Abstract
Background
College is an exciting but also challenging time with an increased risk for mental health issues. Only a minority of the college students concerned get professional help, a problem that might be improvable by internet- and mobile-based interventions (IMIs). However, adherence of IMIs is a concern. While guidance might be a solution, it is resource-intensive, derailing potential implementation on population level. The first aim of this trial is to evaluate the efficacy of the IMI StudiCare Mindfulness (StudiCare-M) for college students with “on demand” and no guidance. The second aim is to examine potential moderators and mediators, contributing to the questions of “how” and “for whom” such interventions work.
Methods
In this three-armed randomized controlled trial, both an unguided and “guidance on demand” (GoD) condition of StudiCare-M are compared to a waitlist control group. StudiCare-M is based on principles of acceptance and commitment therapy and stress management and consists of 7 modules plus two booster sessions. Participants in the GoD condition may ask their e-coach for support whenever needed. A total of 387 college students with moderate to low mindfulness are recruited at 15+ cooperating universities in Germany, Austria, and Switzerland via circular emails. Assessments take place before as well as 1, 2, and 6 months after randomization. The primary outcome is mindfulness. Secondary outcomes include stress, depression, anxiety, interoception, presenteeism, wellbeing, intervention satisfaction, adherence, and potential side effects. Among examined moderators and mediators are sociodemographic variables, pre-treatment symptomatology, treatment expectancy, self-efficacy, cognitive fusion, emotion regulation, and alexithymia. All data will be analyzed according to intention-to-treat (ITT) principles.
Discussion
Providing effective interventions to help college students become more resilient can make a valuable contribution to the health and functionality of future society. If effective under the condition of minimal or no guidance, StudiCare-M offers a low-threshold potentially resource-efficient possibility to enhance college student mental health on a population level. Moderation- and mediation analyses will deliver further insights for optimization of target groups and intervention content.
Trial registration
WHO International Clinical Trials Registry Platform via the German Clinical Studies Trial Register DRKS00014774. Registered on 18 May 2018.
Keywords: College students, University students, Mindfulness, Depression, Anxiety, Internet- and mobile-based interventions, E-health, Efficacy, Moderators and mediators, Guidance on demand
Introduction
The college years are an exhilarating time for students, but also involve multiple stressors such as moving away from home, finding a new peer group, and dealing with academic pressure [1]. As a consequence, this period of life comes with an increased risk for the development of mental health problems [2, 3]. Most mental disorders have their onset by the age of 24, when many young people are attending college [4]. In a recent WHO survey across 21 different countries (n = 1527), almost 25% of interviewed college students met DSM-IV criteria for at least one mental disorder in the last 12 months, with anxiety and mood disorders being the most prevalent [1]. Similar or even higher prevalence rates have been reported in many studies [5–8]. Mental health disorders among college students are related to various negative outcomes, such as poorer academic performance [9, 10] and higher college dropout rates [4, 11]. Unfortunately, less than 30% of the affected students receive minimally adequate treatment [1, 5, 12]. Because early treatment can prevent the onset of mental disorders [13] and thereby contributes to academic success, easily accessible low-threshold prevention and early intervention strategies are needed to help students develop effective coping strategies [14].
Interventions promoting student wellbeing, such as mindfulness trainings, offer a promising way to increase college student mental health. Such interventions train general coping and stress management skills [15] and are arguably less stigma-prone than disorder-specific support offers [16]. In line with assumptions of positive psychology, mindfulness trainings emphasize personal growth and strengthening of resilience rather than the removal of symptoms or disorders [17]. For example, mindfulness has been shown to be associated with enhanced emotion-regulation and therefore represents a protective factor in the face of internal and external stressors [18]. Lately, technology-based mindfulness interventions have been subject of a growing number of studies. Whereas internet and mobile-based interventions (IMIs) have shown to be equally effective as face-to-face treatments for numerous mental health problems [19], they also have several advantages over these traditional formats such as flexibility regarding time and place [20] and anonymous participation [21]. As these interventions can be designed in a cost-effective way, they have the potential to provide a large number of students with effective prevention and treatment options [22]. Finally, IMIs are especially suitable for this population because college students commonly seek health information online and show high acceptance of online mental health interventions [14, 23, 24].
The efficacy of mindfulness-based IMIs has been demonstrated in a number of studies and systematic reviews. In their meta-analysis, Spijkerman, Pots, and Bohlmeijer [25] examined 15 randomized controlled trials investigating adults with various mental disorders as well as healthy populations. Mindfulness interventions were compared to passive control groups (N = 10), active control groups (N = 5), or both (N = 2). The authors found small to medium effect sizes concerning the improvement of depression (g = 0.29, 95% CI 0.13–0.46), anxiety (g = 0.22, 95% CI 0.05–0.39), well-being (g = 0.23, 95% CI 0.09–0.38), and mindfulness (g = 0.32, 95% CI 0.23–0.42). The largest effect was shown for stress (g = .51, 95% CI 0.26–0.75). In another meta-analysis [26] of eight preventive mindfulness-based IMIs for non-clinical populations, similar effects were found compared to mostly (N = 7) passive control groups (g = 0.28–0.43, 95% CI 0.15–0.67) and were even larger at follow-up (g = 0.47–0.70, 95% CI 0.14–1.13). Even though these results are promising, there are still some questions that demand further examination.
To begin with, a majority of studies have used clinical populations, the general population, or employees. RCTs focusing on college student samples suggest comparable efficacy [27–31], but are often compromised by methodological limitations such as small sample sizes [29–31] or no long-term follow-up [27–29, 31]. Consequently, there is a need for large well-designed RCTs to confirm previous findings.
While unguided IMI formats can be effective, evidence suggests superior efficacy of guided IMI. In such guided IMIs, participants receive human support for working through the intervention, e.g., by an e-coach (health professional) giving them feedback and answering their questions. It has been proposed that the superior efficacy of guided IMIs might be due to increased intervention adherence [32]. Adherence is very relevant for mindfulness trainings, as mindfulness skills can only be developed with regular practice [18]. Indeed, unguided mindfulness IMIs have shown intervention dropout rates around 40–60% [29, 33, 34] and seem to be less efficacious then guided ones [25]. However, providing guidance also comes with increased intervention costs and therefore has implications for dissemination and scalability [25, 32]. These barriers might be overcome by using minimal guidance formats that combine the lower costs of unguided IMIs with the lower attrition rates of guided IMIs. One of these formats is called guidance on demand (GoD). In contrast to usual guidance formats, guidance by a therapist or e-coach will only take place when participants ask for it [32]. So far, only two trials examined GoD-IMIs, delivering cognitive behavioral therapy (CBT) to people with social phobia [35] and tinnitus [36]. Contrary to what one would expect, both studies did not find any significant differences in efficacy or adherence of GoD-IMIs compared to unguided IMIs. However, the social phobia trial [35] also found no differences between the GoD and a guided version nor the guided and an unguided version, which is somewhat surprising considering previous evidence for the superiority of guided IMIs [25]. Those results might be explainable by the fact that samples in both trials were highly burdened and therefore very motivated, irrespective of guidance [36]. In line with this, intervention dropout in the two studies was rather low and comparable in all conditions (20–30%). It remains to be examined whether these results can be generalized to mindfulness IMIs designed for non-clinical target-groups typically showing lower adherence.
Finally, the questions of how and for whom exactly mindfulness-based IMIs (as well as IMIs in general) work are yet to be answered [25, 37]. As large-scale RCTs provide an excellent framework for moderation and mediation analyses, they should routinely incorporate such analyses [38]. Therefore, in the current study, we will exploratively examine various potential moderating variables such as sociodemographic variables, pre-treatment symptomatology and treatment expectancy. From theory and current evidence, we also deduced a number of potential variables mediating the effect of mindfulness IMIs on mental health. Those include mindfulness itself [39], cognitive fusion as an aspect of psychological flexibility [39, 40], emotion regulation [41] and clarity about one’s internal experience (operationalized via alexithymia) [42]. Additionally, we will look at self-efficacy, as empowerment and self-management are assumed to be crucial factors for the efficacy of self-help interventions [43].
The current study is an extension of a previous RCT that investigated the efficacy and acceptance of StudiCare Mindfulness (StudiCare-M), a guided internet-based intervention to enhance mindfulness and wellbeing in college students [44]. We will examine whether an adapted unguided as well as a GoD version can be effective, as these would offer affordable options for long-term implementation into student health promotion programs. The specific research questions are:
Are the unguided and GoD versions of StudiCare-M effective in enhancing mindfulness in college students compared to a waitlist control group?
Are these two versions also effective concerning the secondary outcomes depression, anxiety, stress, presenteeism, well-being and interoceptive sensibility?
Are the unguided and GoD versions of StudiCare-M associated with side effects or adverse events?
Are there any differences between the unguided and GoD version of StudiCare-M concerning efficacy, adherence, satisfaction, side effects, or adverse events?
Which factors are associated with, moderate or mediate the effects of StudiCare-M?
Methods
Study design
This multicenter, three-armed randomized controlled trial of parallel design compares the efficacy of an unguided (UG) as well as a “guidance on demand” (GoD) version of the internet-based, preventive intervention StudiCare Mindfulness (StudiCare-M) to a waitlist control group (WL) receiving no intervention (superiority trial; see Fig. 1 for flowchart) within the framework of the StudiCare project funded by BARMER [45]. StudiCare dedicates itself to examining and promoting college students’ well-being offering a broad assortment of internet-based interventions for psychological and behavioral issues (e.g., procrastination, test anxiety, physical activity, depression, substance use, stress [20, 46–50]. It is embedded in the “World Mental Health Survey International College Student” project (WMH-ICS) [51] as well as the “Caring Universities” project [52].
Fig. 1.
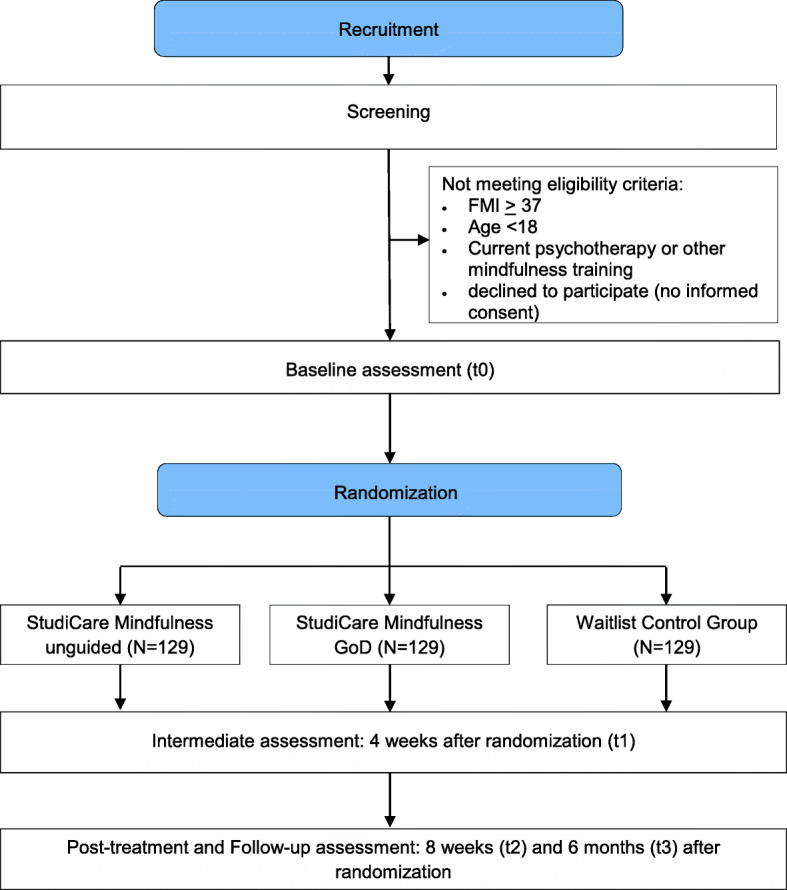
Flow diagram
The two versions of StudiCare-M are further compared to each other on an exploratory level to gain insights on potential differences in effectiveness, adherence, satisfaction side effects, and adverse events. Participants in all intervention arms are informed about and have access to treatment as usual. Use of other support options is monitored to control for potential confounding effects. The present study is conducted and will be reported according to the CONSORT 2010 Statement [53] and the guidelines for executing and reporting internet intervention research [54]. The study protocol follows recommendations of the SPIRIT 2013 Checklist for clinical trial protocols [55].
Eligibility criteria
Participants providing written informed consent further need to meet the following inclusion criteria: (a) age 18 or above, (b) enrolled in university or college, (c) sufficient knowledge of German language (assessed via capability to proceed through enrollment and screening process), (d) internet access, (e) moderate to low mindfulness (Freiburg Mindfulness Inventory FMI ≤ 37); this cutoff was chosen as it represents the medium value of the FMI in subjects from the general population [56]. Participants are excluded from the study if they are undertaking psychotherapy or any kind of mindfulness intervention at the time of the screening.
Setting/recruitment
Recruitment took place from May 2018 to April 2020. The main recruitment channel consists of circular e-mails sent out by more than 15 cooperating colleges in Germany, Austria, and Switzerland to all their students once every semester (for full list of cooperating colleges see StudiCare-Website [45]). Additionally, students are recruited via flyers and posters, social media, student unions, and student counseling. In the circular emails, students are informed about the StudiCare offers and are provided with a link to the StudiCare homepage [45], where they can obtain further information and register for the IMIs. Once registered, they receive an email with a link to the eligibility screening. Depending on which college participants attend, they are either allocated to a parallel local trial at Ulm University which combines StudiCare-M with on-site laboratory and psychophysiological measures [57] or to the present study (all other cooperating colleges). After successfully completing the screening, they receive an email with further information on the study as well as an informed consent form which they are required to send to the study team. When written consent is obtained and the pretest completed, another email with a link to the intervention is sent to participants randomized to one of the intervention groups. Participants are informed that StudiCare-M is not designed to replace psychotherapy, recommended to seek counseling/psychotherapy in case of distinctive mental health problems and provided with further treatment options and contact details. Participants of the WL will receive information on further study procedures as well as on alternative support options they can seek in case of deterioration of wellbeing during the waiting period.
Randomization
After completing baseline assessment, participants are randomly allocated to one of three study groups (unguided, “guidance on demand,” waitlist control group) by an independent researcher not otherwise involved and therefore blinded to all processes of the study. Via an automated, online-based randomization program [58], permuted block randomization is performed with an allocation ratio of 1:1:1 and variable block sizes of 6, 9, and 12 (randomly arranged). As this is an open-label study, blinding of group allocation to participants and e-coaches will not be possible. Consequently, unblinding will not be necessary.
Intervention
The intervention consists of seven weekly core modules of approximately 60 min. each. Additionally, two booster sessions are unlocked 4 and 12 weeks after completion of the seventh module to ensure sustainability of intervention effects. The main goal of the intervention is an increase in mindfulness and psychological flexibility. All modules contain information on stress, wellbeing, and mindfulness with a weekly alternating focus on different subjects such as interoception, dysfunctional thinking, or values and goals. Whereas these contents are provided via text, images, and interactive elements (such as quizzes or conditional content), the intervention also emphasizes the regular practice of mindfulness exercises like body scans and breathing meditations. Each module includes downloadable audio files as well as a mindfulness diary to be practiced in weekly homework assignments. At the beginning of each module, participants are encouraged to review their homework as well as their most and least mindful moments of the week. The content of the intervention is based on elements of acceptance and commitment theory (ACT) [59] as well as stress management principles [60]. ACT teaches acceptance, mindfulness, and value-based living and has found to be effective in the prevention of stress as well as the treatment of various psychological disorders [18]. The intervention was developed by the Department for Clinical Psychology and Psychotherapy, Ulm University. Its efficacy has already been demonstrated in a previous randomized controlled trial [44]. For the current RCT, the intervention was further refined and extended according to participants’ feedback. Table 1 summarizes the topics and contents of each module. The intervention is available to participants on the Minddistrict platform [61], a company specialized in the provision of internet-based health interventions. Participants are able to access the platform via their personal username and password on a 24/7 basis. All transferred data is secured based on ISO27001 and guidelines NEN7510.
Table 1.
Intervention content
| Module | Aims and content | Examples of exercises and assignments |
|---|---|---|
| 1. Being in the here and now | Introducing the concept of mindfulness | Reviewing most and least mindful moments of the day; practicing body scan; taking mindful walk |
| 2. Mindful body perception | Practicing awareness of body signals | Testing one’s heartbeat perception; practicing “heart meditation”; mindful eating and drinking |
| 3. A new perspective on stress | Distancing oneself from stress-inducing thoughts | Identifying former ways of coping with stress; learning techniques to challenge automatic thoughts; meditation exercise |
| 4. Developing beneficial thoughts | Getting to know alternative ways of thinking | Identifying one’s “stress patterns” and developing and internalizing beneficial thoughts; practicing breathing meditation |
| 5. What makes your life valuable? | Identifying one’s values and pursuing one’s goals | Writing a speech for one’s 70th birthday; setting and pursuing goals with the SMART technique; meditation exercise |
| 6. Being mindful towards yourself | Learning how to appreciatively accept one’s personality traits | Exercise to identify different personality traits and corresponding automatic reactions; learning to accept and appreciate all personality traits; loving kindness meditation |
| 7. Training your body and senses | Exercising the ability to enjoy and getting acquainted with the practice of yoga | Mindful chocolate eating exercise; mindful yoga exercises |
| Booster 1 (4 weeks after completion of module 7) | Repeating module 1 to 3 and mindfulness exercises | Choosing favorite mindfulness exercises; setting goals for their implementation in the coming weeks |
| Booster 2 (12 weeks after completion of module 7) | Repeating modules 4 to 7 and ensuring long-term integration of mindfulness into daily life | Reviewing pursuit of goals in the last 2 months; identifying potential barriers and developing solutions |
Guidance and promotion of adherence
Participants randomized to the GoD version of the intervention receive support by an e-coach whenever they desire to. E-Coaches are trained and supervised (by HB, AK) psychologists that give semi-standardized feedback on demand within 2 working days, following an e-coach manual. Whenever participants have questions or wish feedback on their module input, they can contact their personal e-coach via the platform’s message function. Each participant receives a welcoming message by their e-coach as well as a short introduction how to use GoD in the first module. Feedback content is specific to participants’ assignments and includes positive reinforcement, motivation, and encouragement. Actual usage of GoD by participants is documented. Participants in the unguided version of StudiCare-M do not receive any guidance. However, they receive short standardized automated feedback messages after completion of each module to reinforce and motivate them to continue the intervention. In both groups, participants are sent automated standardized e-mails by the Minddistrict platform if they have not logged in for more than 7 days. There will be no special criteria for discontinuing or modifying allocated interventions. However, all participants allocated to one of the two intervention versions will receive a document with descriptions and contact information of additional support offers (e.g., counseling, psychotherapy, emergency room), which they can refer to in case of symptom deterioration or need for more intensive care. Additionally, participants in the GoD version will be individually referred to additional support offers by their e-coaches in case of symptom deterioration. Post-trial care will be provided for both intervention arms in form of two booster sessions unlocking automatically after 1 and 3 months, with optional guidance by an e-coach for the GoD version. Finally, participants of both intervention arms will have unrestricted access to usual treatment options (such as psychotherapy or medication).
SMS coach
In the first module, participants of both IMI trial arms are offered the possibility to sign up for a text message coach. Once signed up, they receive standardized automated text messages every second day for the duration of the core intervention (8 weeks). Messages are designed to remind participants of their homework assignments, motivate them to integrate learned techniques into their daily life, and generally prompt them to be mindful during the day. Text message prompts have been shown to be useful in internet interventions regarding efficiency as well as adherence [62, 63].
Control condition
Participants in the waitlist control group have unrestricted access to usual treatment options (TAU). They receive an information leaflet informing them about alternative support options such as university counseling services, psychotherapy, or helplines as well as the encouragement to seek help in case of any deterioration of wellbeing. After t3 (6 months after randomization), WL participants receive the unguided version of the intervention.
Assessments and outcomes
Assessment takes place before (t0; baseline) as well as 4 weeks (t1; intermediate), 8 weeks (t2; post-treatment), and 6 months (t3; follow-up) after randomization. t1 is an intermediate assessment of a subset of outcomes (see Table 2). All data will be self-reported and collected via the online survey platform “Unipark” [64]. Blinding of outcome assessment will therefore not be possible. To reduce assessment dropout, an email reminder strategy is employed. Participants’ phone numbers are collected on a voluntary basis to have the possibility of reminding them of filling out the surveys. Additionally, a raffle of 10 20-Euro “Amazon” coupons will take place among completers of the t3-survey at the end of the study.
Table 2.
Outcomes and assessment points
| Variables | Measurement | Screening | t0 | t1 | t2 | t3 |
|---|---|---|---|---|---|---|
| Inclusion/exclusion criteria | ||||||
| Inclusion/exclusion criteria | SRQ | x | ||||
| Mindfulness | FMI | x | ||||
| Primary outcome | ||||||
| Mindfulness | FMI | x | ||||
| Secondary outcomes | ||||||
| Mindfulness | FMI | x | x | x | ||
| Depressive symptoms | PHQ-9 | x | x | x | x | |
| Anxiety | GAD-7 | x | x | x | x | |
| Stress | PSS-4 | x | x | x | x | |
| Well-being | WHO-5 | x | x | x | x | |
| Presenteeism | PSS | x | x | x | ||
| Interoceptive sensibility | BPQ | x | x | x | ||
| Self-efficacy | SES | x | x | x | x | |
| Cognitive fusion | CFQ-D | x | x | x | x | |
| Emotion regulation | ERQ | x | x | x | x | |
| Alexithymia | TAS-20 | x | x | x | x | |
| Intervention adherence | Intervention dropout | x | x | x | ||
| Intervention satisfaction | CSQ-8 | x* | ||||
| Side effects of intervention | INEP* | x | x | |||
| Covariates | ||||||
| Demographic variables | SRQ | x | ||||
| Previous experience with mindfulness, use of additional treatment options | SRQ | x | x | x | ||
| Treatment expectation | CEQ | x | ||||
Note. CEQ Client Expectancy Questionnaire, CFQ-D Cognitive Fusion Questionnaire, CSQ-8 Client Satisfaction Questionnaire, German Version ZUF-8, ERQ Emotion Regulation Questionnaire, FMI Freiburg Mindfulness Inventory, GAD-7 Generalized Anxiety Disorder Questionnaire, INEP Inventory for the Assessment of Negative Effects of Psychotherapy, BPQ Body Perception Questionnaire, Awareness Section, PHQ-9 Patient Health Questionnaire, PSS Presenteeism Scale for Students, PSS-4 Short Form Perceived Stress Scale, SES Self-Efficacy Scale, SRQ Self-Report Assessment Questionnaire, TAS-20 Toronto-Alexithymia Scale, WHO-5 World Health Organization Well-Being Index
*Intervention groups only (UG, GoD)
Primary outcome: mindfulness at post-treatment (t2)
The 14-item short scale of the Freiburg Mindfulness Inventory [56] is used to assess mindfulness. The FMI consists of a 4-point scale ranging from 1 = “rarely” to 4 = “almost always.” The short scale demonstrated sensitivity to change [56] and high internal consistency (α = 0.84) [65].
Secondary outcomes
Mindfulness at intermediate assessment (t1) and follow-up (t3)
Mindfulness is also measured after 4 weeks and 6 months.
Depressive symptoms
The depression module of the Patient Health Questionnaire [66] comprises of nine items that are rated on a 4-point scale (0 = “not at all” to 3 = “nearly every day”). The PHQ-9 is a widely used depression screening that has been shown to be a valid instrument [67] with good diagnostic properties and excellent internal consistency (α = 0.89). It has also been evaluated as an online version [68].
Anxiety
The 7-item Generalized Anxiety Disorder Questionnaire [69] is a screening instrument for generalized anxiety disorder and ranges from “not at all” (= 0) to “nearly every day” (= 3). The GAD-7 has been identified to be a reliable and valid measure of anxiety in the general population with a high internal consistency of Cronbach’s α = 0.89 [70].
Stress
The Short Form Perceived Stress Scale (PSS-4), derived from the Perceived Stress Scale [71], will be used to measure the participants’ perceived stress as the degree to which situations in one’s life are rated as stressful (scale ranging from 0 = never to 4 = very often). The psychometric properties of the PSS-4 have been found to be acceptable and reliable across cultures, with α = 0.77 [72].
Well-being
The well-established 5-item World Health Organization Well-Being Index (WHO-5) [73] is used to assess subjective psychological well-being. The scale ranges from “at no time” (= 0) to “all of the time” (= 5). Good psychometric properties of the WHO-5 as a screening tool for depression have been demonstrated among diverse clinical studies. Clinical validity has been identified as very high [74].
Presenteeism
As academic outcomes, presenteeism, loss of productivity, and absenteeism are assessed using a modified version [46] of the Presenteeism Scale for Students [75]. Presenteeism is measured by the subscale for work impairment (Work Impairment Scale; 10 items, scale 1–5, range 10–50). Productivity losses will be assessed by an adaption of the Presenteeism Scale for Students’ work output scale, investigating the current percentage to which participants were able to reach their usual academic productivity (visual analogue scale ranging from 0% = completely unproductive to 100% = full productivity). Additionally, hours of absenteeism are inquired. For the Work Impairment Scale, a Cronbach’s α of 0.90 as well as sufficient test-retest reliability and criterion-related validity could be demonstrated [75].
Interoceptive sensibility
Interoceptive sensibility (IS) is assessed by the awareness section of the Body Perception Questionnaire (BPQ) [76]. The section includes 54 items of subjective identifications of bodily signals on a 5-point scale, ranging from “never” (= 1) to “always” (= 5). High scores reflect poor IS. For the short form of the BPQ, categorical omega coefficients between 0.77 and 0.96 as well as high retest reliability were shown [77].
Subjective side effects and adverse events
The Inventory for the Assessment of Negative Effects of Psychotherapy (INEP) [78] assesses any changes experienced during or after the treatment in the social and/or work environment and whether they are attributed to the psychotherapeutic intervention. Four items are rated on a 7-point bipolar scale (− 3 = “worse,” + 3 = “better”); the others are rated on a 4-point scale (0 = “no agreement,” 3 = “full agreement”). In the present trial, an adapted 22-item version covering possible negative effects associated specifically with online-trainings (e.g., concerns about data protection) will be applied. The original scale has demonstrated high internal consistency with a Cronbach’s α of 0.86 [78].
Intervention satisfaction and adherence
The Client Satisfaction Questionnaire (CSQ-8) [79] is a validated 8-item instrument and is used in a version adapted for the evaluation of IMIs [80]. It comprises of eight items, each with a 4-point scale of specific response alternatives (e.g., 1 = “quite unsatisfied,” 4 = “very satisfied”). Good psychometric properties have been demonstrated including Cronbach’s α between .88 and .92 [81]. To operationalize intervention adherence, the number of completed modules is assessed. “Per protocol” adherence is operationalized by the percentage of participants that completed at least 5 of the 7 modules 8 weeks after randomization (t2). Additionally, quantitative and qualitative data are collected on participants’ satisfaction with various aspects of the intervention (e.g., number and length of modules, SMS-Coach, practicability in daily life) using self-constructed items (e.g., “Which elements did you find particularly helpful?”).
Use of GoD, subscription to SMS coach, and practice of mindfulness exercises
The number of times participants of the GoD condition contact their e-coach will be documented. We will also track whether participants of both intervention groups subscribe to the SMS-coach. Finally, we will assess the weekly time that participants spent practicing the mindfulness exercises introduced to them in the modules (retrospectively at t2).
Potential mediators
Self-efficacy
Perceived general self-efficacy is measured by the 10-item Self-Efficacy Scale [82] on a 4-point response scale from “1 = not at all true” to “4 = very true.” It was used in numerous research projects, where it demonstrated internal consistencies of Cronbach’s α = 0.75–0.91. It has also been proven reliable and valid in various field studies [83].
Cognitive fusion
In ACT, cognitive fusion is defined as the extent to which individuals identify with and are behaviorally regulated by their own thoughts and beliefs. Therefore, it is an important aspect of Psychological Inflexibility, which the intervention aims to reduce. It is assessed with the German version of the Cognitive Fusion Questionnaire [84]. Participants are asked to rate the seven items of the CFQ-D on a 7-point scale ranging from “1 = never true” to “7 = always true.” The CFQ-D has demonstrated good psychometric properties reflected in a Cronbach’s α of 0.95 as well as convergent validity with measures of physical and mental health [84].
Emotion regulation
The Emotion Regulation Questionnaire (ERQ) [85] is used to assess individual differences in habitual use of two emotion regulation strategies, reappraisal and suppression. Participants are required to indicate whether they agree with each statement on a 7-point scale ranging from 1 (= strongly disagree) to 7 (= strongly agree). The ERQ demonstrates good scale score reliability for the suppression (Cronbach’s α = 0.76) as well for the reappraisal factor (Cronbach’s α = 0.74) [86].
Alexithymia
The Toronto-Alexithymia Scale (TAS-20) [87, 88] is used to measure alexithymia. The questionnaire consists of 20 items rated on a 5-point scale (1 = strongly disagree; 5 = strongly agree) with total scores ranging from 20 to 100, reflecting three factor scales: “difficulties identifying feelings” (DIF), “difficulty describing feelings” (DDF), and “externally oriented thinking” (EOF). Higher scores on the different subscales indicate higher levels of alexithymia. The TAS-20 is a valid instrument with good internal consistency (Cronbach’s α = 0.85–0.86) and test-retest reliability [89].
Covariates
To investigate potential effect-modifying influences [90], several sociodemographic as well as other variables are assessed: age, gender, nationality, marital status, study course and number of semesters, previous experience with mindfulness, psychotherapy experience, and use of additional treatment options (such as psychological counseling or psychotherapy) as well as baseline symptomatology. To examine the influence of treatment expectations on outcomes, the Client Expectancy Questionnaire (CEQ) is used, which has demonstrated high internal consistency (α = 0.84–0.85) [91]. It consists of six items which are measured on a 9-point Likert Scale with higher scores representing positive expectations and credibility.
Sample size estimation
Sample size calculations refer to detect an expected increased efficacy of StudiCare-M unguided and GoD compared to WL on the primary outcome mindfulness at t2 (post-treatment). In their meta-analysis, Spijkerman et al. (2015) found a small effect for unguided mindfulness IMIs (g = 0.22, 95% CI 0.10–0.34) and a significantly larger effect for guided IMIs (g = 0.43, 95% CI 0.30–0.56) [25]. The GoD version of our IMI provides the possibility to contact an e-coach at any time. Additionally, both the GoD and unguided versions contain various persuasive e-health technologies and that have been shown to increase efficacy and adherence (e.g., self-monitoring, goal setting, SMS prompts, automatic reminders) [63, 92]. Therefore, we assume an effect size comparable to the effects previously found for guided IMIs of d = 0.40. Originally, a power analysis based on a two-tailed t-test (calculated using G*Power [93]) resulted in a sample size of 133 participants per group. This was documented in the first version of the trial registration. However, in the meantime, a more precise power calculation was done by an independent biostatistician (MM). This calculation indicates that 129 participants per group are required to obtain a power of 1-beta = 90% based on α = .05 (taking into account clustering of participants by university and assuming an ICC = .02). Trial registration was updated accordingly. As data analysis will be based on intention-to-treat (ITT) principles, increasing sample size in order to compensate for drop-outs is not necessary.
Concerning an exploratory comparison between the UG and GoD version of StudiCare-M, the sample size is sufficient to detect a small effect of d = 0.20 as minimal clinically important effect difference with a power of 50% and a significance level of α = .05. This also corresponds to the range of effect sizes of d = 0.02–0.33 found for different outcome measures in the trials of Berger et al. [35] and Rheker et al. [36].
Statistical analyses
All data analyses will be performed after completion of data collection. Interim analysis is not considered necessary as in the context of IMIs, there are no known dangers or harms that could make a trial stop necessary. Analyses will be performed on an intention-to-treat basis. Procedures of imputation will be chosen based on patterns and mechanisms of missingness (e.g., by using multiple imputation). Additionally, per protocol analyses (based on the data of participants that completed at least 5 of the 7 core modules) will be performed to examine the impact of drop-outs on study results. Significance level for all analyses will be defined as alpha = .05. A blinded analysis of primary and secondary outcomes will be conducted by an independent researcher not otherwise involved in the execution of the study (MM).
The primary outcome FMI at t2 will be analyzed by means of linear regression models. Baseline and group will be defined as predictors. Secondary outcomes will be analyzed accordingly. Standardized mean differences with 95% confidence intervals will be calculated post-treatment and follow-up to analyze between-group effect sizes. To obtain the number of participants achieving reliable improvement in mindfulness (FMI), participants will be coded as responders and nonresponders according to the Reliable Change Index (RCI) for t2 and t3 [94]. Additionally, we will investigate potential negative effects on individual level by calculating the number of participants that display a reliable deterioration from t0 to t2 and t3 also using the RCI [95, 96]. For an exploratory examination of associations between adherence, subscription to SMS-coach, actual use of GoD, time spent practicing mindfulness exercises, and the primary outcome FMI at t2 regression analyses will be conducted.
Moderator and mediator analyses
For the exploratory moderator analyses, regression models will be used. In a first step, each potential moderator defined in the “Covariates” section will be tested in a separate regression model. The primary outcome FMI at t2 will be set as dependent variable. Included predictors will be group, the respective moderator variable and the interaction of group and moderator. Subsequently, a comprehensive model of all identified moderators will be estimated.
Mediation analyses will be conducted according to the principles of time-lagged mediation by Cole and Maxwell [97]. This approach will enable the establishment of temporal precedence, an important requirement for the investigation of mechanisms of change [98]. Group will be set as independent variable, whereas the variables defined in the “Potentials mediators” section as well as mindfulness (FMI) will constitute the respective mediating variables. Depression (PHQ-9), stress (PSS-4), and anxiety (GAD-7) will be chosen as outcome variables, as they have been studied most frequently in previous mediation studies in the context of mindfulness interventions [39].
Discussion
Psychological problems in college students are widespread and associated with poorer academic performance. Internet-based interventions (IMIs) for enhancing mindfulness are a promising way to increase student mental health but typically suffer from low adherence, compromising the effectiveness of such interventions. This trial will be the first to examine the effectiveness of a mindfulness IMI without and with guidance-on-demand, comparing both versions to a waitlist control group as well as to each other. Additionally, we will investigate potential moderators and mediators, an area that is still understudied [25, 99].
Despite best efforts, any trial comes with certain limitations that are described in the following.
A common problem with internet-based interventions is high dropout-rates concerning both intervention and assessments. For unguided mindfulness IMIs, intervention dropout rates of 40–60% have been reported suggesting that only about half of the participants complete such interventions [29, 31]. In order to overcome this problem, we will implement the following measures: an optional SMS-Coach prompting participants every other day; the possibility to contact an e-coach any time in the “GoD” condition; and short automated feedback messages after each completed module in the “unguided” condition. Additionally, in order to receive an estimate of the true treatment effect unbiased by assessment dropout, data will be analyzed on ITT basis [100].
Within the framework of our intervention, we will not be able to track the actual amount and frequency of mindfulness practice participants will engage in. Studies have shown that the time spent practicing mindfulness in daily life is an important moderator of intervention efficacy [18]. We will however ask for the average time participants spent practicing at post-assessment and conduct a moderator analysis based on that information. Still, this retrospective assessment might be biased and a future version of StudiCare-M should include immediate tracking of mindfulness practice, e.g., in an accompanying app.
Finally, we will only include college students with moderate to low mindfulness. This approach entails a reduced generalizability of results, not allowing conclusions concerning the efficacy of our IMI for individuals already high on mindfulness. However, we decided to do so because StudiCare-M was designed as an introduction to mindfulness, primarily addressing mindfulness beginners. Consequently, this approach will allow us to evaluate the efficacy of StudiCare-M for those students who need and can therefore benefit from such an intervention the most.
Beside these limitations, our study also offers several strengths.
Many mindfulness studies so far have suffered from samples of limited generalizability. Participants were frequently recruited at only one university or from one study course [30, 34, 101, 102]. Additionally, participants sometimes received money or course credits for study participation [27–29], therefore possibly not representing real students in need. Within the StudiCare project, we are able to overcome these shortcomings. Because participants are mainly recruited via health promotion departments of more than 15 cooperating colleges in Germany, Switzerland, and Austria, we reach students from a great variety of colleges and study courses. Additionally, participants will not receive compensation for their participation reflecting “real life” conditions of health care services (except for a raffle for the long-term follow-up that they will not be informed about until the invitation to the t3 assessment). This approach enables us to learn about real-life demand for and attractiveness of our intervention, important information when it comes to long-term implementation.
So far, only few studies examined the efficacy of IMIs for college students based on acceptance and commitment therapy (ACT) [30, 31, 33, 102]. While mindfulness in general provides useful skills for stress management, ACT goes beyond meditation by emphasizing the concept of psychological flexibility and focusing on values and committed action [59]. These skills might come in specifically handy in a challenging and complex university and subsequent working life. So far, results concerning ACT-IMIs for college students have been promising but have suffered from limitations such as no long-term follow-up [31, 33] or small sample sizes [30, 31]. Our trial is designed to overcome these limitations with a large sample size as well as a 6-month follow-up.
This is one of few studies that examine the effect of “guidance on demand” (GoD) on adherence and efficacy of internet-based interventions [35, 36] and the very first to do so for a mindfulness IMI. As discussed before, mindfulness IMIs could provide an excellent possibility to reach students in need on a population level. Guided IMIs have shown to be most effective but can be associated with increased intervention costs [32]. Consequently, it is worth investigating whether a GoD-IMI for enhancing mindfulness can be effective and well accepted by participants. Additionally, we will compare GoD to an unguided version of StudiCare-M on an exploratory level. Both versions of our intervention are designed according to principles of persuasive design. Therefore, we will gather preliminary evidence on differences between persuasively designed unguided IMIs and IMIs with a minimal form of human guidance concerning effectiveness, adherence, side effects, and adverse events.
While the efficacy of mindfulness IMIs has been shown in many RCTs by now, the questions of how and for whom these interventions work have not been answered sufficiently. Therefore, this RCT will examine a range of different moderators and mediators on an exploratory level in order to contribute to this still understudied area of research [25]. Trials investigating mediators often suffer from methodological limitations such as measuring the proposed mediators, dependent and independent variables only pre- and post-treatment. Consequently, they are unable to establish chronology of change, therefore neglecting a crucial element of investigating mediators [98]. We aim to overcome this shortcoming by conducting an additional assessment at midpoint of the intervention, enabling us to find out at what time changes occur.
One last strength of this RCT is the fact that we will measure potential side effects and adverse events. It has been shown that IMIs can have negative side effects [103–105], but those have been understudied so far [104]. This is especially the case for mindfulness IMIs, where no study so far has investigated such effects. This RCT will therefore contribute to our knowledge on the safety of minimally or unguided mindfulness IMIs, an important precondition for a possible large-scale dissemination of such interventions in the future.
Going to college is associated with high stress levels and an increased risk for the development of mental disorders. Providing effective interventions to college students, helping them improve their coping and stress management skills, can therefore make a valuable contribution to the health and functionality of future society. If effective under the condition of minimal or no guidance, StudiCare-M offers a low-threshold potentially resource-efficient possibility to enhance college student mental health on a population level.
Trial status
This is protocol version number 1.1 as submitted on 26 July 2019 and amended along reviewers’ comments on 23 October 2020. Recruitment began on 21 May 2018 and was completed by 27 April 2020. Data collection will be completed by November 2020. Prior to recruitment start, the trial was registered at the WHO International Clinical Trials Registry Platform via the German Clinical Studies Trial Register: DRKS00014774 (registration date: 18 May 2018; URL: https://www.drks.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00014774). In case of important protocol modifications, trial registration will be updated.
Acknowledgements
We thank Danielle Preuss, Katharina Peip, Christine Schillings, Ronja Gabriel, and Felicitas Weineck for their contributions to the development of StudiCare-M. Thanks to Mathias Harrer for taking care of the StudiCare website and to Fanny Kählke for supporting the establishment of the study administration and assessment processes. Moreover, we would like to thank Francesca Mildenberger and our other study assistants for their support in the development of the intervention, the assessment procedures, and the study administration processes. Special thanks to all cooperating colleges in Germany, Austria, and Switzerland for regularly informing their students about the StudiCare interventions.
Abbreviations
- ACT
Acceptance and commitment therapy
- CBT
Cognitive behavioral therapy
- CONSORT
Consolidated Standards of Reporting Trials
- GoD
Guidance on demand
- IMI
Internet- and mobile-based intervention
- ITT
Intention-to-treat
- RCT
Randomized controlled trial
- StudiCare-M
StudiCare Mindfulness
- TAU
Treatment as usual
- UG
Unguided
- WHO
World Health Organization
- WL
Waitlist control group
Authors’ contributions
AK, DDE, HB, and OP initiated this study. AK, DS, HB, and OP contributed to the design of this study. AK and DS adapted the intervention content and assessment. AK is responsible for recruitment. MM is responsible for the blinded analysis of primary and secondary outcomes and will provide expertise on all other statistical procedures. AK wrote the draft of the manuscript. All authors contributed to the further writing of the manuscript and approved the final version of the manuscript. All authors have agreed to publication and adhere to the authorship guidelines of Trials. No professional writers have been involved.
Funding
The StudiCare Project was initiated by DDE and HB (study sponsors), who are also responsible for proper management, monitoring, and reporting of any study carried out within the project. The project is funded by the BARMER, a major health care insurance company in Germany. BARMER has no role in study design, decision to publish, or preparation of this manuscript. BARMER will not be involved in data collection, analyses, decision to publish, or preparation of future papers regarding the StudiCare project. DDE, HB, and OP will oversee and supervise trial conducting activities and will meet with trial coordinators AK and DS every 3 months (trial steering committee). AK and DS will be responsible for trial organization and management such as recruitment (contact with cooperating universities) and obtaining participant informed consent. A data monitoring committee (DMC) will not be employed as this trial investigates a low-risk intervention. Open Access funding enabled and organized by Projekt DEAL.
Availability of data and materials
All principal investigators will be given full access to the data sets with the exception of MM. He will be responsible for the blinded analysis of the primary and secondary outcomes and hence will only receive a blinded version of the data needed for this analysis. The data set will be stored on password-protected servers of Ulm University with restricted access. External researchers may get access to the final trial dataset (from HB) on request depending on to be specified data security and data exchange regulation agreements. They can obtain the informed consent form in the same way. To ensure confidentiality, data dispersed to any investigator or researcher will be blinded of any identifying participant information. Anonymized results will be published in peer-reviewed journals and presented on international conferences.
Ethics approval and consent to participate
All study procedures have been approved by the ethics committee of the Ulm University (application no. 47/18). Significant changes in the study protocol will be reported to the ethics committee, whereas additional meetings are not considered necessary due to the low-risk nature of the examined intervention. Participants will receive written information on study conditions, data security, publication of anonymized results, voluntariness of participation, and the right to leave the study at all times. They will also be informed that in case of study withdrawal, they will be able to decide whether they want their data to be included in the analysis or to be deleted. Additionally, participants will be asked for permission for the research team to share relevant data with people from regulatory authorities, where necessary. To confirm understanding of the above, written consent is obtained from all participants prior to study entry. This trial will only involve the collection and storage of self-report data, not of biological specimens. Data collection will be pseudonymized and data will only be accessed by authorized study personnel obliged to secrecy. After data collection is completed, personalized information will be deleted and all data will be completely anonymized. According to German law, data will only be shared with parties outside the project team in anonymized form. As there is no anticipated harm for participants of the trial, no compensation will be necessary.
Consent for publication
Not applicable.
Competing interests
AK, DS, and HB were involved in the development of StudiCare-M or its predecessor versions. AK has received fees for lectures/workshops from chambers of psychotherapists and health insurance companies. HB reports to have received consultancy fees and fees for lectures/workshops from chambers of psychotherapists and training institutes for psychotherapists in the e-mental-health context. AK has received fees for lectures/workshops from chambers of psychotherapists and health insurance companies. DDE reports to have received consultancy fees/served in the scientific advisory board from several companies such as Minddistrict, Lantern, Schoen Kliniken, and German health insurance companies. He is stakeholder of the Institute for health training online (GET.ON), which aims to implement scientific findings related to digital health interventions into routine care.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ann-Marie Küchler, Email: ann-marie.kuechler@uni-ulm.de.
Dana Schultchen, Email: dana.schultchen@uni-ulm.de.
Olga Pollatos, Email: olga.pollatos@uni-ulm.de.
Morten Moshagen, Email: morten.moshagen@uni-ulm.de.
David D. Ebert, Email: d.d.ebert@vu.nl
Harald Baumeister, Email: harald.baumeister@uni-ulm.de.
References
- 1.Arnett JJ. Emerging adulthood: a theory of development from the late teens through the twenties. Am Psychol. 2000;55(5):469–480. doi: 10.1037/0003-066X.55.5.469. [DOI] [PubMed] [Google Scholar]
- 2.Hunt J, Eisenberg D. Mental health problems and help-seeking behavior among college students. J Adolesc Health. 2010;46(1):3–10. doi: 10.1016/j.jadohealth.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 3.Stallman HM. Psychological distress in university students: a comparison with general population data. Aust Psychol. 2010;45(4):249–257. doi: 10.1080/00050067.2010.482109. [DOI] [Google Scholar]
- 4.Kessler RC, Foster CL, Saunders WB, Stang PE. Social consequences of psychiatric disorders, I: educational attainment. Am J Psychiatry. 1995;152(7):1026–1032. doi: 10.1176/ajp.152.7.1026. [DOI] [PubMed] [Google Scholar]
- 5.Blanco C, Okuda M, Wright C, Hasin DS, Grant BF, Liu SM, et al. Mental health of college students and their non-college-attending peers: results from the National Epidemiological Study on Alcohol and Related Conditions. Arch Gen Psychiatry. 2008;65(12):1429. doi: 10.1001/archpsyc.65.12.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dyrbye LN, Thomas MR, Shanafelt TD. Systematic review of depression, anxiety, and other indicators of psychological distress among U. S. and Canadian medical students. Acad Med. 2006;81(4):354–373. doi: 10.1097/00001888-200604000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Ibrahim AK, Kelly SJ, Adams CE, Glazebrook C. A systematic review of studies of depression prevalence in university students. J Psychiatr Res. 2013;47(3):391–400. doi: 10.1016/j.jpsychires.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 8.Zivin K, Eisenberg D, Gollust SE, Golberstein E. Persistence of mental health problems and needs in a college student population. J Affect Disord. 2009;117(3):180–185. doi: 10.1016/j.jad.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Eisenberg D, Golberstein E, Hunt JB. Mental health and academic success in college. B E J Econom Anal Policy. 2009;9(1) Available from: https://www.degruyter.com/view/j/bejeap.2009.9.1/bejeap.2009.9.1.2191/bejeap.2009.9.1.2191.xml.
- 10.Bruffaerts R, Mortier P, Kiekens G, Auerbach RP, Cuijpers P, Demyttenaere K, et al. Mental health problems in college freshmen: prevalence and academic functioning. J Affect Disord. 2018;225:97–103. doi: 10.1016/j.jad.2017.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hartley MT. Increasing resilience: strategies for reducing dropout rates for college students with psychiatric disabilities. Am J Psychiatr Rehabil. 2010;13(4):295–315. doi: 10.1080/15487768.2010.523372. [DOI] [Google Scholar]
- 12.Eisenberg D, Hunt J, Speer N. Help seeking for mental health on college campuses: review of evidence and next steps for research and practice. Harv Rev Psychiatry. 2012;20(4):222–232. doi: 10.3109/10673229.2012.712839. [DOI] [PubMed] [Google Scholar]
- 13.National Research Council (US) and Institute of Medicine (US) Committee on the Prevention of Mental Disorders and Substance Abuse Among Children, Youth and YARA and PI . In: Preventing mental, emotional, and behavioral disorders among young people: progress and possibilities. O’Connell ME, Boat T, Warner KE, editors. Washington (DC): National Academies Press; 2009. [PubMed] [Google Scholar]
- 14.Pedrelli P. College students: mental health problems and treatment considerations. Acad Psychiatry. 2015;39(5):503–511. doi: 10.1007/s40596-014-0205-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galante J, Dufour G, Benton A, Howarth E, Vainre M, Croudace TJ, et al. Protocol for the Mindful Student Study: a randomised controlled trial of the provision of a mindfulness intervention to support university students’ well-being and resilience to stress. BMJ Open. 2016;6(11):e012300. doi: 10.1136/bmjopen-2016-012300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corrigan PW, Druss BG, Perlick DA. The impact of mental illness stigma on seeking and participating in mental health care. Psychol Sci Public Interes. 2014;15(2):37–70. doi: 10.1177/1529100614531398. [DOI] [PubMed] [Google Scholar]
- 17.Shapiro S, de Sousa S, Jazaieri H. Mindfulness, mental health, and positive psychology. In: Ivtzan I, Lomas T, editors. Mindfulness in positive psychology. New York: Routledge; 2016. [Google Scholar]
- 18.Carmody J, Baer RA. Relationships between mindfulness practice and levels of mindfulness, medical and psychological symptoms and well-being in a mindfulness-based stress reduction program. J Behav Med. 2008;31(1):23–33. doi: 10.1007/s10865-007-9130-7. [DOI] [PubMed] [Google Scholar]
- 19.Carlbring P, Andersson G, Cuijpers P, Riper H, Hedman-Lagerlöf E. Internet-based vs. face-to-face cognitive behavior therapy for psychiatric and somatic disorders: an updated systematic review and meta-analysis. Cogn Behav Ther. 2018;47(1):1–18. doi: 10.1080/16506073.2017.1401115. [DOI] [PubMed] [Google Scholar]
- 20.Ebert DD, Van Daele T, Nordgreen T, Karekla M, Compare A, Zarbo C, et al. Internet- and mobile-based psychological interventions: applications, efficacy, and potential for improving mental health. Eur Psychol. 2018;23(2):167–187. doi: 10.1027/1016-9040/a000318. [DOI] [Google Scholar]
- 21.Wallin E, Maathz P, Parling T, Hursti T. Self-stigma and the intention to seek psychological help online compared to face-to-face. J Clin Psychol. 2018;74(7):1207–1218. doi: 10.1002/jclp.22583. [DOI] [PubMed] [Google Scholar]
- 22.Ebert DD, Cuijpers P, Muñoz RF, Baumeister H. Prevention of mental health disorders using internet- and mobile-based interventions: a narrative review and recommendations for future research. Front Psychiatry. 2017;8(116):1–16. doi: 10.3389/fpsyt.2017.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Escoffery C, McCormick L, Bateman K. Development and process evaluation of a web-based smoking cessation program for college smokers: innovative tool for education. Patient Educ Couns. 2004;53(2):217–225. doi: 10.1016/S0738-3991(03)00163-0. [DOI] [PubMed] [Google Scholar]
- 24.Ryan ML, Shochet IM, Stallman HM. Universal online interventions might engage psychologically distressed university students who are unlikely to seek formal help. Adv Ment Heal. 2010;9(1):73–83. doi: 10.5172/jamh.9.1.73. [DOI] [Google Scholar]
- 25.Spijkerman MPJ, Pots WTM, Bohlmeijer ET. Effectiveness of online mindfulness-based interventions in improving mental health: a review and meta-analysis of randomised controlled trials. Clin Psychol Rev. 2016;45:102–114. doi: 10.1016/j.cpr.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 26.Jayewardene WP, Lohrmann DK, Erbe RG, Torabi MR. Effects of preventive online mindfulness interventions on stress and mindfulness: a meta-analysis of randomized controlled trials. Prev Med Rep. 2017;5:150–159. doi: 10.1016/j.pmedr.2016.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Messer D, Horan JJ, Turner W, Weber W. The effects of internet-delivered mindfulness training on stress, coping, and mindfulness in university students. AERA Open. 2016;2(1):1–8.
- 28.Nguyen-Feng VN, Greer CS, Frazier P. Using online interventions to deliver college student mental health resources: evidence from randomized clinical trials. Psychol Serv. 2017;14(4):481–489. doi: 10.1037/ser0000154. [DOI] [PubMed] [Google Scholar]
- 29.Kvillemo P, Brandberg Y, Bränström R. Feasibility and outcomes of an internet-based mindfulness training program: a pilot randomized controlled trial. JMIR Ment Health. 2016;3(3):e33. doi: 10.2196/mental.5457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Räsänen P, Lappalainen P, Muotka J, Tolvanen A, Lappalainen R. An online guided ACT intervention for enhancing the psychological wellbeing of university students: a randomized controlled clinical trial. Behav Res Ther. 2016;78:30–42. doi: 10.1016/j.brat.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 31.Levin ME, Haeger JA, Pierce BG, Twohig MP. Web-based acceptance and commitment therapy for mental health problems in college students: a randomized controlled trial. Behav Modif. 2017;41(1):141–162. doi: 10.1177/0145445516659645. [DOI] [PubMed] [Google Scholar]
- 32.Baumeister H, Reichler L, Munzinger M, Lin J. The impact of guidance on Internet-based mental health interventions — a systematic review. Internet Interv. 2014;1(4):205–215. doi: 10.1016/j.invent.2014.08.003. [DOI] [Google Scholar]
- 33.Levin ME, Hayes SC, Pistorello J, Seeley JR. Web-based self-help for preventing mental health problems in universities: comparing acceptance and commitment training to mental health education. J Clin Psychol. 2016;72(3):207–225. doi: 10.1002/jclp.22254. [DOI] [PubMed] [Google Scholar]
- 34.Forbes L, Gutierrez D, Johnson SK. Investigating adherence to an online introductory mindfulness program. Mindfulness (N Y) 2018;9(1):271–282. doi: 10.1007/s12671-017-0772-4. [DOI] [Google Scholar]
- 35.Berger T, Caspar F, Richardson R, Kneubühler B, Sutter D, Andersson G. Internet-based treatment of social phobia: a randomized controlled trial comparing unguided with two types of guided self-help. Behav Res Ther. 2011;49(3):158–169. doi: 10.1016/j.brat.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 36.Rheker J, Andersson G, Weise C. The role of “on demand” therapist guidance vs. no support in the treatment of tinnitus via the internet: a randomized controlled trial. Internet Interv. 2015;2(2):189–199. doi: 10.1016/j.invent.2015.03.007. [DOI] [Google Scholar]
- 37.Harrer M, Adam SH, Baumeister H, Cuijpers P, Karyotaki E, Auerbach RP, et al. Internet interventions for mental health in university students: a systematic review and meta-analysis. Int J Methods Psychiatr Res. 2019;28(2):e1759. doi: 10.1002/mpr.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kraemer HC, Wilson T, Fairburn CG, Argas WS. Mediators and moderators of treatment effects in randomized clinical trials. Arch Gen Psychiatry. 2002;59:877–883. doi: 10.1001/archpsyc.59.10.877. [DOI] [PubMed] [Google Scholar]
- 39.Gu J, Strauss C, Bond R, Cavanagh K. How do mindfulness-based cognitive therapy and mindfulness-based stress reduction improve mental health and wellbeing? A systematic review and meta-analysis of mediation studies. Clin Psychol Rev. 2015;37:1–12. doi: 10.1016/j.cpr.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 40.Lin J, Klatt L-I, McCracken LM, Baumeister H. Psychological flexibility mediates the effect of an online-based acceptance and commitment therapy for chronic pain. Pain. 2018;159(4):663–672. doi: 10.1097/j.pain.0000000000001134. [DOI] [PubMed] [Google Scholar]
- 41.Guendelman S, Medeiros S, Rampes H. Mindfulness and emotion regulation: insights from neurobiological, psychological, and clinical studies. Front Psychol. 2017;8:1–23. doi: 10.3389/fpsyg.2017.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coffey KA, Hartman M, Fredrickson BL. Deconstructing mindfulness and constructing mental health: understanding mindfulness and its mechanisms of action. Mindfulness (N Y). 2010;1(4):235–253. doi: 10.1007/s12671-010-0033-2. [DOI] [Google Scholar]
- 43.Richards D. Self-help: empowering service users or aiding cash strapped mental health services? J Ment Health. 2004;13(2):117–123. doi: 10.1080/09638230410001669246. [DOI] [Google Scholar]
- 44.Küchler A-M, Peip K, Preuss D, Ebert DD, Baumeister H. Efficacy of the internet- and mobile-based intervention “StudiCare Mindfulness” for college students: a randomized controlled trial. Prep.
- 45.StudiCare [Internet]. [cited 2019 Jan 21]. Available from: http://www.studicare.com/.
- 46.Harrer M, Adam SH, Fleischmann RJ, Baumeister H, Auerbach R, Bruffaerts R, et al. Effectiveness of an internet- and app-based intervention for college students with elevated stress: randomized controlled trial. J Med Internet Res. 2018;20(4):1–16. doi: 10.2196/jmir.9293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fleischmann RJ, Harrer M, Zarski A-C, Baumeister H, Lehr D, Ebert DD. Patients’ experiences in a guided internet- and app-based stress intervention for college students: a qualitative study. Internet Interv. 2018;12:130–140. doi: 10.1016/j.invent.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ebert DD, Franke M, Kählke F, Küchler A-M, Bruffaerts R, Mortier P, et al. Increasing intentions to use mental health services among university students. Results of a pilot randomized controlled trial within the World Health Organization’s World Mental Health International College Student Initiative. Int J Methods Psychiatr Res. 2019;28(2):e1754. doi: 10.1002/mpr.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mortier P, Auerbach RP, Alonso J, Bantjes J, Benjet C, Cuijpers P, et al. Suicidal thoughts and behaviors among first-year college students: results from the WMH-ICS Project. J Am Acad Child Adolesc Psychiatry. 2018;57(4):263–273.e1. doi: 10.1016/j.jaac.2018.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Küchler A-M, Albus P, Ebert DD, Baumeister H. Effectiveness of an internet-based intervention for procrastination in college students (StudiCare Procrastination): study protocol of a randomized controlled trial. Internet Interv. 2019;17:100245. doi: 10.1016/j.invent.2019.100245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.The WHO World Mental Health International College Student (WMH-ICS) Initiative [Internet]. [cited 2019 Jan 21]. Available from: https://www.hcp.med.harvard.edu/wmh/college_student_survey.php.
- 52.Caring Universities [Internet]. [cited 2019 Jan 21]. Available from: https://caring-universities.com/.
- 53.Schulz KF, Altman DG, Moher D. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. doi: 10.1136/bmj.c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Proudfoot J, Klein B, Barak A, Carlbring P, Cuijpers P, Lange A, et al. Establishing guidelines for executing and reporting internet intervention research. Cogn Behav Ther. 2011;40(2):82–97. doi: 10.1080/16506073.2011.573807. [DOI] [PubMed] [Google Scholar]
- 55.Chan AW, Tetzlaff JM, Altman DG, Laupacis A, Gøtzsche PC, Krleža-Jerić K, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;158(3):200–209. doi: 10.7326/0003-4819-158-3-201302050-00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Walach H, Buchheld N, Buttenmüller V, Kleinknecht N, Schmidt S. Measuring mindfulness - the Freiburg Mindfulness Inventory (FMI) Pers Individ Dif. 2006;40(8):1543–1555. doi: 10.1016/j.paid.2005.11.025. [DOI] [Google Scholar]
- 57.Schultchen D, Küchler A-M, Schillings C, Weineck F, Karabatsiakis A, Ebert DD, et al. Effectiveness of a guided online mindfulness-focused intervention in a student population: study protocol for a randomised control trial. BMJ Open. 2020;10(3):e032775. doi: 10.1136/bmjopen-2019-032775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sealed Envelope Ltd. Create a blocked randomisation list. [Internet]. 2019 [cited 2019 Jun 6]. Available from: https://www.sealedenvelope.com/.
- 59.Hayes SC, Strosahl KD, Wilson KG. Acceptance and commitment therapy: an experiential approach to behavior change. New York: Guilford Press; 1999. [Google Scholar]
- 60.Kaluza G. Stressbewältigung - Trainingsmanual zur psychologischen Gesundheitsförderung. Berlin Heidelberg: Springer-Verlag; 2011. [Google Scholar]
- 61.Minddistrict [Internet]. [cited 2019 Jan 21]. Available from: https://www.minddistrict.com/.
- 62.Fry JP, Neff RA. Periodic prompts and reminders in health promotion and health behavior interventions: systematic review. J Med Internet Res. 2009;11(2):e16. doi: 10.2196/jmir.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Webb TL, Joseph J, Yardley L, Michie S. Using the Internet to promote health behavior change: a systematic review and meta-analysis of the impact of theoretical basis, use of behavior change techniques, and mode of delivery on efficacy. J Med Internet Res. 2010;12(1):e4. doi: 10.2196/jmir.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Unipark [Internet]. [cited 2019 Jan 21]. Available from: https://www.unipark.com/.
- 65.Heidenreich T, Ströhle G, Michalak J. Achtsamkeit: Konzeptuelle Aspekte und Ergebnisse zum Freiburger Achtsamkeitsfragebogen. Verhaltenstherapie. 2006;16(1):33–40. doi: 10.1159/000091521. [DOI] [Google Scholar]
- 66.Kroenke K, Spitzer RL. The PHQ-9: a new depression diagnostic and severity measure. Psychiatr Ann. 2002;32(9):509–515. doi: 10.3928/0048-5713-20020901-06. [DOI] [Google Scholar]
- 67.Kroenke K, Spitzer RL, Williams JBW. The PHQ-9. Validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Erbe D, Eichert HC, Rietz C, Ebert D. Interformat reliability of the patient health questionnaire: validation of the computerized version of the PHQ-9. Internet Interv. 2016;5:1–4. doi: 10.1016/j.invent.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Spitzer RL, Kroenke K, Williams JB, Löwe B. The GAD-7. A brief measure for assessing generalized anxiety disorder. Arch Intern Med. 2006;166(10):1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 70.Löwe B, Decker O, Müller S, Brähler E, Schellberg D, Herzog W, et al. Validation and standardization of the generalized anxiety disorder screener (GAD-7) in the general population. Med Care. 2008;46(3):266–274. doi: 10.1097/MLR.0b013e318160d093. [DOI] [PubMed] [Google Scholar]
- 71.Cohen S, Williamson G. Perceived stress in a probability sample of the United States. In: Spacapan S, Oskamp S, editors. The social psychology of health. Newbury Park: Sage Publications; 1988. pp. 31–68. [Google Scholar]
- 72.Warttig SL, Forshaw MJ, South J, White AK. New, normative, English-sample data for the Short Form Perceived Stress Scale (PSS-4) J Health Psychol. 2013;18(12):1617–1628. doi: 10.1177/1359105313508346. [DOI] [PubMed] [Google Scholar]
- 73.World Health Organization, Regional Office for Europe P, Unit R. World Health Organization info package: mastering depression in primary care. Frederiksborg; 1998.
- 74.Topp CW, Østergaard SD, Søndergaard S, Bech P. The WHO-5 well-being index: a systematic review of the literature. Psychother Psychosom. 2015;84(3):167–176. doi: 10.1159/000376585. [DOI] [PubMed] [Google Scholar]
- 75.Matsushita M, Adachi H, Arakida M, Namura I, Takahashi Y, Miyata M, et al. Presenteeism in college students: reliability and validity of the presenteeism scale for students. Qual Life Res. 2011;20(3):439–446. doi: 10.1007/s11136-010-9763-9. [DOI] [PubMed] [Google Scholar]
- 76.Porges S. Body Perception Questionnaire. Laboratory of Development Assessment, University of Maryland. 1993. [Google Scholar]
- 77.Cabrera A, Kolacz J, Pailhez G, Bulbena-Cabre A, Bulbena A, Porges SW. Assessing body awareness and autonomic reactivity: factor structure and psychometric properties of the Body Perception Questionnaire-Short Form (BPQ-SF) Int J Methods Psychiatr Res. 2018;27(2):1–12. doi: 10.1002/mpr.1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ladwig I, Rief W, Nestoriuc Y. Welche Risiken und Nebenwirkungen hat Psychotherapie? - Entwicklung des Inventars zur Erfassung Negativer Effekte von Psychotherapie (INEP) Verhaltenstherapie. 2014;24(4):252–263. doi: 10.1159/000367928. [DOI] [Google Scholar]
- 79.Larsen DL, Attkisson CC, Hargreaves WA, Nguyen TD. Assessment of client/patient satisfaction: development of a general scale. Eval Program Plann. 1979;2(3):197–207. doi: 10.1016/0149-7189(79)90094-6. [DOI] [PubMed] [Google Scholar]
- 80.Boß L, Lehr D, Reis D, Vis C, Riper H, Berking M, et al. Reliability and validity of assessing user satisfaction with web-based health interventions. J Med Internet Res. 2016;18(8):1–13. doi: 10.2196/jmir.5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kriz D, Nübling R, Steffanowski A, Rieger J, Schmidt J. Patientenzufriedenheit: Psychometrische Reanalyse des ZUF-8. DRV Schriften. 2008;77:84–85. [Google Scholar]
- 82.Schwarzer R, Jerusalem M. Generalized self-efficacy scale. In: Weinman J, Wright S, Johnston M, editors. Measures in health psychology: a user’s portfolio causal and control beliefs. UK: NFER-Nelson; 1995. pp. 35–37. [Google Scholar]
- 83.Schwarzer R, Mueller J, Greenglass E. Assessment of perceived general self-efficacy on the internet: data collection in cyberspace. Anxiety Stress Coping. 1999;12(2):145–161. doi: 10.1080/10615809908248327. [DOI] [Google Scholar]
- 84.China C, Hansen LB, Gillanders DT, Benninghoven D. Concept and validation of the German version of the Cognitive Fusion Questionnaire (CFQ-D) J Context Behav Sci. 2018;9:30–35. doi: 10.1016/j.jcbs.2018.06.003. [DOI] [Google Scholar]
- 85.Gross JJ, John OP. Individual differences in two emotion regulation processes: implications for affect, relationships, and well-being. J Pers Soc Psychol. 2003;85(2):348–362. doi: 10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- 86.Abler B, Kessler H. Emotion Regulation Questionnaire – Eine deutschsprachige Fassung des ERQ von Gross und John. Diagnostica. 2009;55(3):144–152. doi: 10.1026/0012-1924.55.3.144. [DOI] [Google Scholar]
- 87.Bagby RM, Parker JDA, Taylor GJ. The twenty-item Toronto Alexithymia scale—I. Item selection and cross-validation of the factor structure. J Psychosom Res. 1994;38(1):23–32. doi: 10.1016/0022-3999(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 88.Kupfer J, Brosig B, Brähler E. Toronto-Alexithymie-Skala-20 (TAS-20) Göttingen: Hogrefe; 2001. [Google Scholar]
- 89.Parker JDA, Taylor GJ, Bagby RM. The 20-Item Toronto Alexithymia Scale: III. Reliability and factorial validity in a community population. J Psychosom Res. 2003;55(3):269–275. doi: 10.1016/S0022-3999(02)00578-0. [DOI] [PubMed] [Google Scholar]
- 90.Ebert DD, Gollwitzer M, Riper H, Cuijpers P, Baumeister H, Berking M. For whom does it work? Moderators of outcome on the effect of a transdiagnostic Internet-based maintenance treatment after inpatient psychotherapy: randomized controlled trial. J Med Internet Res. 2013;15(10):1–17. doi: 10.2196/jmir.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Devilly GJ, Borkovec TD. Psychometric properties of the credibility/expectancy questionnaire. J Behav Ther Exp Psychiatry. 2000;31(2):73–86. doi: 10.1016/S0005-7916(00)00012-4. [DOI] [PubMed] [Google Scholar]
- 92.Kelders SM, Kok RN, Ossebaard HC, Van Gemert-Pijnen JEWC. Persuasive system design does matter: a systematic review of adherence to web-based interventions. J Med Internet Res. 2012;14(6):e152. doi: 10.2196/jmir.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Faul F, Erdfelder E, Lang A-G, Buchner A. G*Power: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–191. doi: 10.3758/BF03193146. [DOI] [PubMed] [Google Scholar]
- 94.Jacobson NS, Truax P. Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol. 1991;59(1):12–19. doi: 10.1037/0022-006X.59.1.12. [DOI] [PubMed] [Google Scholar]
- 95.Altman DG. Confidence intervals for the number needed to treat. BMJ. 1998;317(7168):1309–1312. doi: 10.1136/bmj.317.7168.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cook RJ, Sackett DL. The number needed to treat: a clinically useful measure of treatment effect. BMJ. 1995;310(6977):452–454. doi: 10.1136/bmj.310.6977.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cole DA, Maxwell SE. Testing mediational models with longitudinal data: questions and tips in the use of structural equation modeling. J Abnorm Psychol. 2003;112(4):558–577. doi: 10.1037/0021-843X.112.4.558. [DOI] [PubMed] [Google Scholar]
- 98.Kazdin AE. Mediators and mechanisms of change in psychotherapy research. Annu Rev Clin Psychol. 2007;3(1):1–27. doi: 10.1146/annurev.clinpsy.3.022806.091432. [DOI] [PubMed] [Google Scholar]
- 99.Domhardt M, Geßlein H, von Rezori RE, Baumeister H. Internet- and mobile-based interventions for anxiety disorders: a meta-analytic review of intervention components. Depress Anxiety. 2018;19:1–12. doi: 10.1002/da.22860. [DOI] [PubMed] [Google Scholar]
- 100.Gupta S. Intention-to-treat concept: a review. Perspect Clin Res. 2011;2(3):109. doi: 10.4103/2229-3485.83221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cavanagh K, Churchard A, O’Hanlon P, Mundy T, Votolato P, Jones F, et al. A randomised controlled trial of a brief online mindfulness-based intervention in a non-clinical population: replication and extension. Mindfulness (N Y) 2018;9(4):1191–1205. doi: 10.1007/s12671-017-0856-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sagon AL, Danitz SB, Suvak MK, Orsillo SM. The Mindful Way through the Semester: evaluating the feasibility of delivering an acceptance-based behavioral program online. J Context Behav Sci. 2018;9:36–44. doi: 10.1016/j.jcbs.2018.06.004. [DOI] [Google Scholar]
- 103.Rozental A, Boettcher J, Andersson G, Schmidt B, Carlbring P. Negative effects of internet interventions: a qualitative content analysis of patients’ experiences with treatments delivered online. Cogn Behav Ther. 2015;44:1–14. doi: 10.1080/16506073.2014.946077. [DOI] [PubMed] [Google Scholar]
- 104.Boettcher J, Rozental A, Andersson G, Carlbring P. Side effects in Internet-based interventions for social anxiety disorder. Internet Interv. 2014;1(1):3–11. doi: 10.1016/j.invent.2014.02.002. [DOI] [Google Scholar]
- 105.Rozental A, Andersson G, Boettcher J, Ebert DD, Cuijpers P, Knaevelsrud C, et al. Consensus statement on defining and measuring negative effects of Internet interventions. Internet Interv. 2014;1(1):12–19. doi: 10.1016/j.invent.2014.02.001. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All principal investigators will be given full access to the data sets with the exception of MM. He will be responsible for the blinded analysis of the primary and secondary outcomes and hence will only receive a blinded version of the data needed for this analysis. The data set will be stored on password-protected servers of Ulm University with restricted access. External researchers may get access to the final trial dataset (from HB) on request depending on to be specified data security and data exchange regulation agreements. They can obtain the informed consent form in the same way. To ensure confidentiality, data dispersed to any investigator or researcher will be blinded of any identifying participant information. Anonymized results will be published in peer-reviewed journals and presented on international conferences.


