Highlights
-
•
The growth supplements remarkably enhanced the cordycepin production.
-
•
Hypoxanthine and adenosine yielded higher amount of cordycepin.
-
•
The potent growth supplements activated cordycepin biosynthesis pathway.
-
•
Upregulation of RNR, NT5E, ADEK and purA mRNA expression was observed.
Abbreviations: RP-HPLC, Reversed-phase high-performance liquid chromatography; qRT-PCR, Quantitative reverse transcriptase-polymerase chain reaction; mRNA, Messenger ribonucleic acid; cDNA, Complementary deoxyribonucleic acid; dNTP, Deoxyribonucleotide triphosphate; mTOR, Mammalian target of rapamycin; SDA, Sabouraud dextrose agar; MgSO4, Magnesium sulfate; KH2PO4, Potassium dihydrogen phosphate; ANOVA, Analysis of Variance; SEM, Standard error mean
Keywords: Medicinal mushroom, Cordycepin production, Mycelial biomass, Growth supplements, Cordycepin biosynthesis pathway
Abstract
Cordycepin is a crucial bioactive compound produced by the fungus Cordyceps spp. Its therapeutic potential has been recognized for a wide range of biological properties such as anticancer, anti-diabetic, antidepressant, antioxidant, immunomodulation, etc. Moreover, its human random clinical trials depicted a promising anti-inflammatory activity that reduced the airway inflammation remarkably in asthmatic patients. But its overexploitation and low production of cordycepin in naturally growing biomass are insufficient to meet its existing market demand for its therapeutic use. Therefore, strategies for enhancement of cordycepin production in Cordyceps spp. are warranted. However, specifically, wild type Ophiocordyceps sinensis possesses a very low content of cordycepin and has restricted growth in natural mycelial biomass. To overcome these limitations, this study attempted to enhance cordycepin production in its mycelial biomass in vitro under submerged conditions by adding various growth supplements. The effect of these growth supplements was evaluated by reversed-phase high-performance liquid chromatography (RP-HPLC) which demonstrated that among nucleosides- hypoxanthine and adenosine; amino acids-glycine and glutamine; plant hormones- 1-naphthaleneacetic acid (NAA) and 3-indoleacetic acid (IAA); vitamin-thiamine (B1) from each group of growth supplements yielded a higher amount of cordycepin with 466.48 ± 3.88, 380.23 ± 1.78, 434.97 ± 2.32, 269.78 ± 2.92, 227.61 ± 2.34, 226.02 ± 1.69 and 185.26 ± 2.35 mg/L respectively as compared to control with 13.66 ± 0.64 mg/L. Further, at the transcriptional level, quantitative real time-polymerase chain reaction (qRT-PCR) analysis of genes associated with metabolism and cordycepin biosynthesis depicted significant upregulation of major downstream genes- NT5E, RNR, purA, and ADEK which corroborated well with RP-HPLC analysis. Taken together, the present study identified growth supplements as potential precursors to activate the cordycepin biosynthesis pathway leading to improved cordycepin production in O. sinensis.
1. Introduction
O. sinensis (traditionally known as Cordyceps sinensis) is an entomo-pathogenic fungus that belongs to the Ophiocordycipitaceae family classified under the category of medicinal mushrooms [1]. This wild macrofungus exists in different forms such as fruiting bodies and mycelial biomass. Its extract possesses immense medicinal properties due to the presence of bioactive compounds such as cordycepin, cordycepic acid, cordymin, etc. [[2], [3], [4], [5], [6], [7]]. Wild O. sinensis is a rare and endangered species that is found at high altitudes (3000−5000 m) in the Himalayan range [8]. Due to its limited natural resources, increasing demand for its biomass cannot be fulfilled through naturally available fruiting bodies. Therefore, the present study has attempted to cultivate O. sinensis in artificial conditions and enhanced cordycepin production in its biomass. Alternatively, cordycepin could be synthesized by chemical method but the chemical synthesis of cordycepin, as well as its purification is a very complicated and tedious process which requires a large volume of organic solvents to be discharged imposing serious health-related issues to human health and environment. Also, the separation of pure cordycepin compound is hardly achievable rendering with the higher residual of tin with final product suggesting the requirement of improvement in its biosynthesis process [[9], [10], [11]]. Recent reports revealed the production of cordycepin via the utilization of liquid surface and/or submerged fermentation technique [[12], [13], [14]]. But in case of O. sinensis, it is very difficult to produce fruiting bodies that take several months with a lower amount of bio metabolites [15]. Therefore, mycelial biomass production through fermentation technique is a better alternative to produce bioactive compounds like cordycepin. Several studies have claimed that cordycepin is available from both natural and cultured mycelial biomass and can be easily detected by chromatographic techniques [16]. Though naturally growing fruiting bodies or cultured mycelial biomass contain a very low amount of cordycepin but the cordycepin content in mycelial biomass is highly comparable to its amount in naturally growing fruiting bodies [5]. This led us to make an initial attempt to enhance the cordycepin production in its mycelial biomass grown under artificial conditions as compared to naturally growing wild fruiting bodies.
Literature suggests that several strategies have been devised for its biomass production in vitro such as solid and liquid fermentation for mycelial biomass and isolation of bioactive compounds [[17], [18], [19]]. However, submerged fermentation is the most promising method because culture conditions and other parameters can easily be controlled. The optimization of culture conditions for enhanced production of cordycepin by adding growth supplements and additives is easier using submerged fermentation as compared to solid cultivation [17]. Interestingly, artificial mycelial biomass produced through submerged fermentation has similar therapeutic potential and less toxicity as compared to biomass obtained from wild O. sinensis [20] paving ways for its enhanced cultivation of at large scale. In terms of cordycepin production, C. militaris is considered to be more suitable host than O. sinensis due to the presence of higher cordycepin amount in it than O. sinensis. But recently Ji et al. [21] suggested that O. sinensis have more medicinal effects as compared to C. militaris. For instance, O sinensis improves the overall intestinal health by decreasing the pH of gut environment and enhancing the growth of beneficial genera including Phascolarctobacterium and Bifidobacterium, as compared to C. militaris [21]. Intriguingly, Chen et al. [22] reported a higher content of unsaturated fatty acids, peptides and mannitol in natural O. sinensis as compared with cultured C. militaris suggesting O. sinensis to be a better nutritional supplement thereby rationalizing the use of O. sinensis in the present study [22]. However, to confirm whether C. militaris can replace O. sinensis completely needs further investigation.
Cordycepin (3′-deoxyadenosine) is the signature bioactive compound of Cordyceps spp. which is considered as nucleoside antibiotic and possesses immense pharmacological activities such as antimicrobial [23,24], antiviral [25], antioxidant [26], antifungal [27], anti-angiogenesis [28], anti-platelet aggregation [58], immunomodulatory [59], anti-pigmentation [60] and anti tumor [29]. It is also known to interfere with different molecular processes including purine biosynthesis [30,31], DNA/RNA synthesis [32], and mTOR signaling transduction pathway [33]. Based on these in vitro studies, several in vivo preclinical studies have been conducted to evaluate its clinical efficiency in various disorders such as depression [34], steroidogenesis [35], hyperglycemia [36], vascular disorders [37], etc. Even, a human random controlled trial of cordycepin in asthmatic patients depicted that it reduced the airway inflammation by lowering the serum levels of IgE, sICAM-1, IL-4, and MMP-9 [38]. Another clinical trial is being carried out as a combinatorial drug with Pentostatin to treat patients with refractory lymphocytic or chronic myelogenous leukemia (ClinicalTrials.gov Identifier: NCT00003005). Although, cordycepin is also reported to possess an anticancer activity but simultaneously, it exhibits some toxicity towards healthy erythrocytes [39] as well as some impairment in healthy organs in vivo in mice [61]. This encourages researchers to further determine the optimum and non-toxic dose of this drug candidate as its therapeutic applications cannot be discounted. In addition, this molecule has recently been reported to have herbicidal properties which could potentially replace problematic or toxic herbicide such as glyphosate [40]. These diverse biological activities of cordycepin alone necessitate its large scale production as wild growing fruiting bodies cannot accumulate the required amount of cordycepin. Previous studies reported that cordycepin production in C. militaris could be improved by adding different growth supplements such as glycine, adenosine, inosine, thiamine, glutamine and, other growth supplements [[41], [42], [43]]. While there are no reports in the literature for improving cordycepin production in O. sinensis using growth supplements. Hence, we hypothesized that growth supplements can either directly or indirectly participate in the cordycepin biosynthesis pathway to enhance cordycepin production in its mycelial biomass. This incited us to grow O. sinensis under in vitro conditions using submerged fermentation by utilizing different groups of growth supplements. Therefore, to the best of our knowledge, for the first time, this study has shown the effect of different groups of growth supplements on cordycepin production in CS2973 strain isolated from wild growing O. sinensis. Often, the synthesis of bioactive compounds is regulated by an array of genes that get upregulated or downregulated during the biosynthesis pathway in the host. Hence, mRNA expression analysis of genes involved in the cordycepin biosynthesis pathway was also performed to determine the effect of growth supplements on potential target genes whose modulation may improve cordycepin production at large scale avoiding any mutational technologies.
2. Materials and methods
2.1. Collection of wild O. sinensis
Fresh fruiting bodies of O. sinensis were collected from the Pithoragarh region in Uttarakhand, India. CS2973 strain was isolated from these fruiting bodies and confirmed as O. sinensis anamorph both morphologically and through molecular identification, as described previously [44]. Gene sequence information of the isolated strain was deposited at the National Centre for Biotechnology Information (NCBI) under accession no. MG564345. Complete details of isolation and molecular identification of CS2973 strain have been reported in our previous study [44]. Briefly, the CS2973 mycelial culture was grown on a petri plate and inoculum for sub-culturing was prepared by punching out 5 mm potato dextrose agar (PDA) discs using a sterilized cork borer. Further, the discs possessing CS2973 culture was inoculated into 250 mL Erlenmeyer flask with 100 mL of liquid culture medium at 20 °C on a shaking incubator for 10 days. This culture medium was used as inoculum for further expanding the culture [44].
2.2. Submerged culturing of CS2973
The optimal liquid culture medium for culturing of CS2973 was composed of 20 g/L sucrose, 10 g/L corn steep powder, 1 g/L MgSO4, 0.1 g/l KH2PO4. The optimal culture conditions for cordycepin production from mycelial biomass were: temperature-20 °C; pH-6; incubation time-10 days and agitation speed-150 rev min−1. Initially, starter culture was prepared by inoculating liquid media with a 1 cm piece of solid mycelium grown on sabouraud dextrose agar (SDA) medium. The liquid culturing of CS2973 was performed in a 250 mL shaking flask filled with 100 mL culture media inoculated with 5% inoculum from starter culture. The mycelial biomass from these culture flasks was harvested by filtration through Whatman filter paper which was further dried in a hot air oven at 45 °C and ground to powder.
2.3. Supplementation of growth supplements for enhancement in cordycepin production
To increase the cordycepin production in CS2973, four categories of growth supplements were selected and added to the liquid basal culture medium at a working concentration based on literature [42]. The stock solution of each growth supplement was prepared and a working solution was added after filter sterilization through 0.22 μm syringe filters. A list of growth supplements used for enhancement of cordycepin production is given in Table 1.
Table 1.
Different categories of growth supplements for enhancement of cordycepin production in CS2973.
| Growth supplements | |
|---|---|
| (A) Amino acids-1 g/L | (B) Nucleosides-100 mg/L |
| Glycine | Hypoxanthine |
| Glutamine | Adenosine |
| Glutamic acid | Adenine |
| Aspartic acid | Thymidine |
| Aspargine | Thymine |
| Inosine | |
| Uridine | |
| (C) Plant growth hormones-100 mg/L | (D) Vitamins-100 mg/L |
| NAA (1-naphthaleneacetic acid) | B1(thiamine) |
| IAA (3-indoleacetic acid) | B6 (pyridoxine) |
| 2,4- D (2,4-dichlorophenoxyacetic acid | B7 (biotin) |
| BAP (benzyl amino purine) | C (ascorbic acid) |
2.4. Quantification of cordycepin content in mycelial biomass
Cordycepin content in the mycelial biomass of CS2973 was analyzed by RP-HPLC (reversed-phase high-performance liquid chromatography) technique following a reported procedure with slight changes [45]. Briefly, to negate the effect of improved biomass on cordycepin production, equal amount i.e 1 g of dried CS2973 mycelial powder was dissolved in 20 mL of 70 % methanol in distilled water for each treated group for 1 h under sonication to prepare the mycelial extract. This extract was then subjected to centrifugation at 4000 x g for 30 min followed by its filteration using a syringe filter (0.22 μm) prior to injection into RP-HPLC system [45]. The mobile phase consisted of methanol/ultrapure water (10:90) with 10 mmol/L KH2PO4 dissolved in it. The column was set at 40 °C and the flow rate was maintained at 1.0 mL/min to carry out elution. The RP-HPLC system was equipped with a UV detector and the chromatogram was analyzed by recording the absorbance at 261 nm. The standard calibration curve was plotted using standard cordycepin at different concentrations (50, 100, 150, 200, and 250 μg/mL) and used to estimate the cordycepin content in various treated groups of mycelial biomass.
2.5. RNA isolation
RNA isolation was performed using the total RNA isolation kit (Promega Corporation, Wisconsin, USA). In this method, 100 mg of CS2973 mycelia were homogenized in liquid nitrogen followed by the addition of 250 μL RNA lysis buffer and the lysate was vortexed for 30 s. Next, 350 μL of RNA dilution buffer was added and incubated for 5 min at room temperature (RT) followed by centrifugation at 10,000 rev min−1 for 15 min at 4 °C. The aqueous phase present in the upper layer was collected in fresh tube and 200 μL of ethanol was added for RNA precipitation. Further, RNA was eluted following the manufacturer’s protocol The purified RNA was quantified and stored at -80 °C for further use.
2.6. cDNA synthesis
1μg of RNA was used as a template for cDNA synthesis using cDNA synthesis kit (Promega Corporation, Wisconsin, USA) according to the manufacturer’s protocol.After completion of the reaction, cDNA was stored at −20 °C which was further used for qRT-PCR analysis.
2.7. Quantitative mRNA expression analysis of genes involved in cordycepin biosynthetic pathway
50 ng of cDNA was added to the SYBR green real-time master mix (2X) per reaction using 1 pM of each primer specific for genes involved in the biosynthesis of cordycepin. Real-time amplification or expression of each gene for each growth supplement was performed with a Bio-Rad real-time PCR machine (CFX96) at standardized annealing temperature according to the manufacturer’s protocol. qRT-PCR (quantitative real-time polymerase chain reaction) analysis was used to perform mRNA expression analysis of genes involved in cordycepin biosynthesis- PFK, Phosphofructokinase; SAICAR synthetase; AMPD, AMP deaminase; ADEK, Adenylate kinase; NT5E, 5′-Nucleotidase; RNR, Ribonucleotide reductase; purA, Adenylosuccinate synthase; purB, Adenylosuccinate lyase; purC, Phosphoribosyl-aminoimidazole-succinocarboxamide synthase; purl, Phosphoribosyl-formyl-glycinamidine synthase; PRPS, Ribose-phosphate pyrophosphokinase 1 [2,46]. The primer sequence information of these genes along with accession number is provided in Table 2. Each growth supplement was screened for expression of these genes involved in the biosynthetic pathway of cordycepin. The Ct (Threshold cycle) values of gene expression were normalized to the eukaryotic housekeeping gene, 18S ribosomal RNA, and values represented in the graph are the relative fold change of untreated sample as a control which was calculated using a standard delta-delta Ct method:
| Relative fold change = 2^ (-ΔΔCt) |
Where ΔΔCt= (ΔCt of treated - ΔCt of control), ΔCt treated= (Ct of target gene in treated group - Ct of 18 s rRNA gene in treated group) and ΔCt control= (Ct of target gene in control group - Ct of 18 s rRNA gene in control group [56].
Table 2.
Primer sequence information of the genes involved in cordycepin biosynthesis.
| Gene name | Gene symbol | Accession no. | Forward primer | Reverse primer |
|---|---|---|---|---|
| 5′-nucleotidase 1 | NT5E | KP090958 | GCTCGTGTTCCTGCCATAGT | GCTCTACTATCGCCCGAGTG |
| Adenosine kinase | ADEK | KP090962 | CCATTGGGCTCACGAGTCTT | CGTGACGAAAAGACAGTGCC |
| AMP deaminase | AMPD | KP090965 | CCGTGAGCATGTCGAGGAG | GCAGCAAAACACCATCCGTC |
| Ribonucleoside-diphosphate reductase L subunit | RNR | KY435930 | CAGTCCCAGTCGCTCAACAT | TCCTTCACATACGTGGCTCG |
| Phosphoribosyl-formyl-glycinamidine synthase | purl | KP090952 | AGACCTTTGCGCTCGAGAAA | GGTACTGCATCTCGGACTCG |
| Adenylosuccinate synthase | purA | KP090957 | CCGTACCCGAGACTTGACAC | AGGCAGTTGGGATTGACGAG |
| Adenylosuccinate lyase | purB | KP090955 | CCTTTCCAAGTTTGCCTGCC | CGGCCCTAACGTATTCGACA |
| Phosphoribosyl-aminoimidazole-succinocarboxamide synthase | purC | KP090954 | ACATACATCCCGACGATGCC | TTGTCGAAGCTGTCCTGGTC |
| Ribose-phosphate pyrophosphokinase 1 | PRPS | KP090946 | CACGACTACGAGAACCCCAG | TCGGGAATGTGCTTCACGAT |
| 18 s ribosomal RNA | 18 s rRNA | MG642880 | GACGCGTTCGGCACCTTA | TTCAGCCTTGCGACCATAC |
2.8. Statistical analysis
All experiments were performed at least thrice and data were represented as average mean ± SEM. The statistical significance was calculated by one-way or two-way ANOVA to assess the difference between group and their respective controls followed by an appropriate test such as Tukey’s multiple comparison test using Graph Pad Prism version 6.05.
3. Results and discussion
3.1. Hypoxanthine and adenosine enhance cordycepin production
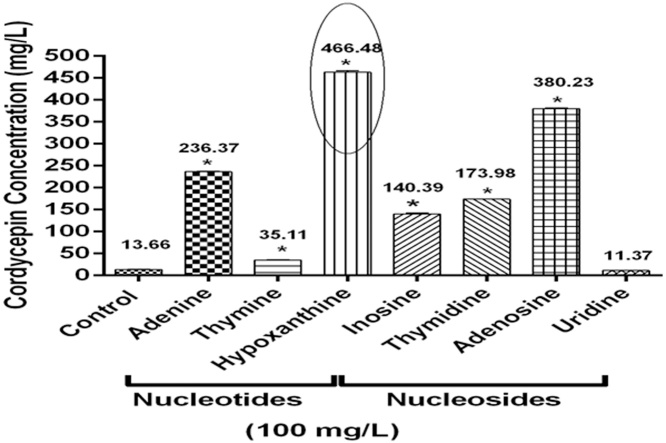
Out of different nucleosides tested for cordycepin production, hypoxanthine depicted maximum amount of cordycepin production (466.48 ± 3.89 mg/L) followed by adenosine (380.23 ± 1.78 mg/L) whereas least amount of cordycepin was obtained with uridine (11.37 ± 0.23 mg/L) as compared to control (13.66 ± 0.64 mg/L) without any supplementation as shown in Fig. 1. However, the contribution of adenine, inosine, and thymidine cannot be neglected as they also induced cordycepin content up to 236.37 ± 1.17, 140.39 ± 2.34, and 173.98 ± 0.33 mg/L respectively. The increased production of cordycepin due to hypoxanthine and adenosine supplementation may be attributed to the fact that cordycepin is a nucleoside analog of adenosine, also adenosine is the main precursor for the biosynthesis of cordycepin which accelerated the cordycepin biosynthesis pathway [47]. Masuda et al. [42] also reported the enhanced cordycepin production using surface culture approach in C. militaris and it was revealed that adenine and adenosine significantly increased the cordycepin content as compared to control in the culture medium.
Fig. 1.
Effect of nucleosides on cordycepin production in CS2973. The graph representing the cordycepin concentration obtained after the addition of different nucleotides and nucleosides to the culture at 100 mg/L concentration. Data was presented as the mean ± SEM of 3 independent experiments. * indicating level of significance accepted at p ≤ 0.05 as compared to control.
3.2. Supplementation of amino acids increases cordycepin production
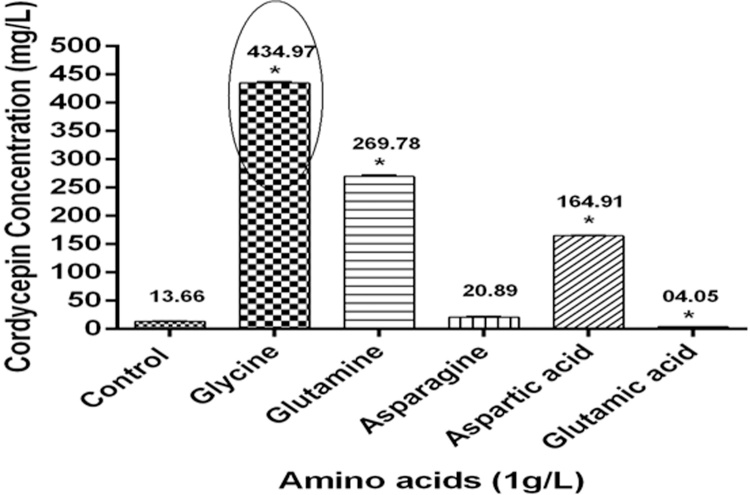
Since amino acids are known to be involved in cordycepin biosynthesis as the nitrogen source, this incited us to utilize amino acids for the enhancement of cordycepin production [48]. The maximum amount of cordycepin (434.97 ± 2.32 mg/L) was obtained when glycine was supplemented at 1 g/L concentration. Glutamine and aspartic acid also significantly increased the cordycepin content 269.78 ± 2.92 and 164.91 ± 1.19 mg/L respectively (Fig. 2). However, on glutamic acid supplementation in the culture medium, the cordycepin amount was reduced to 4.05 ± 0.42 mg/L as compared to control (13.66 ± 0.64 mg/L). Masuda et al. [42] reported that glycine and glutamine amino acids increased the cordycepin content 2.8 and 2.5 times higher respectively using the surface fermentation approach. However, the results depicted that using the submerged fermentation approach, cordycepin content increased 31.8 and 19.7 times using glycine and glutamine amino acids as compared to control. From these results, it can be concluded that glycine and glutamine are the major nitrogen sources required by CS2973 as compared to other amino acids which induced the cordycepin biosynthesis pathway.
Fig. 2.
Effect of amino acids on cordycepin biosynthesis in CS2973.Graph depicting higher cordycepin production in mycelial biomass upon addition of glycine, glutamate, and aspartic acid as compared with control. Data was presented as the mean ± SEM of 3 independent experiments. * indicating significance level at p ≤ 0.05 as compared to control.
3.3. Plant growth hormones- NAA and IAA potentiates cordycepin production
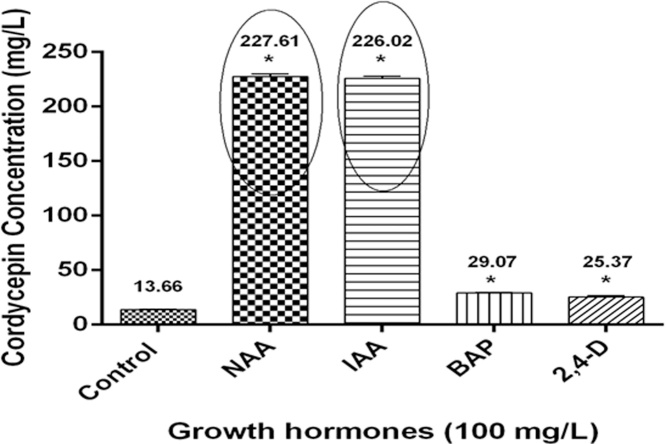
Plant growth hormones play a significant role in cordycepin production in Cordyceps spp. Some of these hormones are the precursors for enzyme synthesis involved in cordycepin biosynthesis [49]. Kang et al. [50] optimized cordycepin production in C. militaris and reported that NAA significantly increased the cordycepin production but utilization of plant growth hormones for cordycepin production in CS2973 has not been explored yet. When plant growth hormones were supplemented to the culture medium, it was observed that NAA and IAA significantly increased the cordycepin content up to 227.61 ± 2.34 and 226.02 ± 1.69 mg/L respectively as compared to control (13.66 ± 0.64 mg/L) as shown in Fig. 3. This increased cordycepin production in mycelial biomass suggested that plant growth hormones stimulate the production of cordycepin in CS2973 which can be utilized at an industrial scale.
Fig. 3.
Effect of plant growth hormones on cordycepin production in CS2973. Cordycepin production was found to be higher in NAA and IAA at a concentration of 100 mg/L as compared to control. Data was presented as the mean ± SEM of 3 independent experiments. The significance level was accepted at p ≤ 0.05 as compared to control.
3.4. Effect of vitamins on cordycepin production
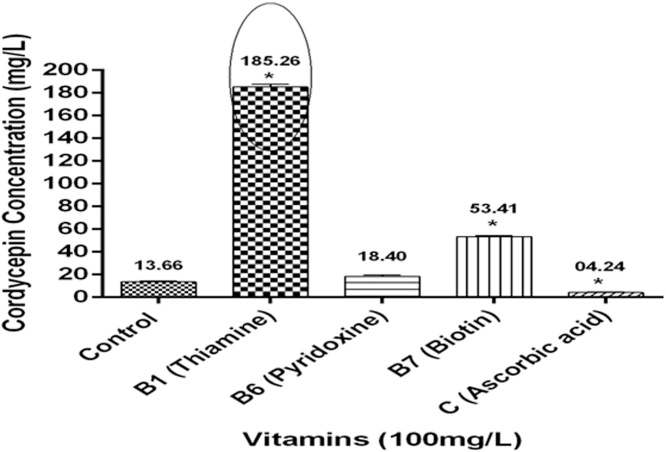
Out of four types of vitamins tested, B1 was found to be the most appropriate vitamin for potentiating the cordycepin production as cordycepin amount raised to 185.26 ± 2.35 mg/L on supplementation at 100 mg/L concentration in liquid medium (Fig. 4). However, biotin also produced 53.41 ± 0.77 mg/L cordycepin content but surprisingly, vitamin C showed a significant reduction in cordycepin content 4.24 mg/L ± 0.31 as compared to control (13.66 ± 0.64 mg/L). Similarly, Kang et al. [50] also reported vitamin B1 as an active growth supplement in C. militaris for cordycepin production.
Fig. 4.
Effect of different vitamins on cordycepin production in CS2973.Graph representing higher cordycepin content in thiamine treated group as compared to control. Data was presented as the mean ± SEM of 3 independent experiments. The significance level was accepted at p ≤ 0.05 as compared to control.
3.5. Differential mRNA expression analysis of genes involved in cordycepin biosynthesis
Several transcriptomic studies have investigated the genome of O. sinensis and C. militaris for identifying the genes involved in the cordycepin biosynthesis pathway to understand its downstream signaling molecular mechanism [2,13,46,57]. An array of genes have been identified in the literature to play a major role in cordycepin production such as ADEK, NT5E, RNR, purA, purC, AMPD, etc [2,57]. A cascade of enzymatic reactions takes place to synthesize cordycepin from its precursors. However, recent reports suggested Cns1 and Cns2 genes encoding for two crucial enzymes to be directly involved in the final steps of cordycepin biosynthesis in C. militaris at the transcriptional level [51,52]. Surprisingly, Xia et al. [52] reported that the Cns1−4 genes are absent in O. sinensis suggesting the role of other unknown genes in this species for cordycepin production. The modulation of the genes or enzymes involved in cordycepin biosynthesis can enhance cordycepin production. For instance, ribonucleotide reductase small subunit, RNRM overexpression increases cordycepin production in C. militaris [46]. However, both RNR large and small subunits are essential for its activity and regulation of product specificity [53]. RNR, NT5E, and ADEK can be utilized as molecular targets to enhance cordycepin production in O. sinensis as these genes have a higher copy number in Cordyceps spp. [46,54]. Hence, in the present study, the growth supplements inducing these cordycepin synthesis related genes expression were considered potent enhancers of cordycepin production.
3.6. Nucleoside-mediated activation of genes related to cordycepin synthesis
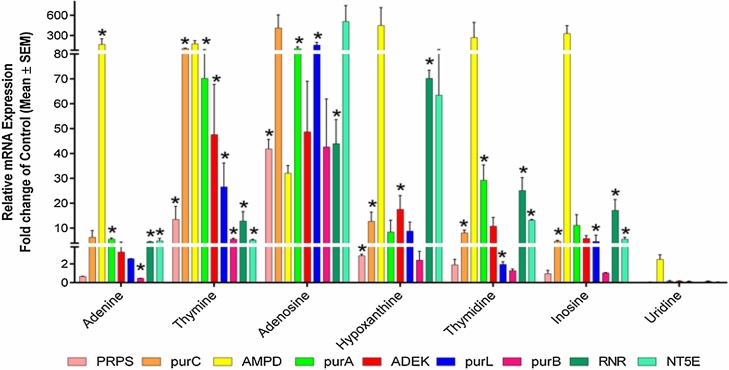
To enhance the cordycepin biosynthesis, nucleosides were supplied to the culture medium. Interestingly, a significant upregulation of RNR (70.19 fold), NT5E (63.43 fold), purA (8.43 fold), and ADEK (17.52 fold) gene expression was observed upon hypoxanthine treatment which are downstream genes involved in cordycepin biosynthesis (Fig. 5). Though, hypoxanthine also induced the significant upregulation of other upstream genes of this pathway, suggesting its continuous requirement for their constitutive expression and importance during the cordycepin synthesis process. Being an analog of cordycepin, the effect of adenosine was also evaluated on cordycepin biosynthesis which also depicted a significant increase in mRNA expression of RNR (43.93 fold), NT5E (508 fold), purA (104.09 fold), and ADEK (48.7 fold) suggesting their essential role in cordycepin biosynthesis. Though there was a significant increase in RNR and NT5E due to other nucleosides also but it was not as high as hypoxanthine and adenosine. In agreement with RP-HPLC observations, uridine supplementation did not show any changes in the expression of any gene involved in the biosynthetic pathway.
Fig. 5.
qRT-PCR analysis to assess nucleotides and nucleosides mediated alteration in genes involved in cordycepin synthesis of CS2973. Graph depicting mRNA expression profile of genes involved in cordycepin biosynthesis for group A (nucleotides and nucleosides) growth supplements. Data was presented as the mean ± SEM of 3 independent experiments. * indicating significance level was accepted at p ≤ 0.05 as compared to control.
3.7. Amino acids-mediated transcriptional changes in mRNA expression of genes responsible for cordycepin synthesis
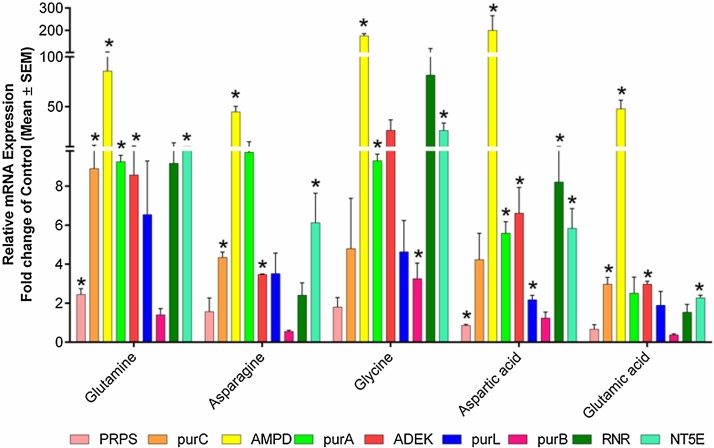
As depicted by RP-HPLC observations of amino acids supplementation in culture medium for cordycepin production, it was observed that glycine, glutamine, and aspartic acid significantly increased the cordycepin production (Fig. 2). To determine the gene expression profile of genes involved in cordycepin biosynthesis using amino acids as growth supplements, qRT-PCR analysis represented that glycine supplementation significantly upregulated the expression of RNR (81.68 fold), NT5E (26.20 fold), purA (9.30 fold) and ADEK (26.25 fold) genes. Glutamine and aspartic acid also showed the increased expression of RNR (9.17 and 8.20 fold), NT5E (9.95 and 5.83 fold), purA (9.25 and 5.59 fold), and ADEK (8.57 and 6.61 fold) genes as compared to control (Fig. 6). These findings suggested that glycine, glutamine, and aspartic acid are better growth supplements for enhancing cordycepin production as their supplementation significantly upregulated the expression of major gene targets (ADEK, RNR, and NT5E) of cordycepin biosynthesis.
Fig. 6.
Amino acids-mediated alterations at the transcriptional level. Graph showing the mRNA expression pattern of genes involved in cordycepin biosynthesis for group B (amino acids) growth supplements. The major downstream genes RNR and NT5E were upregulated in glycine, glutamine, and aspartic acid. Data was presented as the mean ± SEM of 3 independent experiments. The significance level was accepted at p ≤ 0.05 as compared to control.
3.8. Plant growth hormones induced differential expression of genes involved in cordycepin biosynthesis
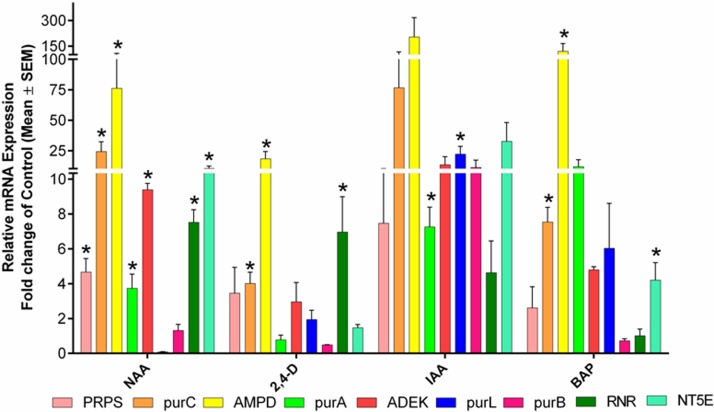
Plant growth hormones can be major inducers for cordycepin synthesis which has not been evaluated until now. The addition of plant growth hormones such as NAA, IAA, 2, 4-D, and BAP in culture medium showed differential expression of genes for cordycepin synthesis. NAA showed the upregulation of RNR (7.51 fold), NT5E (10.59 fold), purA (3.73 fold), and ADEK (9.38 fold) and IAA showed upregulation of RNR (4.63 fold), NT5E (32.79 fold), purA (7.25 fold) and ADEK (13.59 fold) genes as compared to control (Fig. 7). These results suggested that NAA and IAA act as potential growth supplements for increasing cordycepin production in CS2973 as their supplementation also enhanced cordycepin biosynthesis depicted by RP-HPLC.
Fig. 7.
Plant hormones-mediated changes in mRNA expression levels of genes responsible for cordycepin synthesis. Graph representing the mRNA expression pattern of genes involved in cordycepin biosynthesis for group C (plant growth hormones) growth supplements. Data was presented as the mean ± SEM of 3 independent experiments. The significance level was accepted at p ≤ 0.05 as compared to control.
3.9. Effect of vitamins on gene expression of genes involved in cordycepin biosynthesis
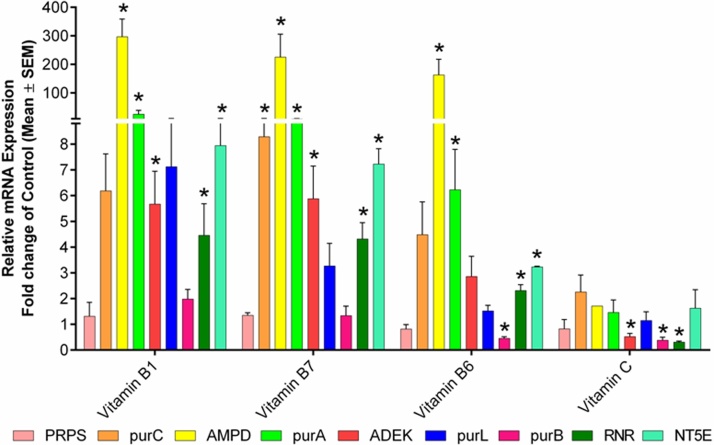
Vitamins, specifically B-complex, play a significant role in the enhancement of cordycepin production in Cordyceps spp. [55]. This led us to evaluate the effect of vitamins on the transcription of genes participating in cordycepin production which has not yet been explored earlier. Four different types of vitamins (B1, B6, biotin, and C) were supplied in the culture medium to study their effect on cordycepin production. The results from the qRT-PCR analysis were corroborated well with the RP-HPLC analysis where B1 significantly increased the cordycepin content present in mycelial biomass of the CS2973. The gene expression profile upon treatment with B1 depicted a significant upregulation of RNR (4.46 fold), NT5E (7.94 fold), purA (25.37 fold), and ADEK (5.67 fold) genes (Fig. 8). So, B1 supplementation majorly increased the expression of purA gene which proved to be a major target for enhancing cordycepin biosynthesis in CS2973. However, Vitamin C did not induce the gene expression which is consistent with the RP-HPLC results suggesting its non-involvement in the pathway.
Fig. 8.
Effect of vitamins on the mRNA expression profile of genes involved in cordycepin biosynthesis. Among group D (vitamins) growth supplements, vitamin B1 and biotin could increase RNR and NT5E gene expression. Data was presented as the mean ± SEM of 3 independent experiments. The significance level was accepted at p ≤ 0.05 as compared to control.
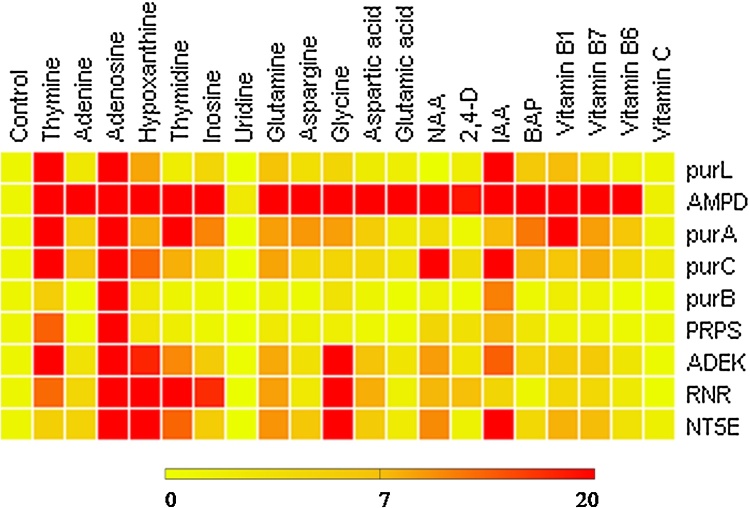
For further evaluation, a heatmap was generated depicting the differential regulation of genes playing role in cordycepin biosynthesis upon the addition of different growth supplements. The results depicted hypoxanthine, adenosine, glycine, IAA, and vitamin B1 serve as better supplements for the enhancement of cordycepin production in mycelial biomass of CS2973 (Fig. 9).
Fig. 9.
Heatmap representing the differential expression of mRNA expression involved in cordycepin biosynthesis: Although many additives activated the signaling mechanism by inducing expression of genes related to cordycepin biosynthesis, but hypoxanthine, adenosine, glycine, and IAA were observed to best growth supplements for enhancement of cordycepin production.
4. Conclusion
Cordycepin possessing a high therapeutic value and market demand have been extensively studied for its pharmacological effect as well as the mode of action mechanistically, but very little attention is paid to enhance the cordycepin biosynthesis. This study deals with the enhancement of bioactive metabolite, cordycepin production in O. sinensis using different growth supplements under submerged fermentation. The RP-HPLC analysis depicted hypoxanthine, adenosine, glycine, glutamine, aspartic acid, NAA, IAA, and thiamine (B1) a better growth supplement to enhance cordycepin production by enhancing RNR, NT5E, ADEK, and purA as compared with other supplements. The mRNA expression analysis provides a comprehensive overview of alterations in genes for cordycepin biosynthesis suggesting the targeted modulation of these genes will enhance cordycepin production. Taken together, this study not only enhanced the cordycepin production at a significant level which can aid in meeting the cordycepin market demand but also facilitates the propagation of this rare and endangered species as well as paves the way for unraveling the mechanism of cordycepin biosynthesis.
CRediT authorship contribution statement
Vikas Kaushik: Conceptualization, Methodology, Data curation, Writing - original draft. Amanvir Singh: Methodology, Writing - review & editing. Aditi Arya: Supervision, Writing - review & editing. Sangeeta Chahal Sindhu: Supervision, Writing - review & editing. Anil Sindhu: Supervision, Conceptualization, Visualization, Writing - review & editing. Ajay Singh: Supervision, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no competing interests.
Acknowledgments
The authors extend their appreciation to the Department of Biotechnology at Deenbandhu Chhotu Ram University of Science and Technology, Murthal, Sonepat, Haryana (India), and Haryana Agro Industries Corporation Limited (HAIC) Murthal, Sonepat, Haryana (India) for financial support and research facilities. The fellowship provided by TEQIP (Technical Education Quality Improvement programme) to Vikas Kaushik is gratefully acknowledged.
References
- 1.Zhang Y., Li E., Wang C., Li Y., Liu X. Ophiocordyceps sinensis, the flagship fungus of China: terminology, life strategy and ecology. Mycology. 2012;3:2–10. [Google Scholar]
- 2.Lin S., Liu Z.Q., Xue Y.P., Baker P.J., Wu H., Xu F., Teng Y., Brathwaite M.E., Zheng Y.G. Biosynthetic pathway analysis for improving cordycepin and cordycepic acid production in Hirsutella sinensis. Appl. Biochem. Biotechnol. 2016;179:633–649. doi: 10.1007/s12010-016-2020-0. [DOI] [PubMed] [Google Scholar]
- 3.Sharma S. Trade of Cordyceps sinensis from high altitudes of the Indian Himalaya: conservation and biotechnological priorities. Curr. Sci. 2004;86:1614–1618. [Google Scholar]
- 4.Valverde M.E., Hernández-Pérez T., Paredes-López O. Edible mushrooms: improving human health and promoting quality life. Int. J. Microbiol. Res. 2015;2015:1–14. doi: 10.1155/2015/376387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Varshney V.K., Pandey A., Kumar A., Rathod D., Kannojia P. Chemical screening and identification of high cordycepin containing cultured isolate (s) of medicinal Chinese caterpillar mushroom, Ophiocordyceps sinensis (Berk.) Int. J. Med. Mushrooms. 2011;13 doi: 10.1615/intjmedmushr.v13.i4.20. [DOI] [PubMed] [Google Scholar]
- 6.Wang J., Liu Y.M., Cao W., Yao K.W., Liu Z.Q., Guo J.Y. Anti-inflammation and antioxidant effect of cordymin, a peptide purified from the medicinal mushroom Cordyceps sinensis, in middle cerebral artery occlusion-induced focal cerebral ischemia in rats. Metab. Brain Dis. 2012;27:159–165. doi: 10.1007/s11011-012-9282-1. [DOI] [PubMed] [Google Scholar]
- 7.Zhou Y., Wang M., Zhang H., Huang Z., Ma J. Comparative study of the composition of cultivated, naturally grown Cordyceps sinensis, and stiff worms across different sampling years. PLoS One. 2019;14 doi: 10.1371/journal.pone.0225750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jang S.H., Kim S.H., Lee H.Y., Jang S.H., Jang H., Chae S.W., Jung S.J., So B.O., Ha K.C., Sin H.S. Immune-modulating activity of extract prepared from mycelial culture of Chinese caterpillar mushroom, Ophiocordyceps sinensis (Ascomycetes) Int. J. Med. Mushrooms. 2015;17:1189–1199. doi: 10.1615/intjmedmushrooms.v17.i12.90. [DOI] [PubMed] [Google Scholar]
- 9.Aman S., Anderson D.J., Conolly T.J., Critall A.J., Ji G.J. From adenosine to 3’-deoxyadenosine: development and scale up. Org. Process Res. Dev. 2000;4:601–605. [Google Scholar]
- 10.Moreau C., Kirchberger T., Swarbick J.M., Bartlett S.J., Fliegert R., Yorgan T., Bauche A., Harneit A., Guse A.H., Potter B.V. Structure-activity relationship of adenosine 5’ diphosphoribose at the transient receptor potential melastatin2 (TRPM2) channel: rational design of antagonists. J. Med. Chem. 2013;56:10079–10102. doi: 10.1021/jm401497a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang S., Liu H., Sun Y., Chen J., Li X., Xu J., Hu Y., Li Y., Deng Z., Zhong S. An effective and convenient synthesis of cordycepin from adenosine. Chem. Pap. 2018;72:149–160. [Google Scholar]
- 12.Shashidhar M.G., Giridhar P., Sankar K.U., Manohar B. Bioactive principles from Cordyceps sinensis: a potent food supplement- A review. J. Funct. Foods. 2013;5:1013–1030. doi: 10.1016/j.jff.2013.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suparmin A., Kato T., Dohra H., Park E.Y. Insight into cordycepin biosynthesis of Cordyceps militaris: comparison between a liquid surface culture and a submerged culture through transcriptomic analysis. PLoS One. 2017;12 doi: 10.1371/journal.pone.0187052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Q., Liu Y., Di Z., Han C.C., Liu Z. The strategies for increasing cordycepin production of Cordyceps militaris by liquid fermentation. Fungal Genom. Biol. 2016;6:134. [Google Scholar]
- 15.Ko Y.F., Lian J.C., Lee C.S., Chiu C.Y., Martel J., Lin C.S., Tseng S.F., Ojcius D.M., Lu C.C., Lai H.C., Young J.D. Isolation, culture and characterization of Hirsutella sinensis mycelium from caterpillar fungus fruiting body. PLoS One. 2017;12 doi: 10.1371/journal.pone.0168734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li S., Li P., Lai C., Gong Y., Kan K.K., Dong T.T., Tsim K.W., Wang Y. Simultaneous determination of ergosterol, nucleosides and their bases from natural and cultured Cordyceps by pressurized liquid extraction and high-performance liquid chromatography. J. Chromatogr. A. 2004;1036:239–243. doi: 10.1016/j.chroma.2004.02.080. [DOI] [PubMed] [Google Scholar]
- 17.Kim H.O., Yun J.W. A comparative study on the production of exopolysaccharides between two entomopathogenic fungi Cordyceps militaris and Cordyceps sinensis in submerged mycelial cultures. J. Appl. Microbiol. 2005;99:728–738. doi: 10.1111/j.1365-2672.2005.02682.x. [DOI] [PubMed] [Google Scholar]
- 18.Leung P.H., Wu J.Y. Effects of ammonium feeding on the production of bioactive metabolites (cordycepin and exopolysaccharides) in mycelial culture of Cordyceps sinensis fungus. J. Appl. Microbiol. 2007;103:1942–1949. doi: 10.1111/j.1365-2672.2007.03451.x. [DOI] [PubMed] [Google Scholar]
- 19.Li R., Jiang X.C., Guan H.S. Optimization of mycelium biomass and exopolysaccharides production by Hirsutella sp. In submerged fermentation and evaluation of exopolysaccharides antibacterial activity. Afr. J. Biotechnol. 2010;9:195–202. [Google Scholar]
- 20.Rathore H., Prasad S., Kapri M., Tiwari A., Sharma S. Medicinal importance of mushroom mycelium: mechanisms and applications. J. Funct. Foods. 2019;56:182–193. [Google Scholar]
- 21.Ji Y., Su A., Ma G., Tao T., Fang D., Zhao L., Hu Q. Comparison of bioactive constituents and effects on gut microbiota by in vitro fermentation between Ophicordyceps sinensis and Cordyceps militaris. J. Funct. Foods. 2020;68 [Google Scholar]
- 22.Chen L., Liu Y., Guo Q., Zheng Q., Zhang W. Metabolomic comparison between wild Ophiocordyceps sinensis and artificial cultured Cordyceps militaris. Biomed. Chromatogr. 2018;32:e4279. doi: 10.1002/bmc.4279. [DOI] [PubMed] [Google Scholar]
- 23.Holliday J.C., Cleaver P., Loomis-Powers M., Patel D. Analysis of quality and techniques for hybridization of medicinal fungus Cordyceps sinensis (Berk.) Sacc. (Ascomycetes) Int. J. Med. Mushrooms. 2004;6:151–164. [Google Scholar]
- 24.Jiang Q., Lou Z., Wang H., Chen C. Antimicrobial effect and proposed action mechanism of cordycepin against Escherichia coli and Bacillus subtilis. J. Microbiol. 2019;57:288–297. doi: 10.1007/s12275-019-8113-z. [DOI] [PubMed] [Google Scholar]
- 25.Zhou X., Gong Z., Su Y., Lin J., Tang K. Cordyceps fungi: natural products, pharmacological functions and developmental products. J. Pharm. Pharmacol. 2009;61:279–291. doi: 10.1211/jpp/61.03.0002. [DOI] [PubMed] [Google Scholar]
- 26.Yue K., Ye M., Zhou Z., Sun W., Lin X. The genus Cordyceps: a chemical and pharmacological review. J. Pharm. Pharmacol. 2013;65:474–493. doi: 10.1111/j.2042-7158.2012.01601.x. [DOI] [PubMed] [Google Scholar]
- 27.Sugar A.M., Maccaffery R.P. Antifungal activity of 3’-deoxyadenosine (cordycepin) Antimicrob. Agents Chemother. 1998;42:1424–1427. doi: 10.1128/aac.42.6.1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoo H.S., Shin J.W., Cho J.H., Son C.G., Lee Y.W., Park S.Y., Cho C.K. Effects of Cordyceps militaris extract on angiogenesis and tumor growth. Acta Pharmacol. Sin. 2004;25:657–665. [PubMed] [Google Scholar]
- 29.Jeong J.W., Jin C.Y., Park C., Hong S.H., Kim G.Y., Jeong Y.K., Lee J.D., Yoo Y.H., Choi Y.H. Induction of apoptosis by cordycepin via reactive oxygen species generation in human leukemia cells. Toxicol. In Vitro. 2011;25:817–824. doi: 10.1016/j.tiv.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 30.Overgaard K.H. The inhibition of 5-phosphoribosyl-1-pyrophosphate formation by cordycepin triphosphate in extracts of Ehrlich ascites tumor cells. Biochim. Biophys. Acta. 1964;80:504–507. doi: 10.1016/0926-6550(64)90154-9. [DOI] [PubMed] [Google Scholar]
- 31.Rottman F., Guarino A.J. The inhibition of phosphoribosylpyrophosphate amidotransferase activity by cordycepin monophosphate. Biochim. Biophys. Acta. 1964;89:465–472. doi: 10.1016/0926-6569(64)90072-0. [DOI] [PubMed] [Google Scholar]
- 32.Holbein S., Wengi A., Decourty L., Freimoser F.M., Jacquier A., DichtlrnaI B. Cordycepin interferes with 3’ end formation in yeast independently of its potential to terminate RNA chain elongation. RNA. 2009;15:837–849. doi: 10.1261/rna.1458909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong Y.Y., Moon A., Duffin R., Barthet-Barateig A., Meijer H.A., Clemens M.J., Moor C.H. Cordycepin inhibits protein synthesis and cell adhesion through effects on signal transduction. J. Biol. Chem. 2010;285:2610–2621. doi: 10.1074/jbc.M109.071159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tianzhu Z., Shihai Y., Juan D. Antidepressant-like effects of cordycepin in a mice model of chronic unpredictable mild stress. Evid. Based Complementary Altern. Med. 2014;2014 doi: 10.1155/2014/438506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leu S.F., Poon S.L., Pao H.Y., Huang B.M. The in vivo and in vitro stimulatory effects of cordycepin on mouse leydig cell steroidogenesis. Biosci. Biotech. Bioch. 2011;75:723–731. doi: 10.1271/bbb.100853. [DOI] [PubMed] [Google Scholar]
- 36.Sun Y., Wang Y.H., Qu K., Zhu H.B. Beneficial effects of cordycepin on metabolic profiles of liver and plasma from hyperlipidemic hamsters. J. Asian Nat. Prod. Res. 2011;13:534–546. doi: 10.1080/10286020.2011.575364. [DOI] [PubMed] [Google Scholar]
- 37.Won K.J., Lee S.C., Lee C.K., Lee H.M., Lee S.H., Fang Z., Choi O.B., Jin M., Kim J., Park T., Choi W.S. Cordycepin attenuates neointimal formation by inhibiting reactive oxygen species–mediated responses in vascular smooth muscle cells in rats. J. Pharm. Sci. 2009;109:403–412. doi: 10.1254/jphs.08308fp. [DOI] [PubMed] [Google Scholar]
- 38.Wang N.Q., Jiang L.D., Zhang X.M., Li Z.X. Effect of dongchong xiacao capsule on airway inflammation of asthmatic patients. Zhongguo Zhong Yao Za Zhi. 2007;32:1566–1568. [PubMed] [Google Scholar]
- 39.Lui J.C., Wong J.W., Suen Y.K., Kwok T.T., Fung K.P., Kong S.K. Cordycepin induced eryptosis in mouse erythrocytes through a Ca2+−dependent pathway without caspase-3 activation. Arch. Toxicol. 2007;81:859–865. doi: 10.1007/s00204-007-0214-5. [DOI] [PubMed] [Google Scholar]
- 40.Quy T.N., Xuan T.D., Andriana Y., Tran H.D., Khanh T.D., Teschke R. Cordycepin isolated from Cordyceps militaris: its newly discovered herbicidal property and potential plant-based novel alternative to glyphosate. Molecules. 2019;24:2901. doi: 10.3390/molecules24162901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Das S.K., Masuda M., Sakurai A., Sakakibara M. Effects of additives on cordycepin production using a Cordyceps militaris mutant induced by ion beam irradiation. Afr. J. Biotechnol. 2009;8:3041–3047. [Google Scholar]
- 42.Masuda M., Urabe E., Honda H., Sakurai A., Sakakibara M. Enhanced production of cordycepin by surface culture using the medicinal mushroom Cordyceps militaris. Enzyme Microb. Technol. 2007;40:1199–1205. [Google Scholar]
- 43.Masuda M., Das S.K., Fujihara S., Hatashita M., Sakurai A. Production of cordycepin by a repeated batch culture of a Cordyceps militaris mutant obtained by proton beam irradiation. J. Biosci. Bioeng. 2011;111:55–60. doi: 10.1016/j.jbiosc.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 44.Kaushik V., Arya A., Sindhu A., Singh A. Identification, optimization of culture conditions and bioactive potential of Chinese caterpillar mushroom Ophiocordyceps sinensis (Ascomycetes) mycelium isolated from fruiting body. Int. J. Med. Mushrooms. 2019;21:931–942. doi: 10.1615/IntJMedMushrooms.2019031841. [DOI] [PubMed] [Google Scholar]
- 45.Yu L., Zhao J., Li S.P., Fan H., Hong M., Wang Y.T., Zhu Q. Quality evaluation of Cordyceps through simultaneous determination of eleven nucleosides and bases by RP‐HPLC. J. Sep. Sci. 2006;29:953–958. doi: 10.1002/jssc.200600007. [DOI] [PubMed] [Google Scholar]
- 46.Han Z.H., Yu-Xian W.A., Xin-Xin T.O., Wallace Y., Jing C.A., Fang W.A., Cheng P.E., Jin-Lin G.U. Overexpression of ribonucleotide reductase small subunit, RNRM, increases cordycepin biosynthesis in transformed Cordyceps militaris. Chin. J. Nat. Med. 2020;18:393–400. doi: 10.1016/S1875-5364(20)30046-7. [DOI] [PubMed] [Google Scholar]
- 47.Liu T., Liu Z., Yao X., Huang Y., Qu Q., Shi X. Identification of cordycepin biosynthesis related genes through de novo transcriptome assembly and analysis in Cordyceps cicade. R. Soc. 2018;5 doi: 10.1098/rsos.181247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kredich N.M., Guarino A.J. Studies on biosynthesis of cordycepin. Biochim. Biophys. Acta. 1961;47:529–534. doi: 10.1016/0006-3002(61)90546-7. [DOI] [PubMed] [Google Scholar]
- 49.Wen T.C., Li G.R., Kang J.C., kang C., Hyde K.D. Optimization of solid state fermentation for fruiting body growth and cordycepin production by Cordyceps militaris. Chiang Mai. J. Sci. 2014;41:858–872. [Google Scholar]
- 50.Kang C., Wen T.C., Kang J.C., Meng Z.B., Li G.R., Hyde K.D. Optimization of large scale culture conditions for the production of cordycepin with Cordyceps militaris by liquid static culture. Transfus. Apher. Sci. 2014;2014 doi: 10.1155/2014/510627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tang J., Qian Z., Wu H. Enhancing cordycepin production in liquid static cultivation of Cordyceps militaris by adding vegetable oil as the secondary carbon source. Bioresour. Technol. 2018;268:60–67. doi: 10.1016/j.biortech.2018.07.128. [DOI] [PubMed] [Google Scholar]
- 52.Xia Y., Luo F., Shang Y., Chen P., Lu Y., Wang C. Fungal cordycepin biosynthesis is coupled with the production of the safeguard molecule pentostatin. Cell Chem. Biol. 2017;24:1479–1489. doi: 10.1016/j.chembiol.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 53.Reichard P. Ribonucleotide reductases: substrate specificity by allostery. Biochem. Biophys. Res. Commun. 2010;396:19–23. doi: 10.1016/j.bbrc.2010.02.108. [DOI] [PubMed] [Google Scholar]
- 54.Kuo H.C., Huang I.C., Chen T.Y. Cordyceps sL (Ascomycetes) species used as medicinal mushrooms are closely related with higher ability to produce cordycepin. Int. J. Med. Mushrooms. 2015;17:1077–1085. doi: 10.1615/intjmedmushrooms.v17.i11.80. [DOI] [PubMed] [Google Scholar]
- 55.Yuan R., Jinchuan M., Yuan X., Jinwen S., Xialong W., Bingli M. The influence of vitamin B1, B6 and 2,4-D on the production of cordycepin in liquid fermentation of Cordyceps militaris. Mycosystema. 2014;33:477–482. [Google Scholar]
- 56.Kaushik K., Das A. Cycloxygenase-2 inhibition potentiates trans-differentiation of Wharton’s jelly–mesenchymal stromal cells into endothelial cells: transplantation enhances neovascularization-mediated wound repair. Cytotherapy. 2019;21:260–273. doi: 10.1016/j.jcyt.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 57.Xiang L., Li Y., Zhu Y., Luo H., Li C., Xu X., Sun C., Song J., Shi L., He L., Sun W. Transcriptome analysis of the Ophiocordyceps sinensis fruiting body reveals putative genes involved in fruiting body development and cordycepin biosynthesis. Genomics. 2014;103:154–159. doi: 10.1016/j.ygeno.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 58.Cho H.J., Cho J.Y., Rhee M.H., Kim H.S., Lee H.S., Park H.J. Inhibitory effects of cordycepin (3'-deoxyadenosine), a component of Cordyceps militaris, on human platelet aggregation induced by thapsigargin. J. Microbiol. Biotechn. 2007;17:1134–1138. [PubMed] [Google Scholar]
- 59.Zhou X., Meyer C.U., Schmidtke P., Zepp F. Effect of cordycepin on interleukin-10 production of human peripheral blood mononuclear cells. Eur. J. Pharmacol. 2002;453:309–317. doi: 10.1016/s0014-2999(02)02359-2. [DOI] [PubMed] [Google Scholar]
- 60.Jin M.L., Park S.Y., Kim Y.H., Park G., Son H.J., Lee S.J. Suppression of α-MSH and IBMX-induced melanogenesis by cordycepin via inhibition of CREB and MITF, and activation of PI3K/Akt and ERK-dependent mechanisms. Int. J. Mol. Med. 2012;29:119–124. doi: 10.3892/ijmm.2011.807. [DOI] [PubMed] [Google Scholar]
- 61.Dalla Rosa L., Da Silva A.S., Gressler L.T., Oliveira C.B., Dambros M.G., Miletti L.C., França R.T., Lopes S.T., Samara Y.N., Da Veiga M.L., Monteiro S.G. Cordycepin (3′-deoxyadenosine) pentostatin (deoxycoformycin) combination treatment of mice experimentally infected with Trypanosoma evansi. Parasitology. 2013;140:663–671. doi: 10.1017/S0031182012001990. [DOI] [PubMed] [Google Scholar]