Abstract
Backgrounds/Aims
Intrahepatic recurrence is frequent result after hepatectomy for hepatocellular carcinoma (HCC). We analyzed the clinical results of patients who had the intrahepatic recurrences of HCC after curative surgical resections.
Methods
From January 2009 to December 2016, 320 patients underwent curative surgical resection for HCC in department of Surgery, Korea Cancer Center Hospital. After surgical resection, 155 patients had suffered HCC recurrence during follow-up period. Among them, 122 patients had only intrahepatic recurrence initially, who were included in this retrospective study. We analyzed about the period of the recurrence after surgery, treatment methods for the recurred tumors, and poor prognostic factors for survival after intrahepatic recurrences.
Results
Among the 122 patients, 83 patients had recurrence within 24 months after surgery. Thirty-eight patients underwent curative treatment for the recurred tumors (re-resection in 18, radiofrequency ablation in 20 patients). Non-curative treatments were performed in 77 patients (TACE in 68 patients, radiotherapy in 9 patients) and conservative management in 7 patients. Five-year survival rate of patients who underwent curative treatment is 86.4% (p≤0.001). Five-year survival rate of non-curative treatment is 55.7% (p≤0.001), conservative management is 0% (p=0.021). Among the clinical factors, non-curative treatment for recurred tumor, AFP level at the time of recurrence, size of recurred tumor were independent poor prognostic factors for survival after intrahepatic recurrences (p<0.001).
Conclusions
For the patients who had intrahepatic recurrent HCC after surgery, aggressive local treatment can improve the prognosis in selective cases. Further study is necessary to validate this retrospective investigation.
Keywords: Hepatocellular carcinoma, Intrahepatic recurrence, Survival
INTRODUCTION
Hepatocellular carcinoma (HCC) is among the most common malignant neoplasms worldwide. The patient outcomes after the resection of HCC have improved because of the development in surgical techniques and perioperative care.1,2 However, the high incidence of intrahepatic recurrence (IHR) remains a major challenge in the treatment of HCC.1 Furthermore, IHR has been extensively studied because it is 6-7 times more common than extrahepatic recurrence.3 Moreover, the liver is the predominant site of the first recurrence after the resection of HCC, and survival is poor after recurrence occurs.4 The cumulative 5-year recurrence rate was 70-80%, and the most common site of recurrence was the remnant liver.5 The 1-year, 3-year, and 5-year overall survival rates after recurrence in patients with intrahepatic nodular recurrence were 91.0%, 71.0%, and 37.5%, respectively. Therefore, it is important to determine how this group of patients should be treated.6
There are some treatment options for IHR of HCC, and surgical resection is the best treatment for IHR.2 Repeat hepatic resection might be the most effective treatment for IHR, and the 5-year survival after re-resection ranged from 31% to 69%.7-9 However, re-resection might be difficult in some cases because of adhesion, modifications in the anatomy, and impaired liver function.10,11
Many studies have evaluated the treatment for IHR of HCC. For example, for patients with a solitary tumor recurrence after resection, repeat hepatectomy, local ablation therapy, and transhepatic arterial chemoembolization (TACE) may contribute to long‐term survival, even if disease recurrence occurs.5 Local thermal and chemical ablation therapies include percutaneous ethanol injection, microwave ablation, and radiofrequency ablation (RFA), all of which are reported to improve the survival rate of patients with IHR.2 The most frequent treatment for IHR is TACE because of the large tumor size, presence of multiple intrahepatic tumors, unfavorable tumor location, or limited residual liver function reserve and poor liver function.5,6,10,11 Moreover, most cases of multiple-diffuse recurrences are inevitably managed with repeated TACE or chemotherapy.12,13
Radiation therapy is a safe and effective treatment for patients with recurrent unresectable HCC.14,15 Recent technological developments in radiation therapy, such as stereotactic body radiation therapy (SBRT) and imaged-guided radiotherapy, have made it possible to deliver a substantial dose of radiation to the tumor and avoid the radiosensitive normal liver in the vicinity.16
The aim of the current study was to analyze the clinical outcomes of patients with IHRs of HCC after surgical resection in our institution.
MATERIALS AND METHODS
Patients
Between January 2009 and December 2016, a total of 320 patients underwent curative surgical resection for HCC in the Department of Surgery, Korea Cancer Center Hospital, Seoul, Republic of Korea. After surgical resection, patients underwent regular blood and imaging tests every 3 months during the first year of follow-up, after which they were examined regularly every 4-6 months during follow-up. A total of 155 patients showed HCC recurrence during the follow-up period. Among the 155 patients, 122 patients had only IHR initially. These 122 patients were included in the current retrospective study (Table 1). We analyzed the period between the primary surgery and recurrence, treatment methods for the recurrent tumors, and poor prognostic factors for survival after IHR for the 122 included patients. We classified these 122 patients into two groups according to the time of recurrence after the primary surgery: 83 patients showed IHR within 24 months after the primary surgery which is early recurrence group, and the remaining 39 patients showed recurrence 24 months after the primary surgery which is late recurrence group (Table 2). We then analyzed the 122 patients for determining the survival rate after the initial recurrence. We analyzed each patient's survival period from the time we found the initial recurrence (IRB no. KIRAMS-2020-04-016).
Table 1.
Number of recurrent patients (number of all cases (n=320))
| Number of recurrent patients during the follow up period | |
|---|---|
| No evidence of disease | 165 |
| Intrahepatic recurrences | 122 |
| Extrahepatic recurrences±intrahepatic recurrences | 33 |
Table 2.
The period of recurrence
| Time period of recurrence from primary operation | Number of cases (n=122) |
|---|---|
| ≤24 months (early recurrence group) | 83 |
| 24 months < (late recurrence group) | 39 |
Clinicopathological variables
The evaluated clinicopathological variables included the time from the primary operation to the first recurrence, the alpha-fetoprotein (AFP) level at the time of recurrence, the size of the recurrent tumor, the number of IHRs, and the presence of cirrhosis.
Curative and non-curative therapy
Surgical resection and RFA are potentially curative treatment modalities for HCC.17 TACE, systemic therapy, and SBRT are non-curative treatments. In the current study, only the initial treatment was used for statistical analysis.
Statistical analysis
We obtained the data from the medical records of patients. The data were analyzed retrospectively, using IBM SPSS statistics (Korean Version 23), Kaplan-Meier analysis, Chi-square analysis and Cox regression analysis. p-values less than 0.05 were considered statistically significant.
RESULTS
Survival rates of patients with intrahepatic recurrence
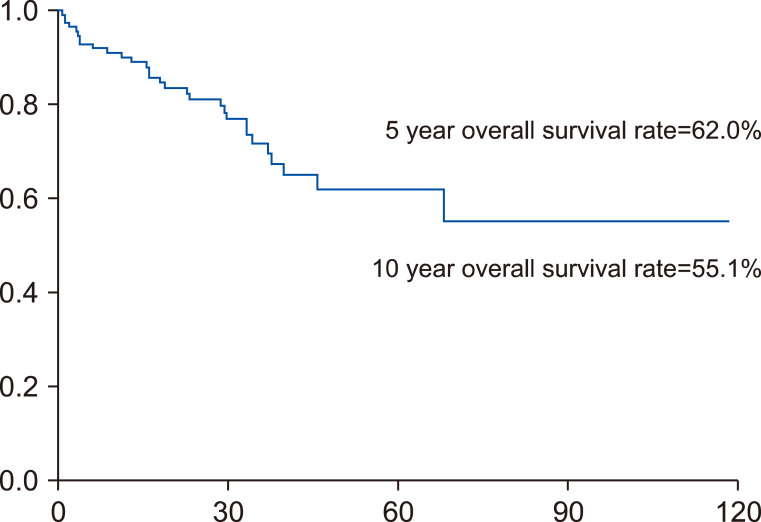
The 5-year overall survival rate of the 122 included patients with initial IHR was 62%, and the 10-year overall survival rate was 55.1% after initial recurrence (Fig. 1).
Fig. 1.
Five-year overall survival rate of 122 patients after initial IHR is 62%, and 10-year overall survival is 55.1%.
Curative and non-curative therapy
Curative treatment for the recurrent tumors was performed for 38 patients (re-resection for 18 patients and RFA for 20). Non-curative treatments were performed for 77 patients (TACE for 68 patients, radiotherapy for 9) and conservative management for 7 (Table 3).
Table 3.
Treatment modality after IHR
| Treatments for IHR | No |
|---|---|
| Curative Tx | |
| Re-resection | 18 |
| RFA | 20 |
| Non-curative Tx | |
| TACE | 68 |
| RT | 9 |
| Conservative care | 7 |
| Total | 122 |
Tx, treatment; RFA, radiofrequency ablation; TACE, transhepatic arterial chemoembolization; RT, radiotherapy
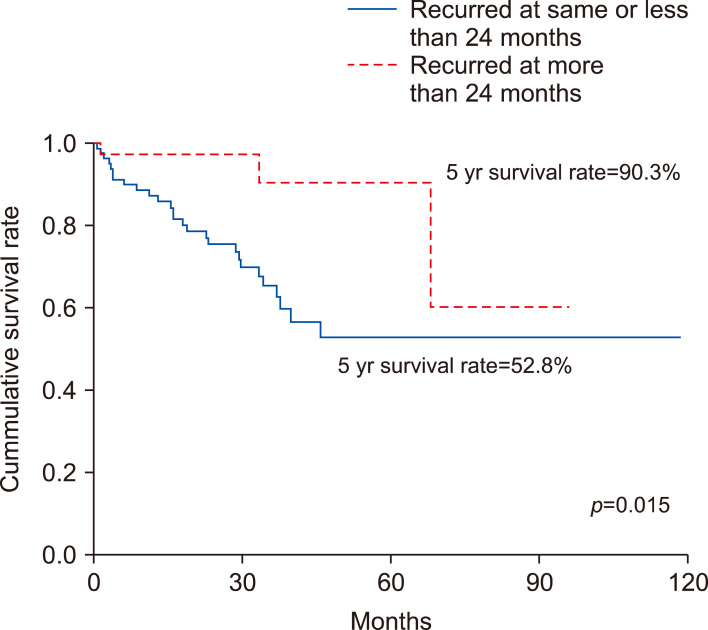
Association between time of recurrence after the primary surgery and survival
The 5-year survival rate was 90.3% for patients of late recurrence group and 52.8% for patients of early recurrence group. The survival rate was higher when the time between the surgery and the recurrence was longer (p=0.015; Fig. 2).
Fig. 2.
The 5-year survival rate was 90.3% for patients of late recurrence group and 52.8% for patients of early recurrence group. The survival rate was higher when the time between the surgery and the recurrence was longer (p=0.015).
Clinical features
We analyzed the clinical features of the patients with IHR according to the period between the primary surgery and recurrence (Table 4). Early recurrence group patients were more likely to have large, multiple recurrent tumors and the AFP level tended to exceed 200 ng/ml. We analyzed the initial treatment for the recurrent tumor as well. Curative treatment was performed for 20 of the 83 patients of early recurrence group and for 18 of the 39 patients in late recurrence group. The curative treatment was more frequently performed in late recurrence group than early recurrence group.
Table 4.
Clinical features of patients with IHR according to the time period of recurrence
| Variables | Number of patients | p-value | |
|---|---|---|---|
| Time period from primary surgery | |||
| ≤24 months | 24 months < | ||
| Size of recurred tumor | 0.189 | ||
| ≤3 cm | 72 | 37 | |
| 3 cm < | 11 | 2 | |
| Number of recurred tumors | <0.001 | ||
| Single | 33 | 30 | |
| Multiple | 50 | 9 | |
| AFP level at the time of recurrence (ng/dl) | 0.031 | ||
| ≤200 | 67 | 37 | |
| 200< | 16 | 2 | |
| Initial treatment for the recurrence | <0.001 | ||
| Curative | 20 | 18 | |
| Non-curative | 57 | 20 | |
| Conservative | 6 | 1 | |
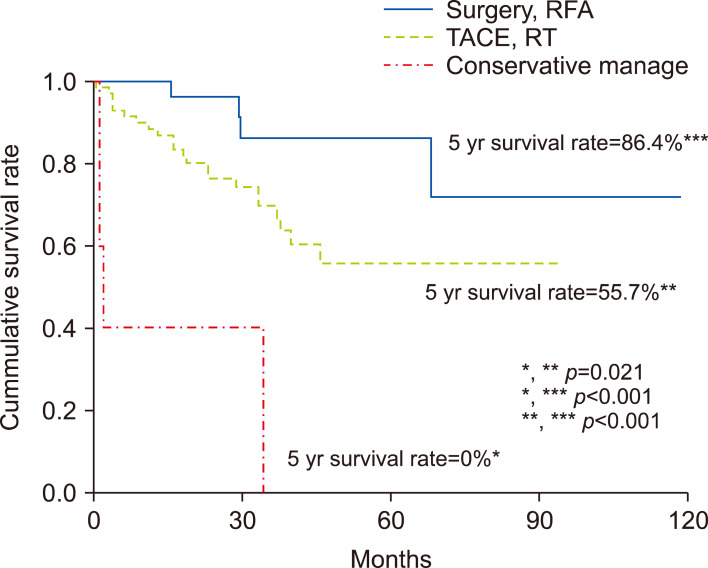
Patient outcomes after the treatment for intrahepatic recurrence
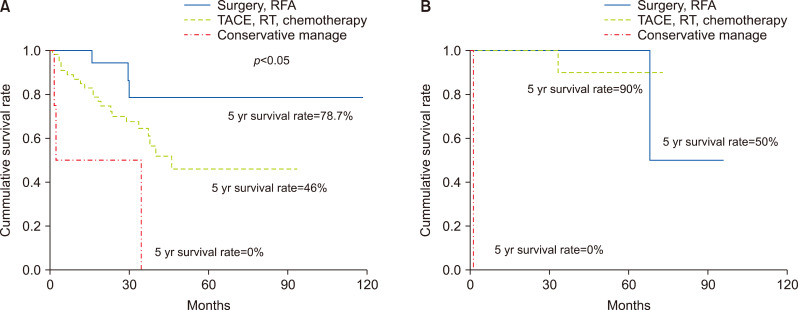
Among the 122 patients with IHR, the survival rate was significantly better for patients who underwent curative therapy than for those who underwent other therapies (Fig. 3). The prognosis was good when curative treatment was performed in early recurrence group. The survival rate according to the method of treatment was significantly different in early recurrence group (p<0.05), however there was no difference in late recurrence group (Fig. 4).
Fig. 3.
Survival rate of patients who had curative treatment was 86.4%. Survival rate of patients who had non-curative treatment was 55.7%, conservative management was 0% (p=0.05).
Fig. 4.
Prognosis is better when performing curative treatment in early recurrence group. (A) The survival rate according to the method of treatment was significantly different in early recurrence group (p<0.05). (B) There was no difference in late recurrence group in late recurrence group.
Independent poor prognostic factors for survival after intrahepatic recurrence
Among the clinical factors, the independent poor prognostic factors for survival after IHRs were non-curative treatment for recurred tumor, AFP level at the time of recurrence, size of recurred tumor (Table 5).
Table 5.
Independent poor prognostic factors for survival after intrahepatic recurrence
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| p-value | p-value | Exp (B) | 95% CI | |
| Age (65 years <) | 0.400 | |||
| Sex | 0.502 | |||
| Period for recurrence (less than 12 months) | 0.015 | 0.330 | ||
| Number (multiple) | 0.004 | 0.181 | ||
| Non-curative treatment for recurred tumor | <0.001 | 0.037 | 0.275 | 0.082-0.925 |
| AFP level at the time of recurrence (200 ng/dl ≤) | <0.001 | 0.002 | 0.255 | 0.112-0.578 |
| Size of recurred tumor (3 cm <) | <0.001 | 0.003 | 0.218 | 0.086-0.556 |
AFP, alpha-fetoprotein
DISCUSSION
In the current study, the survival rate was better after aggressive local treatment than after non-curative treatment and conservative treatment for patients with IHR after the primary surgery. Our results show that curative therapy is indicated for patients with IHR after primary surgery because a good prognosis is predicted. However, the selection bias for curative treatment might have influenced the results of good prognosis. This is because patients who cannot choose re-resection or RFA are more likely to have diffuse or large recurrent tumors, poor liver function, and insufficient remnant liver volume. Moreover, these conditions would have resulted in a bad prognosis. Furthermore, only a few patients who showed recurrence after 24 months might have died after the short follow-up, which may have affected statistical results.
Many studies analyzed the prognostic factors of IHR, which were the presence of portal vein invasion, multiple primary HCC tumors, a high serum AFP level, a large tumor, the presence of cirrhosis, and increased age, among others. Moreover, the number of primary HCC tumors was a significant predictor of disease-free survival after hepatic resection. However, only a few studies have evaluated the prognostic factors of survival after IHR. In a previous study, a period of <1 year to recurrence and the presence of multiple IHRs were unfavorable prognostic factors. Furthermore, a low ICGR (Indocyanine green retention), the absence of cirrhosis, and the absence of portal vein invasion were significantly favorable prognostic factors after recurrence.2,13 In the current study non-curative treatment for recurred tumor, the AFP level at the time of recurrence, and the size of the recurrent tumor were independent poor prognostic factors for survival after IHRs.
Patients are not usually treated with one method but with a combination of various methods. Therefore, it was difficult to analyze the administered treatment because there were many different combinations of treatments. Hence, in the current study, we considered the initial treatment. Curative treatment resulted in better prognosis than non-curative and conservative treatment for patients with IHR after primary resection in the early recurrence group. The survival rate tended to be distinctly better patients who received curative treatment in early recurrence group. Nevertheless, as the follow-up period was relatively short, late recurrence group patients might have shown fewer deaths. Accordingly, the differences in treatment methods were not clearly analyzed.
In summary, the independent poor prognostic factors of patients with intrahepatic recurrence after curative initial surgery are AFP level at the time of recurrence, size of recurred tumor and aggressive local treatment.
In conclusion, aggressive local treatment can improve the prognosis in selected patients with IHR of HCC after surgery. Nevertheless, further studies are necessary to validate the findings of the current retrospective investigation.
Footnotes
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest regarding the publication of this article.
AUTHOR CONTRIBUTIONS
Conceptualization: EHC. Data curation: JL, EHC, SBK, RK. Data analysis: JL, EHC, SBK Methodology: EHC, RK. Project administration: SBK, RK. Visualization: EHC. Writing - original draft: JL, EHC. Writing - review & editing: JL.
REFERENCES
- 1.Kew MC. Recurrence of hepatocellular carcinoma after surgery: can it be avoided? Hepatology. 1996;24:741–742. doi: 10.1002/hep.510240346. [DOI] [PubMed] [Google Scholar]
- 2.Kawano Y, Sasaki A, Kai S, Endo Y, Iwaki K, Uchida H, et al. Prognosis of patients with intrahepatic recurrence after hepatic resection for hepatocellular carcinoma: a retrospective study. Eur J Surg Oncol. 2009;35:174–179. doi: 10.1016/j.ejso.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 3.Hanazaki K, Matsushita A, Nakagawa K, Misawa R, Amano J. Risk factors of intrahepatic recurrence after curative resection of hepatocellular carcinoma. Hepatogastroenterology. 2005;52:580–586. [PubMed] [Google Scholar]
- 4.Cha C, Fong Y, Jarnagin WR, Blumgart LH, DeMatteo RP. Predictors and patterns of recurrence after resection of hepatocellular carcinoma. J Am Coll Surg. 2003;197:753–758. doi: 10.1016/j.jamcollsurg.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Hirokawa F, Hayashi M, Miyamoto Y, Asakuma M, Shimizu T, Komeda K, et al. Appropriate treatment strategy for intrahepatic recurrence after curative hepatectomy for hepatocellular carcinoma. J Gastrointest Surg. 2011;15:1182–1187. doi: 10.1007/s11605-011-1484-z. [DOI] [PubMed] [Google Scholar]
- 6.Choi GH, Kim DH, Kang CM, Kim KS, Choi JS, Lee WJ, et al. Prognostic factors and optimal treatment strategy for intrahepatic nodular recurrence after curative resection of hepatocellular carcinoma. Ann Surg Oncol. 2008;15:618–629. doi: 10.1245/s10434-007-9671-6. [DOI] [PubMed] [Google Scholar]
- 7.Imamura H, Matsuyama Y, Tanaka E, Ohkubo T, Hasegawa K, Miyagawa S, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003;38:200–207. doi: 10.1016/S0168-8278(02)00360-4. [DOI] [PubMed] [Google Scholar]
- 8.Umeda Y, Matsuda H, Sadamori H, Matsukawa H, Yagi T, Fujiwara T. A prognostic model and treatment strategy for intrahepatic recurrence of hepatocellular carcinoma after curative resection. World J Surg. 2011;35:170–177. doi: 10.1007/s00268-010-0794-8. [DOI] [PubMed] [Google Scholar]
- 9.Notake T, Kobayashi A, Shinkawa H, Kawahara T, Shimizu A, Yokoyama T, et al. Nomogram predicting long-term survival after the diagnosis of intrahepatic recurrence of hepatocellular carcinoma following an initial liver resection. Int J Clin Oncol. 2017;22:715–725. doi: 10.1007/s10147-017-1114-1. [DOI] [PubMed] [Google Scholar]
- 10.Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Intrahepatic recurrence after curative resection of hepatocellular carcinoma: long-term results of treatment and prognostic factors. Ann Surg. 1999;229:216–222. doi: 10.1097/00000658-199902000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taura K, Ikai I, Hatano E, Fujii H, Uyama N, Shimahara Y. Implication of frequent local ablation therapy for intrahepatic recurrence in prolonged survival of patients with hepatocellular carcinoma undergoing hepatic resection: an analysis of 610 patients over 16 years old. Ann Surg. 2006;244:265–273. doi: 10.1097/01.sla.0000217921.28563.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsumata T, Kanematsu T, Takenaka K, Yoshida Y, Nishizaki T, Sugimachi K. Patterns of intrahepatic recurrence after curative resection of hepatocellular carcinoma. Hepatology. 1989;9:457–460. doi: 10.1002/hep.1840090320. [DOI] [PubMed] [Google Scholar]
- 13.Shimada K, Sakamoto Y, Esaki M, Kosuge T, Morizane C, Ikeda M, et al. Analysis of prognostic factors affecting survival after initial recurrence and treatment efficacy for recurrence in patients undergoing potentially curative hepatectomy for hepatocellular carcinoma. Ann Surg Oncol. 2007;14:2337–2347. doi: 10.1245/s10434-007-9415-7. [DOI] [PubMed] [Google Scholar]
- 14.Huang WY, Jen YM, Lee MS, Chang LP, Chen CM, Ko KH, et al. Stereotactic body radiation therapy in recurrent hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2012;84:355–361. doi: 10.1016/j.ijrobp.2011.11.058. [DOI] [PubMed] [Google Scholar]
- 15.Jang WI, Kim MS, Bae SH, Cho CK, Yoo HJ, Seo YS, et al. High-dose stereotactic body radiotherapy correlates increased local control and overall survival in patients with inoperable hepatocellular carcinoma. Radiat Oncol. 2013;8:250. doi: 10.1186/1748-717X-8-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jang WI, Bae SH, Kim MS, Han CJ, Park SC, Kim SB, et al. A phase 2 multicenter study of stereotactic body radiotherapy for hepatocellular carcinoma: safety and efficacy. Cancer. 2020;126:363–372. doi: 10.1002/cncr.32502. [DOI] [PubMed] [Google Scholar]
- 17.Livraghi T, Mäkisalo H, Line PD. Treatment options in hepatocellular carcinoma today. Scand J Surg. 2011;100:22–29. doi: 10.1177/145749691110000105. [DOI] [PubMed] [Google Scholar]