ABSTRACT
Background
SARS-CoV-2 which causes coronavirus disease 2019 (COVID-19) binds to angiotensin-converting enyzme 2 (ACE2) and enters the host cell. ACE2 protein is expressed highly in the testis.
Aim
The aim of this study was to compare male reproductive hormones such as total testosterone (TT), luteinizing hormone (LH), follicular stimulant hormone (FSH), and prolactin between patients with COVID-19, age-matched cases with non–COVID-19 respiratory tract infection, and age-matched controls.
Methods
This was a prospective cohort study and included 262 men aged between 20 and 65 years. The study comprised 3 groups including patients with COVID-19 (n = 89), cases with non–COVID-19 respiratory tract infection (n = 30), and age-matched controls (n = 143). All cases were evaluated using TT, LH, FSH, and prolactin. Correlations between TT and clinical parameters of patient groups were investigated using Pearson’s correlation test.
Outcomes
The primary outcome of the study was detection of the difference of TT, FSH, LH, and prolactin levels between the groups. Secondary outcome was to correlate TT and hospitalization time and oxygen saturation on hospital admission (SpO2) of patients.
RESULTS
The mean age of study groups was 49.9 ± 12.5 years, 52.7 ± 9.6 years, and 50 ± 7.8 years, respectively (P = .06). Serum TT levels was median 185.52 ng/dL in patients with COVID-19, median 288.67 ng/dL in patients with non–COVID-19 respiratory tract infection and median 332 ng/dL in control cases, (P < .0001). The proportion of patients with testosterone deficiency in group 1, group 2, and group 3 was 74.2%, 53.3%, and 37.8%, respectively (P < .0001). Serum LH levels (P = 0.0003) and serum prolactin levels (P = .0007) were higher in patients with COVID-19 and patients with non–COVID-19 respiratory tract infection than control cases. Correlation analysis revealed significant negative correlation between serum TT levels and hospitalization time of patients with COVID-19 (r = –0.45, P < .0001). In addition, a significant positive correlation was observed between SpO2 and serum TT levels in patients with COVID-19 ( r = 0.32, P = .0028).
Clinical Implications
Physicians may consider to evaluate male patients with COVID-19 for concomitant androgen deficiency.
Strengths & Limitations
Strengths include the evidence about the alteration of male reproductive hormones under COVID-19. Limitations include the analysis limited to one general hospital, only a single measurement of TT was available, free and bioavailable testosterone levels were not evaluated.
CONCLUSION
This study demonstrates COVID-19 is associated with decreased level of TT and increased level of LH and prolactin. More serious COVID-19 causes more reduction in TT levels and prolongs hospitalization period.
Keywords: COVID-19, Testosterone, Luteinizing Hormone, Prolactin, Follicular Stimulant Hormone
INTRODUCTION
In early December 2019, a new contagious disease were identified in China 1 and caused by as a novel beta coronavirus 2 that has currently been named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).3 It causes respiratory illness named by the World Health Organization as coronavirus disease 2019 (COVID-19) and has become a global viral pandemic and a public health problem of international concern.4
The SARS-CoV-2 infection presents with a broad clinical spectrum, including asymptomatic infection, mild upper respiratory tract infection, to a life-threatening multiorgan failure. This broad clinical presentation is an evidence of the multiple targets of SARS-CoV-2 including digestive, cardiovascular, genitourinary, urinary systems.5–7 Similar to SARS, SARS-CoV-2 recognizes the N-terminal peptidase domain of angiotensin-converting enzyme 2 (ACE2) at the surface of the cell membrane using the S domain and invade the host.8 Based on this relationship between ACE2 and SARS-CoV-2, the susceptibility of SARS-CoV-2 infection is higher in any cells expressing ACE2. Meng-Yuan et al demonstrated that the ACE2 protein had high expression level in the testis, kidney, small intestine, heart, thyroid, and adipose tissue.9,10 Wang et al11 analyzed scRNA-seq data sets obtained from Gene Expression Omnibus and Sequence Read Archive, and showed that ACE2 is highly expressed in spermatogonia, Leydig and Sertoli cells. As a consequence, the testicular tissue may be a target tissue of SARS-CoV-2. After the binding of SARS-CoV-2 to ACE2 and virion membrane fusion, downregulation of ACE2 expression leads to excessive production of angiotensin by the related enzyme ACE.12 A previous study showed that increased replication of coronaviruses downregulates the expression of ACE2.13 Overproduction of angiotensin enhances oxidative stress mechanisms and leads to tissue injury. In sum, these findings may explain the possibility of harmful effects of SARS-CoV-2 on testis, potentially affecting total testosterone (TT) secretion. In analogy to this relationship, it has been shown that the testis may be the target organ for other viruses, including human immunodeficiency virus, mumps, Zika and Ebola causing orchitis, and occasionally decreased TT production and oligospermia.14
Acute respiratory tract infection is another cause of decreased serum TT levels. Muehlenbein et al15 hypothesized that TT levels decreases during acute infection and they evaluated patients with respiratory tract infection. The cases of their study experienced 10% lower salivary TT levels during respiratory tract infection compared with recovered period. In another study by Iglesias et al,16 the authors evaluated serum TT levels in hospitalized patients. Their results demonstrated that serum TT levels were associated with respiratory tract infection and were significantly lower in patients with respiratory tract infection. Moreover, the main cause of hospitalization of hypogonadic patients was respiratory tract infection.
The present study aimed to compare male reproductive hormones such as TT, luteinizing hormone (LH), follicle stimulant hormone (FSH), and prolactin between patients with COVID-19, age-matched cases with non–COVID-19 respiratory tract infection, and age-matched controls.
MATERIALS AND METHODS
Study Design
This study was reviewed and approved by institutional ethics committee, on 27 April 2020 and complied with the Declaration of Helsinki. The information for all patients including demographic data, clinical characteristics, laboratory parameters and outcomes were collected prospectively. 2 authors independently reviewed the data collection forms to ensure that there was no duplicated information. Data were analyzed and interpreted by the authors.
Study Population
The study population comprised 3 groups including patients hospitalized for COVID-19, hospitalized cases with non–COVID-19 respiratory tract infection between 22 March and 22 May 2020 and age-matched controls admitted to the urology outpatient department before January 2020. The first group was patients with COVID-19, the second groups included patients with non–COVID-19 respiratory tract infection, and the third consisted of control cases admitted to urologic outpatient clinic for reproductive function evaluation.
The first inclusion criterion for whole groups was age between 18 and 65 years. Additional inclusion criteria for group 1 were (1) positive reverse-transcription polymerase chain reaction (RT-PCR) test of nasal and pharyngeal swab specimens, (2) typical chest CT findings including ground-glass opacities, particularly on peripheral and lower lobes, crazy paving and bilateral multiple lobular and subsegmental areas of consolidation. Patients with non–COVID-19 respiratory tract infection had final diagnosis of at least 2 negative RT-PCR tests and negative chest CT findings. The exclusion criteria for all study subjects were (1) previous exposure to exogenous testosterone, 5-alpha reductase inhibitors, LH-releasing hormone agonists, dehydroepiandesterone, clomiphene citrate, or other selective estrogen receptor modulators, (2) use of opioid drugs within 3 months before study, (3) diagnosis of prolactinoma and any cancer, (4) history of testicular diseases and surgical interventions known to affect sex hormone levels.
Nasal and pharyngeal swab specimens were collected for the COVID-19 test on the day of admission. RT-PCR assay was used as per the manufacturer’s instructions. The degree of severity (mild, moderate, and severe) was determined in accordance with the COVID-19 Treatment Guidelines published by the National Institutes of Health.17
Clinical, Laboratory, and Imaging Parameters
The recent medical history, clinical symptoms or signs on admission were extracted from electronic medical records. Radiologic assessments included CT, and all laboratory testing was performed in accordance with the clinical care needs of the patient. Laboratory assessments were drawn before any treatment the first morning after admission between 8 and 11 am, after an overnight fast, and consisted of a blood count, C-reactive protein (CRP), procalcitonin, D-dimer, and fibrinogen. After analyzing of blood samples for routine tests required for group-1 and group-2 patients’ needs, the residual serum samples were collected for male hormone profiles detection. The data of serum TT, FSH, LH, and prolactin levels were retrieved from the medical records kept in the hospital. Laboratory analyses were performed in the central laboratory of the hospital with commercially available kits normally used for clinical practice of the hospital. The presence of a radiologic abnormality on the basis of the documentation or description in medical charts was determined.
Definitions
Testosterone deficiency was defined as <300 ng/dL in accordance with the American Urological Association testosterone deficiency guideline.18 Compensated or subclinical hypogonadism was defined as low-normal TT levels (>300 ng/dL) and elevated LH (>9.4 IU/L) as per the European Male Aging Study criteria.19
Outcomes
The primary outcome of the study was detection of the difference of TT, FSH, LH, and prolactin levels between the groups. Secondary outcome was to correlate TT and hospitalization time and oxygen saturation on hospital admission (SpO2) of patients.
Statistical Analysis
Sample size calculation was based on changes in serum TT levels. Type I error α was 0.05, type II error β was 0.10, two-tailed P-value was <0.05, study and control group ratio was 1:1, loss-to-follow-up rate was 10%, and at least 90 patients in each group were studied in the final analysis. Data were checked for suitability for a normal distribution with the Shapiro-Wilk test and expressed as mean (SD) or median [interquartile range (IQR)] values when they did not show a normal distribution or percentage for continuous and categorical variables, respectively. Variables were compared between groups by using the Fisher exact test or chi-square test for categorical variables. Kruskal-Wallis test with Bonferroni correction was used for multiple and pairwise comparisons, for continuous variables. Pearson’s correlation test was used for investigating relationship between continuous variables. All analyses were performed by STATA, version 14 (Stata Corp., College Station, TX, USA). For all the statistical analyses, P < .05 was considered significant.
RESULTS
Demographics and Characteristics of the Study Population
Baseline demographic and clinical characteristics for the patients with COVID-19 (n = 89), those with non–COVID-19 respiratory tract infection (n = 30) and those with neither COVID-19 nor respiratory tract infection (n = 143) presented in Table 1 . Mean age of the study population was 50.28 ± 9.8 years (20-65 years). The mean age of study groups was 49.9 ± 12.5 years, 52.7 ± 9.6 years, and 50 ± 7.8 years, respectively. Cases in 3 study groups were similar of age (P = .06).
Table 1.
Demographic and clinical parameters of study groups
| Sample characteristics | Group 1 (n = 89) | Group 2 (n = 30) | Group 3 (n = 143) | P |
|---|---|---|---|---|
| Age (years) (mean ± SD) | 49.9 ± 12.5 | 52.7 ± 9.6 | 50 ± 7.8 | .06 |
| Symptoms [n (%)] | ||||
| Fever | 48 (54) | 14 (46.7) | n/a | .14 |
| Cough | 44 (49.4) | 7 (23.3) | ||
| Sore throat | 12 (13.5) | 3 (10) | ||
| Myalgia | 29 (32.6) | 5 (16.6) | ||
| Dyspnea | 30 (33.7) | 12 (40) | ||
| Chest pain | 8 (18.56) | 7 (23.3) | ||
| Diarrhea | 3 (3.3) | 2 (6.6) | ||
| Anosmia | 2 (2.2) | 0 (0) |
SD = standard deviation.
Clinical Characteristics of Patients With COVID-19
89 cases infected with COVID-19 were studied (Table 1). Fever (54%) and cough (49.4%) were the most common symptoms. 30 cases (33.7%) had shortness of breath. Moreover, 29 (32.6%) patients had myalgia, 12 patients (13.5%) had sore throat, and 8 patients (18.56%) had chest pain symptoms. During the diagnostic procedure by RT-PCR, we found that 77 patients (86.5%) got a positive result in the first test and 12 patients (13.5%) got a positive result in the second test.
Among 89 patients with COVID-19, 52.8% (47/89) were diagnosed as “mild type”, 33.7% (30/89) as “moderate type”, and 13.5% (12/89) as “severe type”.
Clinical Characteristics of Patients With Non–COVID-19 Respiratory Tract Infection
30 patients had non–COVID-19 respiratory tract infection were studied (Table 1). Fever (46.7%) and dyspnea (40%) were the most common symptoms. 7 cases (23.3%) had chest pain and 7 cases (23.3%) had cough. In addition, 5 (16.6%) patients had myalgia, 3 patients (10%) had sore throat symptoms, and 2 (6.6%) had diarrhea. 21 patients had 2 negative RT-PCR, 8 patients had 3 negative RT-PCR tests, and one patient had 5 negative RT-PCR tests. All patients with non–COVID-19 respiratory tract infection had non-specific CT findings.
Comparison of Inflammation Parameters of Study Groups
The clinical presentation of patients with COVID-19 and patients with non–COVID-19 respiratory tract infection was similar (P = .14) (Table 1). Only the patients with COVID-19 had more cough symptom than the other group (P = .018). The comparison of inflammation parameters are presented in Table 2 . The white blood cell count of the COVID-19 group (median 6.08 109/L, IQR 3.36 109/L) was significantly lower than that of the non–COVID-19 respiratory tract infection (median 8.71 109/L, IQR 4.98 109/L) and control group (median 7.7 109/L, IQR 2.52 109/L) (P = .0001). It was not different between non–COVID-19 respiratory tract infection and control group (P = .07). The lymphocyte count in the COVID-19 group (median 1.4 109/L, IQR 0.83 109/L) was also significantly lower than that in the non–COVID-19 respiratory tract infection (median 2.13 109/L, IQR 1.46 109/L) and control group (median 2.3 g/L, IQR 0.96 g/L) (P = .0001). The lymphocyte count was not different between non–COVID-19 respiratory tract infection and control group (P = .07).
Table 2.
Inflammation parameters of study groups
| Sample characteristics | Group 1 (n = 89) | Group 2 (n = 30) | Group 3 (n = 143) | P |
|---|---|---|---|---|
| Median Hb, g/dL | 14.42 ± 3.37 | 13.13 ± 2.48 | 14.5 ± 1.58 | .0574 |
| Median (IQR) WBC, x 109/L | 6.08 (3.36) | 8.71 (4.98) | 7.7 (2.52) | .0001∗ |
| Median (IQR) LYM, x 109/L | 1.44 (0.83) | 2.13 (1.46) | 2.3 (0.96) | .0001† |
| Median (IQR) CRP, mg/dL | 42.46 (58.97) | 29.93 (68.98) | 0.6 (1.81) | .01‡ |
| Median (IQR) d-dimer, mg/L | 0.54 (0.62) | 0.86 (1.71) | n/a | .007 |
| Median (IQR) procalcitonin, ng/mL | 0.07 (0.13) | 0.07 (0.21) | n/a | .51 |
| Median (IQR) fibrinogen, mg/dL | 496.7 (164.6) | 413.15 (208.2) | n/a | .32 |
Post hoc analyses:
IQR = interquartile range.
1 vs 2 P < .0001; 1vs 3 P = .017; 2 vs3 P = .07.
1 vs 2 P < .0001; 1 vs 3 P = .0017; 2 vs 3 P = .07.
1 vs 2 P = .31; 1 vs 3 P = .0065; 2 vs 3 P = .03.
Hemoglobin levels of patient and control groups were not statistically different (P = .0574) (Table 2). CRP levels were significantly higher in patients with COVID-19 and non–COVID-19 respiratory tract infection than control group (P = .0007 and P = .03). However, COVID-19 and non–COVID-19 respiratory tract infection groups were similar in terms of CRP (P = 1). In addition, the D-dimer, procalcitonin, and fibrinogen levels were not significantly different between group-1 and group 2 (P = .03, P = .51 and P = .32, respectively).
Comparison of Hormonal Level
The comparison of TT levels showed a significant difference between the groups (P = .0001). Serum TT levels decreased from median 332 ng/dL in control cases to median 288.67 ng/dL in patients with non–COVID-19 respiratory tract infection to median 185.52 ng/dL in patients with COVID-19. The proportion of patients with testosterone deficiency in group 1, group 2, and group 3 was 74.2, 53.3, and 37.8%, respectively (P < .0001). 4 cases (4.5%) in the COVID-19 group had compensated or subclinical hypogonadism and only a patient in group 2 (3.33%) had this diagnosis (P = .06). Patients with COVID-19 and patients with non–COVID-19 respiratory tract infection had significantly higher serum LH (P = .0003) and serum PRL (P = .0007) levels compared with control cases. However, there was not a difference between group 1 and group 2 (P = 1 and P = 1). When serum FSH levels were compared, the groups had similar levels of FSH levels (P = .91). The ratio of LH/TT is lowest in patients with COVID-19 (Table 3).
Table 3.
Hormonal levels of study groups
| Sample characteristics | Group-1 (n = 89) | Group-2 (n = 30) | Group-3 (n = 143) | P |
|---|---|---|---|---|
| Median (IQR) TT, ng/dL | 185.52 (179.12) | 288.67 (184.39) | 332 (118) | .0001∗ |
| Median (IQR) LH, U/L | 5.67 (4.52) | 5.39 (2.46) | 4.1 (2.62) | .0002† |
| Median (IQR) prolactin, μg/L | 9.6 (5.59) | 9.61 (9.69) | 7.5 (1.86) | .0009‡ |
| Median (IQR) FSH, U/L | 6.01 (6.17) | 5.65 (6.19) | 6.05 (4.85) | .87 |
| Median LH/T | 0.03 (0.029) | 0.0205 (0.051) | 0.011 (0.011) | .0001§ |
Post hoc analyses:
IQR = interquartile range; TT = total testosterone; LH = luteinizing hormone; FSH = follicle stimulant hormone.
1 vs 2 P = .002; 1 vs 3 P < .0001; 2 vs 3 P = .04.
1 vs 2 P = 1; 1 vs 3 P = .0001; 2 vs 3 P = .01.
1 vs 2 P = 1; 1 vs 3 P = .0007; 2 vs 3 P = .03.
1 vs 2 P = .048; 1 vs 3 P < .0001; 2 vs 3 P < .0001.
The Impact of Severity of COVID-19 on Reproductive Hormones
The comparison of hormone levels of patients with COVID-19 classified into 3 different groups as per disease severity is presented in Table 4 . Despite the serum TT levels of patients with severe illness were lower than other patients, this difference was not statistically different (P = .084). However, serum LH and FSH levels were significantly lower in the patients with severe illness (P = .0348).
Table 4.
Hormonal levels of patients classified according to the severity of the disease
| Sample characteristics | Mild | Moderate | Severe | P |
|---|---|---|---|---|
| Median (IQR) TT, ng/dL | 203.51 (210.01) | 179.61 (105.55) | 82.01 (264.08) | .0837 |
| Median (IQR) LH, U/L | 5.59 (4.3) | 7.32 (4.62) | 3.71 (5.71) | .0107∗ |
| Median (IQR) prolactin, μg/L | 9.05 (5.95) | 10.35 (4.91) | 11.55 (32.02) | .1683 |
| Median (IQR) FSH, U/L | 5.8 (5.76) | 7.59 (7.74) | 3.84 (3.49) | .0348† |
Post hoc analyses:
IQR = interquartile range; TT = total testosterone; LH = luteinizing hormone; FSH = follicle stimulant hormone.
1 vs 2 P = .109; 1 vs 3 P = .1085; 2 vs 3 P = .005.
1 vs 2 P = .165; 1 vs 3 P = .205; 2 vs 3 P = .0182.
Correlation Between TT and Clinical Parameters of Patients
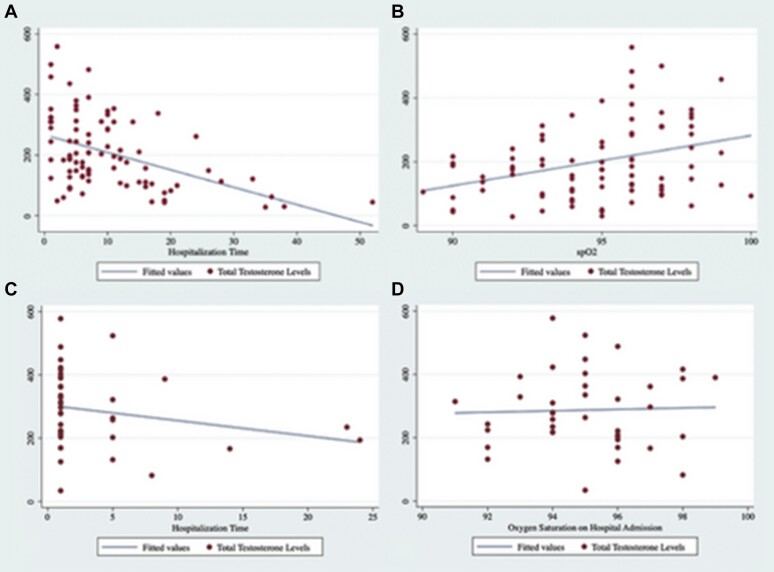
Pearson’s correlation test was performed for investigating any significant relationships between serum TT levels and hospitalization time of patients with COVID-19. Serum TT was found to be negatively associated with hospitalization time of patients with COVID-19 (r = -0.45, P < .0001) (Figure 1 A). In addition, in accordance with the results, a significant positive correlation was observed between SpO2 and serum TT levels in patients with COVID-19 (r = 0.32, P = .0028) (Figure 1B). However, no significant correlations were observed between TT and hospitalization time (Figure 1C) and SpO2 levels (Figure 1D) in patients with non–COVID-19 respiratory tract infection (r = -0.25, P = .21 and r = -0.077, P = .68). Moreover, the correlation between serum TT levels and neutrophil and lymphocyte counts and CRP levels was evaluated and it was not detected any significant association between these parameters. In the present study, only 4 patients with COVID-19 died and the mean levels of serum TT of these patients (116.24) was below the median levels of patients with COVID-19.
Figure 1.
A. Correlation between total testosterone levels and hospitalization time in patients with COVID-19. B. Correlation between total testosterone levels and SpO2 levels in patients with COVID-19. C. Correlation between total testosterone levels and hospitalization time in patients with non–COVID-19 respiratory tract infection. D. Correlation between total testosterone levels and SpO2 levels in patients with non–COVID-19 respiratory tract infection. Figure 1 is available in color online at www.jsm.jsexmed.org.
DISCUSSION
To the best knowledge, despite this article does not present first the evaluation of reproductive hormonal levels in patients with COVID-19, it demonstrates for the first time that lower levels of TT are presented in patients with COVID-19. In addition, the serum TT levels were compared in different groups of patients with COVID-19 classified in accordance with the severity of illness. There was no significant decrease in serum TT levels, even though severity of COVID-19 increased. Serum TT levels were higher in patients with non–COVID-19 respiratory tract infection, but it was lower than controls. The lowest LH/TT ratio was observed in patients with COVID-19. The diagnosis of testosterone deficiency was higher in patients with COVID-19 than in patients with non–COVID-19 respiratory tract infection and control group (P < .00001). However, compensated or subclinical hypogonadism was not different between (P = .06). Moreover, serum LH and prolactin levels were higher in patients with COVID-19 and non–COVID-19 respiratory tract infection. Interestingly, even though the prolactin levels did not differ with the severity of disease, the patients with severe disease had lowest LH levels in comparison with mild and moderate disease patients. Despite of harmful influence of SARS-CoV-2 on LH and TT levels, FSH levels did not differ between patients and control cases. Noteworthy, this study demonstrated a positive relationship between lower TT and longer hospitalization time, thus leading to a prediction of the progression of the disease of patients with COVID-19. Accordingly, lower TT levels are significantly associated with lower SpO2 levels at admission of patients with COVID-19. However, this relationship was not observed in patients with non–COVID-19 respiratory tract infection because of higher serum TT levels in this patient group than in patients with COVID-19. Clinical presentation of patients with COVID-19 did not differ from patients with non–COVID-19 respiratory tract infection. Both of groups presented similar symptoms on admission.
In the literature, there are several studies demonstrated that the testis is susceptible to viral infection.20 In comparison with bacterial infections that normally target accessory glands and epididymis, viruses that circulate in the blood primarily attack testis. It is known that a human immunodeficiency virus (HIV), mumps virus and Zika virus may induce reduction in testosterone production in human, feline leukemia virus in cats, and bluetongue virus in ruminants.20–22 The deleterious effects of viruses involve the direct damage of testicular tissue, destruction of Leydig cells and decreasing testosterone production. Puggioni et al22 showed that bluetongue virus replicated in the endothelial cells of the peritubular areas within the testis. It caused to enhanced type-I interferon response, reduction in testosterone biosynthesis by Leydig cells, and even destruction of Sertoli cells.22 However, a present study by Ma et al23 comparing the sex-related hormones between patients with COVID-19 and controls did not show any difference in serum TT and FSH levels between patients with COVID-19 and control cases. This result is not compatible with the findings of this study because of decreased serum TT and FSH levels of patients with COVID-19 compared with age-matched males.
The testicular function may be affected by multiple pathogenic mechanisms occurring during SARS-CoV-2 infection. ACE2 is highly expressed by Leydig cells and its downexpression by SARS-CoV-2 leads to increase of angiotensin II levels.12 It has been shown that angiotensin II reduced both basal and LH-stimulated testosterone synthesis by Leydig cells, ACE2 may modulate the production of testosterone of these cells and protect testis by limiting angiotensin II detrimental effects.24,25 In this context, changes in LH and TT levels of patients were evaluated in present study and it has been determined that patients with COVID-19 had increased levels of LH and decreased levels of TT compared with patients with non–COVID-19 respiratory tract infection and control cases. Moreover, the lowest LH/TT ratio was calculated in patients with COVID-19. In the literature, several studies suggested that increased LH/TT ratio indicates Leydig cell defects and it is an important marker of primary hypogonadism.26 Primary hypogonadism is a result of factors having negative influence on testicular tissue such as injury, tumor, and infections. This finding supports the impact of harmful effect of SARS-CoV-2 on ACE2 expression and correspondingly increase of angiotensin II and reduction of testosterone production.
Compensated hypogonadism is defined as TT in the normal range and inappropriately high LH.19 In European Male Aging Study including 3,369 community-dwelling middle-aged and older men, they reported that 9.5% of the aforementioned population was classified as compensated hypogonadism. Dutta et al27 presented compensated hypogonadism rates as 12.44% in 225 patients with HIV infection. The rate of compensated hypogonadism in this study was 4.5% in patients with COVID-19. The rate of compensated hypogonadism in patients with COVID-19 was lower than the study by Dutta et al because the disease duration of patients with HIV was 40 months and this long time may influence negatively the hormonal status of patients. However, the rate of patients with COVID-19 is based on acute phase effects of disease and the evaluation of long term effects of COVID-19 on hormonal status may contribute to understand its hormonal impacts.
The serum PRL level also significantly elevated in patients with COVID-19 and in patients with non–COVID-19 respiratory tract infection compared with control cases (P = .0009). However, this elevation reached not to levels of hyperprolactinemia. Therefore this is not a cause of pituitary suppression and decreased TT levels. Serum PRL levels may be influenced by stress and infection.28 In addition, cytokines IL-1, IL-2, and IL-6 stimulate prolactin secretion.29 The increased levels of IL-1, IL-2, and IL-6 in patients with COVID-19 have been demonstrated in several studies.30 This relationship between increased levels of interleukins and suppression of prolactin secretion explained the elevated levels of prolactin in patients with COVID-19.
In the present study, the TT levels correlated negatively with hospitalization time (r = -0.395, P < .0001) and positively with SpO2 levels at admission of patients with COVID-19. This finding demonstrates that serum TT levels is an important factor and predictor for the clinical process of patients. Rastrelli et al31 evaluated the association between TT levels and clinical outcomes in a cohort of patients with COVID-19. They demonstrated that lower TT levels predict poor prognosis in patients with COVID-19. They correlated TT levels with neutrophil and lymphocyte counts and CRP levels and they found a negative correlation between serum TT levels and neutrophil count and CRP levels and a positive correlation between TT levels and lymphocyte count. However, in the present study, serum TT levels did not correlated with neutrophil and lymphocyte counts and CRP levels.
Testosterone deficiency is one possible cause of unexplained anemia in older men because of the lack of stimulating effect of testosterone on erythropoiesis. Despite of lower levels of testosterone in patients with COVID-19, the hemoglobin levels were not different from patients with non–COVID-19 respiratory tract infection and control subjects. The reason for this indifference could be divided into 2 subjects. First of them is long life span of erythrocytes and short incubation period of COVID-19. After 4-6 days’ incubation period, symptoms onset occurred and patients admitted to the hospitals and were included into this study. An erythrocyte has a life span 90-120 days in human circulation. The time interval is not enough to cause in a decrease in hemoglobin levels. In addition, myelosuppressive chemotherapy is an important factor for the treatment-associated anemia and this anemia is detectable at least 4-6 weeks after this therapy due to the life span of erythrocytes in serum.32 The second factor is the required time for the suppression of erythropoiesis by testosterone deficiency. The exact mechanism for the regulation of erythropoiesis by testosterone is not fully understood. Testosterone stimulates erythrocyte production by various mechanisms including stimulation of erythropoietin release, direct stimulatory action on bone marrow erythroid progenitor cells via androgen receptors and iron regulatory protein hepcidin.33 In the light of aforementioned evidences, it is not surprising testicular deficiency leads to anemia. However, the onset of anemia needs a period of time. Hamilton et al, presented a time span for hemoglobin reduction as 1 g/dl after involuntary castration in 6 prisoners as 40 days. In another study of 147 prostate cancer patients receiving combined androgen blockage, hemoglobin levels decreased in all patients from a mean baseline of 14.9 g/dL to means of 13.9 at 1 month.34 With these findings, the indifference of hemoglobin levels between patient and control groups is logical.
This study has several strengths. The study provides the evidence about the alteration of male reproductive hormones under COVID-19. In this study, serum LH and prolactin levels significantly elevated and serum TT levels decreased in patients with COVID-19, which infer to the potential hypogonadism. Because greater portion of patients with COVID-19 were reproductive-aged, cases, who recovered from this disease, should be evaluated with hormonal tests to detect harmful effects of SARS-CoV-2 on reproductive system.
There are several limitations in our study. First, the analysis in our study was limited to one general hospital, but all cases had fulfilled the laboratory diagnosis of COVID-19. Control group included patients who admitted to urology outpatient clinic and evaluated for reproductive function had hormonal abnormalities. As another limitation, only a single measurement of TT was available. Moreover, free and bioavailable testosterone levels were not evaluated. Another limitation was neither semen analysis nor determination of SARS-CoV-2 in semen was performed, which are more clear evidence for SARS-CoV-2–induced testis injury. Psychological stress is another factor effecting serum TT levels. A current study evaluated psychological status of patients admitted with COVID-19 and presented that the predominant emotional state of patients for most days during the stay or for almost all the days during the hospital stay was that of anxiety (92%), remaining worried (96%), and feeling isolated (90%).35 However, the authors could not compare their results with that of existing literature because of limited data on experiences of people with COVID-19. Several studies evaluating the impact of stress on hormonal function in both human and animals confirmed that psychological stress, especially chronic stress, inhibiting role in testosterone production.36 Contrary to these studies presenting the decreasing effect of stress in serum testosterone levels, in the literature, it has been shown psychological stress does not constantly inhibit testosterone secretion.37 In addition to this contradictory studies, a few studies exist which presented that testosterone concentration may be increased at initial stages of acute stress.38,39 Based on the aforementioned findings, it is not possible to associate the reduction of serum TT levels in patients with COVID-19 with their psychological stress in acute phase of the disease. However, even if not the lack of the evaluation of the emotional status of patients and controls is a limitation of the study.
CONCLUSION
This study demonstrates COVID-19 is associated with decreased level of TT and increased level of LH and prolactin. More serious COVID-19 causes more reduction in TT levels and prolongs hospitalization period. In light of these findings, physicians may consider to evaluate male patients with COVID-19 for concomitant androgen deficiency.
STATEMENT OF AUTHORSHIP
Mustafa Kadihasanoglu: Conceptualization, Methodology, Formal Analysis, Writing – Original Draft, Writing – Review & Editing; Semih Aktas: Formal Analysis, Investigation, Data Curation; Emre Yardimci: Formal Analysis, Investigation, Data Curation; Hale Aral: Methodology, Formal Analysis, Investigation, Data Curation, Writing – Review & Editing; Ates Kadioglu: Writing – Review & Editing, Supervision.
Funding
None.
Contributor Information
Mustafa Kadihasanoglu, Department of Urology, Istanbul Training and Research Hospital, Istanbul, Turkey.
Semih Aktas, Department of Urology, Istanbul Training and Research Hospital, Istanbul, Turkey.
Emre Yardimci, Department of Urology, Istanbul Training and Research Hospital, Istanbul, Turkey.
Hale Aral, Istanbul Training and Research Hospital, Department of Biochemistry, Istanbul, Turkey.
Ates Kadioglu, Istanbul University, Istanbul Faculty of Medicine, Department of Urology, Istanbul, Turkey.
REFERENCES
- 1. Huang C., Wang Y., Li X.et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395: 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lu R., Zhao X., Li J.et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 2020;395: 565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhu N., Zhang D., Wang W.et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med 2020;382: 727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Coronavirus disease (COVID-19) outbreak . World Health Organization; 2020. Available from: https://www.who.int. Accessed April 20, 2020.
- 5. Guan W.J., Ni Z.Y., Hu Y.et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med 2020;382: 1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mumm J.N., Osterman A., Ruzicka M.et al. Urinary Frequency as a Possibly Overlooked Symptom in COVID-19 Patients: Does SARS-CoV-2 Cause Viral Cystitis? Eur Urol 2020;78: 624–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Argenziano M.G., Bruce S.L., Slater C.L.et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ 2020;369: m1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yan R., Zhang Y., Li Y.et al. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 2020;367: 1444–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li M.Y., Li L., Zhang Y.et al. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty 2020;9: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Atlas THP . ACE2 2020. Available from: https://www.proteinatlas.org/ENSG00000130234-ACE2. Accessed April 20, 2020
- 11. Wang Z., Xu X.. scRNA-seq Profiling of Human Testes Reveals the Presence of the ACE2 Receptor, A Target for SARS-CoV-2 Infection in Spermatogonia, Leydig and Sertoli Cells. Cells 2020;9: 920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gurwitz D. Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev Res 2020;81: 537–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dijkman R., Jebbink M.F., Deijs M.et al. Replication-dependent downregulation of cellular angiotensin-converting enzyme 2 protein expression by human coronavirus NL63. J Gen Virol 2012;93: Pt 9: 1924–1929. [DOI] [PubMed] [Google Scholar]
- 14. Papadopoulos V., Li L., Samplaski M.. Why does COVID-19 kill more elderly men than women? Is there a role for testosterone? Andrology 2020. 10.1111/andr.12868In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Muehlenbein M.P., Hirschtick J.L., Bonner J.Z.et al. Toward quantifying the usage costs of human immunity: Altered metabolic rates and hormone levels during acute immune activation in men. Am J Hum Biol 2010;22: 546–556. [DOI] [PubMed] [Google Scholar]
- 16. Iglesias P., Prado F., Macías M.C.et al. Hypogonadism in aged hospitalized male patients: prevalence and clinical outcome. J Endocrinol Invest 2014;37: 135–141. [DOI] [PubMed] [Google Scholar]
- 17. C.-T.G. Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health; 2020. Available from: https://www.covid19treatmentguidelines.nih.gov/. Accessed April 20, 2020. [PubMed] [Google Scholar]
- 18. Mulhall J., Trost L., Brannigan R.et al. Evaluation and Management of Testosterone Deficiency. Linthicum, MD: AUA Guideline American Urological Association, 2018. [DOI] [PubMed] [Google Scholar]
- 19. Tajar A., Forti G., O’Neill T.W.et al. Characteristics of secondary, primary, and compensated hypogonadism in aging men: evidence from the European Male Ageing Study. J Clin Endocrinol Metab 2010;95: 1810–1818. [DOI] [PubMed] [Google Scholar]
- 20. Dejucq N., Jégou B.. Viruses in the mammalian male genital tract and their effects on the reproductive system. Microbiol Mol Biol Rev 2001;65: 208–231. first and second pages, table of contents [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stassen L., Armitage C.W., van der Heide D.J.et al. Zika Virus in the Male Reproductive Tract. Viruses 2018;10: 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Puggioni G., Pintus D., Melzi E.et al. Testicular Degeneration and Infertility following Arbovirus Infection. J Virol 2018;92: e01131-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ma L., Xie W., Li D.et al. Evaluation of sex-related hormones and semen characteristics in reproductive-aged male COVID-19 patients. J Med Virol 2020. 10.1002/jmv.26259In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Khanum A., Dufau M.L.. Angiotensin II receptors and inhibitory actions in Leydig cells. J Biol Chem 1988;263: 5070–5074. [PubMed] [Google Scholar]
- 25. Dufau M.L., Khanum A., Winters C.A.et al. Multistep regulation of Leydig cell function. J Steroid Biochem 1987;27: 343–350. [DOI] [PubMed] [Google Scholar]
- 26. Abbaticchio G., Nacucchi O., Giagulli V.A.et al. Exploration of the testis in infertile men. Relationships among serum levels of FSH, LH, 17-alpha-OH-progesterone and testosterone. Andrologia 1990;22: 231–237. [DOI] [PubMed] [Google Scholar]
- 27. Dutta D., Sharma L.K., Sharma N.et al. Occurrence, patterns & predictors of hypogonadism in patients with HIV infection in India. Indian J Med Res 2017;145: 804–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Majumdar A., Mangal N.S.. Hyperprolactinemia. J Hum Reprod Sci 2013;6: 168–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shelly S., Boaz M., Orbach H.. Prolactin and autoimmunity. Autoimmun Rev 2012;11: A465–A470. [DOI] [PubMed] [Google Scholar]
- 30. Tufan A., Avanoğlu Güler A., Matucci-Cerinic M.. COVID-19, immune system response, hyperinflammation and repurposing antirheumatic drugs. Turk J Med Sci 2020;50: 620–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rastrelli G., Di Stasi V., Inglese F.et al. Low testosterone levels predict clinical adverse outcomes in SARS-CoV-2 pneumonia patients. Andrology 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Groopman J.E., Itri L.M.. Chemotherapy-induced anemia in adults: incidence and treatment. J Natl Cancer Inst 1999;91: 1616–1634. [DOI] [PubMed] [Google Scholar]
- 33. Shahani S., Braga-Basaria M., Maggio M.et al. Androgens and erythropoiesis: past and present. J Endocrinol Invest 2009;32: 704–716. [DOI] [PubMed] [Google Scholar]
- 34. Strum S.B., McDermed J.E., Scholz M.C.et al. Anaemia associated with androgen deprivation in patients with prostate cancer receiving combined hormone blockade. Br J Urol 1997;79: 933–941. [DOI] [PubMed] [Google Scholar]
- 35. O’Brien H., Tracey M.J., Ottewill C.et al. An integrated multidisciplinary model of COVID-19 recovery care. Ir J Med Sci 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chichinadze K., Chichinadze N.. Stress-induced increase of testosterone: contributions of social status and sympathetic reactivity. Physiol Behav 2008;94: 595–603. [DOI] [PubMed] [Google Scholar]
- 37. Hjollund N.H., Bonde J.P., Henriksen T.B.et al. Team DFPPS . Reproductive effects of male psychologic stress. Epidemiology 2004;15: 21–27. [DOI] [PubMed] [Google Scholar]
- 38. Wingfield J.C., Sapolsky R.M.. Reproduction and resistance to stress: when and how. J Neuroendocrinol 2003;15: 711–724. [DOI] [PubMed] [Google Scholar]
- 39. Wegner M., Koedijker J.M., Budde H.. The effect of acute exercise and psychosocial stress on fine motor skills and testosterone concentration in the saliva of high school students. PLoS One 2014;9: e92953. [DOI] [PMC free article] [PubMed] [Google Scholar]