 hepatic dysfunctions were reported in 22 of 19 patients. Of these, eight severe cases were reported, and the time of onset was relatively early, ranging from 2 days to 54 days (median 17.8 days) from the start of drug administration. However, as in this case, it may occur after more than six months (208 days). For this reason, it should be noted that the time of onset may be late in some cases.
In these eight severe cases, steroid administration was given for treatment in four out of six cases whose outcomes were known, all of which successfully recovered. Therefore, prompt steroid administration may achieve a good outcome for patients. In the case of grade 3-4 liver dysfunction, The Durvalumab Proper Use Guide (10) recommends durvalumab discontinuation and the administration of PSL 1 to 4 mg/kg/day as the initial steroid dose, or equivalent dose of other steroids. Regarding mPSL, the recommended dose was 0.8-3.2 mg/kg/day. In this case, the patient weighed 65 kg, so the initial dose was 0.92×65 kg ≈60 mg. The dose was reduced by reducing mPSL by 10 mg every week to 40 mg, changing to PSL 35 mg, reducing by 10 mg every week to 25 mg, and finally changing to 5 mg. Good results were also obtained by starting steroid treatment promptly in this case. However, the guideline states that treatment with mycophenolate mofetil is required if steroid treatment does not prove effective (11,12).
In autoimmune hepatitis, it is desirable to maintain low-dose steroids considering the risk of recurrence. However, the decision to maintain low-dose steroids or to eliminate all steroids in immune-related hepatitis depends on the next treatment.
This case was epidermal growth factor receptor (EGFR) mutation positive, therefore, if lung cancer progression is observed in the future, the administration of EGFR tyrosine kinase inhibitor should be considered, together with the administration of cytocidal anti-cancer drugs and other immune checkpoint inhibitors. To prevent an increase in the infectious disease risk due to pancytopenia, we believe it is desirable to be steroid-free at the time that molecular targeted drugs and multicellular anticancer drugs such as those mentioned above are administered.
On the other hand, when using other immune checkpoint inhibitors, including resumption of durvalumab, it is important to determine whether their effects are diminished following steroid treatment. Steroids administered from baseline for the treatment of underlying disorders are known to affect immune checkpoint inhibitor efficacy (13). Conversely, it has been reported that steroids administered for irAE management have no effect on the re-administration of immune checkpoint inhibitors (14). Therefore, considering the effects on cancer treatment and the adverse event benefit-risk balance, the combined administration of low-dose steroids and immune checkpoint inhibitors is to be considered acceptable.
In accordance with the Durvalumab Proper Use Guide (10), we determined whether to continue durvalumab treatment after the normalization of theliver function based on the CTCAE grade of irAE liver injury. If Grade 1 was observed for the first time, then the administration of durvalumab was continued. If Grade 2 or 3 (either ALT 8 times the standard value or less, AST is 8 times the standard value or more, or total bilirubin is 5 times the standard value or less), durvalumab was discontinued until Grade 1 or below. Regardless of the liver function recovering, if Grade 3 (either ALT exceeds 8 times the standard value, AST exceeds 8 times the standard value or total bilirubin exceeds 5 times the standard value) or Grade 4 were met, then the administration was permanently discontinued. Regarding the prognosis after the resumption of immune checkpoint inhibitors, there are reports comparing cases in which immune checkpoint inhibitors were re-administered after irAE onset and those in which they were discontinued (15). In this comparison of cases without a partial response (PR) before irAE onset, cases with re-administration showed better tendencies in both PFS and OS. In addition, no difference was observed in either PFS or OS when comparing cases with PR before irAE onset. However, the aforementioned report was a retrospective study of anti-PD-1 antibody monotherapy, anti-PD-L1 antibody monotherapy and anti-CTLA-4 antibody combination therapy, rather than a prospective study of durvalumab monotherapy. Considering our case was Grade 4 from the first instance, durvalumab administration will be permanently discontinued according to the Appropriate Use Guide. If the patient is Grade 2 or Grade 3 at first, it is necessary to carefully assess durvalumab administration. In such cases, it is essential to consider the benefits of the maintenance effect alongside the risk of irAE recurrence.
As a differential diagnosis in this case, alcoholic liver injury and drug-induced liver injury were ruled out as there had been no increase in alcohol consumption or change to other medication at onset of liver injury. PBC was ruled out as a histological examination of liver biopsy showed no evidence of chronic non-suppurative destructive cholangitis (CNSDC) or granuloma formation, as is seen in PBC. Since blood samples were antinuclear antibody negative and IgG low, it was important to distinguish them from acute-onset autoimmune hepatitis, which often has similar findings. The histology of immune-related hepatitis differs case by case, and three points of similarity with acute-onset autoimmune hepatitis are often exhibited. The three points are conspicuous centrilobular necrosis and inflammation, recognizable plasma cell infiltration and lack of conspicuous fibrosis (16).
Centrilobular necrosis and inflammation were observed in this case; however, focal necrosis within the lobules was observed but not confluent necrosis. Focal necrosis in the lobules was consistent with findings from anti-CTLA4 antibody-induced immune-related hepatitis reported by Kleiner et al., however, the findings in our case were milder than in Kleiner's report (17). On the other hand, plasma cell infiltration was not noticeable.
Although slight lymphocyte infiltration was observed in the portal area, fibrosis was not conspicuous, thus suggesting an acute onset hepatic disorder. Other findings included pigmented macrophages, which ate easily recognizable in acute-onset autoimmune hepatitis. No other patterns which were considered to be easily recognizable, such as emperipolesis, cobblestone appearance of hepatocytes or perivenular necro-inflammatory activity including centrilobular necrosis, were observed (18). Based on the above, although this case presented with symptoms similar to acute-onset liver injury, several points did not correspond to acute-onset autoimmune hepatitis. Therefore, we considered this case to have immune-related hepatitis which is quite distinct from acute-onset autoimmune hepatitis.
Furthermore, the infiltrating lymphocytes in this case were predominantly CD8+ and CD3+ T cells, with few CD4+ and CD20+ cells, and CD8+ cells were found in all zones. Zen et al. previously reported that CD8+ and CD3+ T cells were dominant in immune-related hepatitis with anti-CTLA-4 and anti-PD-1 antibody, and CD8+ cells demonstrated poor zone selectivity. This was in keeping with our findings. Such findings are considered to be important for the diagnosis of immune-related hepatitis, even with the anti-PD-L1 antibody durvalumab (19). We considered that the predominance of CD8+ T cells could help to differentiate this condition from standard autoimmune hepatitis, which is mainly composed of CD4+ cells (20).
Furthermore, anti-PD-L1 antibodies are expected to be widely used in the treatment of various cancers in the future and it is thought that irAE will increase with the increase in drug usage. Hence the early detection and management of irAE is considered to be of high importance.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Guidelines for medical treatment of lung cancer 2019 edition Japan Lung Cancer Society. [Google Scholar]
- 2.Antonia SJ, Villegas A, Daniel D, et al. . Durvalumab after chemoradiotherapy in stageIII non-small-cell lung cancer. N Engl J Med 377: 1919-1929, 2017. [DOI] [PubMed] [Google Scholar]
- 3.Antonia SJ, Villegas A, Daniel D, et al. . Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med 379: 2342-2350, 2018. [DOI] [PubMed] [Google Scholar]
- 4.Osta B. El, Hu F, Sadak R, et al. . Not all immune-checkpoint inhibitors are created equal: meta-analysis and systematic review of immune-related adverse events in cancer trials. Crit Rev Oncol Hematol 119: 1-12, 2017. [DOI] [PubMed] [Google Scholar]
- 5.Ribas A. Tumor immunotherapy directed at PD-1. N Engl J Med 366: 2517-2519, 2012. [DOI] [PubMed] [Google Scholar]
- 6.Chen DS, Irving BA, Hodi FS. Molecular pathways: next-generation immunotherapy- inhibiting programmed death-ligand 1 and programmed death-1. Clin Cancer Res 18: 6580-6587, 2012. [DOI] [PubMed] [Google Scholar]
- 7.Kobayashi T, Iwama S, Yasuda Y, et al. . Patients with antithyroid antibodies are prone to develop destructive thyroiditis by nivolumab: a prospective study. J Endocr Soc 2: 241-251, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hodi FS, Chiarion-Sileni V, Gonzalez R, et al. . Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol 19: 1480-1492, 2018. [DOI] [PubMed] [Google Scholar]
- 9.AstraZeneca MediChannel. IMFINZI EPPV (early post-marketing phase vigilance) final report 2019 [cited 2020 Feb 19]. Available from: http://med.astrazeneca.co.jp/safety/IMF.html
- 10.AstraZeneca MediChannel. Imfinzi Appropriate use guide [cited 2020 Feb 19]. Available from: http://med.astrazeneca.co.jp/medical/product/imf_lc_guide.html
- 11.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) in partnership with the American Society of Clinical Oncology (ASCO): management of immunotherapy-related toxicities. version 1 [Internet]. 2020 [cited 2020 Feb 19]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/immunotherapy.pdf
- 12.Haanen JBAG, Carbonnel F, Robert C, et al. . Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 28: 119-142, 2017. [DOI] [PubMed] [Google Scholar]
- 13.Arbour KC, Mezquita L, Long N, et al. . Impact of baseline steroids on efficacy of programmed cell death-1 and programmed death-ligand 1 blockade in patients with non-small-cell lung cancer. J Clin Oncol 36: 2872-2878, 2018. [DOI] [PubMed] [Google Scholar]
- 14.Horvat TZ, Adel NG, Dang TO, et al. . Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at memorial sloan kettering cancer center. J Clin Oncol 33: 3193-3198, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Santini FC, Rizvi H, Plodkowski AJ, et al. . Safety and efficacy of re-treating with immunotherapy after immune-related adverse events in patients with NSCLC. Cancer Immunol Res 6: 1093-1099, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sue M, Ueno M, Takabatake H, et al. . A case report of pembrolizumab-induced liver injury: the implication of CD8-positive lymphocytes in the immune-related adverse event. Kanzo 59: 571-577, 2018(in Japanese, Abstract in English). [Google Scholar]
- 17.Kleiner D, Berman D. Pathologic changes in ipilimumab-related hepatitis in patients with metastatic melanoma. Dig Dis Sci 57: 2233-2240, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nguyen Canh H, Harada K, Ouchi H, et al. . Acute presentation of autoimmune hepatitis: a multicentre study with detailed histological evaluation in a large cohort of patients. J Clin Pathol 70: 961-969, 2017. [DOI] [PubMed] [Google Scholar]
- 19.Zen Y, Yeh MM. Hepatotoxicity of immune checkpoint inhibitors: a histology study of seven cases in comparison with autoimmune hepatitis and idiosyncratic drug-induced liver injury. Mod Pathol 31: 965-973, 2018. [DOI] [PubMed] [Google Scholar]
- 20.Cassim S, Bilodeau M, Vincent C, et al. . Novel immunotherapies for autoimmune hepatitis. Front Pediatr 5: 8, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]