Abstract
Both phototherapy via photocatalysts and physical puncture by artificial nanostructures are promising substitutes for antibiotics when treating drug-resistant bacterial infectious diseases. However, the photodynamic therapeutic efficacy of photocatalysts is seriously restricted by the rapid recombination of photogenerated electron–hole pairs. Meanwhile, the nanostructures of physical puncture are limited to two-dimensional (2D) platforms, and they cannot be fully used yet. Thus, this research developed a synergistic system of Ag3PO4 nanoparticles (NPs), decorated with black urchin-like defective TiO2 (BU–TiO2-X/Ag3PO4). These NPs had a decreased bandgap compared to BU-TiO2-X, and BU-TiO2-X/Ag3PO4 (3:1) exhibited the lowest bandgap and the highest separation efficiency for photogenerated electron–hole pairs. After combination with BU-TiO2-X, the photostability of Ag3PO4 improved because the oxygen vacancy of BU-TiO2-X retards the reduction of Ag+ in Ag3PO4 into Ag0, thus reducing its toxicity. In addition, the nanospikes on the surface of BU-TiO2-X can, from all directions, physically puncture bacterial cells, thus assisting the hybrid's photodynamic therapeutic effects, alongside the small amount of Ag+ released from Ag3PO4. This achieves synergy, endowing the hybrid with high antibacterial efficacy of 99.76 ± 0.15% and 99.85 ± 0.09% against Escherichia coli and Staphylococcus aureus, respectively, after light irradiation for 20 min followed by darkness for 12 h. It is anticipated that these findings may bring new insight for developing synergistic treatment strategies against bacterial infectious diseases or pathogenic bacterial polluted environments.
Keywords: Antibacterial, Ag3PO4, Defective TiO2, Photocatalytic, Puncture
Graphical abstract
Highlights
-
•
BU-TiO2-X/Ag3PO4 (3:1) hybrid improved the photostability of Ag3PO4.
-
•
BU-TiO2-X/Ag3PO4 (3:1) hybrid exhibited outstanding photodynamic therapeutic effects.
-
•
The nanospikes from all directions on the BU-TiO2-X physically punctured bacterial cells.
-
•
The physical puncture combined with the Ag+ released by Ag3PO4 had long-term bacteriostatic efficacy.
1. Introduction
By 2050, the long-term overuse of antibiotics may lead to a tenfold increase in the rate of mortality from incurable infections [1]. Since bacteria have become resistant to antibiotics, new antibacterial materials and techniques are emerging with increasing speed. It takes only two weeks for bacteria to develop resistance to new antibiotics that have taken several years, and great expense, to be developed [[2], [3], [4]]. Accordingly, innovative, antibiotics-free treatment strategies are expected; bacteria would either have no drug resistance to these treatments or could not develop resistance in a short time. Among new therapeutic methods [[5], [6], [7], [8], [9]], photodynamic therapy (PDT) can effectively inactivate bacteria via the reactive oxygen species (ROS) quickly produced by certain photo-responsive materials under light irradiation. Titanium dioxide (TiO2) is one of most common semiconductor photocatalysts, but its large bandgap of 3.0–3.2 eV restricts its phototherapy application because it can only be excited by ultraviolet (UV) light [10,11]. Although black TiO2, due to the lower bandgap and large amount of oxygen vacancy on its surface [12,13], exhibits better photocatalytic performance than untreated TiO2, the fatal flaw of using a mono-photocatalyst for phototherapy is the rapid recombination of electron–hole pairs [14,15], which reduce ROS yields under light irradiation. A heterojunction composed of two or more semiconductors could benefit the transfer of photo-generated electrons between different components, thus relieving this issue to some extent [[16], [17], [18]].
Trisilver phosphate (Ag3PO4), an important semiconductor with an indirect bandgap (2.36 eV), is attracting the increasing attention in the photocatalytic field because of its great oxidation capacity [19]. However, the reduction power of Ag3PO4 under visible light irradiation is too weak to conduct a photocatalytic reaction, which causes serious photo-corrosion of the Ag3PO4 [[20], [21]]. However, a sacrificial reagent, such as silver nitrate (AgNO3) can suppress the Ag+ of Ag3PO4 to form Ag0, which is conducive to the photostability of Ag3PO4 [22,23]. However, it is unrealistic to provide a sacrificial agent for Ag3PO4 in biologic applications. The Ag3PO4 particle also maintains a relatively large size (0.5–2 μm), which further affects its photocatalytic efficiency because it has a low specific surface area [22]. Loading Ag3PO4 on other semiconductors or two-dimensional (2D) materials can effectively enhance its photostability of and reduce its size [[24], [25], [26]], whereas Ag3PO4-based traditional heterojunction and Z-scheme heterojunction either weaken its oxidation/reduction capacity or decrease the number of photoexcited carriers, which also weakens the photocatalytic performance [[27], [28], [29], [30], [31], [32], [33], [34], [35]].
Recent studies have disclosed that single-mode treatment cannot achieve ideal therapeutic effects for bacterial infectious diseases [36]. Higher concentrations of ROS are essential for effective photodynamic bacteria-killing [37,38], but large amounts of ROS are harmful for normal tissues [7,36]. In addition, both the lifespan and action radius of ROS are very finite [39,40], making them effective only for killing bacteria with which they come into direct contact. A higher photothermal temperature is necessary for inactivating bacteria effectively during photothermal therapy, but such a temperature will inevitably damage normal cells and tissues [6,41]. Therefore, synergistic action through two or more therapeutic modes has been proven to be more effective for killing bacteria—such as photodynamic therapy paired with Ag ions [42,43] or with photothermal action [[44], [45], [46], [47]], as well as combined photodynamic and photothermal therapy paired with Zn2+ [48], Cu2+ [49], etc.
Physical puncture has also been reported to be an effective bacteria-killing mode, which neither produces bacterial resistance nor damages normal tissues and cells [[50], [51], [52]]. A few studies have been carried out to determine whether nanospikes or nanorods on a planar substrate could puncture the bacterial cell envelope [50,53,54]. However, these 2D approaches are not suitable for solution-based three-dimensional (3D) environment. As far as the current authors know, no reports have considered the synergy of phototherapy and mechanical puncture without a planar substrate. Therefore, since TiO2 has various morphologies, including one-dimensional (1D) nanotubes [55], nanorods [56], 2D nanosheets [57], and 3D nanoflowers [58], in addition to its aforementioned outstanding photocatalytic performance, the authors hypothesized that photodynamic therapy and physical puncture may be combined to achieve satisfactory synergistic efficacy without adverse effects.
Based on this hypothesis, the researchers constructed a heterojunction system, in which Ag3PO4 nanoparticles (NPs) were grown in situ on black, urchin-like TiO2-X. This created NPs have large amount of surface oxygen defects (BU–TiO2-X). The synthesis process of BU-TiO2-X/Ag3PO4 is schematically illustrated in Scheme 1. Surface deficiencies such as oxygen vacancy on the surface of urchin-like TiO2 produced by NaBH4, can serve as the nucleation site of Ag3PO4, and a large number of nanospikes on the surface of black urchin-like TiO2-X restricted the growth of Ag3PO4, leading to a higher specific surface area than pure and unloaded Ag3PO4 [15,58,59]. Therefore, black urchin-like TiO2-X/Ag3PO4 NPs, under visible light, can inactivate bacteria in a short period of time. In addition, the extensive nanospikes of the black urchin-like TiO2-X NPs, combined with the Ag+ released by Ag3PO4 in water, inhibit bacterial growth in long term [25].
Scheme 1.
The synthesis process of BU-TiO2-X/Ag3PO4.
2. Experimental
2.1. Materials
Anhydrous ethanol, silver nitrate (AgNO3), disodium hydrogen phosphate (Na2HPO4), hydrogen peroxide (H2O2, 30%) and sodium boron hydride (NaBH4, 98%) were all purchased from Aladdin (Shanghai). Nitric acid (HNO3, 65.0%–69.0%) and sodium hydroxide (NaOH, 96%) were purchased from Jiangtian (Tianjin). TiO2 (Macklin P25) was purchased from Macklin (Shanghai).
2.2. Urchin-like TiO2 NPs fabrication
Urchin-like TiO2 NPs were synthesized via a two-stage hydrothermal reaction with slight modifications [58]. Briefly, TiO2 (Macklin P25) powders (1 g) were dispersed in 60 mL of a NaOH aqueous solution (5 M), continuously stirred for 30 min, and then transferred into a Teflon-lined, stainless-steel autoclave and heated at 120 °C for 24 h. After reaction, the precipitates were collected by centrifugation, and subsequently washed with deionized (DI) water five times. These precipitates were dried at 60 °C in a vacuum for further use. The dried precipitates (0.3 g) were dispersed in a mixture of 57 mL of the NaOH aqueous solution (1 M) and 3 mL of a H2O2 (30%) solution. The suspension was stirred magnetically, ultrasonicated for 30 min, and then transferred into a Teflon-lined, stainless-steel autoclave at 150 °C for 8 h. The precipitates were again collected by centrifugation, washed with DI water five times, and dried at 60 °C in a vacuum to generate urchin-like sodium titanate particles. The urchin-like particles were then treated with excess HNO3 solution (0.1 M) with continuous magnetic stirring to produce urchin-like hydrogen titanate particles. The urchin-like hydrogen titanate particles were then washed with DI water five times and calcined at 400 °C for 1 h to produce the urchin-like TiO2 particles.
2.3. Black urchin-like TiO2-X NP fabrication
0.5 g of urchin-like TiO2 powders were mixed with 0.5 g of NaBH4, and the mixture was thoroughly ground for 30 min under the infrared baking lamp [60,61]. The mixture then was placed in a tubular furnace and heated at a rate of 5 °C min−1 to 350 °C, and then kept for 2 h under an argon atmosphere with subsequent furnace cooling to room temperature. The collected powders were rinsed with ethanol and DI water repeatedly.
2.4. Black urchin-like TiO2-X/Ag3PO4 NPs fabrication
The black urchin-like TiO2-X/Ag3PO4 NPs were synthesized via the following process. Briefly, 0.4 g of synthesized black urchin-like TiO2-X powders were added to 100 mL of ethanol under ultrasonication. A stoichiometric amount of AgNO3 was mixed with the black urchin-like TiO2-X suspension and vigorous stirred for 1 h. During the stirring process, stoichiometric Na2HPO4 dissolved in 20 mL of distilled water, was added, drop by drop, into the above dispersion. The mixture was then continuously stirred for 3 h. The obtained precipitates were separated by centrifugation and washed eight times with ethanol and DI water. The prepared powders were then dried overnight in a vacuum oven at 60 °C. The entire synthesis was carried out in the dark. Pure and unloaded Ag3PO4 was prepared under identical experimental conditions without the presence of black urchin-like TiO2-X.
2.5. Characterizations
The crystal structures of TiO2 NPs and Ag3PO4 were determined using an X-ray diffractometer (XRD; D8 Advanced, Germany) with Cu-Kα as the radiation source (λ = 1.5406 Å). Samples and bacteria morphologies were observed via scanning electron microscope (SEM; Hitachi S-4800, Japan) analysis equipped with an energy-dispersive X-ray spectroscopy (EDS; Oxford X-max20, UK) and a transmission electron microscopy (TEM; JEM-2100F, Japan). The elements composition of the samples were measured via X-ray photoelectron spectroscopy (XPS; Escalab250Xi, Thermofisher, USA), and Raman spectra were conducted by using a Thermo-scientific DXR spectrometer in the range of 100–1000 cm−1.The ultraviolet–visible (UV–Vis) diffuse reflectance (DRS) optical properties of the samples were measured via the Japanese Shimadzu UV-2700 spectrophotometer, with the measurement range set to 200–800 nm and using barium sulfate (BaSO4) as a reference. The steady state photoluminescence (PL) of the samples was examined via a fluorolog-3 fluorescence spectrophotometer (HORIBA), with an excitation wavelength of 365 nm, to illustrate the separation and recombination efficiency of photogenerated electron–hole pairs on irradiated nano-semiconductors. The photocatalytic properties of the samples were investigated via electron spin resonance (ESR; JES-FA200) spectroscopy to detect the ROS by a capture agent (5,5-Dimethyl-1-pyrroline N-oxide).
2.6. Photo-electrochemical performance
Transient photocurrent response measurement and linear sweep voltammetry (LSV) were carried out to further illustrate the photocatalytic performance of the samples under visible light irradiation. Samples were tested on an electrochemical workstation (Gamry Instrument, INTERFACE 1000) in a standard three-electrode system, and the electrolyte was a 0.5 mol L−1 sodium sulfate (Na2SO4) aqueous solution. The photocatalyst samples were created as follows: 8 mg of the samples and 80 μL Nafion were added to 2 mL of ethanol to create a solution under ultrasonication. Then 80 μL of the mixed solution were added to a 10 × 10 mm indium tin oxide (ITO) glass electrode to make a coating.
2.7. Ag ions release
Ag3PO4 and BU-TiO2-X/Ag3PO4 samples soaked in 100 mL of DI water (pH = 7.4), were stored in darkness at 37 °C for 21 days. 3 mL of the solutions were sucked out by a pipette gun at 1, 2, 3, 5, 7, 10, 14, and 21 days and added another 3 mL of DI water as supplementary. Ag+ released concentration was detected via inductively coupled plasma-optical emission spectrometry (ICP-OES; ICAP7000 series).
2.8. In vitro antimicrobial tests
The samples' antimicrobial performances were tested against typical bacteria—Staphylococcus aureus (S. aureus) and Escherichia coli (E. coli)—through the spread plate method. The bacterial suspension was cultured separately in sterile Luria−Bertain (LB) media at 37 °C and was then diluted with LB media to 107 CFUmL-1 before use. After being mixed with 200 μL of diluted bacterial suspension, all samples (control, BU-TiO2-X, Ag3PO4, BU-TiO2-X/Ag3PO4 (6:1), BU-TiO2-X/Ag3PO4 (3:1), BU-TiO2-X/Ag3PO4 (1:1)), at concentrations of 200 ppm, were added to 96-well plates. Each sample was irradiated with double solar intensity for 20 min via the Xenon lamplight to simulate natural light. All samples have matched to samples without irradiation. Moreover, the samples, including the control, BU-TiO2-X, Ag3PO4, BU-TiO2-X/Ag3PO4 (3:1) and BU-TiO2-X/Ag3PO4 (3:1) +light (20 min), were mixed with the bacterial suspension and placed in a constant-temperature shaker set to 160 rpm at 37 °C for 12 h without irradiation. In the end, each 20 μL amount of diluted solution was spread, in a well-distributed fashion, on the agar plate, which was then cultured at 37 °C for 24 h. The antibacterial rate of each plate was calculated by measuring the number of bacterial colonies on the plate according to the following equation:
| Antibacterial ratio (%) = (number of CFUs in control sample - number of CFUs in experimental sample) / (number of CFUs in control sample) × 100% | (1) |
The bacterial morphology changes were observed via SEM. After being treated with the above procedures, the 96-well plate was deposited for 2 h. Then, the bacteria were fixed for 2 h with 200 μL of 2.5% glutaraldehyde in the dark. Subsequently, handled with 30%, 50%, 70%, 90%, and 100% ethanol solutions were applied to dehydrate bacteria, with each step taking 15 min. The bacterial morphology was observed by SEM after drying.
2.9. Simulation computation methods of cell membrane puncture
Cell membrane penetration was conducted in a Finite Element Modeling (FEM) environment (COMSOL Multiphysics), and the process of a bacterium being punctured was simulated by the model of an inflated shell with isotropic elasticity being punctured by a nanospike. The researchers hypothesized that Young's modulus (E) would encompass the entire cell envelope in these simulations and that the modulus of S. aureus and E. coli should be 30 MPa and 5 MPa, respectively [53,62]. The bacterial turgor pressure (ΔP) can be assessed by the modulus of S. aureus and E. coli, and the ratio of the turgor pressure to the modulus can be considered a constant (E/ΔP~100) [63]. The E. coli model was assumed to have a spherocylindrical shape, with a radius of 250 nm and a length of 2000 nm, while S. aureus model was assumed to have a spherical shape, with a radius of 250 nm, according to the statistical data from the SEM results. The researchers hypothesized that the driving force for bacterial deformation would be the centripetal force of BU-TiO2-X and could be written as:
| Fc = MBUω2R, | (2) |
where MBU is the mass of BU-TiO2-X; ω is the angular velocity; and R is the mean moving radius of BU-TiO2-X in the constant-temperature shaker. Based on the above conditions, the driving force could be calculated and was approximately 150 pN. In addition, both E. coli and S. aureus were punctured by a truncated cone nanospike, the mean diameter was 20 nm.
2.10. In vitro cell viability
Cell cytotoxicity was assessed via the water-soluble tetrazole (2-(2-methoxy-4nitrobenzene)-3-(4-nitrobenzene) -5- (2,4-disulfobenzene) -2H-tetrazole monosodium salt) (CCK-8) method using NIH-3T3 cells. All samples and 96-well plates were disinfected with UV light for 30 min. During the cytotoxicity assay, the samples were dipped in the cell culture medium for one, three, and five days, and the leach liquors (cell density 1 × 105 cellsmL-1) were placed in the 96-well plate and cultured for one, three, and five days in a 5% carbon dioxide (CO2) incubator at 37 °C. Then, 10 μL of the CCK-8 solution were added to the cell culture medium and put in the CO2 incubator for 2.5 h. Finally, the cell viability of each sample was measured through a microplate reader (wavelengths of 450 nm), which can obtain the optical density (OD).
2.11. Statistical analysis
All the quantitative data were analyzed by the one-way ANOVA and expressed as means ± standard deviations with n = 3. P values < 0.05 were considered statistically significant.
3. Results and discussion
3.1. Morphologies and structure
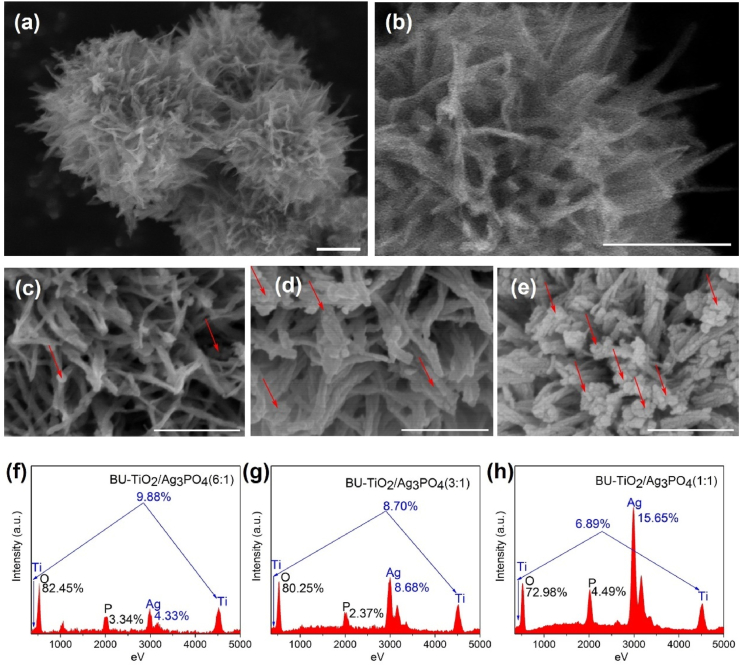
Fig. 1 shows the morphologies of prepared BU-TiO2-X and BU-TiO2-X/Ag3PO4 with different molar ratios of BU-TiO2-X and Ag3PO4 (6:1, 3:1, 1:1). The synthesized BU-TiO2-X particle clearly exhibited an urchin-like structure (Fig. 1(a)) composed of numerous nanospikes (Fig. 1(b)). After in situ growth of Ag3PO4, small nanoparticles appeared uniformly on the surfaces of the nanospikes (marked by red arrows in Fig. 1(c), (d), and (e)), and, as the Ag+ content increased during the in situ loading process, the number of Ag3PO4 nanoparticles also increased, as proven by the corresponding EDS, shown in Fig. 1(f), (g), and (h). The average size of these nanoparticles was about 10 nm. In contrast, the synthesized Ag3PO4 exhibited a far larger size of over 200 nm under the same conditions but without BU-TiO2-X (Fig. S1), indicating that the BU-TiO2-X nanospikes provided many nucleation sites for Ag3PO4 and facilitated the distribution of Ag3PO4 on BU-TiO2-X.
Fig. 1.
Morphologies and elements of synthesized materials: (a) FE-SEM image of BU-TiO2-X; (b) high magnification image of A; (c), (d), (e) FE-SEM images and (f), (g), (h) Energy dispersive spectroscopy of BU-TiO2-X, BU-TiO2-X/Ag3PO4 (6:1, 3:1, 1:1). (Scale bar: 300 nm).
The TEM patterns of U–TiO2, BU-TiO2-X, and BU-TiO2-X/Ag3PO4 are shown in Fig. 2(b). The Ag3PO4 nanoparticles were successfully loading on the surface of nanospikes, which is in accordance with the SEM results. The areas marked by white, dashed circles represent high-resolution TEM (HRTEM) images of U–TiO2, and they reveal that the lattice spacings of 0.354 nm and 0.190 nm corresponded to the anatase (101) plane and (200) plane, respectively [64] (Fig. 2(b)). The HRTEM image of BU-TiO2-X showed that the lattice spacing of 0.354 nm corresponded to the anatase (101) plane, while the HRTEM image of BU-TiO2-X/Ag3PO4 showed that lattice spacings of 0.190 nm and 0.245 nm corresponded to the anatase (200) plane and the Ag3PO4 (211) plane [64]. Some clearly observable oxygen defects and disorders are outlined by the yellow circles. The introduction of surface oxygen defects and disorders restrained the recombination of photogenerated electron–hole pairs via carrier trapping.
Fig. 2.
The micromorphology, crystal structure, and phase composition of synthesized materials: (a) TEM images (Scale bar: 20 nm) and (b) HRTEM images (Scale bar: 2 nm) of U–TiO2, BU-TiO2-X and BU-TiO2-X/Ag3PO4(3:1); (c)XRD patterns of U–TiO2, BU-TiO2-X, Ag3PO4 and BU-TiO2-X/Ag3PO4 (6:1, 3:1, 1:1); (d) Raman spectroscopy of U–TiO2, BU-TiO2-X.
The XRD patterns in Fig. 2(c) show the crystal structure and phase composition of the as-synthesized U–TiO2, BU-TiO2-X, Ag3PO4, and BU-TiO2-X/Ag3PO4 (6:1, 3:1, 1:1) samples. In the XRD pattern for U–TiO2, the characteristic peaks at 2θ values of 25.3°, 37.7°, 48.0°, and 53.8° corresponded to the (101), (004), (200), and (211) crystal planes of the anatase phase [65], indicating that U–TiO2 was successfully formed during the hydrothermal reaction. After U–TiO2 received a reduction treatment in a tubular furnace at 350 °C (Scheme 1), the corresponding peaks became weak and their breadth decreased, indicating that an oxygen-deficient amorphous TiO2−X layer generated, which is in line with the HRTEM results in Fig. 2(b). In the case of the synthesized Ag3PO4 sample, the XRD pattern contained diffraction peaks at 2θ values of 20.8°, 29.7°, 33.3°, 36.6°, 47.8°, 52.7°, 55.0°, 57.2°, and 71.9°, which were assigned to the (110), (200), (210), (211), (310), (222), (320), (321), and (332) crystal planes of Ag3PO4 [19], suggesting that Ag3PO4 was successfully prepared. Meanwhile, the XRD patterns of BU-TiO2-X/Ag3PO4 (6:1, 3:1, 1:1) showed that the peaks intensity was successively increased, indicating that the Ag3PO4 content had also increased in the hybrid. Under visible light for 20 min after three circles, the Ag3PO4 of XRD pattern presented the Ag peaks, while BU-TiO2-X/Ag3PO4 (3:1) still contained no Ag peaks in Fig. S2, indicating that the oxygen vacancy on the surface of BU-TiO2-X can retard the photocorrosion of Ag3PO4 [66]. To further verify the crystalline structure of the as-synthesized U–TiO2 and BU-TiO2-X, Raman spectroscopy was conducted (Fig. 2(d)). The Raman vibration peaks of the U–TiO2, centered at around 143, 197, 394, 515, and 637.0 cm−1, were well ascribed to the Eg, Eg, B1g, A1g + B1g, and Eg characteristic vibration modes of anatase [67]. In the inset of Fig. 3(d), a positive shift in BU-TiO2-X can be observed, indicating that oxygen defects (such as oxygen vacancy) formed on the surface of BU- TiO2-X [67]. And the intensity of the BU-TiO2-X peaks decreased and broadened after the reduction process by NaBH4 because the oxygen vacancies decrease the oxygen atom vibration frequency, indicating that the vibration of the O atom is in connection with oxygen defects [68].
Fig. 3.
(a)XPS survey spectrum of BU-TiO2-X, Ag3PO4, and BU-TiO2-X/Ag3PO4 (3:1); (b) High-resolution scans for Ti 2p electrons of the BU-TiO2-X and BU-TiO2-X/Ag3PO4 (3:1); (c) High-resolution scans for Ag 3d electrons of Ag3PO4 and BU-TiO2-X/Ag3PO4 (3:1); (d) High-resolution scans for O 1s electrons of BU-TiO2-X.
XPS analysis was further carried out to determine the elemental composition and chemical states of the synthesized samples. As shown in Fig. 3(a), the peaks of Ag, P, C, Ti, and O were detected in the BU-TiO2-X/Ag3PO4 (3:1) while only C, Ti, and O were found in the BU-TiO2-x. In addition, the signal intensity of Ag 3d and Ti 2p detected from the hybrid was lower than the corresponding intensity found in the single component, suggesting that the hybrid was successfully prepared. As shown in Fig. 3(b), the high-resolution scan of Ti 2p, detected from the BU-TiO2-X and BU-TiO2-X/Ag3PO4, indicated that Ti 2p contained peaks of Ti 2p1/2 at 464.2 eV, Ti 2p1/2 at 464.8 eV, Ti 2p3/2 at 458.3 eV, and Ti 2p3/2 at 458.9 eV, which belonged to the Ti4+ [69,70]. Compared to BU-TiO2-X, the peaks of the BU-TiO2-X/Ag3PO4 (3:1) sample showed a positive shift in binding energy, indicating an interfacial interaction between Ag3PO4 and BU-TiO2-X. This could be ascribed to the formation of Ti–O–P bonds, which were beneficial for reducing the recombination of the photogenerated electron–hole pairs [58,71,72]. As shown in Fig. 3(c), the high-resolution spectra of Ag 3d, obtained from the Ag3PO4 and BU-TiO2-X/Ag3PO4 (3:1), showed that Ag 3d contained peaks of Ag 3d3/2 at 374.0 eV, Ag 3d3/2 at 374.1 eV, Ag 3d5/2 at 368.0 eV and Ag 3d5/2 at 368.1 eV, which belonged to the Ag+ [73]. The Ag 3d also contained peaks of Ag 3d3/2 at 375.8 eV, Ag 3d3/2 at 374.9 eV, Ag 3d5/2 at 369.6 eV, and Ag 3d5/2 at 369.0 eV, which belonged to the Ag0 [73,74]. Compared with Ag3PO4, the peaks of the Ag0 in the BU-TiO2-X/Ag3PO4 (3:1) sample showed a negative shift in binding energy and a lower intensity, suggesting that the formation of the BU-TiO2-X/Ag3PO4 hybrid can prevent Ag+ from becoming Ag0 [74,75]. In Fig. 3(d), the main peak of O 1s, centered at 529.5 eV, could have been caused by oxygen lattices in BU-TiO2-X. The peak at 531.4 eV for BU-TiO2-X was also ascribed to surface active oxygen that was chemisorbed by oxygen defects (such as oxygen vacancy) [76]. In addition, the atom percentages of Ti and O could be measured using peak areas and the relative sensitivity factor. The stoichiometric ratio of the OTi(O–Ti): Ti2p atoms in the BU-TiO2-X sample was 1.88:1, indicating the appearance of oxygen vacancies after the NaBH4 reduction process [77,78]. These results further demonstrated that Ag3PO4 had been successfully combined with the BU-TiO2-X, which is in line with the XRD results.
3.2. Photocatalytic property
Fig. 4(a) presents the UV–Vis absorption spectra of the synthesized samples. The UV–Vis spectrum of the Ag3PO4 sample indicated that it absorbed sunlight with a wavelength less than 532 nm, corresponding to 2.33 eV of the bandgap energy [19]. The U–TiO2 exhibited an absorption tail of less than 390 nm in the ultraviolet light region, corresponding to 3.20 eV of the bandgap energy. These results are in agreement with previous reports [61,64]. Compared to U–TiO2, BU-TiO2-X exhibited a much wider absorption range, from 410 nm to 800 nm, corresponding to 2.72 eV of the bandgap energy (Fig. 4(b)). The edge potentials of the conduction band (CB) and the valence band (VB) of the photocatalysts could be calculated according to Eqs. (3), (4) [[79], [80], [81]].
| EVB = χ ‒ Ee + 1/2Eg | (3) |
| ECB = EVB − Eg | (4) |
where χ means the absolute electronegativity of the photocatalysts, which can be calculated from the geometric mean of the electronegativity in the constituent atoms (5.75 eV for BU-TiO2-X and 5.96 eV for Ag3PO4) [80]. Eg and Ee are the bandgap values of the photocatalyst and the energy of free electrons, with a value of 4.5 eV (vs Normal Hydrogen Electrode [NHE]). From the above, the calculated CB and VB potential of the synthesized BU-TiO2-X and Ag3PO4 were −0.11 eV, 2.61 eV and 0.30 eV, 2.63 eV vs NHE, respectively. For the BU-TiO2-X/Ag3PO4 (6:1, 3:1, 1:1) samples, the absorption spectra not only exhibited a larger characteristic absorption band edge, but also had a better absorption intensity in the visible region. In fact, BU-TiO2-X enabled the transfer of some photogenerated electrons from Ag3PO4 to BU-TiO2-X because of the oxygen deficiency on the surface of BU-TiO2-X, thus inhibiting electron–hole recombination and enhancing the photocatalytic ability.
Fig. 4.
(a) The UV–vis–NIR absorption curves and (b) band energy gap of synthesized materials; (c) Steady state photoluminescence spectra of U– TiO2, BU-TiO2-X, Ag3PO4, and BU-TiO2-X/Ag3PO4 (3:1); (d) photocurrent curves and (e) linear voltammetry sweep photocurrent curves of BU-TiO2-X, Ag3PO4 and BU-TiO2-X/Ag3PO4 (3:1) under visible light irradiation; ESR spectra of (f) superoxide radical, and (g) hydroxyl radical, obtained from BU-TiO2-X/Ag3PO4 (3:1); (h) The schematic illustration of the photocatalytic mechanism of BU-TiO2-X/Ag3PO4 heterostructure under visible light.
Fig. 4(c) shows the PL spectra of the synthesized samples, which can be used to perceive the recombination and separation efficiency of the photogenerated electron–hole pairs. The intensity of PL emission from the BU-TiO2-X/Ag3PO4 (6:1, 3:1, 1:1) at about 445 nm was far below that of BU-TiO2-X and Ag3PO4, indicating that the combination of BU-TiO2-X and Ag3PO4 is beneficial to the separation of photogenerated electron–hole pairs, which can endow the hybrid with better photocatalytic performance than that of BU-TiO2-X or Ag3PO4 alone. Moreover, the BU-TiO2-X/Ag3PO4 (3:1) showed the lowest intensity of PL emission among three kinds of hybrids. When the molar ratio between BU-TiO2-X and Ag3PO4 is 6:1, the bandgap is further larger than that of other composites, leading to a lower charge excitation efficiency; when the molar ratio between BU-TiO2-X and Ag3PO4 is 1:1, the PL intensity is higher than that of other composites, leading to faster recombination of photogenerated electron–hole pairs. In brief, the molar ratio between BU-TiO2-X and Ag3PO4 plays a critical role in effectively accelerating charge transfer and inhibiting the recombination of photogenerated electron–hole pairs.
Both transient photocurrent response and linear sweep voltammetry (LSV) are significant photoelectrochemical measurements for better understanding electron transfer and separation in the samples under visible light irradiation. As seen in Fig. 4(d), the BU-TiO2-X, Ag3PO4, and BU-TiO2-X/Ag3PO4 (3:1) samples had five repeated photocurrent responses over time (I–t)—on/off cycles, with and without the irradiation of simulated solar light (Xenon lamp light, 0.2 W cm−2). Under the light irradiation, BU-TiO2-X/Ag3PO4 (3:1) exhibited the strongest photocurrent, which was far higher than the photocurrents of Ag3PO4 or BU-TiO2-X alone. The current density vs the potential (J–V) of the synthesized samples was measured under the irradiation of Xenon lamplight with the same power. As Fig. 4(e) indicates, the current density of BU-TiO2-X/Ag3PO4 (3:1) was obviously higher than those of BU-TiO2-X and Ag3PO4, which was well in accordance with the PL results, suggesting that the synthesized hybrid had a much stronger photocatalytic performance. This enhanced photocatalytic performance indicates that photocatalysts can produce more ROS after absorbing photons in appropriate environments, which can endow the material with better antibacterial activity. The electron spin resonance (ESR) spectrum can be used to evaluate the ROS yields of photocatalysts under light irradiation. As shown in Fig. 4(f), obvious six-line peaks were observed, indicating the formation of •O2− [82]. The strong characteristic peaks, with an intensity ratio of 1:2:2:1, are displayed in Fig. 4(g), suggesting the production of large amount of •OH [52]. The above results demonstrated that BU-TiO2-X/Ag3PO4 (3:1) can produce •OH and •O2− under visible light irradiation [15]. The photocatalytic mechanism of the BU-TiO2-X/Ag3PO4 hybrid for generating ROS is schematically illustrated in Fig. 4(h). BU-TiO2-X/Ag3PO4 (3:1) is excited to generate electrons and holes under visible light irradiation. The oxygen vacancy of U–TiO2-X, produced by NaBH4 reduction, leads to local states below the conduction band edge [13], and acts as an electron acceptor to inhibit the recombination of photo-generated charges in BU-TiO2-X/Ag3PO4 (3:1) [48], consequently enhancing the photocatalysis of BU-TiO2-X/Ag3PO4 (3:1). According to the CB and VB potential of synthesized BU-TiO2-x and Ag3PO4, the oxygen vacancy in BU-TiO2-X/Ag3PO4 (3:1) can retard the flow of electrons to Ag+, thus preventing the Ag+ of Ag3PO4 from forming Ag0 compared to Ag3PO4 alone [19], which is in keeping with the XPS results.
3.3. In vitro antibacterial activity
The spread plate results in Fig. S3 present the antibacterial capability of the synthesized samples against E. coli and S. aureus. In Fig. S3(a), it is obvious that there was almost no reduction in E. coli colonies among the synthesized samples compared to that of the control in the dark, indicating that none of the samples had antibacterial activity against E. coli without light irradiation for 20 min. Under the irradiation of Xenon lamp light, the BU-TiO2-X samples exhibited little antibacterial activity, but it was evident that the nanospikes and photodynamic effect of BU-TiO2-X did have some influence on E. coli in the short term. In contrast, under light irradiation, all Ag3PO4 containing samples, due to their photocatalytic properties, reduced bacterial colonies at different levels compared to the control. However, almost no bacterial colonies remained in the group of BU-TiO2-X/Ag3PO4 (3:1) group, suggesting that it had the best antibacterial efficacy against E. coli because of its strongest photocatalytic performance. Under the same conditions, the antibacterial activity of all synthesized samples against S. aureus exhibited the same trends as against E. coli (Fig. S3(b)). The calculated antibacterial efficiencies against E. coli and S. aureus are shown in Fig. 5(a) and (b). The BU-TiO2-X/Ag3PO4 (3:1) sample had the highest antibacterial efficiencies (99.73 ± 0.18% and 98.10 ± 0.09%) against E. coli and S. aureus, respectively, under visible light irradiation for 20 min. This was followed by Ag3PO4, BU-TiO2-X/Ag3PO4 (6:1), and BU-TiO2-X/Ag3PO4 (1:1), with corresponding antibacterial efficiencies of 90.1 ± 1.65%, 95.31 ± 1.17%, 82.58 ± 0.9% against E. coli and 56.85 ± 3.39%, 81.68 ± 1.8%, 89.08 ± 3.09% against S. aureus, respectively. The physical puncture administered by the BU-TiO2-X and the Ag+ released from the Ag3PO4 played significant roles in the samples' antibacterial activity against E. coli and S. aureus, as proven by culturing the bacteria with the samples in the dark for 12 h (Figs. S3(c) and 5(c)). The long-term antibacterial efficiency of BU-TiO2-X/Ag3PO4 (3:1) was superior to BU-TiO2-X or Ag3PO4 alone, and, after visible light irradiation for 20 min, the BU-TiO2-X/Ag3PO4 (3:1) sample had the highest long-term antibacterial efficiency of 99.76 ± 0.15% and 99.85 ± 0.09% against E. coli and S. aureus, respectively, in the dark for 12 h. In addition, the combination of BU-TiO2-X and Ag3PO4 suppressed Ag+ leaching from Ag3PO4 (Fig. 5(d)), indicating that the increasing antibacterial efficiency of the BU-TiO2-X/Ag3PO4 hybrid may be ascribed to physical puncture administered by BU-TiO2-X. The inactivation kinetics of E. coli and S. aureus in the BU-TiO2-X/Ag3PO4 (3:1) sample were investigated via Xenon lamplight in Fig. 5(e) and (f). The antibacterial efficiency of the BU-TiO2-X/Ag3PO4 (3:1) sample against E. coli was obviously higher than that against S. aureus after light irradiation for 15 min. These antibacterial results are mainly ascribed to the samples' photocatalytic performance, physical structure, and Ag+ release, which will be discussed below in the antibacterial mechanism section.
Fig. 5.
The antibacterial activities of the synthesized samples. The antibacterial efficiency of the synthesized samples against (a) E. coli and (b) S. aureus after light irradiation or staying in dark for 20 min; (c) the antibacterial efficiency of the synthesized samples against E. coli and S. aureus after staying in dark for 12 h; (d) long-term release of Ag ions from Ag3PO4 and BU-TiO2-X/Ag3PO4 in 100 mL of DI water (PH = 7.4) at 37 °C; the antibacterial kinetics of (e) E. coli and (f) S. aureus for BU-TiO2-X/Ag3PO4 (3:1) under visible light. The error bars indicate means ± SD, n = 3. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
As shown in Fig. 6, bacterial microcosmic morphologies were observed via SEM, the ultrathin sectional TEM images, and the corresponding EDS. After visible light irradiation for 20 min (Fig. 6(a) and (b)), the E. coli and S. aureus cells had intact cell walls with smooth surfaces and full cytoplasm in the control (without samples or light). With respect to the BU-TiO2-X sample, there were some depressed areas on the bacterial membranes and complete cell walls. In contrast, the E. coli exposed to the Ag3PO4 sample were evidently deformed. Spots of the bacterial membranes had ruptured, and their inner materials had flowed out; however, the bacterial membranes of S. aureus did not rupture. The membranes of most E. coli and S. aureus cells were broken, and the inner content had flowed out, when the cells were exposed to the BU-TiO2-X/Ag3PO4 (3:1) sample. With the BU-TiO2-X sample, after remaining in the dark for 12 h (Fig. 6(c)), the membranes of the E. coli and S. aureus cells showed puncture marks, while the BU-TiO2-X/Ag3PO4 (3:1) sample very obviously ripped and punctured the membranes of both bacterial types. In addition, the ultrathin sectional TEM images and the corresponding EDS (Fig. 6(d) and (e)) further showed the puncturing behavior of BU-TiO2-X/Ag3PO4 (3:1). In the control, the E. coli and S. aureus cells both retained normal-shaped structures with intact bacterial membranes, and EDS showed no signs of Ti or Ag, while the nanospikes on the BU-TiO2-X/Ag3PO4 (3:1) held tight contact with the E. coli and S. aureus cells and even visibly deformed the E. coli cells. The corresponding EDS results also demonstrated that a small amount of ionic Ti and Ag penetrated the E. coli and S. aureus cells, which further proved that BU-TiO2-X/Ag3PO4 (3:1) can puncture the cell membranes. As mentioned above, these results and the spread plate findings corroborated well.
Fig. 6.
The FE-SEM and TEM images of bacteria. (a) E. coli and (b) S. aureus after irradiation by visible light or staying in dark for 20 min; (c) E. coli and S. aureus after staying in dark for 12 h (Scale bar: 500 nm); (d) TEM images of ultrathin section and (e) the corresponding EDS of E. coli and S. aureus.
3.4. Computational modeling of cell membrane puncture
Activation energy theory can be used to describe cell membrane puncture [83]. According to previous research, the rupture of the cell membrane depends on the free energy of deformed cells. When the free energy per unit area is larger than the threshold value [(2.9–8.3) × 10−3 J/m2], cell membrane puncture occurs [53,84]. In the following simulation, the researchers chose the minimum threshold value (2.9 × 10−3 J/m2) as the critical value according to their experimental results. Considering the nanospikes on the surface of the BU-TiO2-X in all directions, the authors simulated the free energy variety of E. coli and S. aureus cells with a nanospike at different angles. At the same time, they assumed that angle(θ) was 0° when the nanospike was perpendicular to the E. coli or S. aureus cells. It is worth noting that when θ goes beyond around 65° for E. coli and around 50° for S. aureus, the bacteria–nanospike system is unstable, and the simulation does not converge. As shown in Fig. 7(a), compared to being punctured with a nanospike at 0°, the E. coli cell generated a higher free energy density of 3.31 × 10−3 J/m2 at 45°. The membrane deformation of the E. coli cell at 45° (outlined by the red circle) is visibly larger than 0°. Under the same simulated condition, the free energy density and membrane deformation of S. aureus exhibited the same trend as E. coli (Fig. 7(b)). The density distribution of the bacterial free energy and the membrane deformation for E. coli and S. aureus at each angle are shown in Multimedia component 1, Multimedia component 2. The free energy value increased as the angle increased for both E. coli and S. aureus (Fig. 7(c)). A puncture criterion (free energy density = 2.9 × 10−3 Jm-2) separated the free energy density–angle plane into green (punctured) and blank regions. Compared to S. aureus, E. coli reached the free energy threshold at about 32°–11° lower than S. aureus. The difference in deformation was further proved by the bacteria's corresponding penetration depth results (Fig. 7(d)). The penetration depth for the E. coli cells was greater than that for S. aureus, indicating that E. coli cells are susceptible to the nanospikes, which is in line with the spread plate results.
Fig. 7.
Computational modeling of cell membrane penetration. The density distribution of bacterial free energy and membrane deformation as the bacteria deform on the nanospikes (d = 20 nm) with two kinds of angles (θ): θ = 0° and θ = 45° for (a) E. coli and (b) S. aureus; (c) the maximum value of bacterial free energy density of E. coli and S. aureus cell punctured by nanospikes rises with increasing θ; (d) The penetration depth of E. coli and S. aureus cell with various θ.
3.5. Antibacterial mechanism
The above results demonstrate that the BU-TiO2-X/Ag3PO4 hybrid had better antibacterial activity against E. coli and S. aureus. The antibacterial mechanisms of the BU-TiO2-X/Ag3PO4 hybrid have three aspects, which are shown in Scheme 2 [52, 58, 85], as follows. (A) ROS (Fig. 4(f) and (g)), such as •OH and •O2−, are generated by the BU-TiO2-X/Ag3PO4 hybrid under visible light; they can disrupt the cell membrane (Fig. 6(a) and (b)) and induce oxidative stress, which causes cell death. (B) Silver ions (Fig. 5(d)), released by the Ag3PO4 NPs, can penetrate cell membranes to inhibit active transport and bacterial metabolism when the concentration reaches a certain level. (C) BU-TiO2-X NPs can puncture bacterial cell walls, leading to deformation and even rupture of the bacterial membranes which causes bacterial death (Fig. 6(c) and (d)). The intense nanospikes, with a mean diameter of 20 nm, on the surface of the BU-TiO2-X NPs provide enough “arms” in all directions.
Scheme 2.
Schematic illustration of possible photocatalysis, physical puncture and released Ag+ mechanisms of BU-TiO2-X/Ag3PO4 heterostructure.
3.6. In vitro cytotoxicity test
As shown in Fig. S4, the Cell Counting Kit-8 (cck-8) assay evaluated cell viabilities on different leach liquors after one, three, and five days of incubation, since the Ag+ released by Ag3PO4 NPs have higher toxicity than Ag NPs according to previous research [86]. Without visible light irradiation, the cell viability of the Ag3PO4 sample was lower than that of the BU-TiO2-X/Ag3PO4 (3:1) sample at the first day. However, the cell viability of the Ag3PO4 sample was obviously higher than that of the BU-TiO2-X/Ag3PO4 (3:1) sample by the third day, suggesting that Ag3PO4 was less stable in water than BU-TiO2-X/Ag3PO4 (3:1). At the fifth day, the cell viability of the Ag3PO4 and BU-TiO2-X/Ag3PO4 (3:1) samples both approximated that of the control sample, indicating that, while Ag3PO4 and BU-TiO2-X/Ag3PO4 (3:1) presented some toxicity in the short term, their cell viability was enhanced and close to 95% in the long term. Thus, the CCK-8 assay proved that BU-TiO2-X/Ag3PO4 (3:1) had good biocompatibility in vitro.
4. Conclusions
BU-TiO2-X/Ag3PO4, with various molar ratio, is controllably designed to enhance its photodynamic therapeutic efficacy to combat E. coli or S. aureus infections through the synergistic antibacterial effects of ROS, nanospikes, and Ag+ release. The cooperation of BU-TiO2-X and Ag3PO4 can decrease their bandgap, inhibit the recombination of photogenerated electron–hole pairs, and effectively promote charge transfer. BU-TiO2-X/Ag3PO4 may have an optimum molar ratio of 3:1 which showed the best antibacterial efficiency (99.76 ± 0.15% and 99.85 ± 0.09%) against E. coli and S. aureus after 20 min of light irradiation followed by darkness for 12 h. The BU-TiO2-X/Ag3PO4 (3:1) heterojunction have great antibacterial efficacy rapidly under visible light. Meanwhile, the physical puncture administered by the nanospikes on the surface of BU-TiO2-X, combined with the Ag+ released by Ag3PO4, have long-term bacteriostatic efficacy. Consequently, BU-TiO2-X/Ag3PO4 has great potential for eliminating infections rapidly and effectively.
CRediT authorship contribution statement
Yingde Xu: Conceptualization, Methodology, Data curation, Writing - original draft. Xiangmei Liu: Conceptualization, Writing - review & editing, Supervision, Project administration. Yufeng Zheng: Conceptualization, Supervision, Project administration. Changyi Li: Methodology. Kelvin Wai Kwok Yeung: Methodology. Zhenduo Cui: Methodology. Yanqin Liang: Methodology. Zhaoyang Li: Methodology. Shengli Zhu: Methodology. Shuilin Wu: Conceptualization, Writing - review & editing, Supervision, Project administration.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgements
This work is jointly supported by the National Science Fund for Distinguished Young Scholars 51925104, National Natural Science Foundation of China nos. 51871162, 51671081, and 81870809, NSFC key program 51631007, Natural Science Fund of Hubei Province, 2018CFA064, RGC/NSFC (N_HKU725-1616), Hong Kong ITC (ITS/287/17, GHX/002/14SZ), as well as Health and Medical Research Fund (No. 03142446).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bioactmat.2020.11.013.
Contributor Information
Xiangmei Liu, Email: liuxiangmei1978@163.com.
Shuilin Wu, Email: shuilinwu@tju.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Hover B.M., Kim S.H., Katz M., Charlop-Powers Z., Owen J.G., Ternei M.A., Maniko J., Estrela A.B., Molina H., Park S., Perlin D.S., Brady S.F. Culture-independent discovery of the malacidins as calcium-dependent antibiotics with activity against multidrug-resistant Gram-positive pathogens. Nat. Microbiol. 2018;3(4):415–422. doi: 10.1038/s41564-018-0110-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lewis K. Platforms for antibiotic discovery. Nat. Rev. Drug Discov. 2013;12(5):371–387. doi: 10.1038/nrd3975. [DOI] [PubMed] [Google Scholar]
- 3.Baker S., Thomson N., Weill F.X., Holt K.E. Genomic insights into the emergence and spread of antimicrobial-resistant bacterial pathogens. Science. 2018;360(6390):733. doi: 10.1126/science.aar3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paudyal M.P., Adebesin A.M., Burt S.R., Ess D.H., Ma Z., Kurti L., Falck J.R. Dirhodium-catalyzed C-H arene amination using hydroxylamines. Science. 2016;353(6304):1144–1147. doi: 10.1126/science.aaf8713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li J., Liu X., Tan L., Liang Y., Cui Z., Yang X., Zhu S., Li Z., Zheng Y., Yeung K.W.K., Wang X., Wu S. Light-activated rapid disinfection by accelerated charge transfer in red phosphorus/ZnO heterointerface. Small Methods. 2019;3(3):1900048. [Google Scholar]
- 6.Tan L., Li J., Liu X., Cui Z., Yang X., Zhu S., Li Z., Yuan X., Zheng Y., Yeung K.W.K., Pan H., Wang X., Wu S. Rapid biofilm eradication on bone implants using red phosphorus and near-infrared light. Adv. Mater. 2018;30(31):1801808. doi: 10.1002/adma.201801808. [DOI] [PubMed] [Google Scholar]
- 7.T. Wei, Q. Yu, H. Chen, Responsive and synergistic antibacterial coatings: fighting against bacteria in a smart and effective way, Adv. Healthcare Mater. 8 (3) 2019 1801381. [DOI] [PubMed]
- 8.Xu J., Cheng X., Tan L., Fu C., Ahmed M., Tian J., Dou J., Zhou Q., Ren X., Wu Q., Tang S., Zhou H., Meng X., Yu J., Liang P. Microwave responsive nanoplatform via P-selectin mediated drug delivery for treatment of hepatocellular carcinoma with distant metastasis. Nano Lett. 2019;19(5):2914–2927. doi: 10.1021/acs.nanolett.8b05202. [DOI] [PubMed] [Google Scholar]
- 9.Qian X., Zheng Y., Chen Y. Micro/nanoparticle-augmented sonodynamic therapy (SDT): breaking the depth shallow of photoactivation. Adv. Mater. 2016;28(37):8097–8129. doi: 10.1002/adma.201602012. [DOI] [PubMed] [Google Scholar]
- 10.Ren Y., Han Y., Li Z., Liu X., Zhu S., Liang Y., Yeung K.W.K., Wu S. Ce and Er co-doped TiO2 for rapid bacteria-killing using visible light. Bioact. Mater. 2020;5(2):201–209. doi: 10.1016/j.bioactmat.2020.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scanlon D.O., Dunnill C.W., Buckeridge J., Shevlin S.A., Logsdail A.J., Woodley S.M., Catlow C.R., Powell M.J., Palgrave R.G., Parkin I.P., Watson G.W., Keal T.W., Sherwood P., Walsh A., Sokol A.A. Band alignment of rutile and anatase TiO2. Nat. Mater. 2013;12(9):798–801. doi: 10.1038/nmat3697. [DOI] [PubMed] [Google Scholar]
- 12.Ji H., Lyu L., Zhang L., An X., Hu C. Oxygen vacancy enhanced photostability and activity of plasmon-Ag composites in the visible to near-infrared region for water purification. Appl. Catal., B. 2016;199:230–240. [Google Scholar]
- 13.Pan X., Yang M.Q., Fu X., Zhang N., Xu Y.J. Defective TiO2 with oxygen vacancies: synthesis, properties and photocatalytic applications. Nanoscale. 2013;5(9):3601–3614. doi: 10.1039/c3nr00476g. [DOI] [PubMed] [Google Scholar]
- 14.Zhang E.L., Fu S., Wang R.X., Li H.X., Liu Y., Ma Z.Q., Liu G.K., Zhu C.S., Qin G.W., Chen D.F. Role of Cu element in biomedical metal alloy design. Rare Met. 2019;38(6):476–494. [Google Scholar]
- 15.Su K., Tan L., Liu X., Cui Z., Zheng Y., Li B., Han Y., Li Z., Zhu S., Liang Y., Feng X., Wang X., Wu S. Rapid photo-sonotherapy for clinical treatment of bacterial infected bone implants by creating oxygen deficiency using sulfur doping. ACS Nano. 2020;14(2):2077–2089. doi: 10.1021/acsnano.9b08686. [DOI] [PubMed] [Google Scholar]
- 16.Dai K., Lv J., Zhang J., Zhu G., Geng L., Liang C. Efficient visible-light-driven splitting of water into hydrogen over surface-fluorinated anatase TiO2 nanosheets with exposed {001} facets/layered CdS–diethylenetriamine nanobelts. ACS Sustain. Chem. Eng. 2018;6(10):12817–12826. Submitted for publication. [Google Scholar]
- 17.Lv J., Zhang J., Liu J., Li Z., Dai K., Liang C. Bi SPR-promoted Z-scheme Bi2MoO6/CdS-diethylenetriamine composite with effectively enhanced visible light photocatalytic hydrogen evolution activity and stability. ACS Sustain. Chem. Eng. 2017;6(1):696–706. [Google Scholar]
- 18.Dai K., Lu L., Liang C., Liu Q., Zhu G. Heterojunction of facet coupled g-C3N4/surface-fluorinated TiO2 nanosheets for organic pollutants degradation under visible LED light irradiation. Appl. Catal., B. 2014;156–157:331–340. [Google Scholar]
- 19.Yi Z., Ye J., Kikugawa N., Kako T., Ouyang S., Stuart-Williams H., Yang H., Cao J., Luo W., Li Z., Liu Y., Withers R.L. An orthophosphate semiconductor with photooxidation properties under visible-light irradiation. Nat. Mater. 2010;9(7):559–564. doi: 10.1038/nmat2780. [DOI] [PubMed] [Google Scholar]
- 20.Ma X., Li H., Wang Y., Li H., Liu B., Yin S., Sato T. Substantial change in phenomenon of “self-corrosion” on Ag3PO4/TiO2 compound photocatalyst. Appl. Catal., B. 2014;158:314–320. [Google Scholar]
- 21.Chai Y., Ding J., Wang L., Liu Q., Ren J., Dai W.L. Enormous enhancement in photocatalytic performance of Ag3PO4/HAp composite: a Z-scheme mechanism insight. Appl. Catal., B. 2015;179:29–36. [Google Scholar]
- 22.Bi Y., Ouyang S., Umezawa N., Cao J., Ye J. Facet effect of single-crystalline Ag3PO4 sub-microcrystals on photocatalytic properties. J. Am. Chem. Soc. 2011;133(17):6490–6492. doi: 10.1021/ja2002132. [DOI] [PubMed] [Google Scholar]
- 23.Bi Y., Ouyang S., Cao J., Ye J. Facile synthesis of rhombic dodecahedral AgX/Ag3PO4 (X = Cl, Br, I) heterocrystals with enhanced photocatalytic properties and stabilities. Phys. Chem. Chem. Phys. 2011;13(21):10071–10075. doi: 10.1039/c1cp20488b. [DOI] [PubMed] [Google Scholar]
- 24.Shao N., Wang J., Wang D., Corvini P. Preparation of three-dimensional Ag3PO4/TiO2@MoS2 for enhanced visible-light photocatalytic activity and anti-photocorrosion. Appl. Catal., B. 2017;203:964–978. [Google Scholar]
- 25.Hong L., Liu X., Tan L., Cui Z., Yang X., Liang Y., Li Z., Zhu S., Zheng Y., Yeung K.W.K., Jing D., Zheng D., Wang X., Wu S. Rapid biofilm elimination on bone implants using near-infrared-activated inorganic semiconductor heterostructures. Adv. Healthcare Mater. 2019;8(19) doi: 10.1002/adhm.201900835. 1900835. [DOI] [PubMed] [Google Scholar]
- 26.Wang Z., Hu T., Dai K., Zhang J., Liang C. Construction of Z-scheme Ag3PO4/Bi2WO6 composite with excellent visible-light photodegradation activity for removal of organic contaminants. Chin. J. Catal. 2017;38(12):2021–2029. [Google Scholar]
- 27.Wang H., Zhang L., Chen Z., Hu J., Li S., Wang Z., Liu J., Wang X. Semiconductor heterojunction photocatalysts: design, construction, and photocatalytic performances. Chem. Soc. Rev. 2014;43(15):5234–5244. doi: 10.1039/c4cs00126e. [DOI] [PubMed] [Google Scholar]
- 28.Tan P., Chen X., Wu L., Shang Y.Y., Liu W., Pan J., Xiong X. Hierarchical flower-like SnSe2 supported Ag3PO4 nanoparticles: towards visible light driven photocatalyst with enhanced performance. Appl. Catal., B. 2017;202:326–334. [Google Scholar]
- 29.Chen F., Yang Q., Li X., Zeng G., Wang D., Niu C., Zhao J., An H., Xie T., Deng Y. Hierarchical assembly of graphene-bridged Ag3PO4/Ag/BiVO4 (040) Z-scheme photocatalyst: an efficient, sustainable and heterogeneous catalyst with enhanced visible-light photoactivity towards tetracycline degradation under visible light irradiation. Appl. Catal., B. 2017;200:330–342. [Google Scholar]
- 30.Zhou P., Yu J., Jaroniec M. All-solid-state z-scheme photocatalytic systems. Adv. Mater. 2014;26(29):4920–4935. doi: 10.1002/adma.201400288. [DOI] [PubMed] [Google Scholar]
- 31.Li X., Zhang J., Huo Y., Dai K., Li S., Chen S. Two-dimensional sulfur- and chlorine-codoped g-C3N4/CdSe-amine heterostructures nanocomposite with effective interfacial charge transfer and mechanism insight. Appl. Catal., B. 2021;280:119452. [Google Scholar]
- 32.Liu L., Hu T., Dai K., Zhang J., Liang C. A novel step-scheme BiVO4/Ag3VO4 photocatalyst for enhanced photocatalytic degradation activity under visible light irradiation. Chin. J. Catal. 2021;42(1):46–55. [Google Scholar]
- 33.Hu T., Dai K., Zhang J., Chen S. Noble-metal-free Ni2P modified step-scheme SnNb2O6/CdS-diethylenetriamine for photocatalytic hydrogen production under broadband light irradiation. Appl. Catal., B. 2020;269:118844. [Google Scholar]
- 34.Mei F., Li Z., Dai K., Zhang J., Liang C. Step-scheme porous g-C3N4/Zn0.2Cd0.8S-DETA composites for efficient and stable photocatalytic H2 production. Chin. J. Catal. 2020;41(1):41–49. [Google Scholar]
- 35.Huo Y., Zhang J., Dai K., Li Q., Lv J., Zhu G., Liang C. All-solid-state artificial Z-scheme porous g-C3N4/Sn2S3-DETA heterostructure photocatalyst with enhanced performance in photocatalytic CO2 reduction. Appl. Catal., B. 2019;241:528–538. [Google Scholar]
- 36.Li J., Liu X., Tan L., Cui Z., Yang X., Liang Y., Li Z., Zhu S., Zheng Y., Yeung K.W.K., Wang X., Wu S. Zinc-doped Prussian blue enhances photothermal clearance of Staphylococcus aureus and promotes tissue repair in infected wounds. Nat. Commun. 2019;10(1):4490. doi: 10.1038/s41467-019-12429-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xie X., Mao C., Liu X., Tan L., Cui Z., Yang X., Zhu S., Li Z., Yuan X., Zheng Y., Yeung K.W.K., Chu P.K., Wu S. Tuning the bandgap of photo-sensitive polydopamine/Ag3PO4/graphene oxide coating for rapid, noninvasive disinfection of implants. ACS Cent. Sci. 2018;4(6):724–738. doi: 10.1021/acscentsci.8b00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mao C., Xiang Y., Liu X., Cui Z., Yang X., Li Z., Zhu S., Zheng Y., Yeung K.W.K., Wu S. Repeatable photodynamic therapy with triggered signaling pathways of fibroblast cell proliferation and differentiation to promote bacteria-accompanied wound healing. ACS Nano. 2018;12(2):1747–1759. doi: 10.1021/acsnano.7b08500. [DOI] [PubMed] [Google Scholar]
- 39.Kim S., Tachikawa T., Fujitsuka M., Majima T. Far-red fluorescence probe for monitoring singlet oxygen during photodynamic therapy. J. Am. Chem. Soc. 2014;136(33):11707–11715. doi: 10.1021/ja504279r. [DOI] [PubMed] [Google Scholar]
- 40.Liang P., Huang X., Wang Y., Chen D., Ou C., Zhang Q., Shao J., Huang W., Dong X. Tumor-microenvironment-responsive nanoconjugate for synergistic antivascular activity and phototherapy. ACS Nano. 2018;12(11):11446–11457. doi: 10.1021/acsnano.8b06478. [DOI] [PubMed] [Google Scholar]
- 41.Li Y., Liu X., Li B., Zheng Y., Han Y., Chen D.F., Yeung K.W.K., Cui Z., Liang Y., Li Z., Zhu S., Wang X., Wu S. Near-infrared light triggered phototherapy and immunotherapy for elimination of methicillin-resistant Staphylococcus aureus biofilm infection on bone implant. ACS Nano. 2020;14(7):8157–8170. doi: 10.1021/acsnano.0c01486. [DOI] [PubMed] [Google Scholar]
- 42.Xie X., Mao C., Liu X., Zhang Y., Cui Z., Yang X., Yeung K.W.K., Pan H., Chu P.K., Wu S. Synergistic bacteria killing through photodynamic and physical actions of graphene oxide/Ag/collagen coating. ACS Appl. Mater. Interfaces. 2017;9(31):26417–26428. doi: 10.1021/acsami.7b06702. [DOI] [PubMed] [Google Scholar]
- 43.Mao C., Xiang Y., Liu X., Cui Z., Yang X., Yeung K.W.K., Pan H., Wang X., Chu P.K., Wu S. Photo-inspired antibacterial activity and wound healing acceleration by hydrogel embedded with Ag/Ag@AgCl/ZnO nanostructures. ACS Nano. 2017;11(9):9010–9021. doi: 10.1021/acsnano.7b03513. [DOI] [PubMed] [Google Scholar]
- 44.Li M., Li L., Su K., Liu X., Zhang T., Liang Y., Jing D., Yang X., Zheng D., Cui Z., Li Z., Zhu S., Yeung K.W.K., Zheng Y., Wang X., Wu S. Highly effective and noninvasive near-infrared eradication of a Staphylococcus aureus biofilm on implants by a photoresponsive coating within 20 min. Adv. Sci. 2019;6(17):1900599. doi: 10.1002/advs.201900599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luo Y., Li J., Liu X., Tan L., Cui Z., Feng X., Yang X., Liang Y., Li Z., Zhu S., Zheng Y., Yeung K.W.K., Yang C., Wang X., Wu S. Dual metal-organic framework heterointerface. ACS Cent. Sci. 2019;5(9):1591–1601. doi: 10.1021/acscentsci.9b00639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Y., Liu X., Tan L., Cui Z., Jing D., Yang X., Liang Y., Li Z., Zhu S., Zheng Y., Yeung K.W.K., Zheng D., Wang X., Wu S. Eradicating multidrug-resistant bacteria rapidly using a multi functional g-C3N4@Bi2S3 nanorod heterojunction with or without antibiotics. Adv. Funct. Mater. 2019;29(20):1900946. [Google Scholar]
- 47.Huang B., Tan L., Liu X., Li J., Wu S. A facile fabrication of novel stuff with antibacterial property and osteogenic promotion utilizing red phosphorus and near-infrared light. Bioact. Mater. 2019;4(1):17–21. doi: 10.1016/j.bioactmat.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li Y., Liu X., Tan L., Cui Z., Yang X., Zheng Y., Yeung K.W.K., Chu P.K., Wu S. Rapid sterilization and accelerated wound healing using Zn2+ and graphene oxide modified g-C3N4 under dual light irradiation. Adv. Funct. Mater. 2018;28(30):1800299. [Google Scholar]
- 49.Han D., Han Y., Li J., Liu X., Yeung K.W.K., Zheng Y., Cui Z., Yang X., Liang Y., Li Z., Zhu S., Yuan X., Feng X., Yang C., Wu S. Enhanced photocatalytic activity and photothermal effects of Cu-doped metal-organic frameworks for rapid treatment of bacteria-infected wounds. Appl. Catal., B. 2020;261:118248. [Google Scholar]
- 50.Jenkins J., Mantell J., Neal C., Gholinia A., Verkade P., Nobbs A.H., Su B. Antibacterial effects of nanopillar surfaces are mediated by cell impedance, penetration and induction of oxidative stress. Nat. Commun. 2020;11(1):1626. doi: 10.1038/s41467-020-15471-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tripathy A., Sen P., Su B., Briscoe W.H. Natural and bioinspired nanostructured bactericidal surfaces. Adv. Colloid Interface Sci. 2017;248:85–104. doi: 10.1016/j.cis.2017.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li J., Tan L., Liu X., Cui Z., Yang X., Yeung K.W.K., Chu P.K., Wu S. Balancing bacteria-osteoblast competition through selective physical puncture and biofunctionalization of ZnO/polydopamine/arginine-glycine-aspartic acid-cysteine nanorods. ACS Nano. 2017;11(11):11250–11263. doi: 10.1021/acsnano.7b05620. [DOI] [PubMed] [Google Scholar]
- 53.Liu L., Chen S., Zhang X., Xue Z., Cui S., Hua X., Yang B., Yan H., Liu C., Wang J., Zhang Z., Yu W., Wu F., Xu W., Lehto V.P., Yue T., Liu Y., Yu Y., Wang T., Wang J. Mechanical penetration of β-Lactam-resistant Gram-negative bacteria by programmable nanowires. Sci. Adv. 2020;6(27) doi: 10.1126/sciadv.abb9593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ivanova E.P., Hasan J., Webb H.K., Gervinskas G., Juodkazis S., Truong V.K., Wu A.H.F., Lamb R.N., Baulin V.A., Watson G.S., Watson J.A., Mainwaring D.E., Crawford R.J. Bactericidal activity of black silicon. Nat. Commun. 2013;4(1):2838. doi: 10.1038/ncomms3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang T., Weng Z., Liu X., Yeung K.W.K., Pan H., Wu S. Controlled release and biocompatibility of polymer/titania nanotube array system on titanium implants. Bioact. Mater. 2017;2(1):44–50. doi: 10.1016/j.bioactmat.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shao J., Sheng W., Wang M., Li S., Chen J., Zhang Y., Cao S. In-situ synthesis of carbon-doped TiO2 single-crystal nanorods with a remarkably photocatalytic efficiency. Appl. Catal., B. 2017;209:311–319. [Google Scholar]
- 57.Li J., Yi D., Zhan F., Zhou B., Gao D., Guo D., Liu S., Wang X., Yao J. Monolayered Ru-1/TiO2 nanosheet enables efficient visible-light-driven hydrogen evolution. Appl. Catal., B. 2020;271:118925. [Google Scholar]
- 58.Wang J., Chen H.J., Hang T., Yu Y., Liu G., He G., Xiao S., Yang B.R., Yang C., Liu F., Tao J., Wu M.X., Xie X. Physical activation of innate immunity by spiky particles. Nat. Nanotechnol. 2018;13(11):1078–1086. doi: 10.1038/s41565-018-0274-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pan X., Xu Y.J. Defect-mediated growth of noble-metal (Ag, Pt, and Pd) nanoparticles on TiO2 with oxygen vacancies for photocatalytic redox reactions under visible light. J. Phys. Chem. C. 2013;117(35):17996–18005. [Google Scholar]
- 60.Kang Q., Cao J., Zhang Y., Liu L., Xu H., Ye J. Reduced TiO2 nanotube arrays for photoelectrochemical water splitting. J. Mater. Chem. 2013;1(18):5766–5774. [Google Scholar]
- 61.Chen X., Liu L., Huang F. Black titanium dioxide (TiO2) nanomaterials. Chem. Soc. Rev. 2015;44(7):1861–1885. doi: 10.1039/c4cs00330f. [DOI] [PubMed] [Google Scholar]
- 62.Jiang H., Sun S.X. Morphology, growth, and size limit of bacterial cells. Phys. Rev. Lett. 2010;105(2) doi: 10.1103/PhysRevLett.105.028101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Malmi-Kakkada A.N., Thirumalai D. Generalized Rayleigh-Plesset theory for cell size maintenance in viruses and bacteria. BioRxiv. 2019 doi: 10.1101/552778. [DOI] [Google Scholar]
- 64.Li Y., Wang P., Huang C., Yao W., Wu Q., Xu Q. Synthesis and photocatalytic activity of ultrafine Ag3PO4 nanoparticles on oxygen vacated TiO2. Appl. Catal., B. 2017;205:489–497. [Google Scholar]
- 65.Liu X., Xing Z., Zhang Y., Li Z., Wu X., Tan S., Yu X., Zhu Q., Zhou W. Fabrication of 3D flower-like black N-TiO2-X@MoS2 for unprecedented-high visible-light-driven photocatalytic performance. Appl. Catal., B. 2017;201:119–127. [Google Scholar]
- 66.Qin J., Huo J., Zhang P., Zeng J., Wang T., Zeng H. Improving the photocatalytic hydrogen production of Ag/g-C3N4 nanocomposites by dye-sensitization under visible light irradiation. Nanoscale. 2016;8(4):2249–2259. doi: 10.1039/c5nr06346a. [DOI] [PubMed] [Google Scholar]
- 67.Naldoni A., Allieta M., Santangelo S., Marelli M., Fabbri F., Cappelli S., Bianchi C.L., Psaro R., Dal Santo V. Effect of nature and location of defects on bandgap narrowing in black TiO2 nanoparticles. J. Am. Chem. Soc. 2012;134(18):7600–7603. doi: 10.1021/ja3012676. [DOI] [PubMed] [Google Scholar]
- 68.Xu Y., Li H., Sun B., Qiao P., Ren L., Tian G., Jiang B., Pan K., Zhou W. Surface oxygen vacancy defect-promoted electron-hole separation for porous defective ZnO hexagonal plates and enhanced solar-driven photocatalytic performance. Chem. Eng. J. 2020;379:122295. [Google Scholar]
- 69.Lu X., Wang G., Zhai T., Yu M., Gan J., Tong Y., Li Y. Hydrogenated TiO2 nanotube arrays for supercapacitors. Nano Lett. 2012;12(3):1690–1696. doi: 10.1021/nl300173j. [DOI] [PubMed] [Google Scholar]
- 70.Guo Y., Chen S., Yu Y., Tian H., Zhao Y., Ren J.C., Huang C., Bian H., Huang M., An L., Li Y., Zhang R. Hydrogen-location-sensitive modulation of the redox reactivity for oxygen-deficient TiO2. J. Am. Chem. Soc. 2019;141(21):8407–8411. doi: 10.1021/jacs.9b01836. [DOI] [PubMed] [Google Scholar]
- 71.McCafferty E., Wightman J.P. Determination of the concentration of surface hydroxyl groups on metal oxide films by a quantitative XPS method. Surf. Interface Anal. 1998;26(8):549–564. [Google Scholar]
- 72.Gopal N.O., Lo H.H., Ke T.F., Lee C.H., Chou C.C., Wu J.D., Sheu S.C., Ke S.C. Visible light active phosphorus-doped TiO2 nanoparticles: an EPR evidence for the enhanced charge separation. J. Phys. Chem. C. 2012;116(30):16191–16197. [Google Scholar]
- 73.Liu Y., Fang L., Lu H., Li Y., Hu C., Yu H. One-pot pyridine-assisted synthesis of visible-light-driven photocatalyst Ag/Ag3PO4. Appl. Catal., B. 2012;115:245–252. [Google Scholar]
- 74.Zhang H., Wang G., Chen D., Lv X., Li J. Tuning photoelectrochemical performances of Ag-TiO2 nanocomposites via reduction/oxidation of Ag. Chem. Mater. 2008;20(20):6543–6549. [Google Scholar]
- 75.Zhu M., Chen P., Liu M. Graphene oxide enwrapped Ag/AgX (X = Br, Cl) nanocomposite as a highly efficient visible-light plasmonic photocatalyst. ACS Nano. 2011;5(6):4529–4536. doi: 10.1021/nn200088x. [DOI] [PubMed] [Google Scholar]
- 76.Fan W., Li H., Zhao F., Xiao X., Huang Y., Ji H., Tong Y. Boosting the photocatalytic performance of (001) bioi: enhancing donor density and separation efficiency of photogenerated electrons and holes. Chem. Commun. 2016;52(30):5316–5319. doi: 10.1039/c6cc00903d. [DOI] [PubMed] [Google Scholar]
- 77.Li S., Qiu J., Ling M., Peng F., Wood B., Zhang S. Photoelectrochemical characterization of hydrogenated TiO2 nanotubes as photoanodes for sensing applications. ACS Appl. Mater. Interfaces. 2013;5(21):11129–11135. doi: 10.1021/am403325a. [DOI] [PubMed] [Google Scholar]
- 78.Michelswirth M., Räkers M., Schäfer C., Mattay J., Neumann M., Heinzmann U. Photocycloaddition of anthracene-functionalized monolayers on silicon (100) surface. J. Phys. Chem. B. 2010;114(10):3482–3487. doi: 10.1021/jp910298a. [DOI] [PubMed] [Google Scholar]
- 79.Li Y., Xu X., Li Y., Ding C., Wu J., Lu A., Ding H., Qin S., Wang C. Absolute band structure determination on naturally occurring rutile with complex chemistry: implications for mineral photocatalysis on both earth and mars. Appl. Surf. Sci. 2018;439:660–671. [Google Scholar]
- 80.Tang C., Liu E., Wan J., Hu X., Fan J. Co3O4 nanoparticles decorated Ag3PO4 tetrapods as an efficient visible-light-driven heterojunction photocatalyst. Appl. Catal., B. 2016;181:707–715. [Google Scholar]
- 81.Ding H., Han D., Han Y., Liang Y., Liu X., Li Z., Zhu S., Wu S. Visible light responsive CuS/protonated g-C3N4 heterostructure for rapid sterilization. J. Hazard Mater. 2020;393:122423. doi: 10.1016/j.jhazmat.2020.122423. [DOI] [PubMed] [Google Scholar]
- 82.Kuppusamy P., Zweier J.L. Characterization of free radical generation by xanthine-oxidase: evidence for hydroxyl radical generation. J. Biol. Chem. 1989;264(17):9880–9884. [PubMed] [Google Scholar]
- 83.Evans E., Heinrich V., Ludwig F., Rawicz W. Dynamic tension spectroscopy and strength of biomembranes. Biophys. J. 2003;85(4):2342–2350. doi: 10.1016/s0006-3495(03)74658-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xie X., Xu A.M., Angle M.R., Tayebi N., Verma P., Melosh N.A. Mechanical model of vertical nanowire cell penetration. Nano Lett. 2013;13(12):6002–6008. doi: 10.1021/nl403201a. [DOI] [PubMed] [Google Scholar]
- 85.Panacek A., Kvitek L., Smekalova M., Vecerova R., Kolar M., Roderova M., Dycka F., Sebela M., Prucek R., Tomanec O., Zboril R. Bacterial resistance to silver nanoparticles and how to overcome it. Nat. Nanotechnol. 2018;13(1):65–71. doi: 10.1038/s41565-017-0013-y. [DOI] [PubMed] [Google Scholar]
- 86.Lai Y., Dong L., Zhou H., Yan B., Chen Y., Cai Y., Liu J. Coexposed nanoparticulate Ag alleviates the acute toxicity induced by ionic Ag+ in vivo. Sci. Total Environ. 2020;723:138050. doi: 10.1016/j.scitotenv.2020.138050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.