Abstract
Tumour‐associated macrophage (TAM) polarization is associated with hepatocellular carcinoma but the molecular mechanism of this polarization is still unknown. Peripheral blood mononuclear cells were induced to differentiate into M0, M1 and M2 macrophages and TAMs. TAMs were transfected with pcDNA3.1‐GAS5, pcDNA3.1‐NC, si‐GAS5, si‐PTEN or si‐Ctrl. A human liver cancer cell line (SMCC‐7721) was incubated with the modified TAM supernatant. Quantitative real‐time PCR and Western blot were performed to detect gene and protein expression. The cell proliferation and invasion properties of the SMCC‐7721 cells were detected by MTT and Transwell assays. GAS5 is up‐regulated in M1 macrophages and down‐regulated in M2 macrophages and TAMs. GAS5 overexpression promoted M1‐like polarization of TAMs and inhibited M2‐like polarization of TAMs. Moreover, GAS5 promoted the expression of PTEN in TAMs. PTEN‐silenced TAM supernatant treatment promoted cell proliferative and invasive properties of the SMCC‐7721 cells and diminished the effect of GAS5‐overexpressed TAM supernatant on the cell proliferation and invasion by SMCC‐7721 cells. Our results demostrared that GAS5 overexpression inhibited M2‐like polarization of TAMs by enhancing PTEN expression, thereby inhibiting cell proliferation and invasion by SMCC‐7721 cells. Thus, our results suggest that GAS5 may be a new therapeutic target for HCC treatment.
Keywords: GAS5, hepatocellular carcinoma, polarization, PTEN, tumour‐associated macrophages
1. INTRODUCTION
Hepatocellular carcinoma (HCC) is one of the most invasive forms of malignant tumours in humans. Because it metastasises and recurs it is ranked as one of the most common causes of cancer ‐ related deaths. 1 , 2 Much progress has been made in diagnosis and treatment, based upon many years of research, but the high rate of recurrence remains a major hurdle to prolonging survival. Thus there is an urgent need to identify effective prevention and treatment. Exploring the mechanism of HCC pathogenesis and metastasis has potentially important value in both early diagnosis and improved prognosis for patients.
HCC is a tumour associated with chronic inflammation. In the inflammatory microenvironment of HCC, the infiltrated inflammatory cells consist mainly of tumour‐associated macrophages (TAMs). Immunology researches have shown that there are two macrophage phenotypes ‐ pro‐inflammatory macrophages (M1) and anti‐inflammatory macrophages (M2). In tumour microenvironments, macrophages can be polarized into M2 macrophages by stimulation with various cytokines, such as IL‐4 and IL‐10. M1 macrophages have the characteristics of pathogen clearance and antineoplasticic activity. In contrast, the main functions of M2 macrophages are immunosuppression and promoting cancer progression, neovascularization and tissue matrix remodelling. 3 , 4 M2 macrophages are further divided into M2a, M2b and M2c. M2a macrophages are involved in antiparasitic, allergic reactions and profibrosis; M2b macrophages mainly participate in immune regulation and activation of T helper 2 cells; M2c macrophages are associated with immunosuppression and tissue repair. 5 , 6 Moreover, TAMs play a crucial role in various stages of HCC by releasing growth factors, cytokines, chemokines and proteinases. 7 Animal experiments show that the progression and metastasis of tumour are suppressed by removing the macrophages. 8 In the tumour microenvironment, the study of the function and molecular mechanism of TAMs in the development and metastasis of HCC will provide new strategies for targeted treatment of HCC.
Long non‐coding RNAs (lncRNAs) play an important role in the occurrence and progression of many tumours. 9 LncRNA growth arrest‐special transcript 5 (GAS5)is a member of GAS family. Previous research has shown that GAS5 has a role in suppressing the occurrence of tumours. 10 But the function of GAS5 in TAMs is still unknown. Phosphatase and tensin homologue deleted from chromosome 10 (PTEN) is an important tumour suppressor, and it inhibits M2‐like polarization of TAMs in tumour microenvironment. 11 Some studies show that GAS5 promotes PTEN expression by target binding with miR‐32‐5p, miR‐103 or miR‐222‐3p. 12 , 13 , 14 Thus, we hypothesized that GAS5 inhibits M2‐like polarization of TAMs by regulating PTEN expression.
2. MATERIALS AND METHODS
2.1. Isolation of peripheral blood mononuclear cells
The peripheral blood was obtained from healthy volunteers (n = 20). The peripheral blood mononuclear cells (PBMCs) were isolated from the peripheral blood by Ficoll‐Hypaque density gradient centrifugation. PBMCs were purified using EasySep™ Human CD14 Positive Selection Kit (StemCell Technologies) according to the manufacturers' instruction. Then, PBMCs were cultured in RPMI‐1640 medium (Sangon Biotech) containing 10% foetal bovine serum (FBS, Sangon Biotech) and 1% penicillin/streptomycin. The cells were incubated in a humidified atmosphere at 37°C and 5% CO2. All participates were informed and gave written consent. All protocols were authorized by the Ethics Committee of the Affiliated Hospital of Xuzhou Medical University.
2.2. Polarization of macrophages
The peripheral blood mononuclear cells were induced to differentiate into different types of macrophages by different treatments. PBMCs were induced to differentiate into M0 macrophages by M‐CSF (50 mg/mL) treatment for 24 hours. PBMCs were treated with 100 ng/mL LPS and IFN‐γ for 24 hours to induce the differentiation of PBMCs into M1‐type macrophages. For M2‐type macrophage polarization, PBMCs were incubated with IL‐4 (20 mg/mL) for 24 hours. For TAM polarization, PBMCs were cultured in RPMI‐1640 medium containing 50% cell supernatant of HepG2 cells and 10% FBS for 7 days.
2.3. Cell culture
The human liver cancer cell line (SMCC‐7721) was purchased from public cell banks (ATCC). SMCC‐7721 cells were cultured in RPMI‐1640 medium containing 10% FBS and 1% penicillin/streptomycin. The cells were incubated in a humidified atmosphere at 37°C and 5% CO2. After TAM polarization, the wild or modified TAMs were washed with PBS for several times and then cultured in new RPMI‐1640 medium for 24 hours. Then, the cell supernatant of the wild or modified TAMs was collected. SMCC‐7721 cells were incubated with the cell supernatant of the wild or modified TAMs for 48 hours.
2.4. Cell transfection
Plasmid vector pcDNA3.1‐GAS5, which could consistently express GAS5, was constructed by RIBOBIO via standard molecular cloning approaches. Small interfering RNAs, specific targeting GAS5 (si‐GAS5) or PTEN (si‐PTEN), and its negative control (NC) were purchased from RIBOBIO. The plasmids were transfected into TAMs using Lipofectamine 2000 Transfection Reagent (Invitrogen). The assay was performed according to the instruction of the test kits.
2.5. Quantitative real‐time PCR (qRT‐PCR)
qRT‐PCR was performed to detect the expression intensity of different genes and cytokines. Total RNA was extracted from the cultured cells using RNAsimple Total RNA Kit (Tiangen). The purity of RNA was detected using a NanoDrop 2000 Spectrophotometer (Thermo Fisher Scientific). The quantity of RNA was determined by agarose gel electrophoresis. Then, the RNA was converted to complementary DNA using PrimeScript™ RT Reagent Kit (Takara). qRT‐PCR was carried out using SYBR Green PCR Mix Kit (Takara) on an ABI 7300 Sequence Detection System (Applied Biosystems) according to the instruction. All the primers were purchased from Takara. The results were analysed using the 2−∆∆ C T method for quantification.
2.6. Western blot
Total protein was extracted from the cells using Tissue or Cell Total Protein Extraction Kit (Sangon Biotech). Equivalent protein from different samples was separated by protein electrophoresis, followed by transformation onto PVDF membranes (Merck Millipore). The membranes were incubated with the anti‐rabbit PTEN (1:1000, Bioswamp) at 4°C overnight after immersed into blocking buffer. After the membranes were washed with TBST for several times, goat anti‐mouse IgG antibody (1:1000, Bioswamp) labelled with horseradish peroxidase was incubated with the membranes as a secondary antibody. Anti‐mouse β‐actin antibody (1:1000, Bioswamp) was used as a reference protein for normalization. The grey levels of the protein bands were examined by ImageJ software.
2.7. MTT assay
An MTT assay was performed to explore the proliferation of the SMCC‐7721 cells at 24 and 48 hours. SMCC‐7721 cells were incubated with the cell supernatant of the wild or modified TAMs. Subsequently, the SMCC‐7721 cells were resuspended in RPMI‐1640 medium containing 10% FBS and the cell density was adjusted to 105/mL. The cell suspension was seeded into 96‐well plates, with 100 µL cell suspension in each well, and incubated for 24 or 48 hours. Then, 10 µL MTT (5 mg/mL) was added to each well, and the cells were cultured for 4 hours in an incubator at 37°C. After that, supernatant of cells was removed and DMSO (100 µL) was added and mixed in each well. The absorbance of samples was detected at 490 nm wavelength using enzyme‐labelled instrument (Thermo Fisher Scientific).
2.8. Transwell assay
SMCC‐7721 cells were incubated with the cell supernatant of the wild or modified TAMs for 48 hours. Subsequently, invasion assay was performed to detect the invasion ability of the SMCC‐7721 cells using the 24‐well Boyden chamber with 8‐µm pore size polycarbonate membrane (Corning). Matrigel was diluted to 1 mg/mL with serum‐free medium and covered on the upper chamber of Boyden chambers. Then, the SMCC‐7721 cell suspension (5 × 104) was seeded into the upper chamber; 600 µL fresh culture medium containing 10% FBS was added into the lower chamber. After cultured at 37°C for 24 hours, invading cells on the bottom surface of the chamber were fixed with 4% paraformaldehyde and stained with 0.5% crystal violet. The invasive cells were observed and counted under an Olympus fluorescence microscope (Olympus).
2.9. Statistical analysis
All experiments were repeated independently at least three times. All values were expressed as mean ± standard deviation and analysed by SPSS 22.0 statistical software (IBM). For comparison of two groups, a two‐tailed Student's t test was used. Comparison of multiple groups was made using a one‐ or two‐way ANOVA P < .05 was considered statistically significant.
3. RESULTS
3.1. GAS5 is up‐regulated in M1 macrophages and down‐regulated in M2 macrophages and TAMs
To investigate the role of GAS5 in polarization of TAMs, we isolated PBMCs from healthy peripheral blood. Then, PBMCs were induced to differentiate into different types of macrophages, including M0, M1 and M2 macrophages and TAMs. We performed qRT‐PCR to detect the expression of GAS5 and macrophage markers in these four types of macrophages. As shown in Figure 1A, GAS5 is highly expressed in M1 macrophages as compared with M0 macrophages. Compared with M0 macrophages, the expression of GAS5 expression was observably reduced in both M2 macrophages and TAMs (Figure 1A). The levels of M1 macrophage markers, TNF‐α and IL‐12, were markedly higher in M1 macrophages than those in M0 macrophages. However, the expression of TNF‐α and IL‐12 was down‐regulated in M2 macrophages and TAMs (Figure 1B and C). Interestingly, the expression of M2 macrophage markers, IL‐10 and IL‐13, were notably decreased in M1 macrophages and significantly enhanced in M2 macrophages and TAMs (Figure 1D and E). Furthermore, we assessed the gene and protein expression of PTEN in the different macrophage phenotypes. We found that the gene and protein expression of PTEN was significantly enhanced in the M1 macrophages with respect to M0 macrophages. Compared with M0 macrophages, M2 macrophages and TAMs exhibited a decrease in the gene and protein expression of PTEN (Figure 1FandG). In addition, SMCC‐7721 cells were incubated with the cell supernatant of the wild TAMs. MTT and transwell assays were performed to explore the cell proliferation and invasion of SMCC‐7721 cells. The cell proliferation and invasion of SMCC‐7721 cells were notably enhanced after TAM supernatant treatment (Figure 1H and I). Therefore, these data indicated that PBMCs were induced into M0, M1 and M2 macrophages and TAMs successfully and that GAS5 was up‐regulated in M1 macrophages and down‐regulated in M2 macrophages and TAMs.
FIGURE 1.
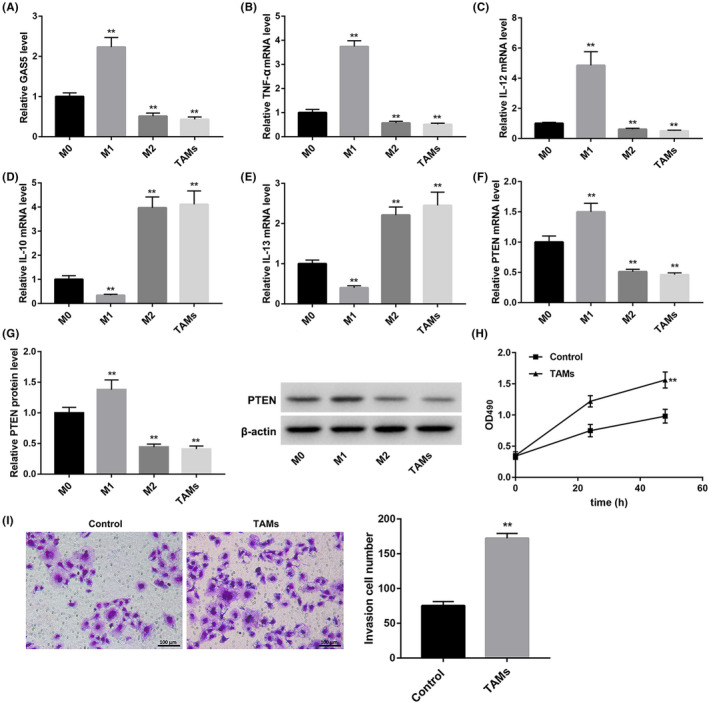
GAS5 is up‐regulated in M1 macrophages and down‐regulated in M2 macrophages and TAMs. PBMCs were induced to differentiate into 4 types of macrophages (M0, M1, M2 macrophages and TAMs) by different treatments. For M0 macrophage polarization, PBMCs were treated with M‐CSF. For M1 macrophage polarization, PBMCs were treated with LPS and IFN‐γ. For M2 macrophage polarization, PBMCs were incubated with IL‐4. For TAM polarization, PBMCs were incubated with 50% HepG2 cell supernatant. The expression of GAS5 (A), TNF‐α (B), IL‐12 (C), IL‐10 (D) and IL‐13 (E) in the 4 types of macrophages was detected by qRT‐PCR. QRT‐PCR (F) and WB (G) were performed to assess the gene and protein expression of PTEN in the 4 types of macrophages. SMCC‐7721 cells were incubated with the cell supernatant of TAMs for 24 or 48 h. H, MTT assay was performed to explore the cell proliferation of SMCC‐7721 cells. I, Transwell assay was performed to estimate the cell invasion of SMCC‐7721 cells. (**P < .01 compared with the M0 or Control group)
3.2. GAS5 participates in polarization of TAMs
To further explore the function of GAS5 in polarization of TAMs, TAMs were transfected with pcDNA3.1‐GAS5 or si‐GAS5 to induce GAS5 overexpression or knockdown in TAMs. Subsequently, we performed qRT‐PCR to estimate the expression of GAS5 in the modified TAMs. GAS5 overexpression caused a boost of GAS5 expression in the TAMs, whereas GAS5 silencing leads to a decrease of GAS5 expression in the TAMs (Figure 2A). Furthermore, the expression of TNF‐α, IL‐12, IL‐10 and IL‐13 in the modified TAMs was detected. GAS5‐overexpressed TAMs exhibited an increase in the expression of TNF‐α and IL‐12, and displayed a decrease in the expression of IL‐10 and IL‐13. However, the expression of TNF‐α and IL‐12 in the TAMs was significantly repressed by GAS5 knockdown. GAS5 silencing notably enhanced the expression of IL‐10 and IL‐13 in the TAMs (Figure 2B‐E). In addition, PTEN was highly expressed in the TAMs after transfected with pcDNA3.1‐GAS5. The expression of PTEN was severely down‐regulated in the GAS5‐silenced TAMs (Figure 2F). Thus, these data implied that GAS5 overexpression promoted M1‐like polarization of TAMs and that inhibited M2‐like polarization of TAMs. However, GAS5 silencing repressed M1‐like polarization of TAMs and enhanced M2‐like polarization of TAMs. And GAS5 promoted the expression of PTEN in TAMs.
FIGURE 2.
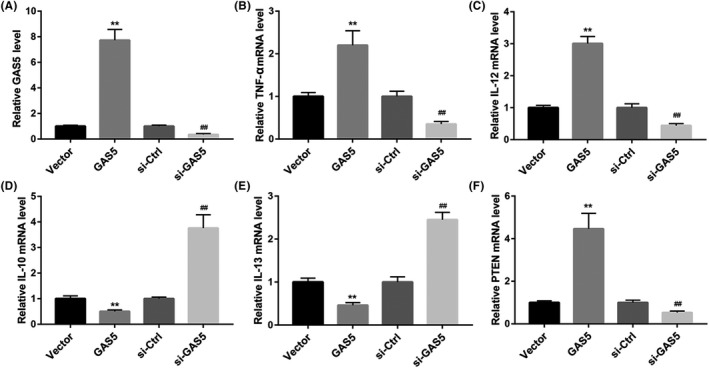
GAS5 overexpression inhibits M2‐like polarization of TAMs, and GAS5 knockdown promotes M2‐like polarization of TAMs. TAMs were transfected with pcDNA3.1‐GAS5, pcDNA3.1‐NC, si‐GAS5 or si‐Ctrl. A, qRT‐PCR was performed to explore the expression of GAS5 in the modified TAMs. The expression of TNF‐α (B), IL‐12 (C), IL‐10 (D), IL‐13 (E) and PTEN (F) in the modified TAMs was detected by qRT‐PCR. (**P < .01 compared with the Vector group, ## P < .01 compared with the si‐Ctrl group)
3.3. GAS5 overexpression inhibits M2‐like polarization of TAMs by regulating PTEN expression
In order to further probe into the involvement of relationship between GAS5 and PTEN in TAMs, TAMs were co‐transfected with pcDNA3.1‐GAS5 or pcDNA3.1‐NC and si‐PTEN or si‐Ctrl. qRT‐PCR data showed that GAS5 overexpression caused an increase in the expression of TNF‐α and IL‐12 in the TAMs. The expression of IL‐10 and IL‐13 in TAMs was notably repressed by GAS5 up‐regulation. PTEN deficiency suppressed the expression of TNF‐α and IL‐12, and promoted the expression of IL‐10 and IL‐13 in the TAMs. Moreover, the influence on the expression of TNF‐α, IL‐12, IL‐10 and IL‐13 conferred by GAS5 overexpression was abolished by PTEN knockdown (Figure 3A‐D). In addition, SMCC‐7721 cells were incubated with the modified TAM supernatant, and the cell proliferation and invasion of SMCC‐7721 cells were analysed by MTT and transwell assays. The cell proliferation and invasion of SMCC‐7721 cells were repressed by GAS5‐overexpressed TAM supernatant treatment. PTEN‐silenced TAM supernatant treatment caused a boost in the cell proliferation and invasion of SMCC‐7721 cells. PTEN‐silenced TAM supernatant treatment diminished the effect of GAS5‐overexpressed TAM supernatant on the cell proliferation and invasion of SMCC‐7721 cells (Figure 3E and F). Taken together, these data demonstrated that GAS5 overexpression inhibited M2‐like polarization of TAMs by regulating PTEN expression.
FIGURE 3.
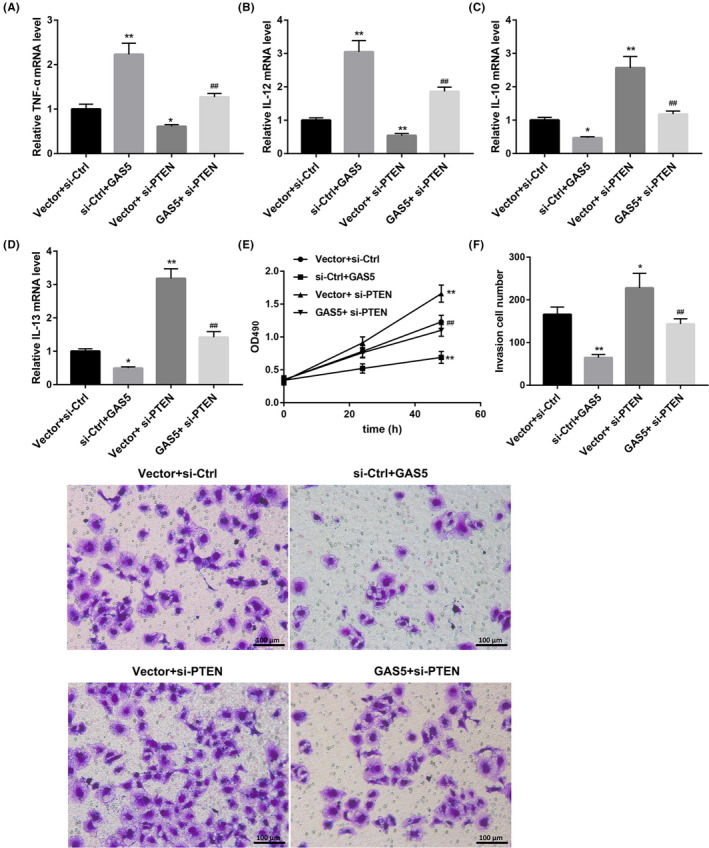
GAS5 overexpression inhibits M2‐like polarization of TAMs by regulating PTEN expression. TAMs were transfected with pcDNA3.1‐GAS5 or pcDNA3.1‐NC and si‐PTEN or si‐Ctrl. The expression of TNF‐α (A), IL‐12 (B), IL‐10 (C) and IL‐13 (D) in the modified TAMs was detected by qRT‐PCR. SMCC‐7721 cells were incubated with cell supernatant of the modified TAMs for 24 or 48 h. E, MTT assay was performed to explore the cell proliferation of SMCC‐7721 cells. F, Transwell assay was performed to detect the cell invasion of SMCC‐7721 cells. (*P < .05 compared with the Vector + si‐Ctrl group, **P < .01 compared with the Vector + si‐Ctrl group, ## P < .01 compared with the Vector + si‐PTEN group)
4. DISCUSSION
Many studies have confirmed the role of GAS5 in various diseases. Previous study has found that GAS5 represses metastasis via miR‐182/ANGPTL1 axis in HCC. 15 GAS5 promotes RECK expression by sponging miR‐135b, thereby suppressing invasion of HCC cells. 16 Corylin represses the progression of HCC by inhibiting GAS5‐mediated epithelial‐mesenchymal transition. 17 The study of Khalid et al has confirmed that PTEN is associated with HCC, and PTEN can be used as a potential prognostic marker for virus‐induced HCC. 18 HRD1 promotes cell proliferation of HCC cells by inhibiting PTEN expression. 19
In our study, we found that TNF‐α and IL‐12 were highly expressed in M1 macrophages, and the expression of IL‐10 and IL‐13 was obviously up‐regulated in M2 macrophages and TAMs. M0 macrophages are unpolarized macrophages, and the expression of various cytokines will not be significantly biased like it is in polarized macrophages. M1 macrophages produce a large number of cytokines, such as IL‐12 and TNF‐α. M1 macrophages exhibit a decrease in IL‐10 expression and show obvious pro‐inflammatory activity. 6 However, M2 macrophages highly express IL‐10, while IL‐12 is down‐regulated, imparting potent anti‐inflammatory properties. 20 Therefore, the expression of IL‐12 and TNF‐α is decreased in M2 macrophages and TAMs compared to M0 macrophages. TAMs exhibited an M2‐like phenotype. Moreover, the expression of GAS5 was up‐regulated in M1 macrophages and was down‐regulated in M2 macrophages and TAMs, suggesting that GAS5 was associated with polarization of TAMs. In addition, TAM supernatant notably promoted the cell proliferation and invasion of SMCC‐7721 cells. Thus, we speculated that GAS5 was associated with polarization of TAMs in the progression of HCC.
Many research studies have shown that lncRNAs play a crucial role in a variety of pathological processes by regulating macrophage polarization. GNAS‐AS1 has a promoting effect on migration and invasion of non–small‐cell lung cancer cells by regulating TAM polarization via the miR‐4319/NECAB3 axis. 21 The NIFK‐AS1/miR‐146a axis reduces the proliferation, migration and invasion of endometrial cancer cells by inhibiting M2‐like polarization of TAMs. 22 CASC2c inhibits M2‐like polarization of TAMs in glioblastoma multiforme. 23 GAS5 participates in polarization of macrophages. NMD pathway–mediated GAS5 down‐regulation results in M2b macrophage polarization in mice. 24 In our study, we investigated the polarization of TAMs from human PBMCs, and we found that GAS5 was associated with polarization of TAMs. GAS5 overexpression promoted M1‐like polarization of TAMs and repressed M2‐like polarization of TAMs. However, GAS5 silencing repressed M1‐like polarization of TAMs and enhanced M2‐like polarization of TAMs. Moreover, GAS5 promoted the expression of PTEN in TAMs. Therefore, these data indicated that GAS5 participated in polarization of TAMs by regulating PTEN expression.
In lung cancer progression, GAS5 overexpression in exosomes inhibits cell proliferation and promotes apoptosis of human umbilical vein endothelial cells by regulating PTEN expression. 25 Up‐regulation of GAS5 suppresses cell proliferation and metastasis of non–small‐cell lung cancer cells by regulating the miR‐205/PTEN axis. 26 GAS5 overexpression inhibits cardiac fibrosis and improves cardiac function via PTEN/MMP‐2 signalling pathway. 27 In our study, we found that PTEN deficiency inhibited M1‐like polarization of TAMs and promoted M2‐like polarization of TAMs. Moreover, PTEN‐silenced TAM supernatant treatment promoted cell proliferation and invasion of SMCC‐7721 cells. PTEN‐silenced TAM supernatant treatment diminished the effect of GAS5‐overexpressed TAM supernatant on the cell proliferation and invasion of SMCC‐7721 cells. Thus, these findings suggested that GAS5 overexpression inhibited M2‐like polarization of TAMs by regulating PTEN expression.
In conclusion, our results proved that GAS5 was associated with TAM polarization. GAS5 overexpression inhibited M2‐like polarization of TAMs in SMCC‐7721 cells by regulating PTEN expression. Overall, our results suggest that GAS5 may be a new therapeutic target for HCC treatment.
ETHICS APPROVAL
All participates were informed and gave written consent. All protocols were authorized by the Ethics Committee of the Affiliated Hospital of Xuzhou Medical University.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Wang X, Li F‐Y, Zhao W, et al. Long non‐coding RNA GAS5 overexpression inhibits M2‐like polarization of tumour‐associated macrophages in SMCC‐7721 cells by promoting PTEN expression. Int. J. Exp. Path. 2020;101:215–222. 10.1111/iep.12374
Xun Wang and Fang‐yuan Li contributed to the work equally and should be regarded as co‐first authors.
Funding information
This study was funded by Xuzhou Science and Technology Bureau Project (grant No. KC17168).
REFERENCES
- 1. El Jabbour T, Lagana SM, Lee H. Update on hepatocellular carcinoma: pathologists' review. World J Gastroenterol. 2019;25(14):1653‐1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pal P, Singh B, Kaur M. Prediction of accuracy for hepatocellular carcinoma patients using cluster based feature ranking. Int J Med Res Health Sci. 2018;7(8):130‐140. [Google Scholar]
- 3. Prenen H, Mazzone M. Tumor‐associated macrophages: a short compendium. Cell Mol Life Sci. 2019;76(8):1447‐1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor‐associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23(11):549‐555. [DOI] [PubMed] [Google Scholar]
- 5. Ricardo SD, van Goor H, Eddy AA. Macrophage diversity in renal injury and repair. J Clin Investig. 2008;118(11):3522‐3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25(12):677‐686. [DOI] [PubMed] [Google Scholar]
- 7. Degroote H, Van Dierendonck A, Geerts A, Van Vlierberghe H, Devisscher L. Preclinical and clinical therapeutic strategies affecting tumor‐associated macrophages in hepatocellular carcinoma. J Immunol Res. 2018;2018:7819520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pathria P, Louis TL, Varner JA. Targeting tumor‐associated macrophages in cancer. Trends Immunol. 2019;40(4):310‐327. [DOI] [PubMed] [Google Scholar]
- 9. Mai H, Zhou B, Liu L, et al. Molecular pattern of lncRNAs in hepatocellular carcinoma. J Exp Clin Cancer Res. 2019;38(1):198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ghaforui‐Fard S, Taheri M. Growth arrest specific transcript 5 in tumorigenesis process: an update on the expression pattern and genomic variants. Biomed Pharmacother. 2019;112:108723. [DOI] [PubMed] [Google Scholar]
- 11. Li N, Qin J, Lan L, et al. PTEN inhibits macrophage polarization from M1 to M2 through CCL2 and VEGF‐A reduction and NHERF‐1 synergism. Cancer Biol Ther. 2015;16(2):297‐306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gao ZQ, Wang JF, Chen DH, et al. Long non‐coding RNA GAS5 suppresses pancreatic cancer metastasis through modulating miR‐32‐5p/PTEN axis. Cell Biosci. 2017;7:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guo C, Song WQ, Sun P, Jin L, Dai HY. LncRNA‐GAS5 induces PTEN expression through inhibiting miR‐103 in endometrial cancer cells. J Biomed Sci. 2015;22:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang XF, Ye Y, Zhao S‐J. LncRNA Gas5 acts as a ceRNA to regulate PTEN expression by sponging miR‐222‐3p in papillary thyroid carcinoma. Oncotarget. 2018;9(3):3519‐3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen F, Li Y, Li M, Wang L. Long noncoding RNA GAS5 inhibits metastasis by targeting miR‐182/ANGPTL1 in hepatocellular carcinoma. Am J Cancer Res. 2019;9(1):108‐121. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16. Yang L, Jiang J. GAS5 Regulates RECK expression and inhibits invasion potential of HCC cells by sponging miR‐135b. Biomed Res Int. 2019;2019:2973289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen CY, Chen C‐C, Shieh T‐M, et al. Corylin suppresses hepatocellular carcinoma progression via the inhibition of epithelial‐mesenchymal transition, mediated by long noncoding RNA GAS5. Int J Mol Sci. 2018;19(2):380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Khalid A, Hussain T, Manzoor S, Saalim M, Khaliq S. PTEN: a potential prognostic marker in virus‐induced hepatocellular carcinoma. Tumour Biol. 2017;39(6):1010428317705754. [DOI] [PubMed] [Google Scholar]
- 19. Liu L, Long H, Wu Y, et al. HRD1‐mediated PTEN degradation promotes cell proliferation and hepatocellular carcinoma progression. Cell Signal. 2018;50:90‐99. [DOI] [PubMed] [Google Scholar]
- 20. Stout RD, Jiang C, Matta B, Tietzel I, Watkins SK, Suttles J. Macrophages sequentially change their functional phenotype in response to changes in microenvironmental influences. J Immunol. 2005;175(1):342‐349. [DOI] [PubMed] [Google Scholar]
- 21. Li Z, Feng C, Guo J, Hu X, Xie D. GNAS‐AS1/miR‐4319/NECAB3 axis promotes migration and invasion of non‐small cell lung cancer cells by altering macrophage polarization. Funct Integr Genomics. 2020;20(1):17–28. [DOI] [PubMed] [Google Scholar]
- 22. Zhou YX, Zhao W, Mao L‐W, et al. Long non‐coding RNA NIFK‐AS1 inhibits M2 polarization of macrophages in endometrial cancer through targeting miR‐146a. Int J Biochem Cell Biol. 2018;104:25‐33. [DOI] [PubMed] [Google Scholar]
- 23. Zhang Y, Feng J, Fu H, et al. Coagulation factor X regulated by CASC2c recruited macrophages and induced M2 polarization in glioblastoma multiforme. Front Immunol. 2018;9:1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ito I, Asai A, Suzuki S, Kobayashi M, Suzuki F. M2b macrophage polarization accompanied with reduction of long noncoding RNA GAS5. Biochem Biophys Res Comm. 2017;493(1):170‐175. [DOI] [PubMed] [Google Scholar]
- 25. Cheng Y, Dai X, Yang T, Zhang N, Liu Z, Jiang Y. Low long noncoding RNA growth arrest‐specific transcript 5 expression in the exosomes of lung cancer cells promotes tumor angiogenesis. J Oncol. 2019;2019:2476175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dong L, Li G, Li Y, Zhu Z. Upregulation of long noncoding RNA GAS5 inhibits lung cancer cell proliferation and metastasis via miR‐205/PTEN axis. Med Sci Monit. 2019;25:2311‐2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu HL, Chen CH, Sun YJ. Overexpression of lncRNA GAS5 attenuates cardiac fibrosis through regulating PTEN_MMP‐2 signal pathway in mice. Eur Rev Med Pharmacol Sci. 2019;23:4414‐4418. [DOI] [PubMed] [Google Scholar]


