Abstract
Background
Epidemiological evidence has linked fine particulate matter (PM2.5) to neurodegenerative diseases; however, the toxicological evidence remains unclear. The objective of this study was to investigate the effects of PM2.5 on neuropathophysiology in a hypertensive animal model. We examined behavioral alterations (Morris water maze), lipid peroxidation (malondialdehyde (MDA)), tau and autophagy expressions, neuron death, and caspase-3 levels after 3 and 6 months of whole-body exposure to urban PM2.5 in spontaneously hypertensive (SH) rats.
Results
SH rats were exposed to S-, K-, Si-, and Fe-dominated PM2.5 at 8.6 ± 2.5 and 10.8 ± 3.8 μg/m3 for 3 and 6 months, respectively. We observed no significant alterations in the escape latency, distance moved, mean area crossing, mean time spent, or mean swimming velocity after PM2.5 exposure. Notably, levels of MDA had significantly increased in the olfactory bulb, hippocampus, and cortex after 6 months of PM2.5 exposure (p < 0.05). We observed that 3 months of exposure to PM2.5 caused significantly higher expressions of t-tau and p-tau in the olfactory bulb (p < 0.05) but not in other brain regions. Beclin 1 was overexpressed in the hippocampus with 3 months of PM2.5 exposure, but significantly decreased in the cortex with 6 months exposure to PM2.5. Neuron numbers had decreased with caspase-3 activation in the cerebellum, hippocampus, and cortex after 6 months of PM2.5 exposure.
Conclusions
Chronic exposure to low-level PM2.5 could accelerate the development of neurodegenerative pathologies in subjects with hypertension.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12989-020-00388-6.
Keywords: Air pollution, Autophagy, Central nervous system toxicity, Particulate matter, Tau
Background
Particulate air pollution has been linked to initiation of neurodegenerative diseases (NGDs) [1, 2]. Results from an aging study cohort indicated that exposure to traffic-related pollution and black carbon was associated with decreases in cognition function [3]. Other results from the Nurses’ Health Study Cognitive Cohort in the US showed that exposure to coarse (with an aerodynamic diameter of 2.5 ~ 10 μm) (PM2.5–10) and fine (PM2.5) particulate matter size fractions was associated with cognitive declines [4]. Exposure to traffic-related air pollution was associated with an increased risk of Parkinson’s disease (PD) [5]. An increased risk of hospital admission for dementia and PD was associated with an increase in PM2.5 as observed in 50 northeastern US cities [6]. These cohort data provide evidence that particulate matter (PM) may be a risk factor contributing to the development of NGDs; however, the underlying pathophysiology remains unclear.
Neuroinflammation is a critical mechanism in the development of NGDs. Our previous study indicated that oxidative stress and inflammation with short-term memory deficiencies occurred after 3 and 6 months of exposure to traffic-related PM1 (< 1 μm in aerodynamic diameter) in Sprague-Dawley rats [7]. Consistently, alterations in motor activity, spatial learning, and memory and emotional behaviors due to exposure to diesel exhaust particles (DEPs) were observed in vivo [8–10]. Acute exposure to DEPs at high concentrations (250 ~ 300 μg/m3) induced oxidative stress and neuroinflammation [8]. Costa and colleague indicated that neurogenesis injury in the hippocampus by DEPs was associated with activation of microglia. In vivo results provided evidence that DEPs caused oxidative stress, inflammation, and cell death in human neuroblastoma cells, which could be associated with regulating tau and autophagy expressions in the brain [11]. Physiological changes, inflammation, oxidative stress, and an unfolded protein response occurred after PM2.5 exposure, the responses of which differed with different size fractions of PM2.5 [12].
Deposition of insoluble proteins in cells of the neuromuscular system was characterized in NGDs. Clinically, tau protein accumulation is considered a biomarker of NGDs in clinical diagnoses [13, 14]. Overexpression of tau can cause its hyperphosphorylation [15], which leads to tau aggregation and the appearance of smaller proteolytic fragments [16]. Regulation of autophagy has a cleansing role in removing tau accumulations from cells, which is believed to be an important function in maintaining the brain’s health [16]. Previous studies observed that PM2.5 caused dysfunction in removing damaged proteins by autophagy [17, 18]. Also, DEPs induced neuroinflammation, oxidative stress, and neurodegenerative-related tau overexpression and regulation by autophagy in human IMR-32 neuroblastoma cells [11]. Autophagy dysfunction can lead to the initiation of NGDs such as Alzheimer’s disease (AD) [19]. However, chronic effects of PM2.5 on tau and autophagy expressions in the brain remain unclear.
During inhalation, PM is able to deposit in the upper airway such as head and nasal regions of humans [20]. For example, ultrafine particles (UFP; ≤100 nm) is mainly deposited by diffusion which makes it more likely to deposit on the olfactory epithelium. Additionally, UFP could translocate to brain via the olfactory epithelium [21, 22]. One study indicated significant hyperphosphorylated tau neurofibrillary tangles, vascular amyloid, neuronal amyloid accumulation, alpha-synuclein aggregates, and neurites in the olfactory bulbs after exposure to high levels of PM2.5 in 179 residents living in Metropolitan Mexico City [23]. Increasing reports suggest that olfactory deficits are associated with early evidence of AD pretangled subcortical and cortical hyperphosphorylated tau.
Cardiovascular diseases and metabolic syndrome are recognized as risk factors for the development of NGDs [24, 25]. A previous study showed that exposure to traffic-related air pollution impaired the brain’s microvascular integrity in a high-fat diet animal model [26]. However, a paucity of evidence is available for neurotoxicity caused by PM2.5 in hypertensive animals. We hypothesized that patient with hypertension is population-at-risk for development of neurodegenerative disease by chronic exposure of PM2.5. The objective of the present study was to investigate the effects of PM2.5 on neuropathophysiology in a hypertensive animal model. We examined behavioral alterations, lipid peroxidation, tau and autophagy expressions, neuronal death, and caspase-3 levels after chronic pulmonary exposure in spontaneously hypertensive (SH) rats.
Results
Characterization of urban PM2.5
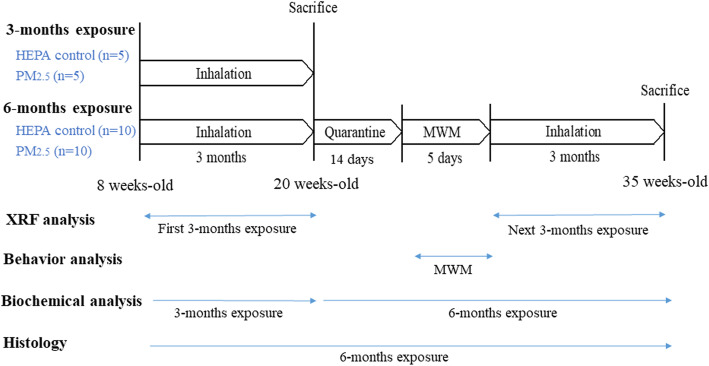
The experimental design is shown in Fig. 1. SH rats were exposed to either 3 or 6 months of urban PM2.5 during the study period. PM2.5 mass concentrations were 8.6 ± 2.5 and 10.8 ± 3.8 μg/m3 μg/m3 for the 3- and 6-month exposure periods, respectively. Meteorological conditions and gaseous pollution were referenced from the nearby EPA Guting air quality monitoring station during the study period (Table S1). The average temperature was 20.2 ± 5.0 °C and relative humidity was 80.8% ± 9.6%. Levels of NO2, SO2, and O3 exposed to the rats (HEPA and PM2.5 groups) were 21.6 ± 6.3, 2.6 ± 1.1, and 26.3 ± 10.7 ppb, respectively. We then determined elements of PM2.5 for the first 3 months and the subsequent 3 months of exposure (Fig. 2). We observed that elements in the first 3 months and subsequent 3 months of exposure respectively accounted for 19.29 and 18.12% of the total PM2.5. Consistently, S, K, Si, and Fe were dominant in the both first 3 months and subsequent 3 months of PM2.5.
Fig. 1.
Overview of the experimental design for investigating the effects of fine particulate matter (PM2.5; PM with an aerodynamic diameter of < 2.5 μm) on neurotoxicity in spontaneously hypertensive (SH) rats. The whole bodies of 8-week-old SH rats in the high-efficiency particulate air (HEPA) and PM2.5 groups were exposed to urban PM2.5 for 3 and 6 months. In the 6-month group, a Morris water maze (MWM) was used to observe behavioral changes in SH rats after 3 months of exposure. After the MWM, rats were followed up for a subsequent 3 months of exposure to PM2.5. Rats were euthanized after 3 or 6 months of exposure for biochemical analyses. The 6-month group was histologically examined. Additionally, urban PM2.5 was collected onto Teflon filters for metal analyses
Fig. 2.
Characterization of elements in particulate matter with an aerodynamic diameter of < 2.5 μm (PM2.5) for an initial 3 months of exposure and a subsequent 3 months of exposure using energy dispersive x-ray fluorescence (ED-XRF) spectrometry. S, K, Si, and Fe were dominant in the first 3-month and subsequent 3-month PM2.5 samples
MWM
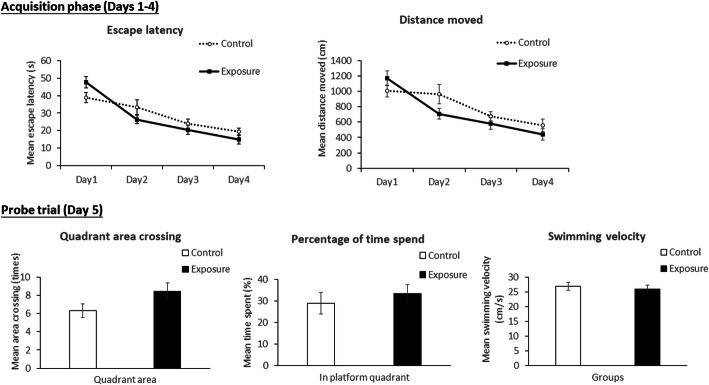
Figure 3 shows the acquisition phase (days 1 ~ 4) and probe trial (day 5) using the MWM to respectively examine spatial learning and memory ability. There were no significant differences in the escape latency or distance moved between the control and exposure groups. For the probe trial, there were no significant differences in the mean area crossing, mean time spent, or mean swimming velocity between the control and exposure groups.
Fig. 3.
Morris water maze for examining spatial learning (acquisition phase, days 1 ~ 4) and memory ability (probe trial, day 5) in SH rats after 3 months of exposure to particulate matter with an aerodynamic diameter of < 2.5 μm (PM2.5; exposure group). There were no significant differences in the mean escape latency, mean distance moved, mean area crossing, mean time spent, or mean swimming velocity between the control (high-efficiency particulate air; HEPA) and exposure groups (PM2.5)
MDA
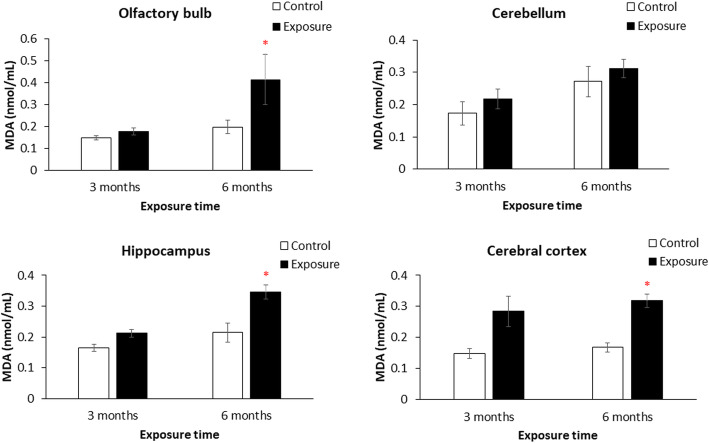
Figure 4 shows MDA levels in the olfactory bulb, cerebellum, hippocampus, and cortex after 3 and 6 months of exposure. There was no significant difference of MDA between the control group and the group with 3 months of exposure. However, MDA levels were significantly higher in the olfactory bulb, hippocampus, and cortex after 6 months of exposure compared to the control group (p < 0.05).
Fig. 4.
Levels of malondialdehyde (MDA) in the olfactory bulb, cerebellum, hippocampus, and cortex after 3 and 6 months of exposure to particulate matter with an aerodynamic diameter of < 2.5 μm (PM2.5; exposure group). Levels of MDA had significantly increased in the olfactory bulb, hippocampus, and cortex after 6 months of PM2.5 exposure compared to the control (high-efficiency particulate air; HEPA). * p < 0.05
Tau expressions
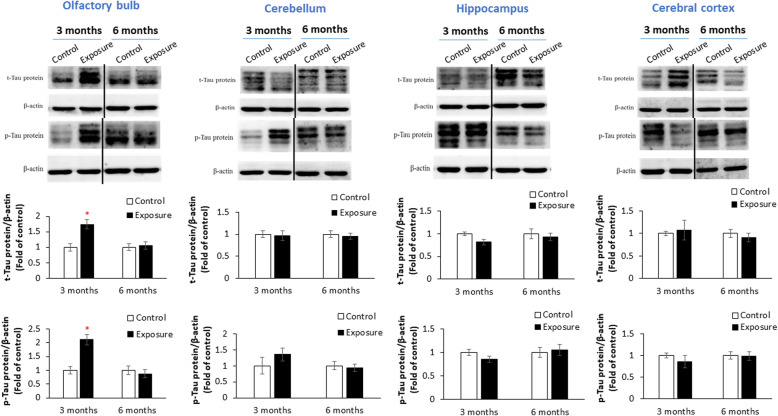
Figure 5 shows expressions of t-tau and p-tau in the olfactory bulb, cerebellum, hippocampus, and cortex after 3 and 6 months of exposure. We observed that 3 months of exposure to PM2.5 caused significant expressions of t-tau and p-tau in the olfactory bulb compared to the control (p < 0.05). However, there were no significant alterations in t-tau or p-tau in the cerebellum, hippocampus, or cortex after PM2.5 exposure. Consistently, we observed no significant difference in t-tau in the cerebellum, hippocampus, or cortex of the rat brain after 6 months of PM2.5 exposure based on IHC observations (Fig. S4).
Fig. 5.
Expressions of total (t)-tau and phosphorylated (p)-tau in the olfactory bulb, cerebellum, hippocampus, and cortex after 3 and 6 months of exposure to particulate matter with an aerodynamic diameter of < 2.5 μm (PM2.5; exposure group). Three months of exposure to PM2.5 caused significant expressions of t-tau and p-tau in the olfactory bulb compared to the control (high-efficiency particulate air; HEPA). * p < 0.05
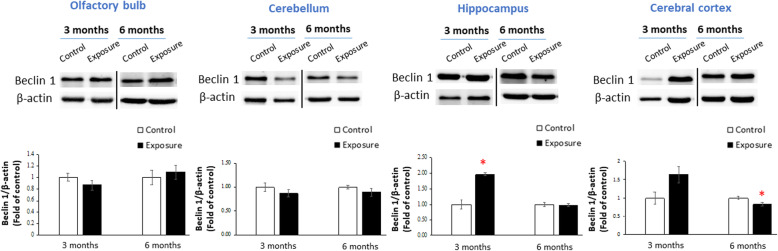
Beclin 1 expression
Figure 6 shows expressions of beclin 1 in the olfactory bulb, cerebellum, hippocampus, and cortex after 3 and 6 months of exposure. We observed that 3 months of exposure to PM2.5 caused significant expression of beclin 1 in the hippocampus compared to the control (p < 0.05), whereas 6 months of exposure to PM2.5 caused a significant decrease in beclin 1 expression in the cortex compared to the control (p < 0.05).
Fig. 6.
Expressions of beclin 1 in the olfactory bulb, cerebellum, hippocampus, and cortex after 3 and 6 months of exposure to particulate matter with an aerodynamic diameter of < 2.5 μm (PM2.5; exposure group). Three months of exposure to PM2.5 (exposure group) caused significant expression of beclin 1 in the hippocampus compared to the control (high-efficiency particulate air; HEPA), whereas 6 months of exposure of PM2.5 significantly decreased the expression of beclin 1 in the cortex compared to the control. * p < 0.05
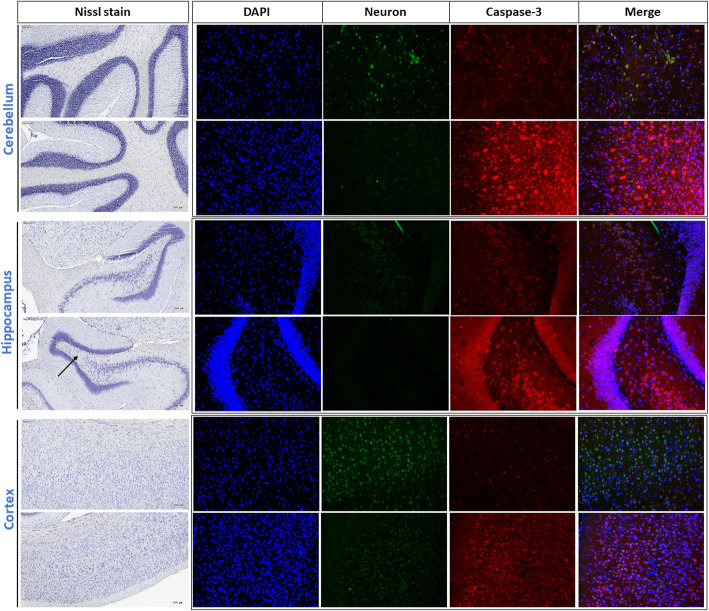
Neuron loss and caspase-3 activation
Figure 7 shows levels of neuron loss and caspase-3 activation based on Nissl staining and IHC in the cerebellum, hippocampus, and cortex after 6 months of exposure. First, the neuron number decreased in the hippocampus after 6 months of PM2.5 exposure (arrow). We further observed that neuron signals were also reduced in the cerebellum, hippocampus, and cortex after 6 months of PM2.5 exposure compared to the control (green color). Next, caspase-3 signals were activated in the cerebellum, hippocampus, and cortex by PM2.5 exposure compared to the control. However, we observed that few neuron cells were co-localized with caspase-3 activation based on merged images in the three brain regions. Results showed that caspase-3 was mainly activated in non-neuron cells.
Fig. 7.
Neuron loss and activation of caspase-3 expression based on Nissl staining and IHC in the cerebellum, hippocampus, and cortex after 6 months of exposure to particulate matter with an aerodynamic diameter of < 2.5 μm (PM2.5; exposure group). For Nissl staining, the neuron number had significantly decreased in the hippocampus after 6 months of PM2.5 exposure (arrow). Neuron signals were also reduced in the cerebellum, hippocampus, and cortex after 6 months of PM2.5 exposure compared to the high-efficiency particulate air (HEPA) control. Caspase-3 signals were significantly activated in the cerebellum, hippocampus, and cortex by PM2.5 exposure compared to the control (HEPA). DAPI, blue; neurons, green; caspase-3, red. The scale bar for Nissl stain is 200 μm. The images for DAPI, neuron and caspase-3 were taken at 10x magnification
Histology
Figure S5 shows results of brain histology in the cerebellum, hippocampus, and cortex after 6 months of exposure to the HEPA control and PM2.5. No significant pathological changes were observed in the three regions of the brain after this exposure.
Discussion
NGDs have been linked to air pollution based on epidemiological evidence. Hypertension is also recognized as a risk factor for NGDs [27, 28]. In the present study, we observed that pulmonary inflammatory infiltration occurred after 3- and 6-months of exposure to PM2.5 and/or HEPA without significant alterations in body weight (Figs. S2 and S3). Subpleural alveolar infiltration of mononuclear cells was observed in the lungs after 3- and 6-months exposure of HEPA and/or PM2.5 (Fig. S2). The observation suggests that the rats exposed to gaseous pollution and/or PM2.5 had adverse effects of histological changes of the lungs after exposure. The results could support the insignificant difference in certain biochemical results observed between PM2.5 and HEPA groups in this study. However, it is still unclear that neurotoxicity is occurred by PM2.5 or gaseous pollutants. In the present study, neurotoxicity was investigated after chronic inhalation of urban PM2.5 and/or gaseous pollution (HEPA) in SH rats. Four major findings are reported in the present study: (1) lipid peroxidation occurred in the olfactory bulb, hippocampus, and cortex after 6 months of exposure to PM2.5; (2) tau proteins (total and phosphorylated) were upregulated in the olfactory bulb by 3 months of exposure to PM2.5; (3) alterations in beclin 1 expression were observed in the hippocampus and cerebellum due to PM2.5 exposure; and (4) neuron loss and caspase-3 activation were induced by 6 months of exposure to PM2.5. Therefore, PM2.5 could accelerate the development of neurodegenerative pathologies in hypertension subjects.
Air pollution may be a risk factor for NGDs. The US Environmental Protection Agency (EPA) estimates that more than 100 million people live in areas that exceed the recommended US EPA air quality levels (Office of Air Quality Planning and Standards and United States, EPA, Air Quality Trends Analysis Group). Notably, chronic exposure to traffic-related PM was reported to increase the risk of NGDs in the US [29] and Canada [30]. In Taiwan, a similar association was identified based on data analyzed from a national population-based cohort study [31]. They showed that 10 years of exposure to PM2.5 and ozone were associated with an increased incidence of AD. To study possible underlying mechanisms in the brain after PM2.5 exposure, a traffic-dominated urban region in Taipei, Taiwan was selected for the experimental site in the present study. The temperature, RH, NOx, SO2, and O3 were referenced from the nearby Guting air quality monitoring station during the study period. Levels of NO2, SO2, and O3 to which rats were exposed were slightly higher than those in our previous study [7]. For PM2.5 exposure, rats were continually exposed to 8.6 and 10.8 μg/m3 PM2.5 for 3 and 6 months, respectively. Average PM2.5 levels in both exposure periods (3 and 6 months) were relatively lower than World Health Organization (WHO) PM2.5 guidelines (25 μg/m3 for the 24-h average) [32]. We further investigated elements in PM2.5, and S, K, Si, and Fe were mainly observed in PM2.5 during the exposure periods. The dominant elements suggest secondary aerosols (e.g., S), soil dust (e.g., Si), and metal/traffic (e.g., Fe) were the main emission sources in the study area [33]. Some elements, such as S and Fe, are recognized as a source for oxygen reactive species (ROS) that are able to cause cellular oxidative damage after inhalation [34, 35]. Increasing evidence indicates that exposure to metal-containing PM2.5 causes neurotoxicity based on human [36] and animal reports [37]. However, effects of pulmonary exposure to low-levels of PM2.5 on neurotoxicity remains unclear.
Chronic exposure to air pollution was observed to change behavior in animals [7] and their offspring [38]. However, the effect of PM2.5 on behavior changes in hypertension is still poorly understood. In the present study, SH rats, an animal model with essential/primary hypertension, was used to study the contributions of PM2.5 on neurotoxicity in hypertension. SH rats is commonly used to study interactions between air pollution and cardiovascular diseases [39–41]. Firstly, we used the MWM to examine spatial learning, according to a previous report [42]. There was no significant difference in spatial learning between the control and exposure groups. Previous reports showed that air pollution exposure caused in vivo behavioral alterations, such as anxiety [43] and short-term memory [7, 44]. Contrarily, another study observed no significant changes in in vivo behaviors after air pollution exposure [45]. Previous studies indicated that hypertension is linked to NGDs [27, 28], leading to memory deficiencies [46, 47]. It is worthy to note that stress could be occurred by behavior examination in rats, affecting pathology change and other biological responses in brain [48]. However, the rats were euthanized after 3 months of MWM in the present study. Thus, the effects of behavior testing on the biochemical results could be insignificant. Together, the insignificant alteration in behavior after air pollution exposure observed in the present study could have resulted from the animal model we used.
Lipid plays an important role of NGDs due to its high concentration in central nervous system, which involved in many central nervous system disorders and injuries that involve deregulated metabolism [49]. Transition metals such as Fe identified in PM2.5 are capable of redox cycling [50] and generate superoxide and hydroxyl radicals [51], through a direct mechanism, the Fenton reaction [34, 52, 53]. When ROS interact with lipids, lipids are degraded by ROS thereby producing lipid peroxidation. Lipid peroxidation is able to disrupt membrane organization and cause the functional loss/modification of proteins and DNA [54]. After 6 months of exposure to PM2.5, we observed significantly increased lipid peroxidation (as determined by MDA) in the olfactory bulb, hippocampus, and cortex of SH rats. Previous reports consistently indicated that PM2.5 exposure in the lungs caused significant lipid peroxidation in different brain regions in vivo [55, 56]. Particulate air pollution is able to cause barrier damage such as nasal epithelium [57], leading to integrity disruption. Olfactory epithelium has recently recognized to be an important target to interact with particulate matter. Oxidative stress and infalammation was occurred by particles between olfactory epithelium and brain [58]. But the responses may delayed depends on proximal and distal brain regions [59]. Repeated injury of these barriers is able to impair their integrity, leading to PM crossing the barriers and entering the brain. Also, oxidative stress is able to directly alter amyloid-β production. The ROS-occurred production of lipid peroxidation may trigger generation of toxic amyloid-β42 species in the brain [60]. Clinically, lipid peroxidation is used as an indicator of ROS that cause NGDs [61]. Together, chronic exposure of PM2.5 causes formation of lipid peroxidation, which may increase the risk of NGDs.
Cellular deposition of insoluble proteins in the neuromuscular system was linked to neurological disease. Tau, for example, was observed to accumulate in intraneurons, which was suggested to be a biomarker of the development of AD [14]. Generally, olfactory sensory neurons are dedicated to processing odor information. Currently, the olfactory system is recognized to decrease in function due to physiological changes during normal aging [62] and NGDs [63]. Olfactory sensory neurons are associated with the development of AD [64–66]. In our study, we observed t-tau and p-tau overexpression in the olfactory bulb after 3 months of PM2.5 exposure in SH rats. A report indicated that activation of microglia caused the increase of p-tau aggregates in the olfactory system [67]. The results from Mexico City study showed that olfactory bulbs is the targets for air pollution, which caused p-tau in olfactory bulb of young city residents [68]. A study showed that the olfactory bulb is nearly equally vulnerable to tau and α-synuclein pathologies in AD with amygdala Lewy bodies [65]. Together, olfactory bulb could be the first target for sub-chronic PM2.5 exposure, leading to p-tau aggregates in SH rats. However, the alteration in t-tau and p-tau of olfactory bulb was not observed by 6-months exposure of PM2.5. The insignificant results could be resulted from the effects of gaseous pollution after chronic exposure in rats. Taken together, PM2.5 exposure causing overexpression of t-tau and p-tau could be associated with the early development of neurotoxicity.
Autophagy is a cleansing mechanism to remove accumulations of tau proteins from the brain. The Bcl-2-interacting protein, beclin 1 (Atg6), and the microtubule-associated protein-1 light chain 3B (LC3B, Atg8) are considered to be major regulators of autophagy [69]. In the present study, we observed that beclin 1 was overexpressed in the hippocampus after 3 months of PM2.5 exposure in SH rats. This observation is in line with our previous report that chronic exposure to traffic-related air pollution activated autophagy expression in the SD rat hippocampus [7]. Additionally, LC3II (conjugated LC3) was significantly upregulated by carbon black and diesel exhaust particles (DEPs), particularly DEPs, in microglial BV2 cells [70]. Autophagy activation could be associated with insignificant alterations in t-tau and/or p-tau of brains of SH rats after exposure. However, we observed downregulated autophagy expression in the cortex after 6 months of PM2.5 exposure in SH rats. Our previous study showed that DEPs did not activate LC3II expression in neuroblastoma cells [11]. The difference could have resulted from cell types distributed in different brain regions or autophagy dysfunction occurring due to chronic PM2.5 exposure.
Notably, we observed neuron loss in the hippocampus of SH rats after 6 months of exposure to PM2.5. We next investigated expressions of neuronal cells in different brain regions. Our observations suggested that neuron cells had decreased in the cerebellum, hippocampus, and cortex after 6 months of exposure to PM2.5. Consistently, our previous study showed that DEPs caused the cell death of BV2 neuroblastomas [11], which may have been related to oxidative stress in the brain after exposure as determined by lipid peroxidation in our current results. Next, we observed that caspase-3 expression occurred in the cerebellum, hippocampus, and cortex after 6 months of exposure to PM2.5. However, caspase-3 expression was co-localized with either neuron cells or other cells in the three brain regions in the present study. Another study indicated that microglial activation occurred due to neuroinflammation by DEPs [70], leading to neuronal cell death [71]. Therefore, it is reasonable to suspect that chronic exposure to PM2.5 can cause the death of neurons and brain cells. Further studies are required to understand interactions between PM2.5 and brain cells and its underlying mechanisms.
We observed lung infiltration after chronic PM2.5 exposure in SH rats, but there were no significant pathological changes in brains of SH rats. A few limitations of this study should be noted. We examined elements of PM2.5 exposure; however, we did not examine the organic fractions of PM2.5. Also, oral uptake of PM2.5 in the rats was not observed. Rats were euthanized using CO2 at each time point, which could induce stress that affect the results. A normotensive and a clean air controls may be required to understand the effects of PM2.5 on the development of NGDs in the hypertensive rat model. In the present study, we did not observe significant behavioral alterations due to PM2.5 exposure in the model. More behavior experiments should be performed in the future works. Other models could have been used, such as aging rats, to examine the role of PM2.5 in NGDs.
Conclusions
In conclusion, neuropathological change was occurred after chronic exposure to low-level PM2.5 in SH rats. Lipid peroxidation, neuron loss, and caspase-3 activation were identified in brains after 6 months of exposure to PM2.5. Tau and beclin 1 overexpressions were caused by 3 months of exposure to PM2.5. Notably, the olfactory bulb may be an important target organ by PM2.5. Our results showed that chronic exposure to low-level PM2.5 could accelerate the development of neurodegenerative pathology in subjects with hypertension.
Materials and methods
Animals
Seven-week-old male SH rats obtained from the National Laboratory Animal Center (Taipei, Taiwan) were used in this study. Rats were housed in plastic cages under a 22 ± 2 °C temperature, 55% ± 10% relative humidity, and a 12/12-h light/dark cycle. Lab Diet 5001 (PMI Nutrition International, USA) and water were provided ad libitum. All animal experiments complied with protocols of the Institutional Animal Care and Use Committee (IACUC) of National Taiwan University (Taipei, Taiwan; approval no. 20130531).
Experimental design
The experimental design is shown in Fig. 1. SH rats in the high-efficiency particulate air (HEPA) and PM2.5 groups were whole-body exposed to urban air pollution for 3 and 6 months between November 2015 and May 2016 in Taipei City, Taiwan. For the 3-month group, rats were immediately decapitation. For the 6-month group, rats were quarantined after 3 months of exposure. Rats were then forced to perform a Morris water maze (MWM) for 5 days. After the MWM, rats were exposed to urban air pollution for the next 3 months. After being euthanized, an animal necropsy was performed, and tissues were collected as described previously [72]. In this study, all rats were euthanized using CO2 at each time point. For 3-month group, there were 5 rats used for biochemical analyses. For 6-month group, there were 6 rats used for biochemical analyses and 4 rats used for pathological observation. The olfactory bulb, cerebellum, cortex, and hippocampus of each rat were obtained and stored at − 80 °C. Also, brain samples were quickly removed from the skull and immersed overnight in 4% formaldehyde at 4 °C for pathological observation. Additionally, urban PM2.5 was collected onto Teflon filters for mass calculation and metal analyses.
Whole-body exposure to urban PM2.5
SH rats were randomly assigned to two groups: (1) a HEPA-filtered air control group (exposed to gaseous pollution only), and (2) a PM2.5 group (exposed to PM2.5 and gaseous pollution). The whole-body exposure system used for rodent PM2.5 exposure was previously described [7, 73], and was located in an urban region with traffic-dominated emission sources (Taipei, Taiwan). Briefly, unconcentrated ambient air was introduced into the whole-body exposure system for 3 and 6 months. Our previous report showed that the coarse (PM2.5–10) and fine (PM2.5) size fractions of PM mass concentration respectively accounted for 0.4 and 99.6% of the whole-body exposure system [73]. Thus, rats were mainly exposed to PM2.5 in this study. Simultaneously, PM was collected onto Teflon filter substrates during the study period (7 days for an interval). Mass concentrations of PM2.5 were obtain from the filters followed by metal analyses. Meteorological conditions and gaseous pollution were referenced from the nearby EPA Guting air quality monitoring station during the study period.
Elemental analysis
Energy dispersive x-ray fluorescence (ED-XRF) spectrometry (on a PANalytical Epsilon 5 ED-XRF analyzer, PANalytical, the Netherlands) was used to determine elemental concentrations in PM2.5 collected on Teflon filters. The ED-XRF procedure was described in a previous report [74]. Mg, Al, Si, S, K, Ca, Ti, V, Cr, Mn, Fe, Ni, Cu, Zn, Ba, and Pb were determined from material collected on Teflon filters. Metal concentrations are presented as a weight percentage of PM2.5 (%).
Morris water maze (MWM)
The MWM was used to examine spatial learning, according to our previous report [42]. Briefly, the MWM consisted of a circular pool with a diameter of 120 cm and a height of 60 cm. A white platform (9 cm in diameter with a rough surface) was submerged 1 cm below the water surface. The pool was filled with water, and the water temperature was kept at 25 °C. The pool was divided into four equal quadrants, and each quadrant was marked with a different visual cue. There was two phases during the study period: an acquisition phase (days 1 ~ 4) and a probe trial phase (day 5) to respectively examine spatial learning and memory abilities. Each trial began by placing a rat in the pool, facing the wall of the tank opposite the midpoint of each quadrant. The sequence of the starting quadrant was randomly assigned. Once the rat had found the platform, it was allowed to stay there for 15 s. If a rat did not find the platform within 60 s, it was gently place on the platform by the experimenter and was allowed to stay there for 15 s. All data were recorded with a video camera-based system (Noldus Etho Vision 3.1, Wageningen, the Netherlands).
Malondialdehyde (MDA) analysis
MDA was extracted from the olfactory bulb, cerebellum, cortex, and hippocampus according to a previously described method with minor modifications [75]. Details of the preparation and liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis are given in Supplymentary Information. Briefly, samples were reacted with 2,4-dinitrophenylhydrazine (DNPH) to derivatize the MDA-DNPH, followed by the LC-MS/MS analysis. MDA-DNPH was separated from other interfering substances on an LC system containing a Thermo Scientific autosampler and a Thermo Scientific Accela LC system with a 1250 quaternary pump (San Joes, CA, USA). A 15-cm (4.6 mm I.D) Syncronis C18 column was packed with 5 μm particles (Thermo Fisher Scientific, Waltham, MA, USA). A Thermo TSQ Quantum Access with a triple-quadrupole mass analyzer was applied to quantitate MDA-DNPH. MS/MS was carried out in selected reaction monitoring (SRM) mode with 1.5 mTorr of argon gas as the collision-induced dissociation (CID) gas. The LC-MS/MS system and data analysis were controlled and processed with Xcalibur software (Thermo Fisher Scientific). Method validation in terms of the linearity, and limits of detection (LODs) and quantification (LOQs), and the matrix effect were performed based on previous research and guidance from US Food and Drug Administration [76, 77]. Since an isotope-labeled internal standard of MDA-DNPH was unavailable, a matrix-matched calibration curve of MDA-DNPH was used to compensate for the matrix effect in SH rat brain samples, and LODs and LOQs were also determined in brain tissues from the control group of SH rats. Spectra of the LC-MS/MS analyses are given in Fig. S1.
Western blot analysis
The Western blot analysis was described previously [7]. Briefly, total tau (t-tau; 1:1000), phosphorylated-tau (p-tau; 1:1000), beclin 1 (1:1000), and β-actin (1:1000) obtained from Cell Signaling (Danvers, MA, US) were immunoreactived with specific proteins. Quantitative data were obtained using Image-Pro vers. 4 (Media Cybernetics, Inc., MD, USA) for Windows. All data were adjusted to the control (multiples of change of the control).
Immunohistochemistry (IHC) of brain tissues
Fixed brain samples were incubated with polyclonal antibodies against t-tau, whereas incubation with phosphate-buffered saline (PBS) served as a negative control. DAPI was used for nuclear staining. Microphotographs were acquired using a Motic Pathology slide scanner (Meyer Instrument, Houston, TX, USA). Also, brain sections were stained with NeuN (GeneTex, San Antonio, TX, USA), the cell apoptosis marker of activated caspase-3 (Cell Signaling Technology, Beverly, MA, USA), and nuclear-staining DAPI (Sigma-Aldrich, St. Louis, MO, USA). For neuron detection, a Nissl staining kit (MDS Analytical Technologies, Sunnyvale, CA, USA) was used to measure Nissl bodies in the cytoplasm of neurons. A fluorescent microscope (EVOS FL imaging system; Thermo Fisher Scientific) was used to obtain NeuN, caspase-3, and DAPI images. Automated microscopy (Tissuefaxs; TissueGnostics, Vienna, Austria) was used to obtain Nissl-stained images. The regions of interest were selected (n = 3 sections per region) based on significant changes and our previous reports [7, 70]. All images were taken under the same exposure time.
Histology
Brain and lung sections were fixed, embedded in paraffin, and sectioned followed by staining with hematoxylin and eosin (H&E). Images were acquired using the Motic Pathology slide scanner. All histological examinations were conducted under light microscopy by a single histopathologist.
Statistical analysis
Data are presented as the mean ± standard deviation (SD). The Wilcoxon rank sum test was used for comparisons between groups. Statistical analyses were performed using SAS 9.2 for Microsoft Windows (SAS Institute, Cary, NC, USA). The level of significance was set to p < 0.05.
Supplementary Information
Additional file 1: Table S1. Meteorological and gaseous data measured by the EPA Guting air quality monitoring stations during the study period. Figure S1. Spectrums of MDA analysed by LC-MS/MS. Figure S2. Effects (3- and 6-months exposure) of PM2.5 on the inflammatory infiltration of lungs in SH rats. Subpleural alveolar infiltration of mononuclear cells was observed in the lungs after 3- and 6-months exposure of HEPA and/or PM2.5. Figure S3. Alteration in body weight of SH rats between HEPA (control) and PM2.5 (exposure) groups after 6-months exposure. Figure S4. IHC images of total Tau (t-Tau) in the cerebellum, hippocampus, and cortex of SH rats after 6 months of exposure to HEPA (control) and PM2.5 (exposure). Scar bar is 50 μm. Figure S5. Chronic effects (6-months exposure) of PM2.5 on the histological changes of cerebellum, hippocampus, and cortex in SH rats. Scale bar: 200 μm.
Acknowledgements
Authors heartedly thank Miss Yi-Syuan Lin, Jen-Ting Chao, Yi-Suuan Chen and Chen-Huan Fu for technical assistance during this project.
Availability of supporting data
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors’ contributions
All authors contributed substantially to the concept and design of the study, drafting of the article, and critically revising the manuscript for important intellectual content. All authors have read and approved the final version of the manuscript for publication.
Funding
This study was funded by the Ministry of Science and Technology of Taiwan (103–2314-B-002-040-MY3 and 106–2314-B-002-218-MY3).
Ethics approval and consent to participate
All the animal protocols were prepared in accordance with the Guide for the Care and Use of Laboratory Animals and were approved by the Laboratory Animal Center at National Taiwan University (Taipei, Taiwan).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hsiao-Chi Chuang, Email: r92841005@ntu.edu.tw.
Hsin-Chang Chen, Email: hsinchang@ntu.edu.tw.
Pei-Jui Chai, Email: r05841017@ntu.edu.tw.
Ho-Tang Liao, Email: arisliao@gmail.com.
Chang-Fu Wu, Email: changfu@ntu.edu.tw.
Chia-Ling Chen, Email: chialing66@tmu.edu.tw.
Ming-Kai Jhan, Email: williamjhan2730@gmail.com.
Hui-I Hsieh, Email: oem@cgh.org.tw.
Kuen-Yuh Wu, Email: kuenyuhwu@ntu.edu.tw.
Ta-Fu Chen, Email: tfchen@ntu.edu.tw.
Tsun-Jen Cheng, Email: tcheng@ntu.edu.tw.
References
- 1.Babadjouni RM, Hodis DM, Radwanski R, Durazo R, Patel A, Liu Q, et al. Clinical effects of air pollution on the central nervous system; a review. J Clin Neurosci. 2017;43:16–24. doi: 10.1016/j.jocn.2017.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Block ML, Calderon-Garciduenas L. Air pollution: mechanisms of neuroinflammation and CNS disease. Trends Neurosci. 2009;32(9):506–516. doi: 10.1016/j.tins.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Power MC, Weisskopf MG, Alexeeff SE, Coull BA, Spiro A, 3rd, Schwartz J. Traffic-related air pollution and cognitive function in a cohort of older men. Environ Health Perspect. 2011;119(5):682–687. doi: 10.1289/ehp.1002767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weuve J, Puett RC, Schwartz J, Yanosky JD, Laden F, Grodstein F. Exposure to particulate air pollution and cognitive decline in older women. Arch Intern Med. 2012;172(3):219–227. doi: 10.1001/archinternmed.2011.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ritz B, Lee PC, Hansen J, Funch Lassen C, Ketzel M, Sorensen M, et al. Traffic-related air pollution and Parkinson’s disease in Denmark: a case-control study. Environ Health Perspect. 2016;124(3):351–6. [DOI] [PMC free article] [PubMed]
- 6.Kioumourtzoglou MA, Schwartz JD, Weisskopf MG, Melly SJ, Wang Y, Dominici F, et al. Long-term PM Exposure and Neurological Hospital Admissions in the Northeastern United States. Environ Health Perspect. 2016;124(1):23–9. [DOI] [PMC free article] [PubMed]
- 7.Shih C-H, Chen J-K, Kuo L-W, Cho K-H, Hsiao T-C, Lin Z-W, et al. Chronic pulmonary exposure to traffic-related fine particulate matter causes brain impairment in adult rats. Part Fibre Toxicol. 2018;245:226–234. doi: 10.1186/s12989-018-0281-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Costa LG, Cole TB, Coburn J, Chang YC, Dao K, Roque PJ. Neurotoxicity of traffic-related air pollution. Neurotoxicology. 2015. [DOI] [PMC free article] [PubMed]
- 9.Gerlofs-Nijland ME, van Berlo D, Cassee FR, Schins RP, Wang K, Campbell A. Effect of prolonged exposure to diesel engine exhaust on proinflammatory markers in different regions of the rat brain. Part Fibre Toxicol. 2010;7:12. doi: 10.1186/1743-8977-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levesque S, Taetzsch T, Lull ME, Kodavanti U, Stadler K, Wagner A, et al. Diesel exhaust activates and primes microglia: air pollution, neuroinflammation, and regulation of dopaminergic neurotoxicity. Environ Health Perspect. 2011;119(8):1149–1155. doi: 10.1289/ehp.1002986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bai KJ, Chuang KJ, Wu SM, Chang LT, Chang TY, Ho KF, et al. Effects of diesel exhaust particles on the expression of tau and autophagy proteins in human neuroblastoma cells. Environ Toxicol Pharmacol. 2018;62:54–59. doi: 10.1016/j.etap.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 12.Guerra R, Vera-Aguilar E, Uribe-Ramirez M, Gookin G, Camacho J, Osornio-Vargas AR, et al. Exposure to inhaled particulate matter activates early markers of oxidative stress, inflammation and unfolded protein response in rat striatum. Toxicol Lett. 2013;222(2):146–154. doi: 10.1016/j.toxlet.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kovacs GG. Molecular Pathological Classification of Neurodegenerative Diseases: Turning towards Precision Medicine. International journal of molecular sciences. 2016;17(2). [DOI] [PMC free article] [PubMed]
- 14.Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT. Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med. 2011;1(1):a006189. doi: 10.1101/cshperspect.a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liazoghli D, Perreault S, Micheva KD, Desjardins M, Leclerc N. Fragmentation of the Golgi apparatus induced by the overexpression of wild-type and mutant human tau forms in neurons. Am J Pathol. 2005;166(5):1499–1514. doi: 10.1016/S0002-9440(10)62366-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chesser AS, Pritchard SM, Johnson GV. Tau clearance mechanisms and their possible role in the pathogenesis of Alzheimer disease. Front Neurol. 2013;4:122. doi: 10.3389/fneur.2013.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lai CH, Lee CN, Bai KJ, Yang YL, Chuang KJ, Wu SM, et al. Protein oxidation and degradation caused by particulate matter. Sci Rep. 2016;6:33727. doi: 10.1038/srep33727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsu YH, Chuang HC, Lee YH, Lin YF, Chen YJ, Hsiao TC, et al. Traffic-related particulate matter exposure induces nephrotoxicity in vitro and in vivo. Free Radic Biol Med. 2019;135:235–244. doi: 10.1016/j.freeradbiomed.2019.03.008. [DOI] [PubMed] [Google Scholar]
- 19.Wolfe DM, Lee JH, Kumar A, Lee S, Orenstein SJ, Nixon RA. Autophagy failure in Alzheimer's disease and the role of defective lysosomal acidification. Eur J Neurosci. 2013;37(12):1949–1961. doi: 10.1111/ejn.12169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.ICRP. Human Respiratory Tract Model for Radiological Protection: ICRP Publication; 1994.
- 21.Garcia GJ, Kimbell JS. Deposition of inhaled nanoparticles in the rat nasal passages: dose to the olfactory region. Inhal Toxicol. 2009;21(14):1165–1175. doi: 10.3109/08958370902882713. [DOI] [PubMed] [Google Scholar]
- 22.Oberdörster G, Sharp Z, Atudorei V, Elder A, Gelein R, Kreyling W, et al. Translocation of inhaled ultrafine particles to the brain. Inhal Toxicol. 2004;16(6–7):437–445. doi: 10.1080/08958370490439597. [DOI] [PubMed] [Google Scholar]
- 23.Calderon-Garciduenas L, Gonzalez-Maciel A, Reynoso-Robles R, Kulesza RJ, Mukherjee PS, Torres-Jardon R, et al. Alzheimer's disease and alpha-synuclein pathology in the olfactory bulbs of infants, children, teens and adults </=40 years in metropolitan Mexico City. APOE4 carriers at higher risk of suicide accelerate their olfactory bulb pathology. Environ Res. 2018;166:348–362. doi: 10.1016/j.envres.2018.06.027. [DOI] [PubMed] [Google Scholar]
- 24.Chakrabarti S, Khemka VK, Banerjee A, Chatterjee G, Ganguly A, Biswas A. Metabolic risk factors of sporadic Alzheimer's disease: implications in the pathology. Pathogen Treat Aging Dis. 2015;6(4):282–299. doi: 10.14336/AD.2014.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hou L, Li Q, Jiang L, Qiu H, Geng C, Hong JS, et al. Hypertension and diagnosis of Parkinson's disease: a meta-analysis of cohort studies. Front Neurol. 2018;9:162. doi: 10.3389/fneur.2018.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suwannasual U, Lucero J, McDonald JD, Lund AK. Exposure to traffic-generated air pollutants mediates alterations in brain microvascular integrity in wildtype mice on a high-fat diet. Environ Res. 2018;160:449–461. doi: 10.1016/j.envres.2017.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kruyer A, Soplop N, Strickland S, Norris EH. Chronic hypertension leads to Neurodegeneration in the TgSwDI mouse model of Alzheimer's disease. Hypertension. 2015;66(1):175–182. doi: 10.1161/HYPERTENSIONAHA.115.05524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carnevale D, Perrotta M, Lembo G, Trimarco B. Pathophysiological links among hypertension and Alzheimer's disease. High Blood Press Cardiovasc Prev. 2016;23(1):3–7. doi: 10.1007/s40292-015-0108-1. [DOI] [PubMed] [Google Scholar]
- 29.Zanobetti A, Dominici F, Wang Y, Schwartz JD. A national case-crossover analysis of the short-term effect of PM2.5 on hospitalizations and mortality in subjects with diabetes and neurological disorders. Environ Health. 2014;13(1):38. doi: 10.1186/1476-069X-13-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen H, Kwong JC, Copes R, Hystad P, van Donkelaar A, Tu K, et al. Exposure to ambient air pollution and the incidence of dementia: a population-based cohort study. Environ Int. 2017;108:271–277. doi: 10.1016/j.envint.2017.08.020. [DOI] [PubMed] [Google Scholar]
- 31.Jung CR, Lin YT, Hwang BF. Ozone, particulate matter, and newly diagnosed Alzheimer's disease: a population-based cohort study in Taiwan. J Alzheimers Dis. 2015;44(2):573–584. doi: 10.3233/JAD-140855. [DOI] [PubMed] [Google Scholar]
- 32.WHO. WHO Air quality guidelines for particulate matter, ozone, nitrogen dioxide and sulfur dioxide: World Health Organization; 2006. Available from: http://www.euro.who.int/Document/E87950.pdf. [PubMed]
- 33.Li Y, Chang M, Ding S, Wang S, Ni D, Hu H. Monitoring and source apportionment of trace elements in PM2.5: implications for local air quality management. J Environ Manag. 2017;196:16–25. doi: 10.1016/j.jenvman.2017.02.059. [DOI] [PubMed] [Google Scholar]
- 34.Chuang HC, Jones TP, Lung SC, BéruBé KA. Soot-driven reactive oxygen species formation from incense burning. Sci Total Environ. 2011;409(22):4781–4787. doi: 10.1016/j.scitotenv.2011.07.041. [DOI] [PubMed] [Google Scholar]
- 35.Ho KF, Wu KC, Niu X, Wu Y, Zhu CS, Wu F, et al. Contributions of local pollution emissions to particle bioreactivity in downwind cities in China during Asian dust periods. Environ Pollut. 2019;245:675–683. doi: 10.1016/j.envpol.2018.11.035. [DOI] [PubMed] [Google Scholar]
- 36.Liu L, Urch B, Szyszkowicz M, Evans G, Speck M, Van Huang A, et al. Metals and oxidative potential in urban particulate matter influence systemic inflammatory and neural biomarkers: a controlled exposure study. Environ Int. 2018;121(Pt 2):1331–1340. doi: 10.1016/j.envint.2018.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ljubimova JY, Braubach O, Patil R, Chiechi A, Tang J, Galstyan A, et al. Coarse particulate matter (PM2.5–10) in Los Angeles Basin air induces expression of inflammation and cancer biomarkers in rat brains. Sci Rep. 2018;8(1):5708. doi: 10.1038/s41598-018-23885-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bernal-Melendez E, Lacroix MC, Bouillaud P, Callebert J, Olivier B, Persuy MA, et al. Repeated gestational exposure to diesel engine exhaust affects the fetal olfactory system and alters olfactory-based behavior in rabbit offspring. Part Fibre Toxicol. 2019;16(1):5. doi: 10.1186/s12989-018-0288-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chang CC, Hwang JS, Chan CC, Cheng TJ. Interaction effects of ultrafine carbon black with iron and nickel on heart rate variability in spontaneously hypertensive rats. Environ Health Perspect. 2007;115(7):1012–1017. doi: 10.1289/ehp.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chang CC, Hwang JS, Chan CC, Wang PY, Hu TH, Cheng TJ. Effects of concentrated ambient particles on heart rate variability in spontaneously hypertensive rats. J Occup Health. 2005;47(6):471–480. doi: 10.1539/joh.47.471. [DOI] [PubMed] [Google Scholar]
- 41.Rohr AC, Kamal A, Morishita M, Mukherjee B, Keeler GJ, Harkema JR, et al. Altered heart rate variability in spontaneously hypertensive rats is associated with specific particulate matter components in Detroit. Michigan Environ Health Perspect. 2011;119(4):474–480. doi: 10.1289/ehp.1002831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen TF, Huang RF, Lin SE, Lu JF, Tang MC, Chiu MJ. Folic acid potentiates the effect of memantine on spatial learning and neuronal protection in an Alzheimer's disease transgenic model. J Alzheimers Dis. 2010;20(2):607–615. doi: 10.3233/JAD-2010-1396. [DOI] [PubMed] [Google Scholar]
- 43.Rao X, Asico LD, Zanos P, Mahabeleshwar GH, Singh Gangwar R, Xia C, et al. Alpha2B-adrenergic receptor overexpression in the brain potentiate air pollution-induced behavior and blood pressure changes. Toxicol Sci. 2019;169(1):95–107. doi: 10.1093/toxsci/kfz025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fonken LK, Xu X, Weil ZM, Chen G, Sun Q, Rajagopalan S, et al. Air pollution impairs cognition, provokes depressive-like behaviors and alters hippocampal cytokine expression and morphology. Mol Psychiatry. 2011;16(10):987–995. doi: 10.1038/mp.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morris-Schaffer K, Merrill A, Jew K, Wong C, Conrad K, Harvey K, et al. Effects of neonatal inhalation exposure to ultrafine carbon particles on pathology and behavioral outcomes in C57BL/6J mice. Part Fibre Toxicol. 2019;16(1):10. doi: 10.1186/s12989-019-0293-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mercado JM, Hilsabeck R. Patient page. Untreated hypertension can lead to memory loss by cutting down on blood flow to the brain. Neurology. 2005;64(8):E28–E29. doi: 10.1212/WNL.64.8.E28. [DOI] [PubMed] [Google Scholar]
- 47.Obisesan TO Hypertension and cognitive function. Clin Geriatr Med. 2009;25(2):259–288. doi: 10.1016/j.cger.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Steimer T. The biology of fear- and anxiety-related behaviors. Dialogues Clin Neurosci. 2002;4(3):231–249. doi: 10.31887/DCNS.2002.4.3/tsteimer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adibhatla RM, Hatcher JF. Role of lipids in brain injury and diseases. Future Lipidol. 2007;2(4):403–422. doi: 10.2217/17460875.2.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gurgueira SA, Lawrence J, Coull B, Murthy GG, Gonzalez-Flecha B. Rapid increases in the steady-state concentration of reactive oxygen species in the lungs and heart after particulate air pollution inhalation. Environ Health Perspect. 2002;110(8):749–755. doi: 10.1289/ehp.02110749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Donaldson K, Brown DM, Mitchell C, Dineva M, Beswick PH, Gilmour P, et al. Free radical activity of PM10: iron-mediated generation of hydroxyl radicals. Environ Health Perspect. 1997;105(Suppl 5):1285–1289. doi: 10.1289/ehp.97105s51285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Senlin L, Zhenkun Y, Xiaohui C, Minghong W, Guoying S, Jiamo F, et al. The relationship between physicochemical characterization and the potential toxicity of fine particulates (PM2.5) in Shanghai atmosphere. Atmos Environ. 2008;42(31):7205–7214. doi: 10.1016/j.atmosenv.2008.07.030. [DOI] [Google Scholar]
- 53.Vidrio E, Jung H, Anastasio C. Generation of hydroxyl radicals from dissolved transition metals in surrogate Lung fluid solutions. Atmos Environ. 2008;42(18):4369–4379. doi: 10.1016/j.atmosenv.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shichiri M. The role of lipid peroxidation in neurological disorders. J Clin Biochem Nutr. 2014;54(3):151–160. doi: 10.3164/jcbn.14-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cole TB, Coburn J, Dao K, Roque P, Chang YC, Kalia V, et al. Sex and genetic differences in the effects of acute diesel exhaust exposure on inflammation and oxidative stress in mouse brain. Toxicology. 2016;374:1–9. doi: 10.1016/j.tox.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zanchi AC, Saiki M, Saldiva PH, Barros HM, Rhoden CR. Hippocampus lipid peroxidation induced by residual oil fly ash intranasal instillation versus habituation to the open field. Inhal Toxicol. 2010;22(1):84–88. doi: 10.3109/08958370902936931. [DOI] [PubMed] [Google Scholar]
- 57.Calderon-Garciduenas L, Torres-Jardon R, Kulesza RJ, Park SB, D'Angiulli A. Air pollution and detrimental effects on children’s brain. The need for a multidisciplinary approach to the issue complexity and challenges. Front Hum Neurosci. 2014;8:613. doi: 10.3389/fnhum.2014.00613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cheng H, Saffari A, Sioutas C, Forman HJ, Morgan TE, Finch CE. Nanoscale particulate matter from urban traffic rapidly induces oxidative stress and inflammation in olfactory epithelium with concomitant effects on brain. Environ Health Perspect. 2016;124(10):1537–1546. doi: 10.1289/EHP134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ajmani GS, Suh HH, Pinto JM. Effects of ambient air pollution exposure on olfaction: a review. Environ Health Perspect. 2016;124(11):1683–1693. doi: 10.1289/EHP136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arimon M, Takeda S, Post KL, Svirsky S, Hyman BT, Berezovska O. Oxidative stress and lipid peroxidation are upstream of amyloid pathology. Neurobiol Dis. 2015;84:109–119. doi: 10.1016/j.nbd.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reed TT. Lipid peroxidation and neurodegenerative disease. Free Radic Biol Med. 2011;51(7):1302–1319. doi: 10.1016/j.freeradbiomed.2011.06.027. [DOI] [PubMed] [Google Scholar]
- 62.Masurkar AV, Devanand DP. Olfactory dysfunction in the elderly: basic circuitry and alterations with Normal aging and Alzheimer's disease. Curr Geriatr Rep. 2014;3(2):91–100. doi: 10.1007/s13670-014-0080-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Doty RL. Olfactory dysfunction in Parkinson disease. Nat Rev Neurol. 2012;8(6):329–339. doi: 10.1038/nrneurol.2012.80. [DOI] [PubMed] [Google Scholar]
- 64.Lachen-Montes M, Gonzalez-Morales A, Zelaya MV, Perez-Valderrama E, Ausin K, Ferrer I, et al. Olfactory bulb neuroproteomics reveals a chronological perturbation of survival routes and a disruption of prohibitin complex during Alzheimer's disease progression. Sci Rep. 2017;7(1):9115. doi: 10.1038/s41598-017-09481-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fujishiro H, Tsuboi Y, Lin WL, Uchikado H, Dickson DW. Co-localization of tau and alpha-synuclein in the olfactory bulb in Alzheimer's disease with amygdala Lewy bodies. Acta Neuropathol. 2008;116(1):17–24. doi: 10.1007/s00401-008-0383-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Franks KH, Chuah MI, King AE, Vickers JC. Connectivity of pathology: the olfactory system as a model for network-driven mechanisms of Alzheimer's disease pathogenesis. Front Aging Neurosci. 2015;7:234. doi: 10.3389/fnagi.2015.00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Carmona-Abellan M, Martinez-Valbuena I, Marcilla I, DiCaudo C, Gil I, Nuñez J, et al. Microglia is associated with p-tau aggregates in the olfactory bulb of patients with neurodegenerative diseases. Neurol Sci. 2020. 10.1007/s10072-020-04686-x. [DOI] [PubMed]
- 68.Calderón-Garcidueñas L, González-Maciel A, Reynoso-Robles R, Kulesza RJ, Mukherjee PS, Torres-Jardón R, et al. Alzheimer's disease and alpha-synuclein pathology in the olfactory bulbs of infants, children, teens and adults ≤ 40 years in metropolitan Mexico City. APOE4 carriers at higher risk of suicide accelerate their olfactory bulb pathology. Environ Res. 2018;166:348–362. doi: 10.1016/j.envres.2018.06.027. [DOI] [PubMed] [Google Scholar]
- 69.Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, et al. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999;402(6762):672–676. doi: 10.1038/45257. [DOI] [PubMed] [Google Scholar]
- 70.Bai KJ, Chuang KJ, Chen CL, Jhan MK, Hsiao TC, Cheng TJ, et al. Microglial activation and inflammation caused by traffic-related particulate matter. Chem Biol Interact. 2019;108762. [DOI] [PubMed]
- 71.Roque PJ, Dao K, Costa LG. Microglia mediate diesel exhaust particle-induced cerebellar neuronal toxicity through neuroinflammatory mechanisms. Neurotoxicology. 2016;56:204–214. doi: 10.1016/j.neuro.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li N, Wang M, Bramble LA, Schmitz DA, Schauer JJ, Sioutas C, et al. The adjuvant effect of ambient particulate matter is closely reflected by the particulate oxidant potential. Environ Health Perspect. 2009;117(7):1116–1123. doi: 10.1289/ehp.0800319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yan YH, CKC C, Wang JS, Tung CL, Li YR, Lo K, et al. Subchronic effects of inhaled ambient particulate matter on glucose homeostasis and target organ damage in a type 1 diabetic rat model. Toxicol Appl Pharmacol. 2014;281(2):211–220. doi: 10.1016/j.taap.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 74.RTI. Standard Operating Procedure for the X-ray Fluorescence Analysis of Particulate Matter Deposits on Teflon Filters. Environmental and Industrial Measurements Division, 2009.
- 75.Tüközkan N, Erdamar H, Seven I. Measurement of Total malondialdehyde in plasma and tissues by high-performance liquid chromatography and Thiobarbituric acid assay. Fırat Tıp Dergisi. 2006;11(2):88–92. [Google Scholar]
- 76.USFDA. Guidance for Industry: Analytical Procedures and Methods Validation. In: Administration FaD, editor.: U.S. Department of Health and Human Services 2000.
- 77.Kohler I, Schappler J, Sierro T, Rudaz S. Dispersive liquid–liquid microextraction combined with capillary electrophoresis and time-of-flight mass spectrometry for urine analysis. J Pharm Biomed Anal. 2013;73:82–89. doi: 10.1016/j.jpba.2012.03.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Meteorological and gaseous data measured by the EPA Guting air quality monitoring stations during the study period. Figure S1. Spectrums of MDA analysed by LC-MS/MS. Figure S2. Effects (3- and 6-months exposure) of PM2.5 on the inflammatory infiltration of lungs in SH rats. Subpleural alveolar infiltration of mononuclear cells was observed in the lungs after 3- and 6-months exposure of HEPA and/or PM2.5. Figure S3. Alteration in body weight of SH rats between HEPA (control) and PM2.5 (exposure) groups after 6-months exposure. Figure S4. IHC images of total Tau (t-Tau) in the cerebellum, hippocampus, and cortex of SH rats after 6 months of exposure to HEPA (control) and PM2.5 (exposure). Scar bar is 50 μm. Figure S5. Chronic effects (6-months exposure) of PM2.5 on the histological changes of cerebellum, hippocampus, and cortex in SH rats. Scale bar: 200 μm.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.