Abstract
In this study, tansy (Tanacetum vulgare L.) in vitro culture was established from seeds collected from natural populations. The multiplication of plantlets was conducted through shoot tips that exhibited potent apical growth and regeneration capacities on basal medium (BM), without the addition of any plant growth regulators (PGRs). PGRs were also omitted for the establishment and cultivation of tansy root cultures. Both abaxial and adaxial leaf surfaces of in vitro micropropagated plantlets were covered with glandular biseriate trichomes. Histochemical staining showed that glandular secretions were rich in lipid and terpene compounds, confirmed by GC-MS analysis of essential oil (EO). In the total EO, similar portions of oxygenated monoterpenes (38.5% m/m) and oxygenated sesquiterpenes (22.6% m/m) were detected. Chemical profiles of methanol extracts of in vitro cultured tansy shoots and roots varied in quantity and quality from those obtained from wild-growingtansy. HPLC analysis indicated that the methanol extracts of in vitro cultured roots were the richest in 3,5-O-dicaffeoylquinic acid (3,5-O-DCQA), in which the concentration was 6 times higher (10.220 mg/g DW) than that in the extract obtained from roots of wild-growing tansy (1.684 mg/g DW). This result is noticeable in the manner of industrial production of biologically active 3,5-O-DCQA that has been shown to have antioxidant, hepatoprotective, antiviral, antimutagenic, and immunomodulatory activity. Biotechnological interventions on secondary metabolite production taking place in trichomes could further enhance the production of some important tansy metabolites and further investigation will be directed toward the elucidation of the pharmaceutical potential of tansy in vitro obtained metabolites, as mixtures or single moieties.
Keywords: Tansy, In vitro cultivation, Essential oil, Methanol extracts, Histochemical analysis, Phytochemical analysis
Introduction
The current pharmacological industry mostly relies on synthetic drugs, due to the intensive development of a computational approach, combinatorial chemistry techniques, and instruments during the second half of the twentieth century (Liu et al. 2017). Despite what was expected, the rate of new chemically synthesized pharmaceuticals reaching the market has continually decreased, with only 24.6% of the 1881 new drugs approved from 1981 to 2019 obtained synthetically (Newman and Cragg 2020). This has forced the pharmaceutical industry recurrently turn to natural products, compounds derived from microorganisms, plants, and animals used in traditional medicine for thousands of years. Among them, secondary metabolites of plants make the most promising pool of potential pharmacological drugs. From the total number of plant species discovered so far, approximately 20% have been screened for biologically active compounds, and about 135 registered drugs have been approved (Newman et al. 2003; David et al. 2015).
Since 1853, when acetylsalicylic acid was first produced from natural salicin isolated from the bark of the white willow (Salix alba), the pharmaceutical industry has been oriented to the chemical synthesis of products isolated from living organisms. Some of the most famous are paclitaxel (Taxol®) from the bark of Taxus brevifolia L. used in the treatment of breast cancer (Cragg 1998), phorbol ester (Prostratin®) isolated from the bark of Homalanthus nutans used for the treatment of lymphoblastic cells infected with HIV-1 (Dias et al. 2012), or chloroquine extracted from the bark of Cinchona officinalis and used to prevent and treat malaria (Plowe 2005). Chloroquine also showed potent antiviral effects (Savarino et al. 2003) and was promoted for the treatment of patients infected with SARS-CoV-2 during the 2019/2020 pandemic spread of this virus (Wang et al. 2020; Devaux et al. 2020).
In parallel, drug formulations of complex partly purified plant extracts have become equally adopted as “single-molecule” medicines. This approach relies on traditional medicine experiences, where complex mixtures of plant compounds were used for the treatment of different health problems without any knowledge about the contained bioactive compounds. In 2008, the first botanical extract, Veregen®, an enriched extract of polyphenols from green tea (Camellia sinensis), was approved in the USA for the treatment of genital warts caused by the human papilloma virus (Scheinfeld 2008). Although there is no doubt that these herbal mixtures provide a synergism of positive effects (Arora and Koul 2014), many of the regulatory approval processes were impeded for various reasons (Chugh et al. 2018). One of the issues is the intrinsic variability of plant material caused by environmental factors (i.e., actual climate, harvesting time, soil composition, altitude, storage conditions) and/or biotic interactions occurring in natural populations before harvest. This can cause significantly altered phytochemical composition of obtained extracts, as well as low yields of targeted products and high abundance and toxicity of some active compounds.
The employment of in vitro cultivation of plants is among the biotechnological solutions favored by researchers. The establishment of in vitro multiplication protocols enables the production of uniform plant material and controlled manipulation with environmental conditions, plant growth regulators, and elicitation strategies that can boost the production and overall yield of phytochemicals. Growing plants under controlled environmental conditions (temperature, light intensity, and duration), on growing media prepared according to strict recipes for plant’s nutritional necessities, showed to be good practice for avoiding the instability in chemical composition that naturally occurs (Robles and Garzino 2000; Demetzos et al. 2002). Strictly aseptic conditions prevent the presence of unexpected compounds synthesized by endophytic fungi and/or bacteria or produced by plants as a result of the interaction with them (David et al. 2015), or harmful residues of pesticides that are often present in plants collected from nature. While intensive wild-crafting and unsustainable harvesting techniques of either whole plants or their particular organs could bring about their persistence and sustainability in natural habitats and even make plant populations threatened (Cordell 2011), in vitro micropropagation techniques offer a proper alternative for the cultivation of valuable plants and large-scale production of targeted metabolites.
Relying on information coming from traditional medicine, we aimed to investigate the phytochemical properties of Tanacetum vulgare L. (tansy, Asteraceae/Compositae). This aromatic perennial herb is a native of Europe. Its generic name, Tanacetum, comes from the Greek word “athanatos” meaning immortality. In ancient times, the unique rejuvenating powers and ability to preserve dead bodies from corruption were ascribed to its long-lasting flowers (Mitich 1992). Since then, tansy has broadly been used in traditional medicines worldwide for the treatment of a variety of medical disorders, such as intestinal worms, rheumatism, colds and fevers, digestive disorders, epileptic seizures, hysteria, gout, kidney problems, and tuberculosis. Many laboratory reports have claimed that essential oils and extracts of tansy display a wide range of biological activities, including antioxidant, antimicrobial, insecticidal, cytotoxic, antivirus, and anti-inflammatory activities (Mordujovich-Buschiazzo et al. 1996; Onozato et al. 2009; Rosselli et al. 2012; Gospodinova et al. 2014; Devrnja et al. 2017). A purified complex extract of flavonoids and phenol carbonic acids from tansy flowers, commercially known as Tanacechol®, is registered in Russia as a choleretic and spasmolytic agent for chronic cholecystitis and biliary dyskinesia (Karabaeva et al. 2015). SeptimebTM, an herbal extract including T. vulgare (tansy), Rosa canina (dog rose), and Urtica dioica (stinging nettle) in addition to selenium, flavonoids, and carotenes, showed positive effects on the reduction of sepsis severity (Pourdast et al. 2017). The weaker version of this mixture, named Setarud (IMOD™), was proposed as an immunomodulator in the treatment of HIV-positive patients (Paydary et al. 2012).
In general, this species is insufficiently investigated since it is endowed with an array of valuable phytochemicals. It is well known for its impressive chemical diversity with more than 30 chemotypes identified by dominant terpenoids (Keskitalo et al. 2001; Rohloff et al. 2004; Wolf et al. 2012). Additionally, there are limited data regarding suitable micropropagation techniques for its in vitro cultivation, and only procedures for the regeneration of petiole and leaf explants and the production of protoplast-derived callus have been reported (Keskitalo et al. 1995). Our previous research (Devrnja et al. 2017) highlighted the phytochemicals in essential oil and methanol extracts from wild-growing tansy plants that manifested strong antioxidant and antimicrobial activity. Special attention was given to its anticancer properties and in vitro results showed a high antiproliferative effect in the micrograms range (up to 100 μg/mL) on human cervical adenocarcinoma (HeLa) cells.
This study aimed to investigate the potential of tansy plants to produce valuable chemicals by in vitro culture. The general aims were to establish both shoot and root cultures of tansy and to analyze the phytochemical composition of obtained essential oil and methanol extracts with histochemical analyses of production sites. Comparing the composition of metabolites mixtures from in vitro grown plants with those obtained from wild-grown parental plants will indicate the sustainability of the proposed thesis that in vitro cultures could provide an efficient tool for the production of innovative pharmaceutical drugs from tansy metabolites in controlled conditions.
Materials and methods
Establishment and maintenance of in vitro shoot and root cultures
Seeds collected from a native population (from the Ada Huja locality, Belgrade, Serbia) were used for in vitro culture establishment. The seeds were washed in tap water containing 2–3 drops of liquid commercial detergent for 30 min and rinsed three times with sterile distilled water. Washed seeds were then surface sterilized using a 30% solution of commercial bleach (4% NaClO) for 30 min, and 15% bleach for 15 min, and subsequently rinsed three times with sterile distilled water for 10 min. Seeds dried on sterile filter paper were aseptically placed in 90-mm Petri dishes (20 seeds per dish) containing 25 mL of basal medium (BM) for germination.
The BM contained MS (Murashige and Skoog 1962) macro and micro mineral salts, 2% sucrose, 100 mg/L myo-inositol, and LS (Linsmaier and Skoog 1965) vitamins, and was solidified with 0.7% (w/v) agar (Torlak, Belgrade, Serbia). The pH of the medium was adjusted to 5.5 before sterilization in an autoclave at 114 °C (80 kPa) for 25 min. The seeds were germinated under controlled conditions of 24 ± 2 °C, 16-h light/8-h dark photoperiod, and under cool white fluorescent light with a photosynthetic photon flux rate of 40 μmol/(m2s), as measured by an LI-1400 DataLogger equipped with an LI-190SA Quantum sensor, LICOR Biosciences.
When the resulting axenic seedlings fully developed 4–5 leaves and a branched root system, they were used as donor material for shoot multiplication. Micro shoots were sub-cultured on fresh BM in glass 100-mL Erlenmeyer flasks every 4 weeks, and material for phytochemical analyses was collected continuously. In parallel, roots excised from the plantlets rooted spontaneously on BM were used for the establishment of in vitro root cultures. Roots were transferred into Erlenmeyer flasks (400 mg in each) with 100-mL liquid half-strength BM (½ BM; with reduced content of MS components) and maintained on a horizontal shaker (90 rpm) under the aforementioned conditions with reduced light (2 μmol/(m2s)). After 4 weeks in culture, roots were removed from the medium and dried on filter paper to remove any adherent culture medium and air-dried for further phytochemical analyses.
Morpho-anatomical analysis
For the investigation of morpho-anatomical structures that produce and accumulate secondary metabolites, the second and third fresh leaves, isolated from shoots cultured in vitro, were used and subjected to scanning electron (SEM) and light microscopy. For SEM, leaf samples were coated with a thin layer of gold in a BAL-TEC SCD 005 (BAL-TEC GmbH, Schalksmühle, Germany) sputter coater. Both adaxial and abaxial surfaces were examined with a JEOL JSM-6390 LV (JEOL, Tokyo, Japan).
For anatomical investigation by a light microscope, the fresh leaves were fixed with FAA (formalin: acetic acid: 70% ethyl alcohol; 10:5:85) for 24 h, dehydrated in a graded ethanol series, subsequently cleared with xylol, and embedded in paraffin wax at 58 °C. Sections (8–10 μm thick) were stained with hematoxylin and photographed using a Zeiss Axiovert (Carl Zeiss GmbH, Jena, Germany) microscope.
Histochemical analysis
To detect the presence of secondary metabolites in secretory cells of trichomes, the second and third fresh leaves were hand-sectioned and the following histochemical staining methods were used: Sudan Black B, Sudan IV, and Sudan Red 7B/hematoxylin for total lipids (Jensen 1962); Nile Blue A for neutral and acidic lipids (Cain 1947); Nadi reagent for terpenoids (David and Carde 1964); periodic acid-Schiff (PAS) reagent for polysaccharides (Jensen 1962); Ruthenium Red for pectins (Johansen 1940); Wagner (Furr and Mahlberg 1981), Dragendorf (Svendsen and Verpoorte 1983) and Ellram (Furr and Mahlberg 1981) reagents for alkaloids; ferric chloride (FeCl3) for polyphenols (Gahan 1984); and Toluidine Blue O for lignins and tannins (Baker 1966). Following the methods of specific authors, standard control procedures were carried out simultaneously for all histochemical methods used. Observations were made on a Zeiss Axiovert microscope. The autofluorescence investigation was carried out using the same instrument.
Isolation and phytochemical analysis of essential oil
Essential oils from the aerial parts of in vitro grown plants were obtained by steam distillation for 2 h in a Clevenger-type apparatus. Before distillation, air-dried plants were blended to a fine powder. The oil distillate was separate from traces of water by extraction with n-hexane and kept at 4 °C until analysis. The oil yield was expressed as mL per 100 g of plant dry weight.
Gas chromatography-mass spectrometry (GC-MS) analysis of essential oils was performed on an Agilent Technologies 7890 A GC system equipped with 5975 CInert MSD and flame ionization detector (FID). The GC system was equipped with the split-splitless injector and HP-5MS capillary column (30 m × 0.25 mm, 0.25-μm film thickness). Helium was the carrier gas, and its flow rate was 3 mL/min. The ion source energy was 70 eV and chromatographic conditions were as follows: column temperature was linearly programmed from 60 to 300 °C (at the rate of 3 °C/min) and held isothermally at 300 °C next 10 min; injector temperature was 250 °C; ion source temperature was 230 °C; and quadrupole temperature was 150 °C.
The constituents were identified by comparison of their mass spectra to those from Wiley275 and NIST/NBS libraries, using different search engines. The experimental values for retention indices were determined by the use of calibrated Automated Mass Spectral Deconvolution and Identification System software (AMDIS ver.2.1., National Institute of Standards and Technology- NIST, Standard Reference Data Program, Gaithersburg, MD, USA), compared to those from available literature (Adams 2007), and used as an additional tool to approve MS findings.
Preparation and phytochemical analysis of methanol extracts
Tansy methanol extracts were prepared from both aerial parts and roots of plants grown in vitro. To compare chemical profiles of extracted compounds, methanol extracts were also made from native tansy plants collected from the Ada Huja locality, Belgrade, Serbia.
For all extracts, 500 mg of air-dried plant material was blended to a fine powder and extracted with 10 mL of methanol in an ultrasonic bath for 20 min. After sonication, the extraction was continued by maceration of tissue for 48 h in the dark at room temperature. Finally, the extracts were filtered through Whatman filter paper No. 1 to a volumetric flask and filled with methanol up to 10 mL. The extracts were stored at room temperature for further analyses.
High-pressure liquid chromatography (HPLC) of the methanol extracts was carried out on an Agilent 1100 chromatograph with a column compartment equipped with a Zorbax SB-C18 analytical column (150 mm × 4.6 mm, 5 μm, Agilent Technologies, Waldbronn, Germany) and diode array detector. The mobile phase consisted of a 1% (v/v) solution of orthophosphoric acid in water (A) and acetonitrile (B). The flow rate of the mobile phase was 0.8 mL/min, and the injection volume was 10 μL. A gradient program was used as follows: 90–75% A, 0–30 min, 75–45% A, 30–40 min, 45–0% A, 40–50 min. The chromatograms were recorded at 280 nm and 360 nm. Identification of 3,5-O-dicaffeoylquinic acid and chlorogenic acid was confirmed by the co-injection method using commercial standard samples purchased from Sigma-Aldrich. The content of 3,5-O-DCQA and chlorogenic acid was determined by the external standard quantification method.
Results
After 15 days, the percentage of germinated tansy seeds was 97.8% (Fig. 1a, b). Grown on BM, seedlings developed 4–5 leaves and a branched root system in 4 weeks (Fig. 1c). The in vitro grown tansy plants displayed a typical tansy phenotype with alternate and pinnately lobed leaf morphology (Fig. 1c). Healthy looking seedlings were used for the establishment of both shoot and root cultures on solid or liquid BM, respectively (Fig. 1d, e). After 4 weeks of cultivation on solid BM, average shoot length was 48.54 ± 0.2 mm, without any lateral branching. The roots cultivated in liquid BM increased their weight 3 times (from an initial 400 mg to 1.2 ± 0.03 g average). After 7 days of air-drying period, the roots’ weight was decreased with average FW/DW index = 6.02 ± 0.05.
Fig. 1.
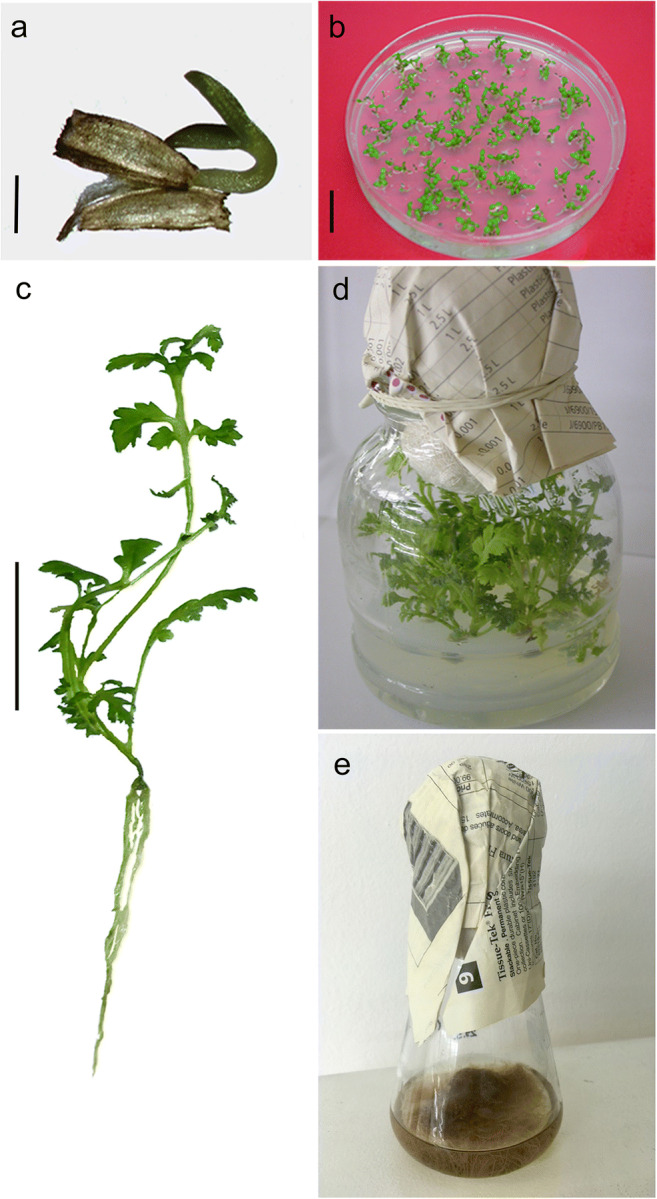
In vitro micropropagation of tansy. a Seedlings observed with binocular magnifier; bar = 1 mm; b Petri dish with germinated seedlings; bar = 2 cm; c Nicely develop tansy seedling with 4–5 leaves and branched root; bar = 2 cm; d Tansy shoots grown on solid medium; e Erlenmeyer with tansy roots grown in liquid medium
Morpho-anatomical and histochemical characteristics of leaves from tansy grown in vitro
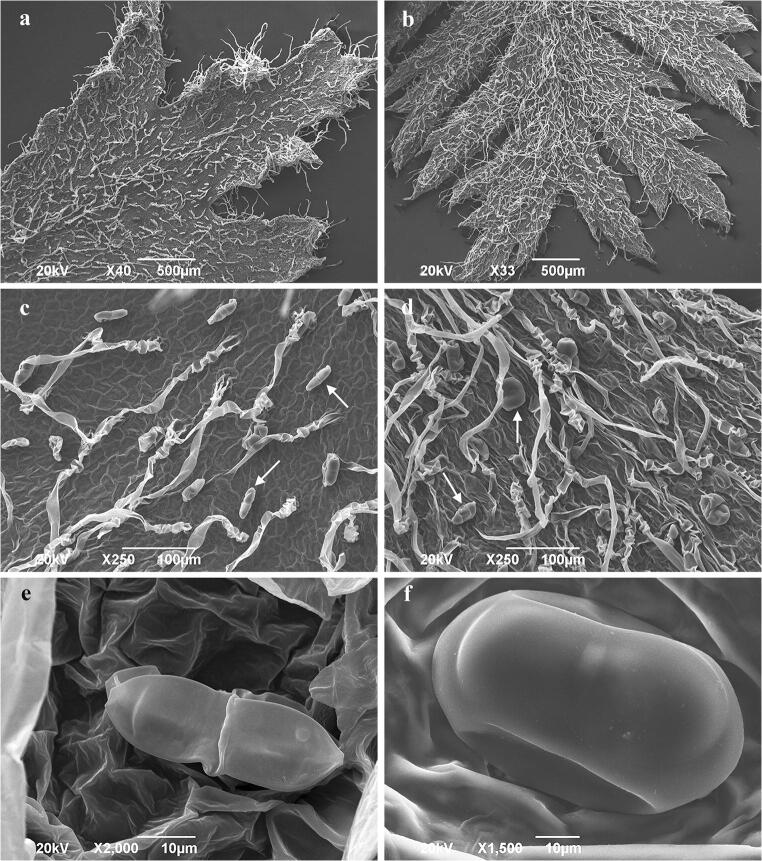
Both adaxial and abaxial surfaces of leaf indumentum consisted of non-glandular and glandular trichomes. Long, uniseriate non-glandular trichomes were more frequent on the abaxial leaf surface (Fig. 2a–d). The biseriate glandular trichomes were observed in tansy grown in vitro (Fig. 2e, f). The surface of immature biseriate glands appears wrinkled, indicative of the close attachment of the cuticle to the secretory upper cell walls (Fig. 2e). During maturation, the gland surface became smoother as the secretory products accumulated within the developing subcuticular space formed by a detachment of the cuticle (Fig. 2f).
Fig. 2.
Scanning electron micrographs of in vitro grown tansy foliar surface. a Adaxial leaf surface; b abaxial leaf surface; c glandular (arrow) and non-glandular trichomes on the adaxial leaf surface; d glandular (arrow) and non-glandular trichomes on the abaxial leaf surface; e biseriate glandular trichome at the beginning of the secretory phase; f mature biseriate glandular trichome on the adaxial leaf surface in the full secretory phase
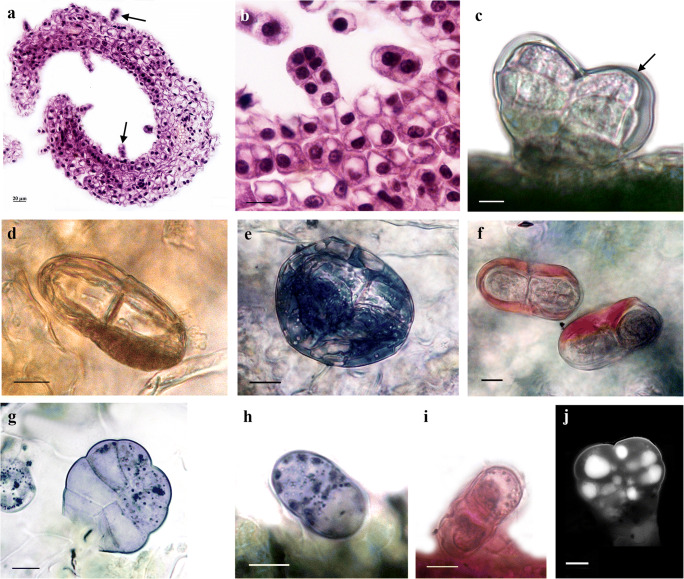
The anatomical investigation of leaf cross-sections by light microscopy revealed a isobilateral leaf type with an upper and lower epidermis with stomata cells, palisade, and spongy mesophyll (Fig. 3a, b). The epidermis consisted of a single layer of cells and was covered with a thin cuticle layer. Photosynthetic tissue was represented as 1–2 layers of palisade tissue cells and 2–3 layers of loosely, spongy mesophyll tissue with closed, collateral vascular bundles (Fig. 3a). Biseriate glandular trichomes were in various stages of development and consisted of two basal cells placed in the epidermis, one pair of cells forming a short stalk and three to four pairs of cells forming the secretory head (Fig. 3b). The cuticle layer over the secretory head expanded and formed the subcuticular space.
Fig. 3.
Structural and histochemical features of leaf glandular trichomes from in vitro grown tansy. a Cross-section of tansy leaf, note: trichomes (arrow) on adaxial and abaxial leaf surface; b young, immature leaf glandular trichomes, note: two basal cells, a short stalk, and secretory head of three pairs cells; c unstained biseriate trichome with subcuticular space (arrow); d orange-brown colored of secretory material in subcuticular space after stained with Sudan Red 7B/hematoxylin; e dark-blue color of lipophilic substance after staining with Sudan black B; f neutral lipids/essential oils stained red, while acid lipids stained blue with Nil blue A; g–h positive reaction with NADI reagent, violet-blue droplets indicate terpene secretion; i positive reaction with PAS; j UV-autofluorescence micrographs of leaf glandular trichomes. Bar = 10 μm
To detect and localize the lipids, terpenoids, phenols, tannins, polysaccharides, pectins, and alkaloids in glandular trichomes, tansy leaves grown in vitro were treated with specific histochemical staining tests (Table 1).
Table 1.
Histochemical analyses perform to identify the main metabolites secreted by leaf biseriate glandular trichomes of in vitro grown tansy
| Metabolite | Reagent | Reaction | Color |
|---|---|---|---|
| Lipids | |||
| Total | Sudan Black B | + | Orange-brown |
| Sudan IV | - | - | |
| Sudan Red 7B/hematoxylin | + | Blue | |
| Neutral and acidic | Nil Blue A | + | Red |
| Terpenoids (essential oils and oleoresins) | NADI reagent | + | Violet-blue |
| Polysaccharides | Periodic acid-Schiff (PAS) | + | Pink-red |
| Pectins | Ruthenium Red | + | Red |
| Alkaloids |
Wagner reagent Dragendorff reagent Ellram reagent |
- - - |
- - - |
| Polyphenols | Ferric chloride | - | - |
| Lignins and tannins | Toluidine Blue O | - | - |
On the in vivo leaf surface, biseriate trichomes with secretions in the subcuticular space were observed (Fig. 3c). The histochemical staining showed that the secretion contained lipophilic substances (Fig. 3d, e). In the glandular secretions localized in the subcuticular space, neutral lipids/essential oils were also detected (Fig. 3f). The terpenoids, represented as dark violet droplets, referred to as essential oils, were localized in the secretory cells and subcuticular space (Fig. 3g, h).
Polysaccharides in the secretory cells and subcuticular space were stained pink-red (Fig. 3i). This reaction was positive with insoluble polysaccharides of non-glandular trichomes and epidermal cell polysaccharides. Drops of secretion localized in the secretory cells of the glandular head emitted intense autofluorescence under UV excitation (Fig. 3j).
Essential oil of in vitro plantlets
Results of the GC-MS analysis of the essential oil are presented in Table 2. The essential oil of in vitro tansy plantlets was composed of 88 volatile compounds of which 42 were identified, representing 72.3% of the total oil. Only four compounds were made up by more than 5% of the total oil and 14 volatiles were present only in traces (< 0.1%). The monoterpenes were the most abundant compounds (40%), followed by sesquiterpenes (33.4%). All identified compounds belonging to six chemical groups: oxygenated monoterpenes (38.5%), monoterpene hydrocarbons (< 0.7%), oxygenated sesquiterpenes (22.6%), sesquiterpene hydrocarbons (10.6%), aromatic hydrocarbons (0.2%), and aromatic alcohols (< 0.2%). The oxygenated monoterpene trans-thujone (22.7%) and oxygenated sesquiterpene neryl-isovalerate (20.6%) were the dominant compounds in the oil.
Table 2.
GC-MS analysis of in vitro grown tansy plantlets
| No. | Compound | KIe | RRT | %m/m | No. | Compound | KIe | RRT | %m/m |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Tricyclen | 919 | 0.456 | tr. | 45 | Spathulenol | 1577 | 2.651 | 0.3 |
| 2 | α-Tujene | 921 | 0.46 | tr. | 46 | Neryl-isovalerate | 1585 | 2.676 | 20.6 |
| 3 | α-Pinene | 932 | 0.481 | tr. | 47 | n.i. | 1589 | 2.69 | 0.3 |
| 4 | Camphene | 946 | 0.514 | 0.1 | 48 | Salvial-4(14)-en-1-on | 1593 | 2.706 | 0.3 |
| 5 | Sabinene | 971 | 0.571 | 0.4 | 49 | n.i. | 1597 | 2.718 | 0.1 |
| 6 | β-Pinene | 974 | 0.58 | tr. | 50 | n.i. | 1603 | 2.737 | 0.1 |
| 7 | Dehydro-1,8 cineole | 986 | 0.614 | tr. | 51 | n.i. | 1605 | 2.746 | 0.1 |
| 8 | α-Terpinene | 1010 | 0.686 | tr. | 52 | n.i. | 1609 | 2.755 | 0.5 |
| 9 | p-Cimene | 1023 | 0.706 | 0.2 | 53 | n.i. | 1613 | 2.771 | 0.2 |
| 10 | 1,8 Cineole | 1029 | 0.725 | 0.5 | 54 | n.i. | 1618 | 2.788 | 0.1 |
| 11 | γ-Terpinene | 1057 | 0.809 | 0.1 | 55 | n.i. | 1830 | 2.824 | 0.6 |
| 12 | Cis-sabinene hydrate | 1065 | 0.834 | 0.1 | 56 | n.i. | 1634 | 2.839 | 0.4 |
| 13 | Terpinolene | 1086 | 0.905 | tr. | 57 | Gossonorol | 1638 | 2.849 | 0.2 |
| 14 | n.i. | 1099 | 0.935 | 0.1 | 58 | n.i. | 1655 | 2.902 | 0.7 |
| 15 | Cis-thujone | 1106 | 0.96 | 0.2 | 59 | n.i. | 1658 | 2.912 | 0.1 |
| 16 | Trans-thujone | 1117 | 1 | 22.7 | 60 | n.i. | 1659 | 2.928 | 0.2 |
| 17 | n.i. | 1133 | 1.059 | 0.1 | 61 | n.i. | 1667 | 2.939 | 0.1 |
| 18 | Trans-pinocarveol | 1135 | 1.075 | 0.1 | 62 | n.i. | 1672 | 2.959 | 0.2 |
| 19 | Camphor | 1142 | 1.093 | 10.1 | 63 | n.i. | 1675 | 2.967 | 0.1 |
| 20 | n.i. | 1154 | 1.133 | 0.1 | 64 | n.i. | 1680 | 2.982 | 0.1 |
| 21 | Cis-chrysanthenol | 1160 | 1.157 | 0.5 | 65 | n.i. | 1686 | 3.004 | 0.2 |
| 22 | Borneol | 1163 | 1.167 | 1 | 66 | n.i. | 1689 | 3.013 | 0.7 |
| 23 | Terpinen 4-ol | 1175 | 1.21 | 0.5 | 67 | n.i. | 1714 | 3.09 | 0.1 |
| 24 | n.i. | 1183 | 1.238 | tr. | 68 | n.i. | 1716 | 3.098 | tr. |
| 25 | α-Terpineol | 1088 | 1.26 | 0.1 | 69 | n.i. | 1719 | 3.105 | tr. |
| 26 | n.i. | 1108 | 1.33 | 0.1 | 70 | n.i. | 1721 | 3.112 | tr. |
| 27 | Bornyl acetate | 1284 | 1.61 | 0.3 | 71 | n.i. | 1724 | 3.121 | tr. |
| 28 | Neryl acetate | 1364 | 1.901 | 2.1 | 72 | n.i. | 1730 | 3.137 | 0.3 |
| 29 | n.i. | 1375 | 1.942 | 0.1 | 73 | n.i. | 1733 | 3.147 | tr. |
| 30 | Modheph-2-ene | 1379 | 1.956 | 0.1 | 74 | n.i. | 1751 | 3.202 | 0.1 |
| 31 | α-Isocomene | 1385 | 1.981 | 0.4 | 75 | n.i. | 1753 | 3.209 | 0.1 |
| 32 | β-Isocomene | 1405 | 2.051 | 0.2 | 76 | n.i. | 1768 | 3.251 | 4.2 |
| 33 | Caryophyllene | 1419 | 2.1 | 0.4 | 77 | n.i. | 1776 | 3.279 | 0.2 |
| 34 | α-Humulene | 1453 | 2.221 | 0.1 | 78 | n.i. | 1781 | 3.299 | 0.2 |
| 35 | β-Pharnesen | 1457 | 2.234 | 1.6 | 79 | n.i. | 1791 | 3.326 | 0.1 |
| 36 | Amorpha 4,7-dien | 1460 | 2.245 | 0.1 | 80 | n.i. | 1825 | 3.423 | 0.1 |
| 37 | cis-muurola-4(14)5-diene | 1463 | 2.255 | 0.1 | 81 | 2-Pentadecanone 6,10,14-trimethyl | 1845 | 3.48 | 0.7 |
| 38 | n.i. | 1476 | 2.299 | tr. | 82 | n.i. | 1901 | 3.65 | 0.9 |
| 39 | Germacrened | 1481 | 2.32 | 1.9 | 83 | n.i. | 1920 | 3.694 | 0.4 |
| 40 | γ-Chimachalen | 1484 | 2.328 | 1.5 | 84 | Methyl hexanoate | 1927 | 3.713 | 0.1 |
| 41 | E-β-Ionon | 1487 | 2.328 | 0.5 | 85 | n.i. | 1953 | 3.788 | 0.9 |
| 42 | Bicyclogermacrene | 1497 | 2.373 | 0.1 | 86 | n.i. | 1974 | 3.849 | 1.6 |
| 43 | γ-Z-Bisabolene | 1517 | 2.441 | 0.1 | 87 | n.i. | 1997 | 3.921 | 10 |
| 44 | β-Sesquiphellandrene | 1525 | 2.469 | 4.2 | 88 | n.i. | 2028 | 3.989 | 0.4 |
| Number of detected compounds | 88 | KIe- Kovats index experimentally determined | |||||||
| Number of identified compounds | 42 | RRT-relative retention time | |||||||
| Identified compounds belonging to | (%) | CI-concentration index | |||||||
| Oxygenated monoterpenes | 38.5 | % m/m-percentage relative to total EO composition | |||||||
| Monoterpene hydrocarbons | < 0.7 | n.i-non-identified | |||||||
| Oxygenated sesquiterpenes | 22.6 | tr.-present in traces (< 0.1%) | |||||||
| Sesquiterpene hydrocarbons | 10.6 | ||||||||
| Aromatic hydrocarbons | 0.2 | ||||||||
Methanol extract compounds
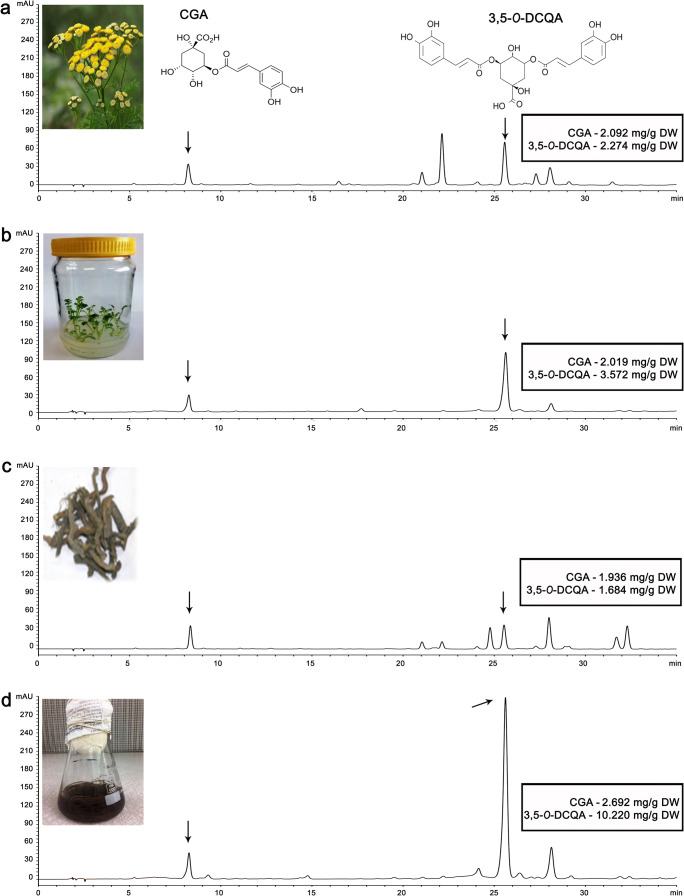
The HPLC chromatograms recorded at 280 nm revealed differences between the chemical compositions of methanol extracts derived from native tansy and from tansy cultured in vitro (Fig. 4). Based on characteristic UV spectra, flavonoids and phenolic acids were detected, while chlorogenic acid (CGA) and 3,5-O-dicaffeoylquinic acid (3,5-O-DCQA) were identified in all extracts tested. The highest content of CGA and 3,5-O-DCQA was recorded in the methanol extract of roots grown in vitro (Fig. 4d). The amount of 3,5-O-DCQA was up to 6 times higher in the methanol extract of in vitro roots (Fig. 4d; 10.220 mg/g DW) compared with methanol extract of native tansy roots (Fig. 4c; 1.684 mg/g DW). The methanol extracts of tansy aerial parts from native (Fig. 4a) and in vitro plants (Fig. 4b) were characterized by relatively similar amounts of the acid (2.274 mg/g DW and 3.579 mg/g DW, respectively).
Fig. 4.
Chromatograms of tansy methanol extracts. Methanol extract of a native tansy herb; b in vitro grown tansy herb; c native tansy roots; d in vitro grown tansy roots. The arrows point to chlorogenic acid (CGA) and 3,5-O-dicaffeoylquinic acid (3,5-O-DCQA) peaks with given amounts of targeting compounds as mg per g of dry weight (DW) for each type of extracts
Discussion
Plant micropropagation is proposed to be the most promising system for the efficient production of useful secondary metabolites under controlled cultivation conditions. In vitro culture allows rapid production of genetically identical pathogen- and contaminant-free plants through clonal propagation, using relatively small amounts of space, supplies, and time. In this study, tansy in vitro culture was established from seeds collected from natural populations, whose composition of secondary metabolites in essential oils and methanol extracts were determined in our previous study (Devrnja et al. 2017). Successful multiplication of plantlets was conducted through shoot tips that exhibited potent apical growth and regeneration capacities on BM without the addition of any PGRs. It has been previously reported that exogenously applied PGRs, especially from the cytokinin and auxin groups, may impair genetic stability and lead to somaclonal variation (Rani and Raina 2000; Bairu et al. 2007), which is considered to be undesirable in the production of plant material via in vitro culture, especially if it will be used for secondary metabolite isolation (Bairu et al. 2007; Passinho-Soares et al. 2017). Moreover, for the establishment and propagation of tansy in vitro root cultures, PGR supplementation was also unnecessary, and roots increased their mass 3 times in 4 weeks in PGR-free liquid BM. For roots detached from the spontaneously rooted micropropagated shoots and further in vitro maintenance, auxins were considered a necessary treatment. Roots that were excised and propagated in PGR-free medium, often turned brown and died soon after being removed from the “mother” plant (Stanišić et al. 2019).
Light and SEM microscopy of the leaf surface of in vitro micropropagated tansy plants revealed the presence of glandular biseriate trichomes randomly distributed on both adaxial and abaxial leaf surfaces. These were surrounded by long non-glandular ones, which are known to be typical for all species belonging to the Asteraceae family (Ciccarelli et al. 2007). Glandular trichomes were also present on the tansy stems. In many cases, glandular trichomes were abundant on both in vitro gentle leaf surfaces, while in aged ex vitro leaves, these structures were only present on the abaxial surface (Bandyopadhyay et al. 2004). Frequent in vitro sub-culturing practices in tansy, which occur every 4 weeks, could be responsible for retarding plantlets in the juvenile stage and prevent trichome distribution related to the maturation process.
The presence of storage glands is intrinsically associated with terpenoid metabolism (Maffei 2010), which was confirmed by histochemical analysis of in vitro tansy leaves. Glandular secretions were rich in lipid and terpene compounds, which was also confirmed by GC-MS analysis of EO. Tansy biseriate trichomes contained a storage subcuticular space in which volatile compounds were retained in their liquid state, like in other representatives of the Asteraceae family (Turner et al. 2000). The morphology of these trichomes is adapted for the fast release of volatile defense compounds upon herbivory attack or other mechanical damage. During the first decades of the twenty-first century, special attention was given to the investigation of site-specific regulation of synthesis machinery for plant secondary metabolites (Lange et al. 2011; Tissier 2012a; Sultana et al. 2015). Impressive progress has been made in the identification of promoters driving trichome-specific gene expression, which could be used to control trichome density and, consequently, to improve the productivity of EOs or even some particular constituents (Tissier 2012b). The recorded presence of numerous, highly metabolically active biseriate trichomes on the surface of in vitro grown tansy could be the starting point for the utilization of obtained knowledge.
A review of the available literature found no data referring to phytochemical analyses of EOs from in vitro tansy cultures. Essential oil constituents of native tansy, of which the seeds were taken for the establishment of in vitro cultures presented in this study, were predominantly oxygenated monoterpenes, with trans-chysantenyl acetate as a dominant compound (Devrnja et al. 2017). Interestingly, in vitro cultivation significantly affected EO composition that may be favored by the high chemical plasticity reported for tansy (Lawrence 2000). Like in some other reports (Arumugam et al. 2020), in EO obtained from in vitro cultivated plants, a significantly greater number of chemical compounds was detected, as compared to that of wild-grown tansy (88 vs. 65, respectively). In vitro cultivation conditions also enhanced the accumulation of sesquiterpenes with respect to EO from field-grown “mother” plants, with a similar portion of identified monoterpene and sesquiterpene compounds. Although EO chemistry is genotype determined, the plasticity of secondary metabolism allows plants to alter their defenses in response to environmental and/or physiological-stress circumstances (Usano-Alemany et al. 2016; Kirimer et al. 2017). Strikingly, the dominant sesquiterpene from in vitro tansy, neryl-isovalerate, present with 20.6% in total EO, was not detected in native tansy plants. Neryl-isovalerate is a registered food flavoring agent and adjuvant (Joint FAO/WHO Expert Committee 1997; EU Commission 2012). Additionally, the dominant compounds in the EO of in vitro tansy, trans-thujone and camphor, were identified in a much higher portion (22.7% and 10.1%, respectively) than in the oil of native tansy (9.04% and 4.9%, respectively). At this point, the observed differences in EO metabolite production in tansy trichomes affected by cultivation practice are hard to explain. Several authors have reported that these quantity-quality variations induced by in vitro culture conditions could be accounted for by the requirement of some metabolites for a specified environmental factor, such as light or temperature, for highly differentiated in vitro cells/tissues or for cells/tissues in a specified stage of development (Dörnenberg and Knorr 1997; Avato et al. 2005; Batista et al. 2016; Passinho-Soares et al. 2017). Variation in EO composition could also be the consequence of different ontological stages, with in vitro plants being in the juvenile-stage by definition and mature native tansy plants in the flowering stage. The elevated prevalence of camphor in micropropagated plants, compared to field-grown plants, was also observed in Salvia officinalis (Avato et al. 2005), which was explained by the correlation of camphor levels with juvenile stages of development of micropropagated plants relying on earlier works by Croteau et al. (1981, 1987), who have shown that the presence of camphor in the oil decreases with the age of the plant. Although the specific growing conditions could alter the chemotype of “mother” plants, in vitro cultivation could offer a supply of constant EO, unrelated to environmental conditions, time of the year, and presence of unwanted biotic interactions. Biotechnological interventions on secondary metabolites production taking place in trichomes could further enhance the production of some important metabolites in tansy EO. Guided by a similar goal, Sultana et al. (2015) used the Tanacetum cinerariifolium promoter of chrysanthemol synthase (TcCHS), coding for the first enzyme in the pyrethrum biosynthesis pathway, fused to a GFP (Green Fluorescent Protein) and GUS (β-glucuronidase) reporter genes, to transform Chrysanthemum morifolium and Nicotiana tabacum plants. The accumulation of pyrethrum was detected only in glandular trichomes of both species.
Quantity and quality variations were reflected also in the chemical profile of methanol extracts of in vitro cultured tansy herbs and roots compared to wild-grown tansy. In both in vitro and native plant material, extracts were rich in phenolics and flavonoids. The strong antioxidant, antimicrobial, and anticancer potential of tansy extracts obtained from wild-grown plants were attributed to these compounds (Devrnja et al. 2017). The most striking differences were observed for 3,5-O-DCQA, one of the dicaffeoylquinic acid derivatives with two caffeic acid moieties substituted at the 3 and 5 positions of the quinic acid. Results indicated that the methanol extract of in vitro cultured roots was the richest in 3,5-O-DCQA content, whose concentration was 6 times higher than that in the extract obtained from roots of wild-growing tansy. This is a noticeable result in the manner of industrial production of biologically active 3,5-O-DCQA, which has been shown to have antioxidant, hepatoprotective, antimutagenic, and immunomodulatory activity (Peluso et al. 1995; Tatefuji et al. 1996; Basnet et al. 1996; Yoshimoto et al. 2002; Juan-Badaturuge et al. 2009; Kim et al. 2012, 2017). It has also been reported that 3,5-O-DCQA improves pancreatic function in type 2 diabetic mice (Yin et al. 2018) and is a potent inhibitor of HIV-1 integrase, an enzyme that is essential for viral replication and subsequent HIV infection of humans (Tamura et al. 2006).
Conclusions
The establishment of an efficient and inexpensive method for in vitro multiplication of tansy is a promising starting point for the introduction of high-capacity bioreactors and mass micropropagation of shoots and roots for stabile secondary metabolites production. In addition, well-developed protocols open up new directions for research that could affect secondary metabolite production by the manipulation of chemical and physical cultivation conditions, application of precursors and/or elicitors, or genetic transformation that would affect the expression of genes from the biosynthetic pathway of the target compound. The significance of these results is even higher since studies related to the in vitro root cultures or leaf secretory structures of Tanacetum vulgare are not available to our knowledge. EO and methanol extract obtained from in vitro grown tansy qualitatively and quantitatively differed from the previously analyzed wild-grown plants, being the source of new mixtures shown to be rich in biologically active compounds. Further investigation will be directed toward the optimization of in vitro cultivation conditions, including elicitation, for obtaining maximum production of tansy secondary metabolites and the elucidation of their pharmaceutical potential, as mixtures or single moieties.
Author statement
Nina Devrnja collected samples and data, carried out the experiments, preformed data analyses, and prepared the original draft. Dijana Krstić Milošević and Vele Tešević performed chemical analyses of the samples. Dušica Janošević performed histochemical analyses. Branka Vinterhalter contributed in in vitro culturing. Jelena Savić contributed in designing the experiment and critically reviewed the manuscript. Dušica Ćalić designed the experiment.
Funding
This research was funded by the Ministry of Education, Science and Technological Development of the Republic of Serbia (Grant Nos. 451-03-68/2020-14/200007; 451-03-68/2020-14/ 200178).
Compliance with ethical standards
Conflict of interests
The authors declare that they have no conflict of interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Adams RP. Identification of essential oil components by gas chromatography/mass spectrometry. 4. Carol Stream: Allured Publishing Corporation; 2007. p. 469. [Google Scholar]
- Arora N, Koul AA. ‘Complex solution’ to a ‘complex problem’: tackling the complexity of cancer with botanicals. Eur J Cancer Prev. 2014;23:568–578. doi: 10.1097/CEJ.0000000000000045. [DOI] [PubMed] [Google Scholar]
- Arumugam G, Sinniah UR, Swamy MK, Lynch PT (2020) Micropropagation and essential oil characterization of Plectranthus amboinicus (Lour.) Sprengel, an aromatic medicinal plant. In Vitro Cell Dev Biol Plant. 10.1007/s11627-020-10056-1
- Avato P, Fortunato IM, Ruta C, D’Elia R. Glandular hairs and essential oils in micropropagated plants of Salvia officinalis L. Plant Sci. 2005;169:29–36. [Google Scholar]
- Bairu MW, Stirk WA, Doležal K, Van Staden J. Optimizing the micropropagation protocol for the endangered Aloe polyphilla: can meta-topolin and its derivatives serve as replacement for benzyladenine and zeatine? Plant Cell Tissue Organ Cult. 2007;90:1523. [Google Scholar]
- Baker JR. Cytological technique: the principles underlying routine methods (5th Edition) London: Methuen & Co; 1966. [Google Scholar]
- Bandyopadhyay T, Gangopadhyay G, Poddar R, Mukherjee KK. Trichomes: their diversity, distribution and density in acclimatization of teak (Tectona grandis L.) plants grown in vitro. Plant Cell Tissue Organ Cult. 2004;78:113–121. [Google Scholar]
- Basnet P, Matsushige K, Hase K, Kadota S, Namba T. Four di-0-caffeoyl quinic acid derivatives from propolis. Potent Hepatoprotective activity in experimental liver injury models. Biol Pharm Bull. 1996;19:1479–1484. doi: 10.1248/bpb.19.1479. [DOI] [PubMed] [Google Scholar]
- Batista DS, de Castro KM, da Silva AR, Teixeira ML, Sales TA, Soares LI, das Graças Cardoso M, de Oliveira Santos M, Viccini LF, Otoni WC. Light quality affects in vitro growth and essential oil profile in Lippia alba (Verbenaceae) Vitro Cell Dev Biol Plant. 2016;52:276–282. [Google Scholar]
- Cain AJ. The use of Nile Blue in the examination of lipoids. J Cell Sci. 1947;88:383–392. [Google Scholar]
- Chugh NA, Bali S, Koul A. Integration of botanicals in contemporary medicine: road blocks, checkpoints and go-ahead signals. Integr Med Res. 2018;7:09–125. doi: 10.1016/j.imr.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccarelli D, Garbari F, Pagni AM. Glandular hairs of the ovary: a helpful character for Asteroideae (Asteraceae) taxonomy? Ann Bot Fenn. 2007;44:1–7. [Google Scholar]
- Cordell GA. Sustainable medicines and global health care. Planta Med. 2011;77:1129–1138. doi: 10.1055/s-0030-1270731. [DOI] [PubMed] [Google Scholar]
- Cragg GM. Paclitaxel (Taxol): a success story with valuable lessons for natural product drug discovery and development. Med Res Rev. 1998;18:315–331. doi: 10.1002/(sici)1098-1128(199809)18:5<315::aid-med3>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Croteau R, Felton M, Karp F, Kjonaas R. Relationship of camphor biosynthesis to leaf development in sage (Salvia officinalis) Plant Physiol. 1981;67:820–824. doi: 10.1104/pp.67.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croteau R, El-Bialy H, Dehal SS. Metabolism of monoter penes. Metabolic fate of (+)-camphor in sage (Salvia offcinalis) Plant Physiol. 1987;84:649–653. doi: 10.1104/pp.84.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David R, Carde JP. Coloration différentelle des inclusions lipidiques et terpéniques des pseudophylles du pin maritime au moyen du réactif Nadi. Comptes Rendus de l`Académie des Sciences. Paris. 1964;258:1338–1340. [Google Scholar]
- David B, Wolfender JL, Dias DA. The pharmaceutical industry and natural products: historical status and new trends. Phytochem Rev. 2015;14:299–315. [Google Scholar]
- Demetzos C, Angelopoulou D, Perdetzoglou D. A comparative study of the essential oils of Cistus salviifolius in several populations of Crete (Greece) Biochem Syst Ecol. 2002;30:651–665. doi: 10.1016/s0305-1978(00)00071-5. [DOI] [PubMed] [Google Scholar]
- Devaux CA, Rolain JM, Colson P, Raoult D (2020) New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? Int J Antimicrob Agents 105938. Adv Online Publ. 10.1016/j.ijantimicag.2020.105938 [DOI] [PMC free article] [PubMed]
- Devrnja N, Anđelković B, Aranđelović S, Radulović S, Soković M, Krstić-Milošević D, Ristić M, Ćalić D. Comparative studies on the antimicrobial and cytotoxic activities of Tanacetum vulgare L. essential oil and methanol extracts. S Afr J Bot. 2017;111:212–221. [Google Scholar]
- Dias DA, Urban S, Roessner U. A historical overview of natural products in drug discovery. Metabolites. 2012;2:303–336. doi: 10.3390/metabo2020303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dörnenberg H, Knorr D. Challenges and opportunities for metabolite production from plant cell and tissue cultures. Food Technol. 1997;51:47–54. [Google Scholar]
- EU Commission (2012) Commission Implementing Regulation (EU) No 872/2012 of 1 October 2012 adopting the list of flavouring substances provided for by Regulation (EC) No 2232/96 of the European Parliament and of the Council, introducing it in Annex I to Regulation (EC) No 1334/2008 of the European Parliament and of the Council and repealing Commission Regulation (EC) No 1565/2000 and Commission Decision 1999/217/EC Text with EEA relevance. https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32012R0872. Accessed 27 Mar 2020
- Furr M, Mahlberg PG. Histochemical analyses of laticifers and glandular trichomes in Cannabis sativa. J Nat Prod. 1981;44:153–159. [Google Scholar]
- Gahan PB. Plant histochemistry and cytochemistry: an introduction. Florida: Academic press; 1984. p. 301. [Google Scholar]
- Gospodinova Z, Antov G, Angelova S, Krasteva M. In vitro antitumor potential of Bulgarian Tanacetum vulgare L. on human breast adenocarcinoma cells. Inter J Pharma Sci. 2014;4:468–472. [Google Scholar]
- Jensen WA. Botanical histochemistry: principles and practice. San Francisco: W.H. Freeman & Co.; 1962. p. 408. [Google Scholar]
- Johansen D. Plant microtechnique. London: McGraw-Hill Publishing Company, Ltd.; 1940. p. 523. [Google Scholar]
- Joint FAO/WHO Expert Committee (1997) Evaluation of certain food additives and contaminants: forty-ninth report of the Joint FAO/WHO Expert Committee on Food additives, Report TRS884-JECFA 49/64. Rome, Italy. https://apps.who.int/food-additives-contaminants-jecfa-database/chemical.aspx?chemID=5165. Accessed 27 Mar 2020
- Juan-Badaturuge M, Habtemariam S, Jackson C, Thomas MJK. Antioxidant principles of Tanacetum vulgare L. aerial parts. Nat Prod Commun. 2009;4:1561–1564. [PubMed] [Google Scholar]
- Karabaeva VV, Vichkanjva SA, Sidelnikova GF, Dgumayan AR, Terentyeva TL. A new look of the results researcy of using Tanatsehol in the conditions of district clinic. Вопросы биологической, медицинской и фармацевтической химии. 2015;7:26–29. [Google Scholar]
- Keskitalo M, Kanerva T, Pehu E. Development of in vitro procedures for regeneration of petiole and leaf explants and production of protoplast-derived callus in Tanacetum vulgare L. (Tansy) Plant Cell Rep. 1995;14:261–266. doi: 10.1007/BF00233646. [DOI] [PubMed] [Google Scholar]
- Keskitalo M, Pehu E, Simon JE. Variation in volatile compounds from Tansy (Tanacetum vulgare L.) related to genetic and morphological differences of genotypes. Biochem Syst Ecol. 2001;29:267–285. doi: 10.1016/s0305-1978(00)00056-9. [DOI] [PubMed] [Google Scholar]
- Kim JY, Lee HK, Hwang BY, Kim S, Yoo JK, Seong YH. Neuroprotection of Ilex latifolia and caffeoylquinic acid derivatives against excitotoxic and hypoxic damage of cultured rat cortical neurons. Arch Pharm Res. 2012;35:1115–1122. doi: 10.1007/s12272-012-0620-y. [DOI] [PubMed] [Google Scholar]
- Kim Y, Kim JT, Park J, Son HJ, Kim EY, Lee YJ, Rhyu MR. 4, 5-Di-O-Caffeoylquinic Acid from Ligularia fischeri Suppresses Inflammatory Responses through TRPV1 Activation. Phytother Res. 2017;31:1564–1570. doi: 10.1002/ptr.5885. [DOI] [PubMed] [Google Scholar]
- Kirimer N, Mokhtarzadeh S, Demirci B, Goger F, Khawar KM, Demirci F. Phytochemical profiling of volatile components of Lavandula angustifolia Miller propagated under in vitro conditions. Ind Crop Prod. 2017;96:120–125. [Google Scholar]
- Lange BM, Mahmoud SS, Wildung MR, Turner GW, Davis EM, Lange I, Baker RC, Boydston RA, Croteau RB. Improving peppermint essential oil yield and composition by metabolic engineering. Proc Natl Acad Sci U S A. 2011;108:16944–16949. doi: 10.1073/pnas.1111558108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence BM. Progress in essential oils: tansy oil. Perfume Flavor. 2000;25:33–47. [Google Scholar]
- Linsmaier EM, Skoog F. Organic growth factor requirements of tobacco tissue cultures. Physiol Plant. 1965;18:100–127. [Google Scholar]
- Liu R, Li X, Lam KS. Combinatorial chemistry in drug discovery. Curr Opin Chem Biol. 2017;38:117–126. doi: 10.1016/j.cbpa.2017.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei ME. Sites of synthesis, biochemistry and functional role of plant volatiles. S Afr J Bot. 2010;76:612–631. [Google Scholar]
- Mitich LW. Tansy. Weed Technol. 1992;6:242–244. [Google Scholar]
- Mordujovich-Buschiazzo P, Balsa EM, Busciazzo HO, Mandrile E, Rosella M, Schinella G, Fioravanti D. Anti-inflammatory activity of Tanacetum vulgare. Fitoterapia. 1996;67:319–322. [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant. 1962;15:473–497. [Google Scholar]
- Newman DJ, Cragg GM. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J Nat Prod. 2020;83:770–803. doi: 10.1021/acs.jnatprod.9b01285. [DOI] [PubMed] [Google Scholar]
- Newman DJ, Cragg GM, Snader KM. Natural products as sources of new drugs over the period 1981-2002. J Nat Prod. 2003;66:1022–1037. doi: 10.1021/np030096l. [DOI] [PubMed] [Google Scholar]
- Onozato T, Nakamura CV, Cortez DA, Filho BP, Ueda-Nakamura T. Tanacetum vulgare: antiherpes virus activity of crude extract and the purified compound parthenolide. Phytother Res. 2009;23:791–796. doi: 10.1002/ptr.2638. [DOI] [PubMed] [Google Scholar]
- Passinho-Soares HC, David JP, de Santana JR, David JM, Rodrigues FDM, Mesquita PR, de Oliveira FS, Bellintani MC. Influence of growth regulators on distribution of trichomes and the production of volatiles in micropropagated plants of Plectranthus ornatus. Rev Bras. 2017;27:679–690. [Google Scholar]
- Paydary K, Emamzadeh-Fard S, Khorram Khorshid HR, Kamali K, SeyedAlinaghi S, Mohraz M. Safety and efficacy of Setarud (IMODTM) among people living with HIV/AIDS: a review. Recent Pat Antiinfect Drug Discov. 2012;7:66–72. doi: 10.2174/157489112799829756. [DOI] [PubMed] [Google Scholar]
- Peluso G, De Feo V, De Simone F, Bresciano E, Vuotto ML. Studies on the inhibitory effects of caffeoylquinic acids on monocyte migration and superoxide anion production. J Nat Prod. 1995;58:639–646. doi: 10.1021/np50119a001. [DOI] [PubMed] [Google Scholar]
- Plowe CV. Antimalarial drug resistance in Africa: strategies for monitoring and deterrence. Malaria: drugs, disease and post-genomic biology. Curr Top Microbiol Immunol. 2005;295:55–79. doi: 10.1007/3-540-29088-5_3. [DOI] [PubMed] [Google Scholar]
- Pourdast A, Sanaei M, Jafari S, Mohammadi M, Khalili H, Shafiee G, Ahadi Z, Rostami M, Alizad S, Heshmat R, Mohraz M. Effect of SeptimebTM as a new natural extract on severe sepsis: A randomized clinical trial. Caspian J Inter Med. 2017;8:35–43. [PMC free article] [PubMed] [Google Scholar]
- Rani V, Raina SN. Genetic Fidelity of Organized Meristem-Derived Micropropagated Plants: A Critical Reappraisal. In Vitro Cell Dev Biol Plant. 2000;36:319–330. [Google Scholar]
- Robles C, Garzino S. Infraspecific variability in the essential oil composition of Cistus monspeliensis leaves. Phytochemistry. 2000;53:71–75. doi: 10.1016/s0031-9422(99)00460-4. [DOI] [PubMed] [Google Scholar]
- Rohloff J, Mordal R, Dragland S. Chemotypical Variation of Tansy (Tanacetum vulgare L.) from 40 Different Locations in Norway. J Agric Food Chem. 2004;52:1742–1748. doi: 10.1021/jf0352430. [DOI] [PubMed] [Google Scholar]
- Rosselli S, Bruno M, Raimondo FM, Spadaro V, Varol M, Koparal AT, Maggio A. Cytotoxic effect of eudesmanolides isolated from flowers of Tanacetum vulgare ssp. siculum. Molecules. 2012;17:8186–8195. doi: 10.3390/molecules17078186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savarino A, Boelaert JR, Cassone A, Majori G, Cauda R. Effects of chloroquine on viral infections: an old drug against today's diseases? The Lancet. Infect Dis Ther. 2003;3:722–727. doi: 10.1016/s1473-3099(03)00806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinfeld N. Sinecatechins. Drugs Future. 2008;33:27–30. [Google Scholar]
- Stanišić M, Ćosić T, Savić J, Krstić-Milošević D, Mišić D, Smigocki A, Ninković S, Banjac N. Hairy root culture as a valuable tool for allelopathic studies in apple. Tree Physiol. 2019;39:888–905. doi: 10.1093/treephys/tpz006. [DOI] [PubMed] [Google Scholar]
- Sultana S, Hu H, Gao L, Mao J, Luo J, Jongsma MA, Wang C. Molecular cloning and characterization of the trichome specific chrysanthemyl diphosphate/chrysanthemol synthase promoter from Tanacetum cinerariifolium. Sci Hortic. 2015;185:193–199. [Google Scholar]
- Svendsen AB, Verpoorte R (1983) Chromatography of alkaloids, part a: thin-layer chromatography. J Chromatogr Library, 23A, Elsevier, Amsterdam, Oxford, New York
- Tamura H, Akioka T, Ueno K, Chujyo T, Okazaki K, King PJ, Robinson WE., Jr Anti-human immunodeficiency virus activity of 3,4,5-tricaffeoylquinic acid in cultured cells of lettuce leaves. Mol Nutr Food Res. 2006;50:396–400. doi: 10.1002/mnfr.200500216. [DOI] [PubMed] [Google Scholar]
- Tatefuji T, Izumi N, Ohta T, Arai S, Ikeda M, Kurimoto M. Isolation and identification of compounds from brazillian propolis which enhance macrophage spreading and mobility. Biol Pharm Bull. 1996;19:966–970. doi: 10.1248/bpb.19.966. [DOI] [PubMed] [Google Scholar]
- Tissier A. Glandular trichomes: what comes after expressed sequence tags? Plant J. 2012;70:51–68. doi: 10.1111/j.1365-313X.2012.04913.x. [DOI] [PubMed] [Google Scholar]
- Tissier A (2012b) Trichome specific expression: promoters and their applications. In: Transgenic plants – advances and limitations. In Tech Open Access Company, Rijeka, Croatia pp. 353–378
- Turner GW, Gershenzon J, Croteau RB. Distribution of peltate glandular trichomes on developing leaves of peppermint. Plant Physiol. 2000;124:655–663. doi: 10.1104/pp.124.2.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usano-Alemany J, Palá-Paúl J, Herráiz-Peñalver D. Essential oil yields and qualities of different clonal lines of Salvia lavandulifolia monitored in Spain over four years of cultivation. Ind Crop Prod. 2016;80:251–261. [Google Scholar]
- Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, Shi Z, Hu Z, Zhong W, Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf VC, Gassmann A, Clasen BM, Smith AG, Müller C. Genetic and chemical variation of Tanacetum vulgare in plants of native and invasive origin. Biol Control. 2012;61:240–245. [Google Scholar]
- Yin XL, Xu BQ, Zhang YQ. Gynura divaricata rich in 3, 5−/4, 5-dicaffeoylquinic acid and chlorogenic acid reduces islet cell apoptosis and improves pancreatic function in type 2 diabetic mice. Nutr Metab. 2018;15:73. doi: 10.1186/s12986-018-0310-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto M, Yahara S, Okuno S, Islam MS, Ishiguro K, Yamakawa O. Antimutagenicity of mono-, di-, and tricaffeoylquinic acid derivatives isolated from sweetpotato (Ipomoea batatas L.) leaf. Biosci Biotechnol Biochem. 2002;66:2336–2341. doi: 10.1271/bbb.66.2336. [DOI] [PubMed] [Google Scholar]