Abstract
A survey of ixodid ticks parasitizing white-tailed deer (Odocoileus virginianus) and nilgai antelope (Boselaphus tragocamelus) was completed during the 2018–2019 public hunt season on the Laguna Atascosa National Wildlife Refuge (Cameron County, Texas) and the East Foundation's El Sauz Ranch in nearby Willacy County (Texas). Anocenter nitens was the predominant tick species identified with 5% of these ticks collected from nilgai. All life stages were encountered in high numbers on white-tailed deer, indicating that deer may be a primary host in this region. Amblyomma maculatum and Amblyomma inornatum were identified from both hosts, while Ixodes scapularis was encountered only on white-tailed deer. This is the first published record of A. inornatum on nilgai. A subset of ticks was used in PCR assays to detect Rickettsia spp., family Anaplasmataceae, Borrelia spp., and Theileria-Babesia spp. Borrelia spp. were not detected in any of the ticks analyzed. Rickettsia parkeri was detected in three A. maculatum adult ticks from deer, Rickettsia sp. endosymbiont sequences were present in all I. scapularis ticks, and Rickettsia amblyommatis was detected in three A. inornatum adult ticks from deer. Sequence analysis of Anaplasmataceae-positive amplicons from A. nitens and A. maculatum had low percent identity to published Anaplasma spp. sequences, suggesting a unique Anaplasma sp. may be circulating in the population. Anaplasma platys was detected from A. nitens larvae and an Ehrlichia sp. Delta strain was present in A. maculatum, both of unknown pathogenicity towards deer. Theileria cervi was detected in all stages of A. nitens ticks, and positive ticks originated from 27 of 31 deer and a single nilgai sampled from throughout the survey site. The primary vector for T. cervi is absent from this region, suggesting T. cervi is possibly maintained by a different tick species.
Keywords: Anocenter nitens, Horse tick, Amblyomma maculatum, Amblyomma inornatum, Rickettsia parkeri, Theileria cervi, Rickettsia amblyommatis
Graphical abstract
Highlights
-
•
Anocenter nitens predominates on white-tailed deer and nilgai in southern Texas.
-
•
Theileria cervi was detected in Anocenter nitens ticks from deer and a single nilgai.
-
•
Three Theileria cervi genotypes were detected in this region of southern Texas.
-
•
Unique Anaplasma sp. detected in A. nitens; low identity to known Anaplasma sp.
-
•
Amblyomma inornatum ticks were identified on nilgai; a first record on this host.
1. Introduction
The border region of south Texas shares a tropical tick fauna with Mexico that is unlike that found in the rest of the USA. Cattle fever ticks (Rhipicephalus (Boophilus) spp.), vectors of bovine babesiosis, are prevalent in Mexico and found intermittently in the USA only in the counties bordering the Rio Grande (Lohmeyer et al., 2011). Beginning in 2014 however, a population became established on sylvatic hosts in the coastal areas of Cameron and Willacy counties (Texas) (Lohmeyer et al., 2018; Olafson et al., 2018). While cattle fever ticks are one-host ticks that use bovines as their preferred hosts, white-tailed deer (Odocoileus virginianus Zimmerman; hereafter referred to as deer) have long been known as secondary hosts (Pound et al., 2010), and evidence suggests that deer are neither susceptible to infection nor serve as reservoirs of Babesia bovis, the causative agent of bovine babesiosis (Ueti et al., 2015). Nilgai antelope (Boselaphus tragocamelus Pallas; hereafter referred to as nilgai), are even more concerning as they are competent hosts of the tick vector and are vehicles for tick dissemination and, as a bovine, are potential reservoirs of bovine babesiosis (Foley et al., 2017; Lohmeyer et al., 2018). The dynamics of cattle fever tick populations relative to other tick fauna that occupy this landscape is understudied, as are the parasites that may be harbored by these tick populations. Surveys of ticks on wild pigs (Sus scrofa), deer, and nilgai identified a variety of tick species that share this environment, including Amblyomma maculatum Koch, Amblyomma mixtum Koch, Amblyomma tenellum Koch, Ixodes scapularis Say, Dermacentor variabilis Say, and Dermacentor halli McIntosh (Corn et al., 2016; Feria-Arroyo et al., 2014; Olafson et al., 2018; Sanders et al., 2011), but molecular surveillance for parasites is limited.
For this study, deer and nilgai antelope were sampled for tick infestations at the Laguna Atascosa National Wildlife Refuge (LANWR) in Cameron County, Texas and on the East Foundation's El Sauz Ranch in Willacy County, Texas. A subsample of these tick specimens was then screened for bacteria and piroplasms of concern to human and animal health.
2. Materials and methods
2.1. Study site and tick collections
Deer and nilgai were censused for tick infestations at the Laguna Atascosa National Wildlife Refuge (LANWR; Cameron County, Texas). Animals were harvested from Units 1, 2, 3, 5, 6, and 8 as part of the open public hunts (Fig. 1). Deer and nilgai were also inspected at East Foundation's El Sauz Ranch (ESR) located approx. 35 km north of LANWR Units 3 and 5. This combined census effort was part of surveillance for invasive cattle fever ticks, and harvested (LANWR) or live-captured (ESR) animals were scratch-inspected for ticks by inspectors from the US Department of Agriculture (USDA)-Animal Plant Health Inspection Service-Veterinary Services-Cattle Fever Tick Eradication Program and the Texas Animal Health Commission. If harvested, carcasses were typically field dressed, could be many hours post-mortem, and had been transported to the check station from the harvest site. If live-captured, animals were scratch-inspected for ticks prior to release. The unit within the LANWR where each animal was taken was recorded, and the position was based on hunter recall. Ticks collected from each individual host were placed in a glass vial, and the vial was marked with a unique identifier for the deer or nilgai. Vials of ticks were delivered to the USDA-Agricultural Research Service, Cattle Fever Tick Research Lab (Edinburg, Texas) where ticks per individual host were enumerated and identified to species using taxonomic keys (Estrada-Pena et al., 2005; Guzmán-Cornejo et al., 2011; Keirans and Clifford, 1978; Nava et al., 2014; Yunker et al., 1986). The ticks were subsequently archived in absolute ethanol and stored at room temperature.
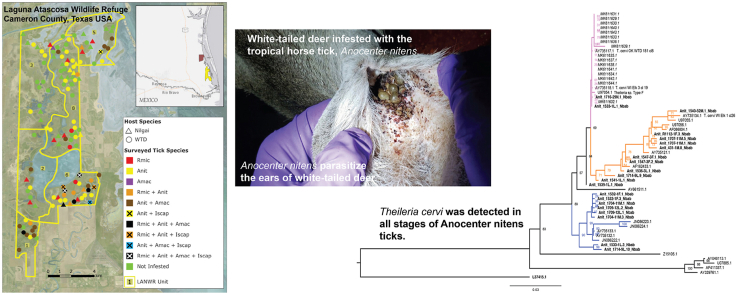
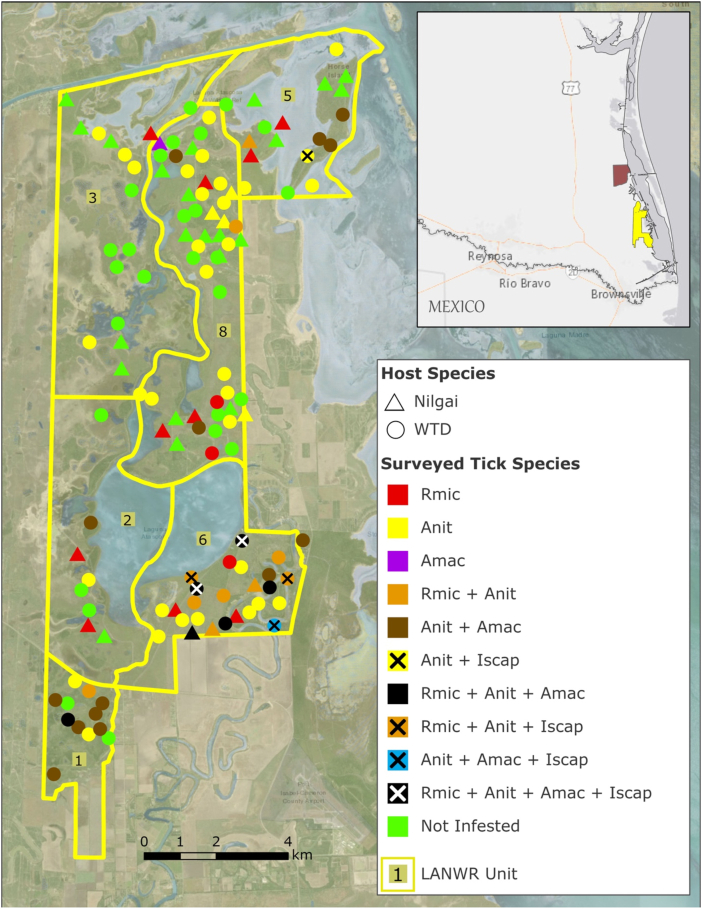
Fig. 1.
Prevalence of ixodid ticks on white-tailed deer and nilgai on the Laguna Atascosa Wildlife Refuge (LANWR) in Cameron County (Texas). The LANWR is divided into Units, outlined in yellow. Deer and nilgai were harvested from the indicated units during the public hunt season in 2018–2019. Deer are identified by circles, nilgai are identified by triangles. Ixodid tick species collected either singly or in combination from these hosts are indicated by a different fill color presented in the legend. Animals from which no ticks were collected are shaded in green (‘Not infested’). Rmic: Rhipicephalus (Boophilus) microplus; Anit: Anocenter nitens; Amac: Amblyomma maculatum; Iscap: Ixodes scapularis. Inset: The LANWR units are shaded in yellow, while the East Foundation's El Sauz Ranch located north of the LANWR is shaded in maroon.
2.2. Tick specimens for molecular analysis
Genomic DNA (gDNA) was isolated from a sub-sample of ticks collected from the deer and nilgai described in Section 2.1. Anocenter nitens Neumann gDNA was isolated from adults (N = 235; 84 female, 151 male), nymphs (N = 115) and 55 larval samples with 1–15 larvae comprising each sample. These were collected from 31 deer hosts, while A. nitens gDNA was also isolated from adults (N = 70; 32 female, 38 male), nymphs (N = 12) and two larval samples collected from five nilgai hosts. All A. nitens ticks were collected from hosts in the LANWR. gDNA was isolated from A. maculatum collected from seven deer hosts in the LANWR (N = 16; 3 females, 12 males, 1 nymph) and four deer live-captured at the ESR (N = 17; 13 females, 4 males)., gDNA was isolated from Ixodes scapularis Say adults obtained from six deer hosts in the LANWR (N = 29; 23 females, 6 males) and live-captured deer on ESR (N = 8 ticks, all female, of 52 total deer inspected). Amblyomma inornatum Banks adults obtained from three deer hosts in the LANWR (N = 3 ticks; 1 female, 2 males) were used for gDNA isolation. Ethanol was decanted from individual vials housing the tick samples. The ticks were then rinsed in three changes of Tris-EDTA, pH 8.0, dried on paper towels, and placed in Petri dishes. All specimens were dried under a chemical fume hood for 5 h, after which each adult or nymph was placed into a Kimble® DNAse/RNase-free, 1.5 ml reaction tube. Dried A. nitens larvae were either isolated individually or pooled into a total of 55 larval samples. Each tube was chilled in liquid nitrogen, and the tick or larval pool was macerated using an individual, liquid nitrogen-cooled, disposable Kimble® pestle. Tick gDNA was isolated using the DNEasy® Blood and Tissue Kit (Qiagen, Germantown, MD), and gDNAs were eluted from the column in 100 μl Tris HCl, pH 8.0. Samples were stored at −20 °C until further analysis.
2.3. Tick-borne bacteria and piroplasm detection by PCR
Tick gDNA samples were tested for presence of Rickettsia spp., family Anaplasmataceae, Borrelia spp., and Theileria-Babesia spp. DNA using PCR assays to target the Rickettsia surface cell antigen, sca0 (rompA) (Regnery et al., 1991), family Anaplasmataceae heat shock protein groEL (Tabara et al., 2007), Theileria-Babesia 18S SSU rRNA spanning the V4 region (Oosthuizen et al., 2008; Ueti et al., 2015), and Borrelia flagellin flaB (Barbour et al., 1996). Oligonucleotide primer sequences, reaction conditions, and cycling parameters are summarized in the supplementary file (Table S1). Assays were conducted in a 20 μl reaction volume with Platinum™ Taq DNA Polymerase (ThermoFisher Scientific, Waltham MA). Anocenter nitens adult, nymph, and larval sample gDNAs were initially screened in pools. Each pool was comprised of 5 μl tick gDNA from up to ten individuals/larval samples in a total volume of 50 μl. An aliquot of the pooled gDNA (5 μl) was used as template in an initial screen for parasite detection. If a positive amplicon was detected, individual tick or larval sample gDNAs that comprised the pool were further screened to assess the number of positive ticks or larval samples (2 μl gDNA in a 20 μl reaction volume). Amblyomma maculatum, A. inornatum, and I. scapularis ticks were screened individually using a 2 μl gDNA aliquot in a 20 μl reaction volume.
Positive control DNAs were included in all diagnostic PCR assay runs. Template gDNA was procured from the American Type Culture Collection (Manassas, VA) for Rickettsia rickettsii (NR-48826) and Borrelia burgdorferi (35210D-5.) Also used were template from an Ehrlichia chaffeensis-positive Amblyomma americanum adult female tick (EC-16-TN; 2 μl) kindly provided by Dr. Rebecca Trout Fryxell (University of Tennessee, Knoxville) and gDNA isolated from whole blood of a Theileria cervi-infected white-tailed deer doe. A no-template, water control was included with each diagnostic PCR assay run.
Amplicons were prepared for sequencing using the DNA Clean-and-Concentrator kit (Zymo Research), and these samples were submitted to Genscript (Piscataway, NJ) for bidirectional Sanger sequencing. Given the number of family Anaplasmataceae- and Theileria-Babesia-positive A. nitens ticks, at least one individual tick amplicon per host animal was sequenced in full length to validate identity. A larger, Theileria 18S rRNA SSU fragment (1660–1665 bp) was amplified from selected A. nitens ticks to obtain further information about the isolate types circulating in this population. A hemi-nested approach was used to amplify the fragment from individual ticks, and primer sequences, reaction conditions, and cycling parameters are summarized in the supplementary file (Table S1). Assays were conducted in a 20 μl reaction volume with 50× Advantage® High Fidelity 2 Polymerase (Takara, Mountain View CA). A number of these amplicons were polymorphic at numerous positions, thus were ligated to the pCR™4-TOPO™ plasmid vector (ThermoFisher Scientific), cloned in One Shot™ TOP10 Chemically Competent E. coli (ThermoFisher Scientific), and the resulting plasmids of at least three clones were sequenced using multiple primers to span the full length of the clone (Table S1). All sequences were submitted to the GenBank repository. These include: the smaller T. cervi 18S SSU rRNA amplicons from LANWR A. nitens ticks (MW008539-MW008550) and the larger (1660–1665 bp) 18S SSU rRNA cloned fragments from LANWR A. nitens ticks (MW008518-MW008538). Also submitted were: A. nitens groEL amplicons from adult (MW008554) and larval (MW0 08555) templates, A. maculatum groEL from ESR adults (MW008557-MW008558) and a LANWR adult (MW008556), A. inornatum sca0 amplicon from LANWR adults (MW241133), I. scapularis sca0 amplicons from adults (MW008552) and the A. maculatum sca0 amplicon from LANWR and ESR adults (MW008551).
2.4. Phylogenetic analysis
Nucleotide sequences from representative Theileria and Babesia 18S SSU rRNAs were obtained from GenBank (publicly available database) and aligned with Theileria sequences (1660–1665 bp) from A. nitens using the MUSCLE algorithm (Edgar, 2004). Similarly, representative sequences annotated as rickettsial endosymbionts of Ixodes spp. and Amblyomma spp. were obtained and aligned with the Rickettsia sp. sequences from I. scapularis and A. maculatum obtained in this study. Alignments were used to construct a maximum likelihood phylogeny with the web server version of IQ-TREE software (Trifinopoulos et al., 2016) with best-fit substitution model (Kalyaanamoorthy et al., 2017) and branch support assessed with 10,000 replicates of UFBoot bootstrap approximation (Hoang et al., 2018). The Theileria 18S SSU rRNA phylogenetic tree was rooted using Toxoplasma gondii (L37415) as an outgroup, as it belongs to a different class of apicomplexa. The Rickettsia spp. tree was rooted at midpoint.
3. Results
3.1. Prevalence of ixodid ticks on white-tailed deer and nilgai in southern Texas
A total of 90 deer and 42 nilgai were harvested as part of the 2018-19 public hunts on the LANWR. The location within the LANWR where deer and nilgai were harvested, along with color coding for the tick species identified per host is presented in Fig. 1. A summary of the number of hosts on which various tick species were identified either singly or in combination with other tick species is presented in Table 1, Table 2, respectively. There were 27 deer and 22 nilgai from which no ticks were found (‘Not Infested’, Fig. 1). Thirty-five deer were infested with one species of tick, while 28 deer were infested with between two and four species of ticks. Fifteen nilgai were infested with one species of tick, and four nilgai were infested with two or three species of ticks.
Table 1.
Numbers of white-tailed deer and nilgai hosts from the Laguna Atascosa National Wildlife Refuge infested with one species of tick. Numbers of female and male hosts found infested per tick species are listed. Also listed are the number of hosts from which no ticks were identified ('Not Infested').
| Species of tick identified Number of female (F) or male (M) hosts found infested with tick species |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Host |
Total Hosts |
Hosts Not Infested |
Rhipicephalus microplus |
Anocenter nitens |
Amblyomma maculatum |
Amblyomma inornatum |
Ixodes scapularis |
|||||||
| F | M | F | M | F | M | F | M | F | M | F | M | F | M | |
| Nilgai | 24 | 13 | 15 | 7 | 5 | 5 | 3 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| Deer | 26r | 36 | 11 | 16 | 0 | 3 | 15 | 17 | 0 | 0 | 1 | 0 | 0 | 0 |
Table 2.
Records of white-tailed deer and nilgai hosts from the Laguna Atascosa Wildlife Refuge infested with multiple species of ticks.
| Host |
Total |
Rm/An/Ama |
Rm/Ana |
An/Isa |
An/Ama |
Rm/An/Isa |
Rm/An/Am/Isa |
An/Am/Isa |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | M | F | M | F | M | F | M | F | M | F | M | F | M | F | M | |||
| Nilgai | 1 | 3 | 1 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Deer | 4 | 24 | 1 | 2 | 1 | 4 | 0 | 1 | 2 | 12 | 0 | 2 | 0 | 2 | 0 | 1 | ||
Rm: Rhipicephalus microplus; An: Anocenter nitens; Am: Amblyomma maculatum; Is: Ixodes scapularis.
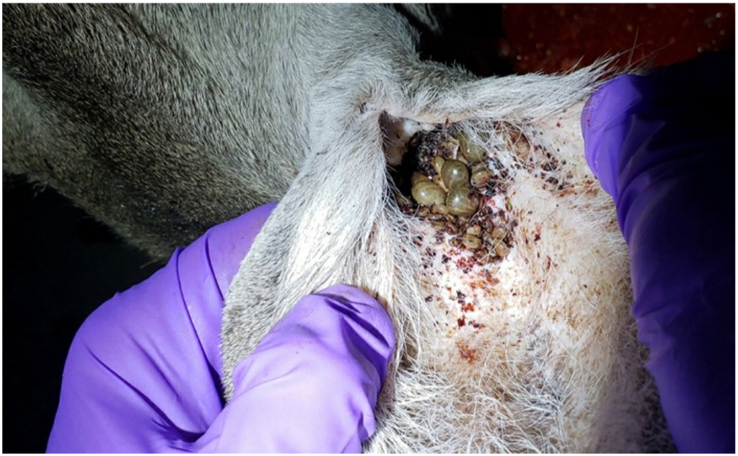
The predominant tick species collected from these hosts was A. nitens with a total of 3129 ticks, comprising 275 adult females, 435 adult males, 386 nymphs, and 2033 larvae. Of these, 39 females, 50 males, 24 nymphs, and 50 larvae were collected from eight of the nilgai. These animals were harvested from all public hunt Units of the LANWR and, as in horses, the preferred site of A. nitens infestation on deer was in the ears (Fig. 2). Rhipicephalus (B.) microplus Canestrini ticks (N = 285) were also identified from both hosts in the LANWR and comprised 71 adult females, 84 adult males, and 130 nymphs. These hosts were harvested from Units 1, 2, 5, 6, and 8 (Fig. 1). A total of 47 A. maculatum ticks comprising 19 adult females, 24 adult males, and 2 nymphs were collected from 19 white-tailed deer, while one adult female and one adult male were collected separately from two nilgai. These hosts were harvested from Units 1, 2, 5, 6, and 8 (Fig. 1). Thirty-one female and six male I. scapularis ticks were collected from deer in Units 5 and 6 and none were identified from nilgai hosts (Fig. 1). Two adult female and two adult male A. inornatum ticks were identified on three deer and one nilgai from Units 1, 5, 6, and 8 of the LANWR.
Fig. 2.
Anocenter nitens, the tropical horse tick, infesting a white-tailed deer. As in horses, A. nitens ticks preferentially infest the ears of this cervid host. Photo Credit: Emma Mitchell
3.2. Rickettsial and piroplasm DNA detected in ixodid ticks collected from white-tailed deer and nilgai in southern Texas
Results of molecular screening assays are summarized in Table 3. Borrelia sp. was not detected in any of the ticks sampled. All A. nitens ticks were negative for Rickettsia sp. Approximately 21% of A. nitens samples were Anaplasmataceae-positive (N = 103/489) and represent ticks collected from 17 deer hosts in the LANWR. The proportion of Anaplasmataceae-positive A. nitens were similar between adult females (0.39) and males (0.40) with a lower proportion of positives detected from nymphs (0.15) and larval samples (0.07). Sequence data was obtained for at least one individual tick amplicon per host animal. On one of the hosts, 39 of 57 ticks were Anaplasmataceae-positive. Five representative sequences were obtained from this host for a total of 22 Anaplasmataceae-positive amplicon sequences. Seventeen of these sequences, all from adult ticks, were 100% identical to one another and displayed 81–82% nucleotide sequence identity to Anaplasma bovis and Anaplasma phagocytophylum groEL sequences in GenBank (e.g., GenBank MH255900, MH255905). The remaining five amplicons were 100% identical to one another and 99% identical to Anaplasma platys groEL isolates (e.g., GenBank MN202021), all amplicons of which were from larval templates.
Table 3.
Detection of tick-borne parasites harbored by ixodid ticks collected from white-tailed deer and nilgai hosts in south Texas.
| Tick Species (Host) |
Anaplasmatacae sp. groEL positive |
Rickettsia sp. rompA positive |
Theileria-Babesia sp. 18S SSU rRNA positive |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Female |
Male |
Nymphs |
Larval Pools |
Female |
Male |
Nymphs |
Larval Pools |
Female |
Male |
Nymphs |
Larval Pools |
||
| # positive/ total number (proportion positive of total) |
# positive/ total number (proportion positive of total) |
# positive/ total number (proportion positive of total) |
|||||||||||
|
Anocenter nitens (White-tailed deer) |
40/ 84 (0.47) | 41/ 151 (0.27) | 15/ 115 (0.13) | 7/ 55 (0.13) | 0/ 84 - |
0/ 151 - |
0/ 115 - |
0/ 55 - |
16a/ 84 (0.19) | 60 a/ 151 (0.40) | 15/ 115 (0.13) | 48/ 55 (0.87) | |
| (Nilgai) | 0/ 32 - |
0/ 38 - |
0/ 12 - |
0/ 2 - |
0/ 32 - |
0/ 38 - |
0/ 12 - |
0/ 2 - |
2/ 32 (0.06) | 6/ 38 (0.16) | 0/ 12 - |
0/ 2 - |
|
|
Amblyomma maculatum (White-tailed deer) |
6/ 16 (0.38) | 2/ 16 (0.13) | 0/ 1 - |
n/a - |
2/ 16 (0.13) | 2/ 16 (0.13) | 0/ 1 - |
n/a - |
0/ 16 - |
0/ 16 - |
0/ 1 - |
n/a - |
|
|
Ixodes scapularis (White-tailed deer) |
0/ 31 - |
0/ 6 - |
n/a - |
n/a - |
31/ 31 (1.00) | 6/ 6 (1.00) | n/a - |
n/a - |
0/ 31 - |
0/ 6 - |
n/a - |
n/a - |
|
Of these positive A. nitens ticks, two adult females and six adult males were collected from a single nilgai host.
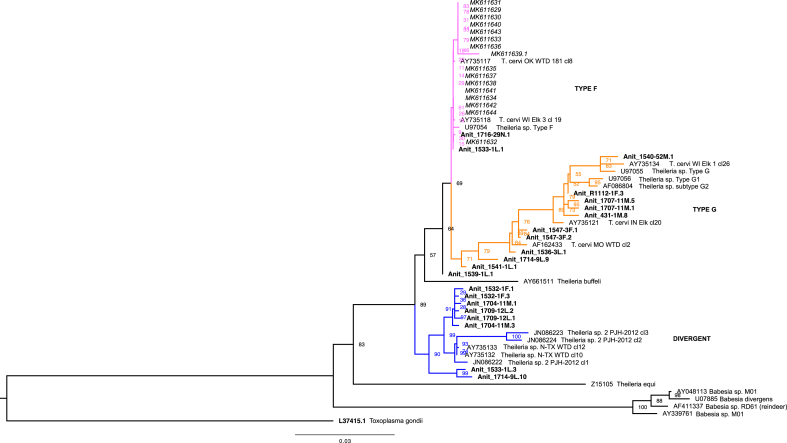
Theileria-Babesia-positive A. nitens ticks were detected in a higher proportion than Anaplasmatacae-positive A. nitens (N = 147/489, 0.30). These represented specimens collected from 27 deer and a single nilgai from the LANWR (summarized in Table S2). The highest proportion of Theileria-Babesia-positive ticks were from adult males (0.45) and larval samples (0.33) with similar proportions of positives detected in adult females (0.12) and nymphs (0.10). Two adult female and six adult male A. nitens ticks (of 24 total) were positive on the single nilgai. Sequence analyses of these smaller 430–440 bp 18S SSU rRNA fragments indicated they aligned with 95–100% nucleotide sequence identity to Theileria cervi and unclassified Theileria sp. isolates originating from cervid hosts, including white-tailed deer and elk (e.g., GenBank JN086224, U97055). These data further indicated the presence of polymorphisms, prompting isolation and sequencing of a larger, 18S SSU rRNA fragment (1660–1670 bp in size). Cloned sequences were 95–100% identical to each other and further confirmed identification of Theileria sp. and T. cervi. A phylogenetic analysis of the A. nitens Theileria sp. sequences and those in the public domain supported clustering of the A. nitens representatives with Type G and Type F T. cervi, as well as with a divergent Theileria sp. reported from white-tailed deer in north Texas (Fig. 3). Co-detection of Anaplasma sp. and Theileria sp. was observed in 38 of the A. nitens ticks, over half (21/38, 0.55) of which were adult males followed by eight females (0.21), three nymphs (0.08), and six larval samples (0.16).
Fig. 3.
Phylogentic analysis of Theileria sp. fragments from Anocenter nitens ticks. Representative Type F, Type G, and ‘divergent’ Theileria sp. sequences were identified from individual A. nitens ticks collected from white-tailed deer and a single nilgai host (bold labels). A maximum-likelihood tree was constructed using Toxoplasma gondii as the outgroup, as it is from a different axpicomplexan class than Theileria. Branch support was assessed with 10,000 replicates of UFBoot bootstrap replication, and bootstrap percentages are indicated at each branch point in the tree. GenBank accession numbers and annotated identification for sequences used in the comparative analysis are indicated on the branch labels. Accession numbers in italics are those T. cervi sequences from white-tailed deer on the East Foundation's San Antonio Viejo Ranch in Starr and Jim Hogg Counties, Texas (Yu et al., 2020).
All A. maculatum ticks were negative for Theileria-Babesia spp., while eight A. maculatum ticks were positive for family Anaplasmatacae sp. Sequence data of the amplicons for seven ticks collected from four different deer displayed 98–99% sequence identity to an Ehrlichia sp. Delta strain, closely related to Ehrlichia muris (GenBank MT681330). These four deer were live-captured and released from ESR. Data from one tick had 82% sequence identity to An. bovis and An. phagocytophylum groEL from livestock (e.g., GenBank MH255900, MH255905), and this was from a deer in the LANWR. A diagnostic amplicon for Rickettsia sp. sca0 (rompA) was detected in four adult ticks, and sequence data from three adults (1 female, 2 males) displayed 99% identity to Rickettsia parkeri (e.g., GenBank MG574938). Each of the R. parkeri-positive ticks were obtained from separate deer, two deer of which were from Unit 1 of the LANWR (Fig. 1) and one of which was from ESR. Sequence data from one female tick had 99% identity to uncultured Rickettsia sp. and rickettsial endosymbionts from ixodid ticks (e.g., GenBank KX077194). Rickettsia parkeri was co-detected with the Ehrlichia sp. Delta strain in one of the three R. parkeri-positive ticks, while the A. bovis-like sequence was co-detected in another R. parkeri-positive tick. The one rickettsial endosymbiont was co-detected with the Ehrlichia sp. Delta strain in a single A. maculatum tick.
The three A. inornatum ticks were negative for Borrelia sp., family Anaplasmatacae, and Theileria-Babesia sp., but a diagnostic amplicon for Rickettsia sp. sca0 was detected in two of the three ticks. Sequence data from both ticks displayed 99–100% nucleotide sequence identity to Rickettsia amblyommatis sca0 (e.g., GenBank MN336348).
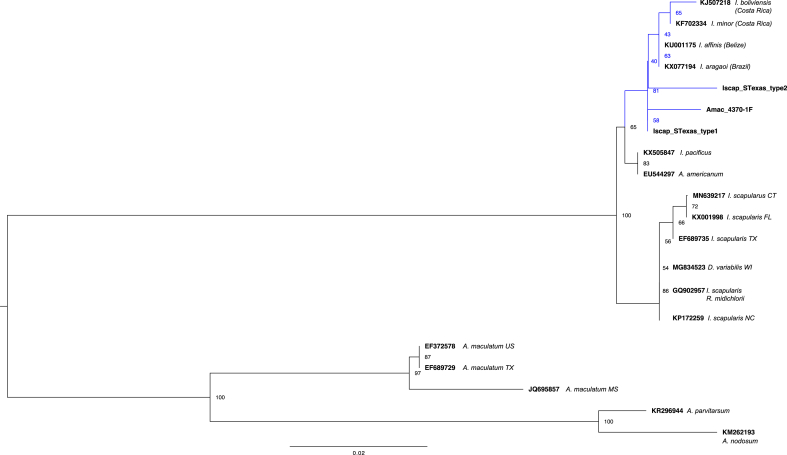
All I. scapularis ticks were negative for Borrelia sp., Anaplasmatacae sp., and Theileria-Babesia sp. However, a diagnostic amplicon for Rickettsia sp. sca0 (rompA) was detected in all 37 I. scapularis ticks representing ticks from both LANWR and ESR. Sequence data obtained for each tick displayed 99–100% nucleotide sequence identity to rickettsial endosymbionts reported from ixodid ticks (e.g., GenBank KX077194). Comparison of the I. scapularis sequences identified two highly similar genotypes with five polymorphic nucleotide positions (Iscap_STexas_type1 and _type 2). These were used for phylogenetic analysis along with the Am. maculatum endosymbiont sequence (Amac_4370-1F), which was identical to Iscap_STexas_type1 and is represented in the phylogeny (Fig. 4).
Fig. 4.
Phylogentic analysis of sca0 (rompA) sequences from putative Rickettsia sp. endosymbionts of Amblyomma maculatum and Ixodes scapularis ticks collected from white-tailed deer in southern Texas. This is a maximum-likelihood tree that is rooted at midpoint. Branch support was assessed with 10,000 replicates of UFBoot bootstrap replication, and bootstrap percentages are indicated at each branch point in the tree. Sequences from GenBank used in the comparative analysis were annotated as rickettsial endosymbionts. Accession numbers and tick species from which sequence was identified are included on the branch label.
4. Discussion
White-tailed deer are known reservoirs of tick-borne pathogens, including E. chafeensis (Lockhart et al., 1997), Ehrlichia ewingii (Yabsley et al., 2002), Ehrlichia spp. (Yabsley et al., 2008) and Anaplasma spp. (Dugan et al., 2006). It is unclear what role nilgai may have in this capacity. Documenting the various tick species parasitizing these hosts expands what we know of the tick fauna in southern Texas that share a landscape with the invasive cattle fever tick, which was found singly and in combination with between one to three different tick species in the LANWR (Table 1, Table 2). Screening these tick specimens further provides insight into parasites circulating within these ungulate populations in this unique region of Texas. The most common tick encountered on deer at the LANWR was the tropical horse tick, A. nitens. We follow Borges et al. (1998) in recognizing Anocentor as a genus distinct from Dermacentor. It is the vector of equine babesiosis and equine piroplasmosis (Pfeifer Barbosa et al., 1995; Schwint et al., 2008), and it is the most important ectoparasite of horses and donkeys in Latin America (Borges et al., 2000). This species is distributed throughout Latin America as far south as Argentina and on the islands of the Caribbean. It expanded into Florida in 1958 but is otherwise found in the USA only in southern Texas (Drummond et al., 1969; Scoles et al., 2011). It is a one-host tick, and aside from equines, has been reported as incidental on cattle and dogs. There are single records on deer in Mexico (Guzmán-Cornejo et al., 2011) and Costa Rica (Carreno et al., 2001), Nelson et al. (2017) reported them parasitizing deer in the US Virgin Islands, and Szabo et al. (2003) found them infesting marsh deer, Blastocerus dichotomus Illiger, in Brazil. Inasmuch as equids, cattle and dogs are not native to the New World, the finding of this tick in all stages and in great numbers on deer in southern Texas suggests that the latter are the native hosts. Additional surveys of cervid populations in countries throughout Latin America would better inform this host association.
Amblyomma maculatum, the Gulf Coast tick, was also collected from deer in this study at both the LANWR and ESR. It occurs across the southern USA from Virginia to California then south to Paraguay (Lado et al., 2018). As its name implies, this species is native to the Gulf coast of Louisiana and Texas, and only within 160 km of the coast, according to Bishopp and Hixson (1936). However, in the latter half of the twentieth century climate change and growth of the livestock industry resulted in an expansion into most of the southern USA (Paddock and Goddard, 2015; Sonenshine, 2018; Teel et al., 2010). Amblyomma maculatum is an important pest of cattle and dogs (Cooley and Kohls, 1944) and is another tick that prefers the ears as the bite site. A three-host tick, the larvae infest primarily birds (Teel et al., 1998). The native nymphal host is likely coyote (Canis latrans Say), and native adult host in the USA is presumably white-tailed deer as they have been reported on deer from essentially every state in their range (Nadolny and Gaff, 2018; Teel et al., 2010), including Texas (Samuel and Trainer, 1970). Amblyomma maculatum is the alternate reservoir of Hepatozoon americanum, the causative agent of wasting disease in dogs (Johnson et al., 2009). Experimentally they can transmit Panola Mountain Ehrlichiosis for which deer are the susceptible reservoir in nature (Loftis et al., 2016), and Cowdria ruminatium, the etiological agent of heartwater (Mahan et al., 2000), which has not yet been reported in the USA.
Deer are the typical host of adult black-legged ticks, I. scapularis, which is a three-host tick (Wilson et al., 1990). Ixodes scapularis is the primary vector of Borrelia burgdorferi, causative agent of Lyme disease, over the northeastern and mideastern U.S. (Keirans et al., 1996; Oliver et al., 1993). While the distribution map by Dennis et al. (1998) does not show it extending into southern Texas, Feria-Arroyo et al. (2014) confirm that the tick is present on white-tailed deer in this region, including in Cameron County, and in adjoining northern Mexico. Ixodes scapularis is also known to transmit Babesia odocoilei, a parasite originally described from white-tailed deer that has been detected in Texas herds (Waldrup et al., 1989, 1990).
While only four specimens were identified, A. inornatum was found on deer and a single nilgai from the LANWR. This species was originally described from southern Texas, but its range includes most of Mexico (Cooley and Kohls, 1944; Guzmán-Cornejo et al., 2011). A 3-host tick, it has been found on rabbits, dogs, cattle, deer, and humans. The present is the first report on nilgai. In Mexico it has been found on both species of peccary (Pecari tajacu and Tayassu pecari), so the latter may be the typical native host. There are no records of pathogens transmitted by this tick, but Medlin et al. (2015) detected Rickettsia sp., Borrelia sp., and Ehrlichia sp. in questing A. inornatum from Webb County (Texas).
Rickettsial parasites have been detected in ixodid ticks sampled from white-tailed deer, including A. phagocytophilum, R. parkeri, E. ewingii, and rickettsial endosymbionts (Baldridge et al., 2009; Lockwood et al., 2018; Mays et al., 2016; Trout Fryxell et al., 2015). Of the tick specimens screened from our study region, Anaplasma sp. were identified from A. nitens, while Anaplasma sp., Ehrlichia sp., and Rickettsia sp. were identified in A. maculatum ticks. Only Rickettsia sp. were detected in A. inornatum and I. scapularis ticks. Identities were based on nucleotide sequence similarity of the PCR amplicons to the GenBank database, and >99% identity with 100% coverage was indicative of a significant match. Thus, an only 80% sequence identity of the groEL amplicons from 17 adult A. nitens and one A. maculatum to that of A. bovis/A. phagocytophylum suggests that a unique Anaplasma sp. is circulating in these white-tailed deer. Use of additional gene targets for further characterization is warranted, given the high proportion of A. nitens adults that tested positive (81/103, 79%). Anaplasma platys, identified from A. nitens larval samples, is a known pathogen of dogs and is transovarially and transstadially transmitted in Rhipicephalus sanguineus Latreille (Llanes and Rajeev, 2020; Snellgrove et al., 2020). Anaplasma platys has been previously reported from cervids, including a recent survey of white-tailed deer from the East Foundation's San Antonio Viejo Ranch (SAVR) in Starr and Jim Hogg counties of southern Texas (Li et al., 2016; Yu et al., 2020). Twenty percent of A. maculatum ticks, all from ESR, were positive for an Ehrlichia sp. Delta strain, recently reported from an Amblyomma triste Koch tick in Argentina (Cicuttin et al., 2020). As A. platys and the latter Ehrlichia sp. are of unknown pathogenicity in deer, these data serve as records of their circulation in this region.
Targeting the Rickettsia sp. sca0 (rompA) gene, no Rickettsia sp. were detected in A. nitens ticks. Rickettsia parkeri, the causative agent of R. parkeri rickettsiosis, was detected in 9% of the screened A. maculatum ticks, the species known to transmit this pathogen in the USA (Paddock et al., 2004). These ticks were from both the LANWR and ESR. Rickettsia parkeri was reported in A. maculatum larvae from small mammals in southeastern Texas (Castellanos et al., 2016), and Paddock et al. (2020) detected R. parkeri in adult A. maculatum collected from a road-kill mule deer (Odocoileus hemionus) in western Texas; the current study expands the records to positive A. maculatum ticks from cervids in southern Texas. Medlin et al. (2015) reported Candidatus Rickettsia amblyommii (now Rickettsia amblyommatis) from questing A. inornatum adults in Webb County, Texas, and our detection of R. amblyommatis from A. inornatum ticks on-host in this study indicates circulation in this region. Rickettsia amblyommatis is commonly detected in A. americanum, and it is considered the most widely distributed spotted fever group rickettsia in the Americas because of the diversity and range of ticks from which it has been detected (Karpathy et al., 2016). Rickettsial endosymbiont sca0 (ompA) sequences were amplified from one Am. maculatum and all 37 I. scapularis ticks screened. The sequences isolated in this study form a clade with public database entries annotated as rickettsial endosymbionts from Ixodes affinis Neumann, Ixodes aragaoi Fonsec, Ixodes minor Neumann, and Ixodes boliviensis Neumann and are a sister clade to representative endosymbiont sequences from Ixodes pacificus Cooley & Kohls and A. americanum. This clade is distinct from that housing rickettsial endosymbionts of I. scapularis, and it is on a separate lineage from rickettsial endosymbionts previously reported from A. maculatum, Amblyomma parvitarsum Neumann, and Amblyomma nodosum Neumann (Fig. 4). Detection of rickettsial endosymbionts is not uncommon in ticks collected from free-ranging deer and small mammals or arthropods in general (Castellanos et al., 2016; Trout Fryxell et al., 2015; Weinert et al., 2015), and their role in tick biology is still being explored (Hunter et al., 2015). The implication of co-detection of this endosymbiont with the Ehrlichia sp. Delta strain in Am. maculatum is unclear, as the pathogenicity of both rickettsial species is unknown in deer.
Theileria cervi, identified in a high proportion of A. nitens ticks but in neither A. maculatum nor I. scapularis in this study, is a hemoprotozoan parasite transmitted by A. americanum ticks to white-tailed deer (Kuttler et al., 1967). Robinson et al. (1967) surveyed deer from throughout Texas and documented that more than half of the 1600 deer sampled were positive for Theileria by blood smear analysis. Detected in both free-ranging and farmed cervids (Cauvin et al., 2019; Samuel and Trainer, 1970), T. cervi is considered somewhat non-pathogenic; however, its pathogenicity can be accentuated by the physiological state of the host, (i.e., poor nutrition, heavy tick burden, infection; (Robinson et al., 1967; Yabsley et al., 2005). Interestingly, while A. americanum is the demonstrated primary vector of T. cervi (Kuttler et al., 1967), none were collected during this surveillance effort. Samuel and Trainer (1972) described A. americanum from deer in San Patricio County (Texas), which is approx. 200 km north of the LANWR, and an A. americanum adult tick was recorded from a single nilgai host at ESR (DBT, unpublished); however, they are not typically reported from this area (Corn et al., 2016). This suggests T. cervi is maintained in the population by a different tick vector, and it appears to associate with a high prevalence of A. nitens. Other tick species, e.g., R. (B.) microplus, A. mixtum and A. tenellum, are encountered in this region as well (Corn et al., 2016; Olafson et al., 2018), but whether they harbor T. cervi is currently unknown.
Different genotypes of Theileria 18S SSU rRNA have been defined from various bovine and cervid hosts and geographic regions (Chae et al., 1998, 1999a), resulting in three different types (Type F, Type G, and ‘divergent’) from white-tailed deer, elk (Cervus canadensis), and mule deer in the USA (Cauvin et al., 2019; Chae et al., 1998, 1999b; Wood et al., 2013). A recent survey of deer on the SAVR (Starr and Jim Hogg Counties, Texas) indicated approx. 7% of 245 animals were positive for T. cervi (Yu et al., 2020). Analysis of the 16 publicly available T. cervi sequences from Yu et al. (2020) indicate a single genotype (Type F), while sequence data from A. nitens ticks in the current study indicate that all three cervid genotypes (Type F, G, and ‘divergent’) are circulating within this Cameron County (Texas) deer population (Fig. 3). Further, detection and confirmation of the T. cervi sequence from adult A. nitens collected from a single nilgai host suggests circulation of the piroplasm in this exotic ungulate species. Deer infested with T. cervi -positive A. nitens ticks were distributed across the units within LANWR and, using positive ticks as an indicator of parasite circulation, suggests a much higher prevalence (27/31, 87%) of T. cervi in deer of this region.
5. Conclusions
Southern Texas shares an ungulate fauna with northern Mexico: nilgai antelope, collared peccaries, white-tailed deer. As such, these ungulates can transport species of ticks across the international border, and with them associated pathogens. Among those of concern are bovine babesiosis, equine babesiosis, and anaplasmosis. Bovid and cervid hosts can sustain populations of various tick species in this region, thus the wildlife-livestock interface in southern Texas is of concern because of the potential for transmission of pathogens to livestock. Further, presence of the invasive cattle fever tick in this region is likely a result of dissemination by alternative hosts, such as deer and nilgai, impacting tick eradication efforts (Lohmeyer et al., 2018; Pound et al., 2010). Surveying ticks from deer and nilgai offer an opportunity to identify pathogens that may be circulating in these hosts. This study contributed records of the tick fauna parasitizing deer and nilgai and documented the circulation of sylvatic piroplasmas, anaplasmas and rickettsias in southern Texas.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgements
We are especially grateful to Emma Mitchell for diligence in scratch-inspection for ticks at the LANWR, and for permission to reproduce the photograph of A. nitens in the ear of its cervine host. We are also appreciative of personnel at the USDA-APHIS-Veterinary Services-Cattle Fever Tick Eradication Program and Texas Animal Health Commission. This work was supported by the USDA-ARS, Project 3094-32000-042-00-D. This is manuscript number 057 of the East Foundation. This article reports the result of research only. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the USDA. The USDA is an equal opportunity provider and employer.
Footnotes
Note: Nucleotide sequence data reported in this paper are available in the GenBank™ database.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ijppaw.2020.11.002.
Appendix A. Supplementary data
References
- Baldridge G.D., Scoles G.A., Burkhardt N.Y., Schloeder B., Kurtti T.J., Munderloh U.G. Transovarial transmission of Francisella-like endosymbionts and Anaplasma phagocytophilum variants in Dermacentor albipictus (Acari: ixodidae) J. Med. Entomol. 2009;46:625–632. doi: 10.1603/033.046.0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour A.G., Maupin G.O., Teltow G.J., Carter C.J., Piesman J. Identification of an uncultivable Borrelia species in the hard tick Amblyomma americanum: possible agent of a Lyme disease-like illness. J. Infect. Dis. 1996;173:403–409. doi: 10.1093/infdis/173.2.403. [DOI] [PubMed] [Google Scholar]
- Bishopp F.C., Hixson H. Biology and economic importance of the Gulf Coast tick. J. Econ. Entomol. 1936;20:1068–1076. [Google Scholar]
- Borges L.M., Labruna M.B., Linardi P.M., Ribeiro M.F. Recognition of the tick genus Anocentor Schulze, 1937 (Acari: ixodidae) by numerical taxonomy. J. Med. Entomol. 1998;35:891–894. doi: 10.1093/jmedent/35.5.891. [DOI] [PubMed] [Google Scholar]
- Borges L.M., Oliveira P.R., Ribeiro M.F. Seasonal dynamics of Anocentor nitens on horses in Brazil. Vet. Parasitol. 2000;89:165–171. doi: 10.1016/s0304-4017(00)00204-1. [DOI] [PubMed] [Google Scholar]
- Carreno R.A., Durden L.A., Brooks D.R., Abrams A., Hoberg E.P. Parelaphostrongylus tenius (Nematoda: protostrongylidae) and other parasites of white-tailed deer (Odocoileus virginianus) in Costa Rica. Comp. Parasitol. 2001;68:177–184. [Google Scholar]
- Castellanos A.A., Medeiros M.C., Hamer G.L., Morrow M.E., Eubanks M.D., Teel P.D., Hamer S.A., Light J.E. Decreased small mammal and on-host tick abundance in association with invasive red imported fire ants (Solenopsis invicta) Biol. Lett. 2016;12:20160463. doi: 10.1098/rsbl.2016.0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauvin A., Hood K., Shuman R., Orange J., Blackburn J.K., Sayler K.A., Wisely S.M. The impact of vector control on the prevalence of Theileria cervi in farmed Florida white-tailed deer, Odocoileus virginianus. Parasites Vectors. 2019;12:100. doi: 10.1186/s13071-019-3344-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae J., Lee J., Kwon O., Holman P.J., Waghela S.D., Wagner G.G. Nucleotide sequence heterogeneity in the small subunit ribosomal RNA gene variable (V4) region among and within geographic isolates of Theileria from cattle, elk and white-tailed deer. Vet. Parasitol. 1998;75:41–52. doi: 10.1016/s0304-4017(97)00183-0. [DOI] [PubMed] [Google Scholar]
- Chae J.S., Allsopp B.A., Waghela S.D., Park J.H., Kakuda T., Sugimoto C., Allsopp M.T., Wagner G.G., Holman P.J. A study of the systematics of Theileria spp. based upon small-subunit ribosomal RNA gene sequences. Parasitol. Res. 1999;85:877–883. doi: 10.1007/s004360050651. [DOI] [PubMed] [Google Scholar]
- Chae J.S., Waghela S.D., Craig T.M., Kocan A.A., Wagner G.G., Holman P.J. Two Theileria cervi SSU RRNA gene sequence types found in isolates from white-tailed deer and elk in North America. J. Wildl. Dis. 1999;35:458–465. doi: 10.7589/0090-3558-35.3.458. [DOI] [PubMed] [Google Scholar]
- Cicuttin G.L., De Salvo M.N., Perez P.D., Silva D., Felix M.L., Venzal J.M., Nava S. A novel Ehrlichia strain (Rickettsiales: Anaplasmataceae) detected in Amblyomma triste (Acari: ixodidae), a tick species of public health importance in the Southern Cone of America. Pathog. Glob. Health. 2020;114:318–322. doi: 10.1080/20477724.2020.1795579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooley R.A., Kohls G.M. The genus Amblyomma (Ixodidae) in the United States. J. Parasitol. 1944;30:77–111. [Google Scholar]
- Corn J.L., Duhaime R.A., Alfred J.T., Mertins J.W., Leland B.R., Sramek R.L., Moczygemba J.D., Shaw D.W. Survey for ticks on feral swine within a cattle fever tick-infested landscape in Texas, USA. Syst. Appl. Acarol. 2016;21:1564–1570. [Google Scholar]
- Dennis D.T., Nekomoto T.S., Victor J.C., Paul W.S., Piesman J. Reported distribution of Ixodes scapularis and Ixodes pacificus (Acari: ixodidae) in the United States. J. Med. Entomol. 1998;35:629–638. doi: 10.1093/jmedent/35.5.629. [DOI] [PubMed] [Google Scholar]
- Drummond R.O., Whetstone T.M., Ernst S.E., Gladney W.J. Laboratory study of Anocentor nitens (Neumann) (Acarina: ixodidae), the tropical horse tick. J. Med. Entomol. 1969;6:150–154. doi: 10.1093/jmedent/6.2.150. [DOI] [PubMed] [Google Scholar]
- Dugan V.G., Yabsley M.J., Tate C.M., Mead D.G., Munderloh U.G., Herron M.J., Stallknecht D.E., Little S.E., Davidson W.R. Evaluation of white-tailed deer (Odocoileus virginianus) as natural sentinels for Anaplasma phagocytophilum. Vector Borne Zoonotic Dis. 2006;6:192–207. doi: 10.1089/vbz.2006.6.192. [DOI] [PubMed] [Google Scholar]
- Edgar R.C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada-Pena A., Venzal J.M., Mangold A.J., Cafrune M.M., Guglielmone A.A. The Amblyomma maculatum Koch, 1844 (Acari: ixodidae: Amblyomminae) tick group: diagnostic characters, description of the larva of A. parvitarsum Neumann, 1901, 16S rDNA sequences, distribution and hosts. Syst. Parasitol. 2005;60:99–112. doi: 10.1007/s11230-004-1382-9. [DOI] [PubMed] [Google Scholar]
- Feria-Arroyo T.P., Castro-Arellano I., Gordillo-Perez G., Cavazos A.L., Vargas-Sandoval M., Grover A., Torres J., Medina R.F., de Leon A.A., Esteve-Gassent M.D. Implications of climate change on the distribution of the tick vector Ixodes scapularis and risk for Lyme disease in the Texas-Mexico transboundary region. Parasites Vectors. 2014;7:199. doi: 10.1186/1756-3305-7-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley A.M., Goolsby J.A., Ortega S.A., Jr., Ortega S.J., Perez de Leon A., Singh N.K., Schwartz A., Ellis D., Hewitt D.G., Campbell T.A. Movement patterns of nilgai antelope in South Texas: implications for cattle fever tick management. Prev. Vet. Med. 2017;146:166–172. doi: 10.1016/j.prevetmed.2017.08.002. [DOI] [PubMed] [Google Scholar]
- Guzmán-Cornejo C., Robbins R.G., Guglielmone A.A., Montiel-Parra G., Pérez T.M. The Amblyomma (Acari: ixodida: ixodidae) of Mexico: identification keys, distribution and hosts. Zootaxa. 2011;2998:16–38. [Google Scholar]
- Hoang D.T., Chernomor O., von Haeseler A., Minh B.Q., Vinh L.S. UFBoot2: improving the ultrafast bootstrap approximation. Mol. Biol. Evol. 2018;35:518–522. doi: 10.1093/molbev/msx281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter D.J., Torkelson J.L., Bodnar J., Mortazavi B., Laurent T., Deason J., Thephavongsa K., Zhong J. The Rickettsia endosymbiont of Ixodes pacificus contains all the genes of de novo folate biosynthesis. PLoS One. 2015;10 doi: 10.1371/journal.pone.0144552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E.M., Panciera R.J., Allen K.E., Sheets M.E., Beal J.D., Ewing S.A., Little S.E. Alternate pathway of infection with Hepatozoon americanum and the epidemiologic importance of predation. J. Vet. Intern. Med. 2009;23:1315–1318. doi: 10.1111/j.1939-1676.2009.0375.x. [DOI] [PubMed] [Google Scholar]
- Kalyaanamoorthy S., Minh B.Q., Wong T.K.F., von Haeseler A., Jermiin L.S. ModelFinder: fast model selection for accurate phylogenetic estimates. Nat. Methods. 2017;14:587–589. doi: 10.1038/nmeth.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpathy S.E., Slater K.S., Goldsmith C.S., Nicholson W.L., Paddock C.D. Rickettsia amblyommatis sp. nov., a spotted fever group Rickettsia associated with multiple species of Amblyomma ticks in North, Central and South America. Int. J. Syst. Evol. Microbiol. 2016;66:5236–5243. doi: 10.1099/ijsem.0.001502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keirans J.E., Clifford C.M. The genus Ixodes in the United States: a scanning electron microscope study and key to the adults. J. Med. Entomol. Suppl. 1978;2:1–149. doi: 10.1093/jmedent/15.suppl2.1. [DOI] [PubMed] [Google Scholar]
- Keirans J.E., Hutcheson H.J., Durden L.A., Klompen J.S.H. Ixodes (Ixodes) scapularis (Acari: ixodidae): redescription of all active stages, distribution, hosts, geographical variation, and medical and veterinary importance. J. Med. Entomol. 1996;33:297–318. doi: 10.1093/jmedent/33.3.297. [DOI] [PubMed] [Google Scholar]
- Kuttler K.L., Robinson R.M., Bell R.R. Tick transmission of theileriasis in a white-tailed deer. Bull. Wildl. Dis. Assoc. 1967;3:182–183. [Google Scholar]
- Lado P., Nava S., Mendoza-Uribe L., Caceres A.G., Delgado-de la Mora J., Licona-Enriquez J.D., Delgado-de la Mora D., Labruna M.B., Durden L.A., Allerdice M.E.J., Paddock C.D., Szabo M.P.J., Venzal J.M., Guglielmone A.A., Beati L. The Amblyomma maculatum Koch, 1844 (Acari: ixodidae) group of ticks: phenotypic plasticity or incipient speciation? Parasites Vectors. 2018;11:610. doi: 10.1186/s13071-018-3186-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Chen Z., Liu Z., Liu J., Yang J., Li Q., Li Y., Luo J., Yin H. Molecular survey of Anaplasma and Ehrlichia of red deer and sika deer in Gansu, China in 2013. Transbound Emerg. Dis. 2016;63:e228–e236. doi: 10.1111/tbed.12335. [DOI] [PubMed] [Google Scholar]
- Llanes A., Rajeev S. First Whole Genome Sequence of Anaplasma Platys, an Obligate Intracellular Rickettsial Pathogen of Dogs. Pathogens. 2020;vol. 9 doi: 10.3390/pathogens9040277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart J.M., Davidson W.R., Stallknecht D.E., Dawson J.E., Howerth E.W. Isolation of Ehrlichia chaffeensis from wild white-tailed deer (Odocoileus virginianus) confirms their role as natural reservoir hosts. J. Clin. Microbiol. 1997;35:1681–1686. doi: 10.1128/jcm.35.7.1681-1686.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood B.H., Stasiak I., Pfaff M.A., Cleveland C.A., Yabsley M.J. Widespread distribution of ticks and selected tick-borne pathogens in Kentucky (USA) Ticks Tick Borne Dis. 2018;9:738–741. doi: 10.1016/j.ttbdis.2018.02.016. [DOI] [PubMed] [Google Scholar]
- Loftis A.D., Kelly P.J., Paddock C.D., Blount K., Johnson J.W., Gleim E.R., Yabsley M.J., Levin M.L., Beati L. Panola mountain Ehrlichia in Amblyomma maculatum from the United States and Amblyomma variegatum (Acari: ixodidae) from the caribbean and africa. J. Med. Entomol. 2016;53:696–698. doi: 10.1093/jme/tjv240. [DOI] [PubMed] [Google Scholar]
- Lohmeyer K.H., May M.A., Thomas D.B., Perez de Leon A.A. Implication of nilgai antelope (Artiodactyla: bovidae) in reinfestations of Rhipicephalus (Boophilus) microplus (Acari: ixodidae) in south Texas: a review and update. J. Med. Entomol. 2018;55:515–522. doi: 10.1093/jme/tjy004. [DOI] [PubMed] [Google Scholar]
- Lohmeyer K.H., Pound J.M., May M.A., Kammlah D.M., Davey R.B. Distribution of Rhipicephalus (boophilus) microplus and Rhipicephalus (boophilus) annulatus (Acari: ixodidae) infestations detected in the United States along the Texas/Mexico border. J. Med. Entomol. 2011;48:770–774. doi: 10.1603/me10209. [DOI] [PubMed] [Google Scholar]
- Mahan S.M., Peter T.F., Simbi B.H., Kocan K., Camus E., Barbet A.F., Burridge M.J. Comparison of efficacy of American and African Amblyomma ticks as vectors of heartwater (Cowdria ruminantium) infection by molecular analyses and transmission trials. J. Parasitol. 2000;86:44–49. doi: 10.1645/0022-3395(2000)086[0044:COEOAA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Mays S.E., Houston A.E., Trout Fryxell R.T. Specifying pathogen associations of Amblyomma maculatum (Acari: ixodidae) in western Tennessee. J. Med. Entomol. 2016;53:435–440. doi: 10.1093/jme/tjv238. [DOI] [PubMed] [Google Scholar]
- Medlin J.S., Cohen J.I., Beck D.L. Vector potential and population dynamics for Amblyomma inornatum. Ticks Tick Borne Dis. 2015;6:463–472. doi: 10.1016/j.ttbdis.2015.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadolny R.M., Gaff H.D. Natural history of Amblyomma maculatum in Virginia. Ticks Tick Borne Dis. 2018;9:188–195. doi: 10.1016/j.ttbdis.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nava S., Beati L., Labruna M.B., Caceres A.G., Mangold A.J., Guglielmone A.A. Reassessment of the taxonomic status of Amblyomma cajennense ( ) with the description of three new species, Amblyomma tonelliae n. sp., Amblyomma interandinum n. sp. and Amblyomma patinoi n. sp., and reinstatement of Amblyomma mixtum, and Amblyomma sculptum (Ixodida: ixodidae) Ticks Tick Borne Dis. 2014;5:252–276. doi: 10.1016/j.ttbdis.2013.11.004. [DOI] [PubMed] [Google Scholar]
- Nelson S.L., Durden L.A., Reuter J.D. Rhipicephalus microplus and Dermacentor nitens (Acari: ixodidae) coparasitize white-tailed deer on St. John, U.S. Virgin Islands. J. Med. Entomol. 2017;54:1440–1443. doi: 10.1093/jme/tjx112. [DOI] [PubMed] [Google Scholar]
- Olafson P.U., Thomas D.B., May M.A., Buckmeier B.G., Duhaime R.A. Tick vector and disease pathogen surveillance of nilgai antelope ( Boselaphus tragocamelus) in southeastern Texas, USA. J. Wildl. Dis. 2018;54:734–744. doi: 10.7589/2017-09-239. [DOI] [PubMed] [Google Scholar]
- Oliver J.H., Jr., Chandler F.W., Jr., Luttrell M.P., James A.M., Stallknecht D.E., McGuire B.S., Hutcheson H.J., Cummins G.A., Lane R.S. Isolation and transmission of the Lyme disease spirochete from the southeastern United States. Proc. Natl. Acad. Sci. U. S. A. 1993;90:7371–7375. doi: 10.1073/pnas.90.15.7371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosthuizen M.C., Zweygarth E., Collins N.E., Troskie M., Penzhorn B.L. Identification of a novel Babesia sp. from a sable antelope (Hippotragus Niger Harris, 1838) J. Clin. Microbiol. 2008;46:2247–2251. doi: 10.1128/JCM.00167-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paddock C.D., Goddard J. The evolving medical and veterinary importance of the Gulf Coast tick (Acari: ixodidae) J. Med. Entomol. 2015;52:230–252. doi: 10.1093/jme/tju022. [DOI] [PubMed] [Google Scholar]
- Paddock C.D., Hecht J.A., Green A.N., Waldrup K.A., Teel P.D., Karpathy S.E., Johnson T.L. Rickettsia parkeri (rickettsiales: rickettsiaceae) in the sky islands of west Texas. J. Med. Entomol. 2020;57:1582–1587. doi: 10.1093/jme/tjaa059. [DOI] [PubMed] [Google Scholar]
- Paddock C.D., Sumner J.W., Comer J.A., Zaki S.R., Goldsmith C.S., Goddard J., McLellan S.L., Tamminga C.L., Ohl C.A. Rickettsia parkeri: a newly recognized cause of spotted fever rickettsiosis in the United States. Clin. Infect. Dis. 2004;38:805–811. doi: 10.1086/381894. [DOI] [PubMed] [Google Scholar]
- Pfeifer Barbosa I., Bose R., Peymann B., Friedhoff K.T. Epidemiological aspects of equine babesioses in a herd of horses in Brazil. Vet. Parasitol. 1995;58:1–8. doi: 10.1016/0304-4017(94)00704-g. [DOI] [PubMed] [Google Scholar]
- Pound J.M., George J.E., Kammlah D.M., Lohmeyer K.H., Davey R.B. Evidence for role of white-tailed deer (Artiodactyla: cervidae) in epizootiology of cattle ticks and southern cattle ticks (Acari: ixodidae) in reinfestations along the Texas/Mexico border in south Texas: a review and update. J. Econ. Entomol. 2010;103:211–218. doi: 10.1603/EC09359. [DOI] [PubMed] [Google Scholar]
- Regnery R.L., Spruill C.L., Plikaytis B.D. Genotypic identification of rickettsiae and estimation of intraspecies sequence divergence for portions of two rickettsial genes. J. Bacteriol. 1991;173:1576–1589. doi: 10.1128/jb.173.5.1576-1589.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson R.M., Kuttler K.L., Thomas J.W., Marburger R.G. Theileriases in Texas white-tailed deer. J. Wildl. Manag. 1967;31:455–459. [Google Scholar]
- Samuel W.M., Trainer D.O. Amblyomma (Acarina: ixodidae) on white-tailed deer, Odocoileus virginianus (Zimmermann), from south Texas with implications for theileriasis. J. Med. Entomol. 1970;7:567–574. doi: 10.1093/jmedent/7.5.567. [DOI] [PubMed] [Google Scholar]
- Samuel W.M., Trainer D.O. Lipoptena mazamae Rondani, 1878 (Diptera: hippoboscidae) on white-tailed deer in southern Texas. J. Med. Entomol. 1972;9:104–106. doi: 10.1093/jmedent/9.1.104. [DOI] [PubMed] [Google Scholar]
- Sanders D.M., Schuster A.L., McCardle P.W., Strey O.F., Blankenship T.L., Teel P.D. Ixodid ticks associated with feral swine in Texas. J. Vector Ecol. 2011;38:361–373. doi: 10.1111/j.1948-7134.2013.12052.x. [DOI] [PubMed] [Google Scholar]
- Schwint O.N., Knowles D.P., Ueti M.W., Kappmeyer L.S., Scoles G.A. Transmission of Babesia caballi by Dermacentor nitens (Acari: ixodidae) is restricted to one generation in the absence of alimentary reinfection on a susceptible equine host. J. Med. Entomol. 2008;45:1152–1155. doi: 10.1603/0022-2585(2008)45[1152:tobcbd]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Scoles G.A., Hutcheson H.J., Schlater J.L., Hennager S.G., Pelzel A.M., Knowles D.P. Equine piroplasmosis associated with Amblyomma cajennense ticks, Texas, USA. Emerg. Infect. Dis. 2011;17:1903–1905. doi: 10.3201/eid1710.101182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snellgrove A.N., Krapiunaya I., Ford S.L., Stanley H.M., Wickson A.G., Hartzer K.L., Levin M.L. Vector competence of Rhipicephalus sanguineus sensu stricto for Anaplasma platys. Ticks Tick Borne Dis. 2020;11:101517. doi: 10.1016/j.ttbdis.2020.101517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenshine D.E. Range expansion of tick disease vectors in North America: implications for spread of tick-borne disease. Int. J. Environ. Res. Publ. Health. 2018;15:478. doi: 10.3390/ijerph15030478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo M.P., Labruna M.B., Pereira M.C., Duarte J.M. Ticks (Acari: ixodidae) on wild marsh-deer (Blastocerus dichotomus) from Southeast Brazil: infestations before and after habitat loss. J. Med. Entomol. 2003;40:268–274. doi: 10.1093/jmedent/40.3.268. [DOI] [PubMed] [Google Scholar]
- Tabara K., Arai S., Kawabuchi T., Itagaki A., Ishihara C., Satoh H., Okabe N., Tsuji M. Molecular survey of Babesia microti, Ehrlichia species and Candidatus neoehrlichia mikurensis in wild rodents from Shimane Prefecture, Japan. Microbiol. Immunol. 2007;51:359–367. doi: 10.1111/j.1348-0421.2007.tb03923.x. [DOI] [PubMed] [Google Scholar]
- Teel P.D., Hopkins S.W., Donahue W.A., Strey O.F. Population dynamics of immature Amblyomma maculatum (Acari: ixodidae) and other ectoparasites on meadowlarks and northern bobwhite quail resident to the coastal prairie of Texas. J. Med. Entomol. 1998;35:483–488. doi: 10.1093/jmedent/35.4.483. [DOI] [PubMed] [Google Scholar]
- Teel P.D., Ketchum H.R., Mock D.E., Wright R.E., Strey O.F. The Gulf Coast tick: a review of the life history, ecology, distribution, and emergence as an arthropod of medical and veterinary importance. J. Med. Entomol. 2010;47:707–722. doi: 10.1603/me10029. [DOI] [PubMed] [Google Scholar]
- Trifinopoulos J., Nguyen L.T., von Haeseler A., Minh B.Q. W-IQ-TREE: a fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016;44:W232–W235. doi: 10.1093/nar/gkw256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trout Fryxell R.T., Steelman C.D., Szalanski A.L., Billingsley P.M., Williamson P.C. Molecular detection of Rickettsia species within ticks (Acari: ixodidae) collected from Arkansas United States. J. Med. Entomol. 2015;52:500–508. doi: 10.1093/jme/tjv027. [DOI] [PubMed] [Google Scholar]
- Ueti M.W., Olafson P.U., Freeman J.M., Johnson W.C., Scoles G.A. A virulent Babesia bovis strain failed to infect white-tailed deer (Odocoileus virginianus) PLoS One. 2015;10 doi: 10.1371/journal.pone.0131018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldrup K.A., Kocan A.A., Barker R.W., Wagner G.G. Transmission of Babesia odocoilei in white-tailed deer (Odocoileus virginianus) by Ixodes scapularis (Acari: ixodidae) J. Wildl. Dis. 1990;26:390–391. doi: 10.7589/0090-3558-26.3.390. [DOI] [PubMed] [Google Scholar]
- Waldrup K.A., Kocan A.A., Qureshi T., Davis D.S., Baggett D., Wagner G.G. Serological prevalence and isolation of Babesia odocoilei among white-tailed deer (Odocoileus virginianus) in Texas and Oklahoma. J. Wildl. Dis. 1989;25:194–201. doi: 10.7589/0090-3558-25.2.194. [DOI] [PubMed] [Google Scholar]
- Weinert L.A., Araujo-Jnr E.V., Ahmed M.Z., Welch J.J. The incidence of bacterial endosymbionts in terrestrial arthropods. Proc. Biol. Sci. 2015;282:20150249. doi: 10.1098/rspb.2015.0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M.L., Litwin T.S., Gavin T.A., Capkanis M.C., Maclean D.C., Spielman A. Host-dependent differences in feeding and reproduction of Ixodes dammini (Acari: ixodidae) J. Med. Entomol. 1990;27:945–954. doi: 10.1093/jmedent/27.6.945. [DOI] [PubMed] [Google Scholar]
- Wood J., Johnson E.M., Allen K.E., Campbell G.A., Rezabek G., Bradway D.S., Pittman L.L., Little S.E., Panciera R.J. Merogonic stages of Theileria cervi in mule deer (Odocoileus hemionus) J. Vet. Diagn. Invest. 2013;25:662–665. doi: 10.1177/1040638713501173. [DOI] [PubMed] [Google Scholar]
- Yabsley M.J., Loftis A.D., Little S.E. Natural and experimental infection of white-tailed deer (Odocoileus virginianus) from the United States with an Ehrlichia sp. closely related to Ehrlichia ruminantium. J. Wildl. Dis. 2008;44:381–387. doi: 10.7589/0090-3558-44.2.381. [DOI] [PubMed] [Google Scholar]
- Yabsley M.J., Quick T.C., Little S.E. Theileriosis in a white-tailed deer (Odocoileus virginianus) fawn. J. Wildl. Dis. 2005;41:806–809. doi: 10.7589/0090-3558-41.4.806. [DOI] [PubMed] [Google Scholar]
- Yabsley M.J., Varela A.S., Tate C.M., Dugan V.G., Stallknecht D.E., Little S.E., Davidson W.R. Ehrlichia ewingii infection in white-tailed deer (Odocoileus virginianus) Emerg. Infect. Dis. 2002;8:668–671. doi: 10.3201/eid0807.020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S., Modarelli J., Tomecek J.M., French J.T., Hilton C., Esteve-Gasent M.D. Prevalence of common tick-borne pathogens in white-tailed deer and coyotes in south Texas. Int. J. Parasitol. Parasites Wildl. 2020;11:129–135. doi: 10.1016/j.ijppaw.2020.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunker C.E., Keirans J.E., Clifford C.M., Easton E.R. Dermacentor ticks (Acari: ixodoidea: Ixodidae) of the New World: a scanning electron microscope atlas. Proc. Entomol. Soc. Wash. 1986;88:609–627. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.