Abstract
Objective
To evaluate the clinical course of and risk factors for arterial thrombotic events in adult inpatients with coronavirus disease 2019 (COVID-19).
Methods
All consecutive adult patients admitted for COVID-19 infection in a referral center in France and discharged from the hospital between April 1 and April 30, 2020, were included. All arterial thrombotic events that occurred through discharge were considered for analysis. Epidemiologic, demographic, clinical, laboratory, treatment, and outcome data were extracted from electronic medical records with use of a standardized data collection form.
Results
Overall, 531 COVID-19+ patients were analyzed. Among them, 30 (5.6%) experienced arterial thrombotic events. Arterial thrombotic events in the setting of COVID-19 infection happened at a median of 11 (5-20) days after the first symptoms of infection; occurred in high-risk patients according to traditional cardiovascular risk factors; had an atypical pattern, such as thrombosis of the aorta, upper limb, or renal arteries or cerebral microvasculopathy in 7 (23.3%) cases; and were associated with an in-hospital mortality rate of 40%. Arterial thrombotic events increased the risk of death by 3-fold in COVID-19+ patients (hazard ratio, 2.96; 95% CI, 1.4 to 4.7; P=.002). A subdistribution survival hazard model showed that a concentration of D-dimer above 1250 ng/mL increased the risk of arterial thrombotic events in COVID-19+ patients by more than 7 (subdistribution hazard ratio, 7.68; 95% CI, 2.9 to 20.6; P<.001).
Conclusion
A dramatically high rate of in-hospital death was observed in patients who suffered arterial thrombotic events in the setting of COVID-19 infection. A D-dimer level above 1250 ng/mL at entry may identify COVID-19+ patients at risk for arterial thrombotic events.
Abbreviations and Acronyms: COVID-19, coronavirus disease 2019; CRP, C-reactive protein; CT, computed tomography; CVE, cardiovascular event; HR, hazard ratio; RT-PCR, reverse transcriptase polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2
Patients with coronavirus disease 2019 (COVID-19) are at increased risk of thrombosis.1 Given the rapidly growing pandemic, there is an urgent need to measure the rate of thrombotic manifestations associated with COVID-19 and to analyze the impact on survival. Most studies in the field have reported on venous thrombosis and focused on patients hospitalized in intensive care units. The primary goal of this study was to assess the incidence of and risk factors for arterial thrombotic events in all hospitalized patients with COVID-19 in a referral center in France during 1 month.
Methods
Patients
All consecutive adult patients admitted for COVID-19 infection at Bichat Hospital, Paris, and discharged from the hospital between April 1 and April 30, 2020, were retrospectively reviewed. Bichat Hospital, Paris, is a referral center in France for patients with suspected or confirmed emerging infectious diseases.2 Inclusion criteria for COVID-19 inpatients were age older than 18 years, diagnosis of COVID-19 according to World Health Organization interim guidance, and positive response for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) on reverse transcriptase polymerase chain reaction (RT-PCR) testing of a respiratory sample (nasopharyngeal swab or invasive respiratory sample). COVID-19 infection was considered despite a negative SARS-CoV-2 RT-PCR test result at time of admission in patients who had close contact with a confirmed COVID-19 case before onset of symptoms, symptoms suggestive of COVID-19 infection (cough, fever, shortness of breath, sudden onset of anosmia, ageusia, or dysgeusia), typical computed tomography (CT) scan appearance (“crazy paving” pattern), and no alternative diagnosis. Patients not admitted because of mild clinical symptoms or who were transferred to another hospital or in whom COVID-19 infection was not confirmed after review were excluded. The International Classification of Diseases, Tenth Revision code for COVID-19 infection (U071) was used to screen patients. Data were extracted from the French Diagnosis Related Groups-based information system database. Information regarding eligibility conditions, exclusion criteria, and epidemiologic, demographic, clinical, laboratory, treatment, and outcome data were extracted from electronic medical records using a standardized data collection form.
Routine blood tests included at least complete blood count, electrolyte values, creatinine concentration, liver tests, lactate dehydrogenase level, C-reactive protein (CRP) level, D-dimer level, and ferritin activity. Laboratory confirmation for SARS-CoV-2 was obtained at the Bichat Hospital Virology Department, Paris, France. RT-PCR assays were performed in accordance with the protocol established by the World Health Organization (Coronavirus Disease [COVID-19]) Technical Guidance: Laboratory Testing for 2019-nCoV in Humans).
The severity of COVID-19 was analyzed at the time of admission on the basis of the extent (<10%, between 10% and 25%, between 25% and 50%, and >50%) of lung involvement on CT scan. All consecutive adult COVID-19–negative patients (Supplemental Table, available online at http://www.mayoclinicproceedings.org) admitted for arterial thrombotic events at Bichat Hospital, Paris, and discharged from the hospital between March 1 and March 31, 2020, were used as controls (Supplemental Figure 1, available online at http://www.mayoclinicproceedings.org).
Arterial Thrombotic Events
International Classification of Diseases, Tenth Revision codes for arterial thrombotic events were used to screen patients and controls, including I21.x, I236, I240, I513, I63.x, I65.x, I66.x, I676, I74.x, I788, H348, and H349. All arterial thrombotic events that occurred through discharge were considered for analysis. Extensive screening for conventional cardiovascular risk factors (age, sex, smoking status, body mass index, dyslipidemia, past cardiovascular event [CVE], high blood pressure, diabetes mellitus) was undertaken in all cases.
Ethical Statement
Our study is a human noninterventional study in which participants were assigned to a diagnosis strategy within current practice. The study involved products with a marketing authorization that are prescribed in the usual manner and used in accordance with authorizations by French agencies. Epidemiologic methods were used to analyze the data, and information used in the study was collected for clinical care. According to the Public Health French Law (French Research Standard MR-004), approval from an Institutional Review Board and written consent are not required for human noninterventional studies. For ethical consideration, however, patients were informed that data collected in medical records might be used for research study in accordance with the privacy rule. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki. Our study involves personal health data and has been authorized by the Commission nationale de l’informatique et des libertés (declaration number 2218921v0).
Statistical Analyses
Categorical variables are presented as number (percentage). Quantitative variables are presented as median (interquartile range). Odds ratio and hazard ratio (HR) are presented with 95% CIs. Factors associated with the presence of arterial thrombotic events on univariate and multivariable logistic regression procedures were analyzed. Data were compared between groups using the χ 2 (or Fisher) test for categorical variables and the Mann-Whitney U test for continuous variables. To perform the multivariate logistic regression procedure, we used all clinically relevant variables that had a level of significance <.20 as covariates. The onset of 2 major outcomes—death and arterial thrombotic events—from admission to last follow-up was considered. By use of the Kaplan-Meier method, the survival curve associated with death was plotted according to arterial thrombotic event status, and the survival curve associated with arterial thrombotic events was plotted according to the D-dimer level at admission. We calculated univariate HRs using Cox proportional hazard models. We used a subdistribution hazard model to analyze survival without an arterial thrombotic event, according to the presence of a high D-dimer level and considering death as a competing risk. All tests were two sided, and P values <.05 were considered to indicate a significant association. All the analyses were made with R software version 3.4.4 (R Foundation for Statistical Computing).
Results
Characteristics of Patients
A consecutive 531 patients with COVID-19 infection were admitted in our national referral center during a 1-month period (Supplemental Figure 1). The SARS-CoV-2 RT-PCR test result was positive on a respiratory sample in 499 (94%) cases. The median time between first symptoms and infection diagnosis was 7 (3 to 10) days.
At admission, 351 (66.1%) patients were male with a median age of 66.1 (52.8-77.1) years. Most patients had chronic comorbidities. More than 40% (217/531 patients) had 3 or more major cardiovascular risk factors besides advanced age and male sex, including smoking, overweight, dyslipidemia, past CVE, high blood pressure, and diabetes.
At admission, a thoracic CT scan showed pneumonia in all patients. Disease extension on chest CT scan was between 25% and 50% in 236 (44.4%) patients and more than 50% in 58 (10.9%) patients. Serum CRP level was high in 482 patients (90.8%) at admission and higher than 50 mg/dL in most cases (70.3%). Seventy (13.2%) patients had critical illness requiring mechanical ventilation. The median duration from admission to discharge was 9 (5 to 13) days. The time from admission to last follow-up was 20 (11-36) days. Overall in-hospital death was 17.5% (93/531; Table 1 ).
Table 1.
| All COVID-19+ (N=531) | COVID-19+ AT− (n=501 [94.4%]) | COVID-19+ AT+ (n=30 [5.6%]) | P | |
|---|---|---|---|---|
| Age (y) | 66.1 (52.8-77.1) | 65.7 (52.4-76.6) | 70.7 (57-79.5) | .24 |
| Male sex | 351 (66.1) | 327 (65.3) | 24 (80.0) | .14 |
| Laboratory confirmation of SARS-CoV-2 | 499 (94) | 472 (94.2) | 27 (90) | >.99 |
| Time of first symptoms to diagnosis (d) | 7 (3-10) | 7 (3-10) | 6 (2-8) | .74 |
| Comorbidities | ||||
| Cancerd | 46 (8.7) | 43 (8.6) | 3 (10) | .61 |
| ESRD | 35 (6.6) | 31 (6.2) | 4 (13.3) | .25 |
| HIV infection | 19 (4.1) | 18 (4.1) | 1 (3.3) | >.99 |
| Inflammatory disorderse | 38 (7.2) | 35 (7.0) | 3 (10) | .37 |
| Solid organ transplantf | 31 (5.8) | 29 (5.8) | 2 (6.7) | >.99 |
| Cardiovascular risk factors | ||||
| High blood pressure | 283 (53.4) | 265 (53.0) | 18 (60.0) | .56 |
| Smoking | 172 (34.2) | 159 (33.5) | 13 (43.3) | .30 |
| Diabetes | 173 (32.6) | 164 (32.8) | 9 (30.0) | .91 |
| Dyslipidemia | 171 (32.3) | 158 (31.7) | 13 (43.3) | .26 |
| Past CVE | 115 (21.7) | 101 (20.2) | 14 (46.7) | .001 |
| BMI (kg/m2) | 26.5 (24-30.7) | 26.7 (24-30.8) | 25.2 (23.6-28.4) | .22 |
| BMI >25 kg/m2 | 256 (48.2) | 242 (48.3) | 14 (46.7) | >.99 |
| Atrial fibrillation | 47 (8.9) | 43 (8.6) | 4 (13.3) | .58 |
| Treatment at admission | ||||
| Antiplatelet | 137 (25.8) | 125 (25.0) | 12 (40.0) | .11 |
| Statin | 138 (26.1) | 126 (25.3) | 12 (40.0) | .09 |
| ACE inhibitors or ARB | 183 (34.7) | 170 (34.2) | 13 (43.3) | .41 |
| Lung involvement on CT scan | ||||
| <10% | 67 (12.6) | 65 (13.0) | 2 (6.7) | .36 |
| 10%-25% | 177 (33.3) | 168 (33.5) | 9 (30.0) | .69 |
| 25%-50% | 180 (33.9) | 172 (34.3) | 8 (26.7) | .39 |
| >50% | 58 (10.9) | 51 (10.2) | 7 (23.3) | .02 |
| Critical illnessg | 70 (13.2) | 64 (12.8) | 6 (20.0) | .39 |
| Treatment received | ||||
| Antiviral drugs | 162 (30.6) | 149 (29.9) | 13 (43.3) | .18 |
| Hydroxychloroquine | 52 (9.8) | 50 (10) | 2 (6.7) | .76 |
| Steroids | 260 (49.1) | 244 (48.9) | 16 (53.3) | .78 |
| Anti–IL-1 | 87 (16.4) | 78 (15.6) | 9 (30.0) | .07 |
| Anti–IL-6 | 19 (3.6) | 18 (3.6) | 1 (3.3) | >.99 |
| Anticoagulant treatment | 267 (50.3) | 252 (50.3) | 15 (50) | >.99 |
| Laboratory findings | ||||
| Hemoglobin (g/dL) | 12.9 (11.5-14.2) | 12.9 (11.5-14.2) | 13 (11.5-14) | .65 |
| Hematocrit (%) | 37.9 (34.1-41) | 37.9 (34.1-41) | 38.6 (34.9-40.7) | .79 |
| Leukocytes (109/L) | 6.7 (4.9-8.9) | 6.8 (4.9-8.8) | 6.0 (4.4-9.7) | .97 |
| Neutrophils (109/L) | 4.9 (3.3-6.9) | 5.0 (3.3-6.9) | 4.3 (3.1-7.8) | .97 |
| Lymphocytes (109/L) | 0.99 (0.70-1.4) | 0.98 (0.71-1.38) | 1.09 (0.58-1.67) | .68 |
| Monocytes (109/L) | 0.50 (0.36-0.71) | 0.50 (0.36-0.70) | 0.60 (0.37-0.77) | .38 |
| Platelets (109/L) | 203 (160-266) | 203 (159-264) | 217 (179-310) | .26 |
| APTT (sec) | 34.1 (30.4-39.6) | 33.9 (30.4-39.4) | 36.7 (30.4-41.9) | .19 |
| PT (sec) | 11.3 (11.3-11.3) | 11.3 (11.3-11.3) | 11.3 (11.3-11.3) | .86 |
| Fibrinogen (g/L) | 5.4 (4.6-6.3) | 5.5 (4.6-6.3) | 4.9 (4.2-5.5) | .02 |
| D-dimer (ng/mL)h | 869 (525-1359) | 836 (514-1320) | 1860 (1268-7409) | <.001 |
| CRP (mg/L) | 78 (33-133) | 78 (33-133) | 109 (18-127) | .71 |
| Creatinemia (μm/L) | 79 (63-118) | 79 (63-117) | 90 (72-183) | .04 |
| LDH (U/L) | 320 (245-398) | 316 (247-391) | 378 (230-485) | .10 |
| AST (U/L) | 38 (24-61) | 38 (24-60) | 54 (27-94) | .03 |
| ALT (U/L) | 33 (23-50) | 33 (23-50) | 36 (23-60) | .70 |
| Ferritinemia (μg/L) | 74 (338-1359) | 737 (343-1350) | 870 (275-1500) | .59 |
| Follow-up | ||||
| Length of hospitalization (d) | 9 (5-13) | 9 (5-13) | 13 (8.2-24.7) | .005 |
| Length of follow-up (d) | 20 (11-36) | 20 (11-36) | 18 (9.75-39) | .96 |
| In-hospital deathi | 93 (17.5) | 81 (16.2) | 12 (40.0) | .002 |
ACE, angiotensin-converting enzyme; ALT, alanine transaminase; APTT, activated partial thromboplastin time; ARB, angiotensin II receptor blocker; AST, aspartate transaminase; AT, arterial thrombotic events; BMI, body mass index, calculated as weight in kilograms divided by height in meters squared; COVID-19, coronavirus disease 2019; CRP, C-reactive protein; CT, computed tomography; CVE, cardiovascular event; ESRD, end-stage renal disease; HIV, human immunodeficiency virus; IL, interleukin; LDH, lactate dehydrogenase; PT, prothrombin time; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
SI conversion factors: To convert hemoglobin values to g/L, multiply by 10; to convert fibrinogen values to μmol/L, multiply by 3401 to convert U/L values to μkat/L, multiply by 0.0167; to convert ferritin values to pmol/L, multiply by 0.445038.
Categorical variables are presented as number (%). Continuous variables are presented as median (interquartile range).
Cancer was considered when it was diagnosed less than 5 years before the COVID-19 infection. It included prostate cancer (n=8), breast cancer (n=8), colon cancer (n=7), low-grade non-Hodgkin lymphoma (n=7), skin cancer (n=5), myelodysplastic syndrome (n=4), lung cancer (n=2), endometrial/ovarian cancer (n=2), liver cancer (n=1), pancreatic cancer (n=1), and bladder cancer (n=1).
Inflammatory disorders were considered when corticosteroid use was required. They included asthma (n=13) and chronic autoimmune/inflammatory systemic diseases (n=25).
Solid organ transplant included kidney (n=19), heart (n=8), lung (n=3), and liver (n=1) transplants.
Critical illness was defined as mechanical ventilation. Anti–IL-1 treatment was anakinra; anti–IL-6 treatment was tocilizumab. Antiviral drugs included lopinavir/ritonavir (n=155) and remdesivir (n=7). Anticoagulant treatment was given at prophylactic or therapeutic dose in 217 (81.3%) and 50 (18.7%) patients, respectively.
The reference concentration of D-dimer at our institution is less than 234 ng/mL.
In-hospital death was defined as death at discharge.
Arterial Thrombotic Events in the Setting of COVID Infection
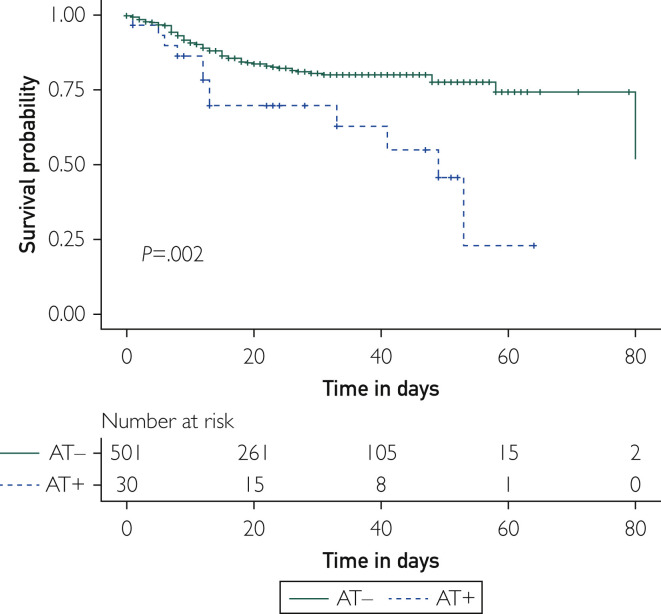
Thirty COVID-19+ patients (5.6%) experienced arterial thrombotic events that occurred 2 (0-11) days after admission and 11 (5-20) days after first symptoms of COVID-19 infection. An arterial thrombotic event was the first symptom of COVID-19 infection in 1 (3.3%) patient and the main reason for admission in 12 (40%) patients. Arterial thrombotic events consisted of myocardial infarction (n=9 [30%]), stroke (n=8 [26.7%]), and acute or subacute limb ischemia (n=6 [20%]). Interestingly, age, sex, high blood pressure, smoking, diabetes, dyslipidemia, past CVE, and body mass index were not statistically different between COVID-19+ and COVID-19− control in-hospital patients with arterial thrombotic events (Supplemental Table). However, despite a similar risk for a CVE, COVID-19+ patients displayed atypical arterial thrombotic events (n=7 [23.3%]), including massive aortic thrombosis (n=2), splenic infarction (n=1), renal artery thrombosis (n=1), diffuse cerebral microvasculopathy (n=1), and upper extremity arterial thrombosis (n=1), that were not observed in the control group (Supplemental Table). In-hospital death was dramatically higher in COVID-19+ patients with arterial thrombotic events compared with COVID-19− arterial thrombotic event controls (40% vs 3.1%; P<.001) and COVID-19+ patients with no arterial thrombotic events (40% vs 16.2%; P=.002). Overall, in the setting of COVID-19 infection, the Cox model showed that arterial thrombotic events increased the risk of death by 3-fold (HR, 2.96; 95% CI, 1.4 to 4.7; P=.002; Figure 1 ).
Figure 1.
Overall survival in COVID-19+ patients according to arterial thrombotic event (AT) status. Kaplan-Meier curves represent survival without in-hospital death according to AT status. Time 0 was the day of admission. Follow-up ended at discharge. The solid line represents the outcome in patients without AT (AT−). The dashed lines represent the outcomes in patients with AT (AT+). Analysis was performed on 531 patients.
Association Between D-Dimer Levels and Arterial Thrombotic Events in COVID-19 Infection
Considering its poor prognosis, we aimed to identify risk factors for arterial thrombotic events in COVID-19+ patients. By comparing the characteristics of COVID-19+ patients with and without arterial thrombotic events, we observed that a past CVE and a higher plasma level of D-dimer were both associated with arterial thrombotic events (Table 1). In the multivariable analysis, only D-dimer level was associated with arterial thrombotic events in COVID-19+ patients (Table 2 ).
Table 2.
| OR (95% CI) | P | |
|---|---|---|
| Age | 1.007 (0.970-1.045) | .71 |
| Male sex | 1.409 (0.464-5.251) | .31 |
| Past CVE | 2.659 (0.898-7.521) | .07 |
| Lung involvement | 1.947 (0.719-5.850) | .20 |
| D-dimerc | 1.003 (1.000-1.007) | .04 |
CVE, cardiovascular event; OR, odds ratio.
A multivariable logistic regression procedure was performed considering clinically relevant factors identified in univariate analysis with a P value <.2.
D-dimer values were obtained 1 (0-3) day after admission and 2 (1-14) days before onset of an arterial thrombotic event in COVID-19+ patients and were available for analysis in 351 patients.
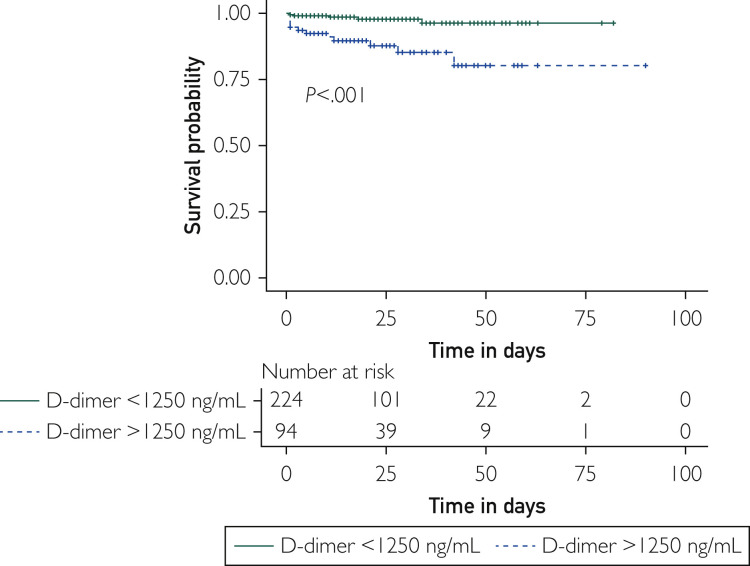
D-dimer was measured in plasma 1 [0-3] day after admission and 2 (1-14) days before arterial thrombotic event onset. The corresponding receiver operating characteristics curve showed that D-dimer level is predictive for arterial thrombotic events in COVID-19+ patients (area under the curve, 0.77; 95% CI, 0.66 to 0.88; P<.001), with a plasma D-dimer level of 1267 ng/mL showing a sensitivity of 76.2% (95% CI, 54.9% to 89.4%) and a specificity of 72.7.% (95% CI, 67.7% to 77.2%) for arterial thrombotic events (Supplemental Figure 2, available online at http://www.mayoclinicproceedings.org). Accordingly, the subdistribution survival hazard model considering death as a competing event showed that a D-dimer level above 1250 ng/mL measured in plasma early after admission is strongly associated with the occurrence of arterial thrombotic events during follow-up (subdistribution HR, 7.68; 95% CI, 2.9 to 20.6; P<.001; Figure 2 ).
Figure 2.
Survival without arterial thrombotic events in COVID-19+ patients according to D-dimer level measured in plasma at baseline. Kaplan-Meier curves represent the survival without an arterial thrombotic event according to D-dimer level (>1250 or <1250 ng/mL) measured in plasma early after admission. Time 0 was the day of admission. Follow-up ended at discharge. The solid line represents the outcome in patients with D-dimer level below 1250 ng/mL. The dashed lines represent the outcomes in patients with D-dimer level above 1250 ng/mL. D-dimer values were obtained 1 (0-3) day after admission and 2 (1-14) days before onset of an arterial thrombotic event and were available for analysis in 318 patients. The reference concentration of D-dimer at our institution is less than 234 ng/mL.
Discussion
Previous studies varied in the incidence of thrombosis in patients with COVID-19, but all suggested a heightened risk, particularly for deep venous thrombosis and pulmonary embolism.3 , 4 Few studies, however, specifically focused on arterial thrombotic events in COVID-19+ patients. A previously published study reported an incidence of 11.1% of arterial thrombotic events among a consecutive 3334 hospitalized COVID-19 patients in 4 hospitals in New York City.5 In our referral center in France, we found a lower arterial thrombotic event rate of 5.6%, more consistent with other published studies in Spain and Italy.6 , 7
D-dimer level at admission was high and consistent with an early distinct coagulation disorder associated with COVID-19. Evidence for abnormal coagulation parameters associated with COVID-19 appeared in early reports from China.8 Disseminated intravascular coagulation could be ruled out because D-dimer levels were increased far out of proportion with any abnormalities in prothrombin time, activated partial thromboplastin time, fibrinogen level, or platelet count (Table 1).9 , 10 Elevated levels of D-dimer are common in critically ill patients.11 , 12 D-dimer levels are not routinely measured otherwise but are now routinely assessed in symptomatic patients with COVID-19. Whether the elevated D-dimer levels and thrombotic manifestations are caused by a specific COVID-19 coagulopathy or merely related to coagulation activation associated with severe inflammation is unknown. In our study, in contrast to previous reports,13 , 14 arterial thrombotic events were not associated with inflammatory markers such as CRP.
Elevated D-dimer levels at admission are known to be associated with increased mortality in COVID-19.1 , 5 , 13 , 15 , 16 In our study, D-dimer level was also independently and strongly associated with arterial thrombotic events. According to our findings, D-dimer levels at admission may also identify patients at high risk for arterial thrombotic events. Low-molecular-weight heparin was associated with lower mortality in patients with markedly elevated D-dimer levels.17 D-dimer has also been used to guide anticoagulation.18 Noteworthy in our series, arterial thrombotic events occurred although most patients were receiving anticoagulation according to current recommendations.19
Both the fact that numerous arterial events were unusual—such as thrombosis of the aorta, upper limb, or renal arteries and cerebral microvasculopathy—and the strikingly high risk of death compared with arterial thrombotic event control patients with no COVID-19 infection are suggestive of a COVID-19–specific arterial vasculopathy. COVID-19 infection causes cytokine storm, hypoxic injury, hypercoagulability, increased platelet activity, and endothelial damage.1 , 20, 21, 22, 23
In our study, COVID-19+ patients with arterial thrombotic events shared the same cardiovascular risk burden as the general population. COVID-19 infection may act as an acute second hit in patients at otherwise high risk for CVE according to traditional risk factors.
Our study has several limitations. First, it is a retrospective single-center observational study. Second, around 6% of patients have highly probable but not microbiologically confirmed COVID-19 infection. In clinical practice, however, many patients presented with a negative RT-PCR test result for COVID-19, even though they had typical lung manifestations and a highly suspected contact history. In our study, patients with a negative RT-PCR response had recent exposure, symptoms highly suggestive of COVID-19 infection, typical CT scan appearance, and no alternative diagnosis. In those cases, we considered the negative RT-PCR response a false-negative result and believed that including those patients would more accurately reflect the “real-life” management of COVID-19 patients. Third, arterial thrombotic events occurred in a small number of patients, limiting statistical power. Last, laboratory data were not uniformly available for each evaluated laboratory parameter including D-dimer. On the other hand, because it helped to determine the risk factors and prognosis of arterial thrombotic events in patients with COVID-19, the comparison with patients with a concomitant arterial thrombotic event without COVID-19 infection as a control group was strong.
Conclusion
The acute arterial vasculopathy related to COVID-19 is diverse and associated with a high risk of death. Elevated D-dimer levels at entry may help predict arterial thrombotic events and recognize high-risk patients.
Footnotes
For editorial comment, see page 274
Potential Competing Interests: G.D. received travel fees from AstraZeneca, Bayer, and BMS and speaker’s or consulting fees from Amgen, AstraZeneca, Bayer, BMS, Janssen, Sanofi, and Terumo. F.X.L. received travel fees from Astellas, Eumedica, and MSD and speaker’s or consulting fees from Gilead and MSD. K.S. received travel fees from GSK and Roche and consulting fees from BMS. The other authors declare no conflict of interest.
Supplemental material can be found online at http://www.mayoclinicproceedings.org. Supplemental material attached to journal articles has not been edited, and the authors take responsibility for the accuracy of all data.
Supplemental Online Material
References
- 1.Connors J.M., Levy J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peiffer-Smadja N., Lucet J.C., Bendjelloul G. Challenges and issues about organizing a hospital to respond to the COVID-19 outbreak: experience from a French reference centre. Clin Microbiol Infect. 2020;26(6):669–672. doi: 10.1016/j.cmi.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cui S., Chen S., Li X., Liu S., Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18(6):1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bilaloglu S., Aphinyanaphongs Y., Jones S., Iturrate E., Hochman J., Berger J.S. Thrombosis in hospitalized patients with COVID-19 in a New York City health system. JAMA. 2020;324(8):799–801. doi: 10.1001/jama.2020.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cantador E., Núñez A., Sobrino P. Incidence and consequences of systemic arterial thrombotic events in COVID-19 patients. J Thromb Thrombolysis. 2020;50(3):543–547. doi: 10.1007/s11239-020-02176-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lodigiani C., Iapichino G., Carenzo L. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taylor F.B., Jr., Toh C.H., Hoots W.K., Wada H., Levi M. Scientific Subcommittee on Disseminated Intravascular Coagulation (DIC) of the International Society on Thrombosis and Haemostasis (ISTH). Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2001;86(5):1327–1330. [PubMed] [Google Scholar]
- 10.Toh C.H., Hoots W.K. SSC on Disseminated Intravascular Coagulation of the ISTHJ. The scoring system of the Scientific and Standardisation Committee on Disseminated Intravascular Coagulation of the International Society on Thrombosis and Haemostasis: a 5-year overview. Thromb Haemost. 2007;5(3):604–606. doi: 10.1111/j.1538-7836.2007.02313.x. [DOI] [PubMed] [Google Scholar]
- 11.Goldhaber S.Z. The perils of D-dimer in the medical intensive care unit. Crit Care Med. 2000;28(2):583–584. doi: 10.1097/00003246-200002000-00056. [DOI] [PubMed] [Google Scholar]
- 12.Shorr A.F., Thomas S.J., Alkins S.A., Fitzpatrick T.M., Ling G.S. D-dimer correlates with proinflammatory cytokine levels and outcomes in critically ill patients. Chest. 2002;121(4):1262–1268. doi: 10.1378/chest.121.4.1262. [DOI] [PubMed] [Google Scholar]
- 13.Al-Samkari H., Karp Leaf R.S., Dzik W.H. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood. 2020;136(4):489–500. doi: 10.1182/blood.2020006520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grimnes G., Isaksen T., Tichelaar Y.I.G.V., Brox J., Brækkan S.K., Hansen J.B. C-reactive protein and risk of venous thromboembolism: results from a population-based case-crossover study. Haematologica. 2018;103(7):1245–1250. doi: 10.3324/haematol.2017.186957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thachil J., Tang N., Gando S. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18(5):1023–1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kollias A., Kyriakoulis K.G., Dimakakos E., Poulakou G., Stergiou G.S., Syrigos K. Thromboembolic risk and anticoagulant therapy in COVID-19 patients: emerging evidence and call for action. Br J Haematol. 2020;189(5):846–847. doi: 10.1111/bjh.16727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iba T., Levy J.H., Thachil J., Wada H., Levi M. Scientific and Standardization Committee on DIC of the International Society on Thrombosis and Haemostasis. The progression from coagulopathy to disseminated intravascular coagulation in representative underlying diseases. Thromb Res. 2019;179:11–14. doi: 10.1016/j.thromres.2019.04.030. [DOI] [PubMed] [Google Scholar]
- 21.Iba T., Levy J.H., Wada H., Thachil J., Warkentin T.E., Levi M., Subcommittee on Disseminated Intravascular Coagulation Differential diagnoses for sepsis-induced disseminated intravascular coagulation: communication from the SSC of the ISTH. J Thromb Haemost. 2019;17(2):415–419. doi: 10.1111/jth.14354. [DOI] [PubMed] [Google Scholar]
- 22.Levi M., Thachil J., Iba T., Levy J.H. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7(6):e438–e440. doi: 10.1016/S2352-3026(20)30145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang L., Feng X., Zhang D. Deep vein thrombosis in hospitalized patients with COVID-19 in Wuhan, China: prevalence, risk factors, and outcome. Circulation. 2020;142(2):114–128. doi: 10.1161/CIRCULATIONAHA.120.046702. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.