Abstract
Oxidative stress is a critical element in relationship to the pathophysiology of Duchenne muscular dystrophy (DMD). In the mice the diaphragm (DIA) is most resembles the dystrophic human pathology. In this study we have evaluated the consequences of a synthetic antioxidant (tempol) on oxidative stress parameters in the DIA muscle of mdx mice. The mdx mice were separated into two groups: mdx, the control group receiving intraperitoneal (i.p.) injections of saline solution (100 µL), and mdxT, the treated group receiving i.p. injections of tempol (100 mg/kg). The tempol‐treated group showed reduced oxidative stress markers, decreasing the dihydroethidium reaction (DHE) area; autofluorescent lipofuscin granules; and 4‐hydroxynonenal (4‐HNE)‐protein adduct levels. DIA muscle of mdx mice. At the same time, the manganese‐superoxide dismutase 2 (SOD2) levels were increased in the tempol‐treated group. In addition, the tempol‐treated group showed reduced levels of glutathione‐disulphide reductase (GSR), glutathione peroxidase 1 (GPx1) and catalase (CAT) in immunoblots. The tempol‐treated group has also shown lower relative gene expression of SOD1, CAT and GPx than the non‐treated group. Our data demonstrated that tempol treatment reduced oxidant parameters and increased anti‐oxidant SOD2 levels in the DIA muscle of mdx mice, which may contribute to the normalization of the redox homeostasis of dystrophic muscles.
Keywords: diaphragm muscle, dystrophic muscles, mdx mice, oxidative stress, redox homeostasis
1. INTRODUCTION
Several studies have demonstrated that oxidative stress plays a critical function in the pathogenesis of Duchenne muscular dystrophy (DMD). 1 , 2 , 3 DMD is the most frequent and lethal muscular dystrophy, affecting 1 in 3000‐6000 male children, 3 and at present, there is no efficient therapy for this dystrophy. 4
Mitochondria are one of the main sources of reactive oxygen species (ROS). Hydroxyl radical, superoxide anion, hydrogen peroxide and singlet oxygen constitute the ROS, and when produced in excess or not properly controlled, it becomes toxic. 5 , 6 There is feed‐forward looping; the high levels of ROS produced by the mitochondrial electron transport chain damage the mitochondria and consequently lead to even greater production of ROS and mitochondrial injury. 6
Considering that chronic oxidative stress performs a critical function in DMD pathogenesis, 3 the use of anti‐oxidant therapies to slow the progression of dystrophic pathologies seems fully justified. Enzymatic and non‐enzymatic antioxidant defence systems are involved against free radical–mediated tissue or cellular damage and metabolizing free radicals to non‐radical products. 3 Moreover, antioxidant therapy might help empower the antioxidant defence system against ROS damage. Different authors have shown that tempol (4‐hydroxy‐2,2,6,6‐tetramethylpiperidine‐N‐oxyl), a redox cycling, metal‐independent, membrane‐permeable anti‐oxidant that mimics the function of superoxide dismutase (SOD), 7 , 8 is able to diminish the oxidative stress–mediated cell damage in several pathologic conditions. 9 , 10
Specifically in terms of DMD, it has been demonstrated that tempol treatment can improve diaphragm functional capacity; the metabolic enzyme activities 11 ; and the dystrophic phenotype, by reducing myonecrosis and inflammatory process in the diaphragm and biceps brachii muscles in mdx mice. 11 , 12 Although the positive effects of tempol on mdx mice muscles have been assigned to its anti‐oxidant function, 7 , 8 the precise nature of this anti‐oxidant action in the dystrophic muscle is still unclear. A previous study 11 has seen indirect evidence of the anti‐oxidant action of tempol in the mdx muscle, demonstrating recovery of aerobic and glycolytic enzyme activities after treatment in vivo. These data motivated us to test whether tempol has anti‐oxidant properties on the diaphragm muscle. To test this in, this study we analysed the consequences of tempol treatment on oxidative stress and enzymatic anti‐oxidant defence system in the dystrophic diaphragm muscle of mdx mice in the acute phase of degeneration. In the acute phase of degeneration, from about 3 weeks of postnatal age, muscles of mdx mice undergo an acute bout of severe necrosis and inflammation. 13 In addition, the NADPH oxidase, which is upregulated during the period of oxidative stress (in 19‐day‐old mdx mice), was identified as being a major source of ROS production in mdx muscles. 14
2. METHODS
2.1. Animals
This study used both male and female mdx mice (C57BL/10‐Dmdmdx/PasUnib), a model for DMD. Since diaphragm muscles of male and female mdx mice are equally affected by dystrophic characteristics, 15 they were randomly selected. Some male and female wild‐type mice (C57BL/10ScCr/PasUnib) were used for certain focused experiments. The research protocol was approved by the institutional Ethics Committee on the Use of Animals (CEUA) of State University of Campinas (UNICAMP) (# 3937‐1). Animals were housed and cared for following the guidelines of the Brazilian College for Animal Experimentation (COBEA). Mice chow and water were provided ad libitum, and the animals were kept in a temperature‐controlled room (25°C ± 0.5) and relative humidity (55% ± 1) with 12‐hour light/dark cycles.
2.2. Experimental design
The wild‐type mice (14 days old) were used as normal controls (Ctrl group; n = 10) and received no treatment. The Ctrl group was subdivided for the proposed analysis: histopathological oxidative stress (n = 5) and Western blotting (n = 5).
The mdx mice (14 days old) were randomly divided into two groups: mdx (n = 17), as used as a control (sham), receiving intraperitoneal administration (i.p.) of saline solution (100 µL); and mdxT (n = 17), the treated group, receiving i.p. administration of tempol (100 mg/kg) dissolved in 100 µL of saline solution, according to previous studies. 7 , 12 Each group was subdivided for the proposed analysis: histopathological oxidative stress (n = 5), Western blotting (n = 5) and gene expression (n = 7). From the 14th day of postnatal life, before muscle degeneration/regeneration cycles in mdx mice begin, 13 , 16 , 17 mdx mice were weighed and treated daily until the 28th day of postnatal life. Different studies report that massive acute muscle degeneration occurs abruptly in 3‐ to 4‐week‐old mdx animals. 16 , 17 The animals were weighed to calculate the correct dose according to body weight.
After treatment time course, all animals were weighed and euthanized through a mixture of ketamine hydrochloride (130 mg/kg; Francotar; Virbac) and xylazine hydrochloride (6.8 mg/kg; 2% Virbaxil; Virbac), and the diaphragm (DIA) muscle was extracted and immediately weighed in an analytical balance (Tecnal 6K, Class I). After collecting the DIA muscle, they were first frozen in isopentane at –90°C cooled in liquid nitrogen; then, they were inserted in liquid nitrogen at –159°C and stored in a biofreezer at –70°C until being processed.
2.3. Histopathological assessment of oxidative stress (n = 5 per group)
For quantification of the number of autofluorescent granules of lipofuscin, muscle samples were analysed using serial unfixed cryosections (8 µm) of the DIA muscle. Quantification was performed in a fluorescent inverted microscope (Nikon, Eclipse TS100/TS100F) connected to a Nikon camcorder at 20× magnification connected to a computer using the NIS‐elements AR Advances Research software in each cross section (4‐5 sections per muscle).
Serial cryosections (8 µm) were incubated with 5 μL of dihydroethidium (DHE; 37291; Sigma‐Aldrich) for the quantification of the ROS levels, as previously reported. 18 DHE staining presents a bright red emission on fluorescence microscopy. The intensity of reactive DHE by muscle area was quantified in a fluorescent inverted microscope (Nikon, Eclipse TS100/TS100F) connected to a Nikon camcorder at 20× magnification connected to a computer by measuring pixels in a specific range (70 ± 255 wavelength). The equipment was adjusted to eliminate interference from background fluorescence using the NIS‐elements AR Advances Research software.
2.4. Western blotting (n = 5 per group)
Muscle sample extracts were washed thrice with PBS before the addition of lysis buffer (1% Triton, 10 mmol/L sodium pyrophosphate, 100 mmol/L NaF, 10 µg/mL aprotinin, 1 mmol/L phenylmethanesulphonyl fluoride and 0.25 mmol/L Na3VO4).
Cell detritus were removed by centrifugation at 13 500 g for 20 minutes at 4°C, and the cleared lysate was subjected to SDS‐PAGE. The Bradford method was used to determine the total protein content. Total protein from cell lysate (30 µg) was stacked on 6%–15% SDS‐polyacrylamide gels.
The proteins were transferred from gels to nitrocellulose membranes by electrophoresis (Mini Trans‐Blot® electrophoretic transfer cell ‐ Bio‐Rad). A blocking buffer was used in all membranes for 2 hours at room temperature. Membranes were incubated with appropriated primary antibodies overnight at 4°C with gentle shaking: 4‐HNE (goat polyclonal; Santa Cruz Biotechnology); monoclonal anti‐catalase (mouse; Sigma‐Aldrich); anti‐SOD‐2 (rabbit; Sigma‐Aldrich); anti‐GSR (rabbit; Sigma‐Aldrich); anti‐GPx1 (rabbit; Sigma‐Aldrich); and glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH; rabbit polyclonal; Santa Cruz Biotechnology).
Peroxidase‐conjugated secondary antibodies purified mouse or rabbit IgG antibody (KPL) were used to incubate membranes for 2 hours at room temperature. Membranes were washed 3 × 10 minutes with TBST after both incubations. Glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) was used as a control protein loading. All membranes were revealed using the Clarity Western ECL Substrate (Bio‐Rad).
GeneTools software (SynGene—A Division of Synoptics) was used for band intensity quantification.
2.5. Gene expression (mRNA) by quantitative real‐time QPCR (n = 7 per group)
Total RNA from mdx muscle was extracted using Trizol reagent according to the manufacturer's instruction. Complementary DNA (cDNA) first‐strand synthesis was performed with 2 µg total RNA using MultiScribe™ Reverse Transcriptase Kit (ThermoFisher Scientific) following the manufacturer's instructions. qPCRs were performed with Fast SYBR Green Master Mix (ThermoFisher Scientific) using 1 µL of the cDNA reaction and 300 nmol/L of each primer in a 7500 Fast Real‐Time PCR system (Applied Biosystems). Gene expression levels were analysed according to the ΔΔCT method. 19 RPL39 was used as a reference gene. Primer sequences (Exxtend, Oligo Solutions) are listed in Table 1.
Table 1.
qRT‐PCR primer sequences (5′–3′), forward (F) and reverse (R)
| Gene name | Primer sequences |
|---|---|
| SOD1 |
F: 5′‐GCGGTGAACCAGTTGTGTTG‐3′ R: 5′‐CTGCACTGGTACAGCCTTGT’3′ |
| CAT |
F: 5′‐CTCGCAGAGACCTGATGTCC‐3′ R: 5′‐GACCCCGCGGTCATGATATT‐3′ |
| GPx1 |
F: 5′‐TCCAGTATGTGTGCTGCTCAT‐3′ R: 5′‐TTCATCTCGGTGTAGTCCCG‐3′ |
| GR |
F: 5′‐GGGGCTCACTGAAGACGAAG‐3′ R: 5′‐TCACAGCGTGATACATCGGG‐3′ |
| RPL |
F: 5′‐CAAAATCGCCCTATTCCTCA‐3′ R: 5′‐AGACCCAGCTTCGTTCTCCT‐3′ |
Abbreviations: CAT, catalase; GPx1, glutathione peroxidase 1; GR, glutathione reductase; RPL39, ribosomal protein L39; SOD1, manganese‐superoxide dismutase 1.
2.6. Statistical analysis
Data are expressed as mean ± standard deviation (SD). Statistical analysis for direct comparison between means of groups was performed by ANOVA, followed by Tukey test used for multiple statistical comparisons between groups. Statistical analysis for direct comparison between two groups was performed by Student's t test. The significance level of 5% (P ≤ .05) was used. We used the GraphPad Prism 5 software package (GraphPad Software).
3. RESULTS
3.1. Body weight and DIA muscle weight
All experimental groups showed weight gain during the experimental period: 45.6% in the Ctrl mice group (n = 10), 41.5% in the mdx mice group (n = 17) and 39.1% in the mdxT mice group (n = 17; Table 2). This was expected, since the animals were young and growing.
Table 2.
Body weight and DIA weight of Ctrl and mdx mice
| Group | Sex (n) | Initial body weight (g) | Final body weight (g) | Body weight (g) | DIA weight (g) | |
|---|---|---|---|---|---|---|
| M | F | |||||
| Ctrl | 7 | 3 | 7.71 ± 0.25 | 14.17 ± 0.72 ### | 6.46 ± 0.52 | 0.063 ± 0.011 |
| mdx | 8 | 9 | 7.22 ± 0.84 | 12.35 ± 1.31 ### | 5.14 ± 0.80 | 0.050 ± 0.015 |
| mdxT | 10 | 7 | 6.88 ± 0.96 | 11.30 ± 2.74*, ### | 3.89 ± 1.01* | 0.061 ± 0.008 |
Values are expressed as mean ± standard deviation (SD). Wild‐type C57BL/10 mice (Ctrl), saline‐treated mdx mice (mdx) and tempol‐treated mdx mice (mdxT). Initial body weight: 1st day of treatment with tempol treatment; final body weight: 14th day of tempol treatment; body weight: body weight gain in the treatment period; DIA weight: weight of diaphragm muscle. Values are reported as grams (g).
P ≤ .05 vs Ctrl group (one‐way ANOVA with Tukey's post hoc test)
P ≤ .0001 vs initial weight of the same group (paired Student's t test)
The tempol‐treated mdx group presented a lower final body weight compared to the Ctrl group (Table 2). However, the DIA muscle weight did not differ significantly between experimental groups (Table 2).
3.2. Tempol effects on the oxidative stress markers
The mdx group showed a significant increase in oxidative stress by greatly increasing the DHE area; the autofluorescent lipofuscin granules; and the 4‐hydroxynonenal (4‐HNE)‐protein adduct levels compared to the Ctrl group (Figure 1A‐F). 4‐Hydroxynonenal (4‐HNE) is a well‐known biomarker of oxidative stress and lipid peroxidation. 20 , 21
Figure 1.
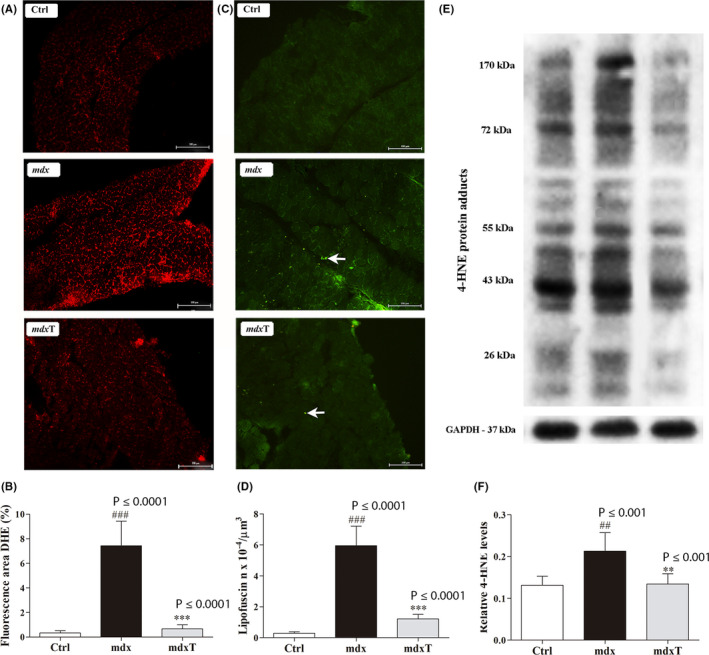
Tempol effects on the oxidative stress markers in the wild‐type and dystrophic diaphragm muscle. A, Diaphragm (DIA) cross sections showing dihydroethidium (DHE) fluorescence in wild‐type C57BL/10 (Ctrl), saline‐treated mdx mice (mdx) and tempol‐treated mdx mice (mdxT). Scale bar: 100 µm. B, The graphs show the DHE staining area (%) in wild‐type C57BL/10 (Ctrl), mdx and mdxT groups. C, DIA cross sections showing autofluorescent lipofuscin granules (white arrow) in wild‐type C57BL/10 (Ctrl), mdx and mdxT groups. Scale bar: 100 µm. D, The graphs show the number of lipofuscin granules × 10−4/µm3 in wild‐type C57BL/10 (Ctrl), mdx and mdxT groups. E, Western blotting analysis of 4‐hydroxynonenal (4‐HNE)‐protein adducts in wild‐type C57BL/10 (Ctrl), mdx and mdxT groups. Bands corresponding to protein (top row), and glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH; used as a loading control) (bottom row), are shown. F, The graphs show protein levels in the crude extracts of DIA muscle of wild‐type C57BL/10 (Ctrl), mdx and mdxT groups. All values expressed as mean ± standard deviation (SD). ## P ≤ .001 vs Ctrl group, ### P ≤ .0001 vs Ctrl group, **P ≤ .001 vs Ctrl group ***P ≤ .0001 vs mdx Ctrl group (one‐way ANOVA with Tukey's post hoc test)
The tempol‐treated group showed a reduction in oxidative stress markers. The DIA muscles showed a reduction of 91.3% in the DHE area on the mdxT group when compared to the saline‐treated mdx group (Figure 1B). The mdxT group showed a reduction of 79.4% in the number of lipofuscin granules in the DIA muscle compared to the mdx group (Figure 1D). The whole band of 4‐HNE protein adducts (proteins from 17 to 150 kDa) was examined in both groups. The mdxT group has shown a reduction of 29.3% in the 4‐HNE protein adduct levels compared to the mdx group (Figure 1F). Note the DHE staining area, the number of lipofuscin granules and 4‐hydroxynonenal (4‐HNE)‐protein adducts reaching levels close to Ctrl in the mdxT groups (Figure 1B, D, F).
3.3. Tempol effects on the enzymatic antioxidant defence system
The mdx group showed a significant increase in GSR, GPx1 and catalase levels (by 41.8%, 74.1% and 90.5%, respectively) compared to the Ctrl group (Figure 2A, B, D).
Figure 2.
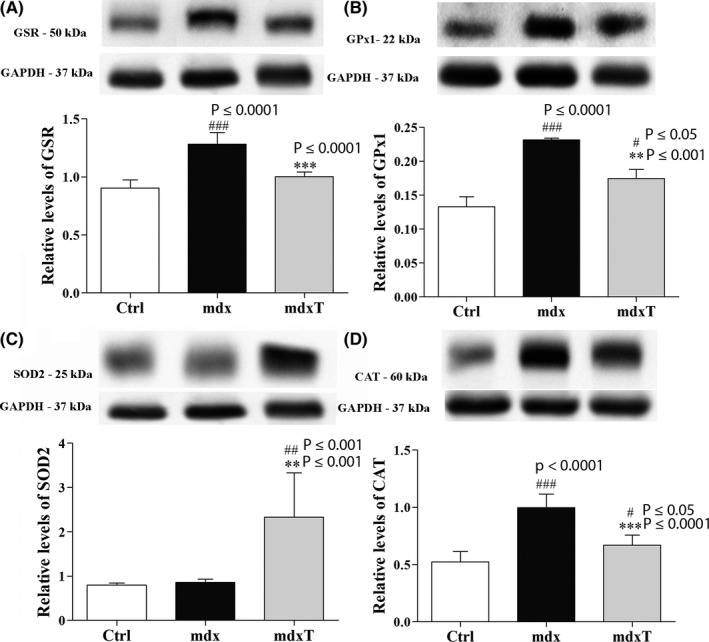
Tempol effects on enzymatic antioxidant system levels in the wild‐type and dystrophic diaphragm muscle. Western blotting analysis of (A) glutathione reductase (GR), (B) glutathione peroxidase (GPx1), (C) manganese‐superoxide dismutase (SOD2) and (D) catalase, content in the diaphragm (DIA) from wild‐type C57BL/10 (Ctrl), saline‐treated mdx mice (mdx) and tempol‐treated mdx mice (mdxT). Bands corresponding to protein (top row) and glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH; used as a loading control) (bottom row) are shown. The graphs show protein levels in the crude extracts of DIA muscle of wild‐type C57BL/10 (Ctrl), mdx and mdxT groups. All values expressed as mean ± standard deviation (SD). # P ≤ .05 compared vs mdx group, ## P ≤ .001 vs Ctrl group, ### P ≤ .0001 vs Ctrl group, **P ≤ .001 vs Ctrl group ***P ≤ .0001 vs mdx Ctrl group (one‐way ANOVA with Tukey's post hoc test)
The tempol‐treated mdx group showed a significant reduction in GSR, GPx1 and catalase levels (by 21.9%, 24.6% and 32.8%, respectively) compared to the saline‐treated mdx group (Figure 2A, B, D). The mdxT group presented significantly higher levels of SOD2 compared to the Ctrl and mdx group (by 193.5% and 173.2%, respectively) (Figure 2C). Note the GSR levels close to Ctrl in mdxT groups (Figure 2A).
3.4. Tempol effects on enzymatic antioxidant defence system gene expression
The reduction in SOD1, CAT and GPx1 gene expression in tempol‐treated mdx mice was found to be significant compared to the saline‐treated mdx mice (Figure 3A‐C). There was no change in GSR gene expression between experimental groups (Figure 3D).
Figure 3.
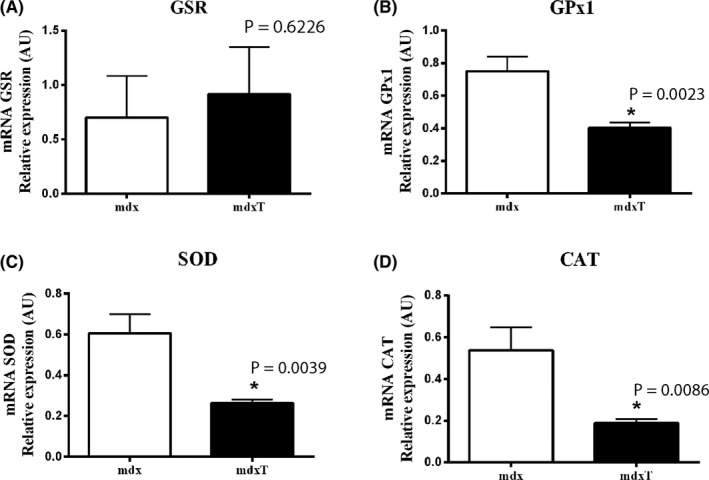
Tempol effects on enzymatic antioxidant system expression in the dystrophic diaphragm muscle. Gene expression of (A) glutathione reductase (GSR), (B) glutathione peroxidase (GPx1), (C) manganese‐superoxide dismutase (SOD) and (D) catalase (CAT) measured by qRT‐PCR in the diaphragm (DIA) from saline‐treated mdx mice (mdx) and tempol‐treated mdx mice (mdxT). All values expressed as mean ± standard deviation (SD). *P ≤ .05 compared vs mdx group (unpaired Student's t test with Welch's correction)
4. DISCUSSION
Impaired enzymatic anti‐oxidant responses in mdx mice and muscle biopsies of DMD patients enhance levels of oxidized glutathione and promoted higher protein oxidation. 3 The diaphragm muscle (DIA) from mdx mice is what most resembles the dystrophic human pathology, 22 expressing increased oxidative stress markers (such as DHE, lipofuscin granules and the 4‐HNE protein adduct) and reduction in SOD and catalase levels. 23 , 24
Differing from the diaphragm muscle, limb muscles from mdx mice have high levels and activity of antioxidant enzymes, such as SOD, catalase and GPx. 25 The mild limb muscle phenotype shown in mdx mice has been attributed to this variance. 11
Considering the anti‐oxidant potential of tempol, 11 , 12 , 26 this study evaluated for the first time its effects on redox homeostasis in the diaphragm muscle of mdx mice.
It is well documented that ROS promote proteolysis and autophagy‐mediated protein breakdown in skeletal muscles. 27 Recently, it has been shown that increased SOD2 in rat diaphragm contributes to the prevention of contractile dysfunction and consequent loss of strength, as well as preventing proteolysis, suggesting that SOD2 plays a role in MuRF1 and Atrogin‐1 mRNA. 28 In addition, SOD2 overexpression in the diaphragm provides partial protection against mitochondrial uncoupling, proving to be capable of protecting against mitochondrial dysfunction. 28
Furthermore, it has been shown to restore diaphragm capacity in mdx mice after tempol supplementation. 11 This finding was associated with increased citrate synthase and lactate dehydrogenase enzyme activity levels compared to wild‐type mice.
A striking finding in the current investigation is that the tempol‐treated group showed significantly higher levels of an anti‐oxidant enzyme (SOD2) in the mdx diaphragm. On the other hand, relative mRNA expression of SOD1, catalase and GPx1 was significantly lower in tempol‐treated mdx mice.
The principal anti‐oxidant defence mechanism is a dismutation of superoxide anions to oxygen and hydrogen peroxide catalysed by SOD. 29 There are three isoforms of SOD (1, 2 and 3) in mammals, and only SOD2 (manganese‐superoxide dismutase) is located in the mitochondrial matrix. 29 Previous studies using mice lacking in SOD2 have shown significant oxidative injury to mitochondria. The complete loss of SOD2 protein in the mutant mouse (Sod−/−) led to mouse death within 20 days after birth. 30 Already, the mutant mouse (Sod2+/−) has shown increased oxidative stress. 30 In contrast, SOD2 overexpression in mice may protect against oxidative stress, apoptosis and fibrosis. 31
A myriad of anti‐oxidants has been used in both DMD patients and animal models. A cell‐permeative salen–manganese compounds (EUK), synthetic superoxide dismutase (SOD) and catalase mimetics have been shown to eliminate both O2·− and H2O2. EUK‐134 [manganese 3‐methoxy N, N′‐bis(salicylidene)ethylenediamine chloride] has been shown to prevent redox stress‐induced muscle wasting and weakness in various pathological conditions. 32 , 33 The EUK‐134 was efficient in reducing the levels of the oxidative stress markers, inflammation and indicators of muscle damage in mdx diaphragm. However, muscle function was partially recovered. 32
N‐acetylcysteine (NAC) is a dietary anti‐oxidant and precursor to glutathione supplementation. 34 A previous study has shown that NAC improved mdx diaphragm functional capacity, reduced fibrosis and immune cell infiltration in mdx diaphragm. 35 NAC also increased Nrf2 mRNA expression in mdx diaphragm and had no adverse effect on ventilation, inspiratory pressure and respiratory muscle EMG or growth measures in mdx mice. 35 NAC was also able to promote overexpression of SOD2, endothelial nitric oxide synthase (eNOS) and matrix metalloproteinase 3 (MMP3) in rat kidneys. 36
In agreement with our results, previous studies also showed that tempol upregulated SOD2 activity and that this increase was accompanied by oxidative stress reduction. 37 , 38 Tempol can act by catalysing superoxide anion, promoting an increased hydroxyl radical (OH) level generation from hydrogen peroxide (H2O2). 9 Despite that, tempol also may protect cells from oxidative damage by catalase‐like activity, generating H2O and O2 from H2O2 and preventing ∙OH production. 9 , 39 These tempol actions may explain the oxidative stress marker reduction and justify the content or expression reduction in the other enzymatic anti‐oxidant defence system components, such as GSR, GPX1 and catalase, observed in our study.
The lower mRNA relative expression of SOD1, CAT and GPx1 in the diaphragm muscle of tempol‐treated mdx mice strengthens its anti‐oxidant action. As SOD‐1 converts superoxide to hydrogen peroxide that can cause lipid peroxidation, SOD‐1 may have a pathogenic rather than protective role in dystrophinopathy, despite functioning as an anti‐oxidant enzyme. 13 , 40 The high SOD2 level and the ROS reduction can be explained by the inhibition of transcriptional pathways of mRNA of SOD1, CAT and GPx1 at the time of analysis, since real‐time quantitative PCR (qPCR) may identify small quantities of nucleic acids associated with a specific time point inside the biological material. 41
Loss of diaphragm function resulting from extensive muscular wasting and weakness is a major problem in humans, leading to death. 13 Tempol shows promise to improve muscle function by decrease myonecrosis, inflammatory response and oxidative stress in the dystrophic diaphragm 12 supporting their use as a potential pharmacological strategy for DMD patients.
Long‐term administration of tempol attenuates post–infarct ventricular dysfunction and sympathetic activity in rats. 42 It is a more positive effect since the cardiac muscles in DMD are also affected by chronic heart failure. 43
The long‐term treatment using different dietary antioxidants (vitamin E, glutathione, melatonin, strawberry extract, ß‐carotene, alpha‐tocopherol, ascorbic acid, rutin, selenium and zinc) showed no side effects in mice. 44 However, many antioxidants have adverse effects on humans. 45
Therefore, more studies are necessary to unveil the molecular basis of tempol, as well as eventual side effects of its chronic use. 46
In summary, our results suggest that tempol treatment significantly changed SOD2 protein content leading to an improvement of anti‐oxidant status and contributing to the normalization of redox homeostasis in the dystrophic diaphragm muscle of mdx mice.
CONFLICT OF INTEREST
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
AUTHOR CONTRIBUTIONS
TAH conducted the study. TAH, DSM, GLR, HNMS, CC, CCL, ECLP and RF analysed the data and performed the statistical analysis. EM participated in the design of the study and coordination. EM, TAH, RF and ECLP helped to draft the manuscript. All authors read and approved the final manuscript.
ACKNOWLEDGEMENTS
We thank Mrs Deirdre Jane Donovan Giraldo for the English revision of the manuscript.
Hermes TDA, Mizobuti DS, da Rocha GL, et al. Tempol improves redox status in mdx dystrophic diaphragm muscle. Int J Exp Path. 2020;101:289–297. 10.1111/iep.12376
Funding information
This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, grant 17/01638‐0) and by Coordenação de Pessoal de Nível Superior‐Brasil (CAPES)—Finance Code 001. TAH and CC were the recipients of a CNPq and CAPES fellowship. DSM, GLR, HNM and CCL were the recipient of a CAPES fellowship.
REFERENCES
- 1. Whitehead NP, Yeung EW, Allen DG. Muscle damage in mdx (dystrophic) mice: role of calcium and reactive oxygen species. Clin Exp Pharmacol Physiol. 2006;33(7):657‐662. [DOI] [PubMed] [Google Scholar]
- 2. Canton M, Menazza S, Di Lisa F. Oxidative stress in muscular dystrophy: from generic evidence to specific sources and targets. J Muscle Res Cell Motil. 2014;35(1):23‐36. [DOI] [PubMed] [Google Scholar]
- 3. Choi I, Lim HoTae, Estrellas K, et al. Concordant but varied phenotypes among Duchenne muscular dystrophy patient‐specific myoblasts derived using a human iPSC‐based model. Cell Rep. 2016;15(10):2301‐2312. [DOI] [PubMed] [Google Scholar]
- 4. Andrews JG, Wahl RA. Duchenne and Becker muscular dystrophy in adolescents: current perspectives. Adolesc Health Med Ther. 2018;9:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mailloux RJ. Mitochondrial antioxidants and the maintenance of cellular hydrogen peroxide levels. Oxid Med Cell Longev. 2018;2018:7857251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bolisetty S, Jaimes EA. Mitochondria and reactive oxygen species: physiology and pathophysiology. Int J Mol Sci. 2013;14(3):6306‐6344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bernardy CCF, Zarpelon AC, Pinho‐Ribeiro FA, et al. Tempol, a superoxide dismutase mimetic agent, inhibits superoxide anion‐induced inflammatory pain in mice. Biomed Res Int. 2017;2017:9584819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Batinić‐Haberle I, Rebouças JS, Spasojević I. Superoxide dismutase mimics: chemistry, pharmacology, and therapeutic potential. Antioxid Redox Signal. 2010;13(6):877‐918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wilcox CS. Effects of tempol and redox‐cycling nitroxides in models of oxidative stress. Pharmacol Ther. 2010;126(2):119‐145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Francischetti IMB, Gordon E, Bizzarro B, et al. Tempol, an intracellular antioxidant, inhibits tissue factor expression, attenuates dendritic cell function, and is partially protective in a murine model of cerebral malaria. PLoS One. 2014;9(2):e87140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Burns DP, Ali I, Rieux C, Healy J, Jasionek G, O'Halloran KD. Tempol supplementation restores diaphragm force and metabolic enzyme activities in mdx mice. Antioxidants (Basel). 2017;6(4):101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hermes TA, Mâncio RD, Macedo AB, et al. Tempol treatment shows phenotype improvement in mdx mice. PLoS One. 2019;14(4):e0215590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grounds MD, Radley HG, Lynch GS, Nagaraju K, De Luca A. Towards developing standard operating procedures for pre‐clinical testing in the mdx mouse model of Duchenne muscular dystrophy. Neurobiol Dis. 2008;31(1):1‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Whitehead NP, Yeung EW, Froehner SC, Allen DG. Skeletal muscle NADPH oxidase is increased and triggers stretch‐induced damage in the mdx mouse. PLoS One. 2010;5(12):e15354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Van Putten M, Putker K, Overzier M, et al. Natural disease history of the D2‐mdx mouse model for Duchenne muscular dystrophy. FASEB J. 2019;33(7):8110‐8124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dangain J, Vrbova G. Muscle development in mdx mutant mice. Muscle Nerve. 1984;7(9):700‐704. [DOI] [PubMed] [Google Scholar]
- 17. Tanabe Y, Esaki K, Nomura T. Skeletal muscle pathology in X chromosome‐linked muscular dystrophy (mdx) mouse. Acta Neuropathol. 1986;69(1–2):91‐95. [DOI] [PubMed] [Google Scholar]
- 18. Whitehead NP, Pham C, Gervasio OL, Allen DG. N‐Acetylcysteine ameliorates skeletal muscle pathophysiology in mdx mice. J Physiol. 2008;586(7):2003‐2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real‐time quantitative PCR and the 2(‐Delta Delta C(T)) Method. Methods. 2001;25(4):402‐408. [DOI] [PubMed] [Google Scholar]
- 20. Esterbauer H, Cheeseman KH. Determination of aldehydic lipid peroxidation products: malonaldehyde and 4‐hydroxynonenal. Methods Enzymol. 1990;186:407‐421. [DOI] [PubMed] [Google Scholar]
- 21. Weber D, Milkovic L, Bennett SJ, Griffiths HR, Zarkovic N, Grune T. Measurement of HNE‐protein adducts in human plasma and serum by ELISA‐Comparison of two primary antibodies. Redox Biol. 2013;1:226‐233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yucel N, Chang AC, Day JW, Rosenthal N, Blau HM. Humanizing the mdx mouse model of DMD: the long and the short of it. NPJ Regen Med. 2018;3:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. TeA H, Macedo AB, Fogaça AR, et al. Beneficial cilostazol therapeutic effects in mdx dystrophic skeletal muscle. Clin Exp Pharmacol Physiol. 2016;43(2):259‐267. [DOI] [PubMed] [Google Scholar]
- 24. Mâncio RD, Hermes TDA, Macedo AB, et al. Vitamin E treatment decreases muscle injury in mdx mice. Nutrition. 2017;43–44:39‐46. [DOI] [PubMed] [Google Scholar]
- 25. Kaczor JJ, Hall JE, Payne E, Tarnopolsky MA. Low intensity training decreases markers of oxidative stress in skeletal muscle of mdx mice. Free Radic Biol Med. 2007;43(1):145‐154. [DOI] [PubMed] [Google Scholar]
- 26. Shateri H, Ranjbar A, Kheiripour N, et al. Tempol improves oxidant/antioxidant parameters in testicular tissues of diabetic rats. Life Sci. 2019;221:65‐71. [DOI] [PubMed] [Google Scholar]
- 27. Powers SK, Morton AB, Ahn B, Smuder AJ. Redox control of skeletal muscle atrophy. Free Radic Biol Med. 2016;98:208‐217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Morton AB, Smuder AJ, Wiggs MP, et al. Increased SOD2 in the diaphragm contributes to exercise‐induced protection against ventilator‐induced diaphragm dysfunction. Redox Biol. 2019;20:402‐413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Powers SK, Ji LL, Kavazis AN, Jackson MJ. Reactive oxygen species: impact on skeletal muscle. Compr Physiol. 2011;1(2):941‐969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Van Remmen H, Salvador C, Yang H, Huang TT, Epstein CJ, Richardson A. Characterization of the antioxidant status of the heterozygous manganese superoxide dismutase knockout mouse. Arch Biochem Biophys. 1999;363(1):91‐97. [DOI] [PubMed] [Google Scholar]
- 31. Silva JP, Shabalina IG, Dufour E, et al. SOD2 overexpression: enhanced mitochondrial tolerance but absence of effect on UCP activity. EMBO J. 2005;24(23):4061‐4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim JH, Lawler JM. Amplification of proinflammatory phenotype, damage, and weakness by oxidative stress in the diaphragm muscle of mdx mice. Free Radic Biol Med. 2012;52(9):1597‐1606. [DOI] [PubMed] [Google Scholar]
- 33. Himori K, Abe M, Tatebayashi D, et al. Superoxide dismutase/catalase mimetic EUK‐134 prevents diaphragm muscle weakness in monocrotalin‐induced pulmonary hypertension. PLoS One. 2017;12(2):e0169146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Matera MG, Calzetta L, Cazzola M. Oxidation pathway and exacerbations in COPD: the role of NAC. Expert Rev Respir Med. 2016;10(1):89‐97. [DOI] [PubMed] [Google Scholar]
- 35. Burns DP, Drummond SE, Bolger D, et al. N‐acetylcysteine decreases fibrosis and increases force‐generating capacity of mdx Diaphragm. Antioxidants (Basel). 2019;8(12):581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ceylan B, Ozansoy M, Kılıç Ü, et al. N‐acetylcysteine suppresses colistimethate sodium‐induced nephrotoxicity via activation of SOD2, eNOS, and MMP3 protein expressions. Ren Fail. 2018;40(1):423‐434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhao B, Pan Y, Wang Z, Tan Y, Song X. Intrathecal administration of tempol reduces chronic constriction injury‐induced neuropathic pain in rats by increasing SOD activity and inhibiting NGF expression. Cell Mol Neurobiol. 2016;36(6):893‐906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hou L, Guo J, Xu F, Weng X, Yue W, Ge J. Cardiomyocyte dimethylarginine dimethylaminohydrolase 1 attenuates left‐ventricular remodeling after acute myocardial infarction: involvement in oxidative stress and apoptosis. Basic Res Cardiol. 2018;113(4):28. [DOI] [PubMed] [Google Scholar]
- 39. Abouzied MM, Eltahir HM, Taye A, Abdelrahman MS. Experimental evidence for the therapeutic potential of tempol in the treatment of acute liver injury. Mol Cell Biochem. 2016;411(1–2):107‐115. [DOI] [PubMed] [Google Scholar]
- 40. Rando TA, Crowley RS, Carlson EJ, Epstein CJ, Mohapatra PK. Overexpression of copper/zinc superoxide dismutase: a novel cause of murine muscular dystrophy. Ann Neurol. 1998;44(3):381‐386. [DOI] [PubMed] [Google Scholar]
- 41. Bustin SA, Benes V, Garson JA, et al. The MIQE guidelines: minimum information for publication of quantitative real‐time PCR experiments. Clin Chem. 2009;55(4):611‐622. [DOI] [PubMed] [Google Scholar]
- 42. Shi Z, Chen A‐D, Xu Y, et al. Long‐term administration of tempol attenuates postinfarct ventricular dysfunction and sympathetic activity in rats. Pflugers Arch. 2009;458(2):247‐257. [DOI] [PubMed] [Google Scholar]
- 43. Meyers TA, Townsend D. Cardiac pathophysiology and the future of cardiac therapies in duchenne muscular dystrophy. Int J Mol Sci. 2019;20(17):4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Meydani M, Lipman RD, Han SN, et al. The effect of long‐term dietary supplementation with antioxidants. Ann N Y Acad Sci. 1998;854:352‐360. [DOI] [PubMed] [Google Scholar]
- 45. Sarangarajan R, Meera S, Rukkumani R, Sankar P, Anuradha G. Antioxidants: friend or foe? Asian Pac J Trop Med. 2017;10(12):1111‐1116. [DOI] [PubMed] [Google Scholar]
- 46. Chiarotto GB, Cartarozzi LP, Perez M, et al. Tempol improves neuroinflammation and delays motor dysfunction in a mouse model (SOD1 G93A) of ALS. J Neuroinflammation. 2019;16(1):218. [DOI] [PMC free article] [PubMed] [Google Scholar]


