Summary
Gastric cancer is a common and high‐incidence malignant gastro‐intestinal cancer that seriously threatens human life. Evidence suggests that microRNAs (miRNAs) play an essential role in regulating the occurrence and development of gastric cancer, but the possible mechanisms and effects remain to be further explored. In the present study, a new tumour suppresser function of miR‐484 was identified in gastric cancer. The expression of miR‐484 was obviously decreased, and the expression of CCL‐18 was obviously increased in gastric cancer tissues and cell lines. In addition, upregulation of miR‐484 suppressed cell proliferation, migration and invasion, and induced cell cycle arrest in G1 phase and cell apoptosis in gastric cancer cells. Besides, miR‐484 mimics could block the PI3K/AKT signalling pathway. Moreover, CCL‐18 was confirmed as a direct target of miR‐484 by binding its 3′‐UTR, and over‐expression of CCL‐18 could restore the effects of miR‐484 on the growth and metastasis of gastric cancer. Finally, in vivo experiments showed that over‐expression of miR‐484 inhibited the subcutaneous tumorigenicity of gastric cancer cells, and the inhibition was blocked after over‐expression of CCL‐18. To conclude, miR‐484 expression was downregulated in gastric cancer tissues and cells and played an anti‐cancer role in the occurrence and development of gastric cancer, which may be achieved by inhibiting the expression of transcription factor CCL‐18 and blocking the PI3K/AKT pathway.
Keywords: CCL‐18, gastric cancer, metastasis, microRNA‐484, PI3K/AKT signalling, proliferation
1. INTRODUCTION
Gastric cancer is a common high‐incidence malignant gastrointestinal form of cancer that poses serious threat to human life. At present, there are about 980 000 new cases and 730 000 deaths worldwide each year, and both morbidity and mortality are the third highest among malignant tumours. 1 In China, the incidence and mortality of gastric cancer rank it first in malignant tumours of digestive system and second in malignant tumours, and the mortality from gastric cancer accounts for 23.18% of all malignant tumours. 2 Currently, endoscopy, X‐ray, B‐mode ultrasonography, CT, MRI and exfoliative cytology are the main methods for clinic diagnosis of gastric cancer, but these diagnostic methods have some deficiencies when trying to make an early diagnosis of gastric cancer. 3 This lack of specific and sensitive screening indicators is the most important factor in hampering early diagnosis of gastric cancer, and this leads to the fact that the majority of patients with gastric cancer are in the middle and late stages at the time of clinical diagnosis, ultimately resulting in poor clinical efficacy of treatment and thus of poor prognosis. 4 Therefore, it is of both theoretical significance and direct clinical guidance significance to study the molecular mechanisms of the occurrence, development, invasion and metastasis of gastric cancer. Early diagnosis, molecular typing, and treatment strategy selection are essential in order to develop more accurate prognostic prediction of gastric cancer.
In recent years, the functional study of microRNAs (miRNA) has provided a promising direction for exploring the occurrence of gastric cancer and finding new diagnostic markers and therapeutic targets. 5 , 6 miRNAs belong to endogenous small single stranded non‐coding RNA molecules with a length of 18‐22 nucleotides and can regulate gene expression by directly degrading mRNAs or inhibiting post‐transcriptional protein translation via binding to the 3′ untranslated region (3′‐UTR) of target mRNAs. 7 , 8 Since the discovery of miRNAs in 1993, there has been evidence that changes in the expression of miRNAs were closely related to tumours including gastric cancer. 9 Many miRNAs, acting as either anti‐oncogenes or oncogenes have been shown to exert essential effects on occurrence, development, spread and metastasis of different cancers. At present, there are abnormal regulated miRNAs in gastric cancer, which not only participate in the proliferation, apoptosis, migration and migration of gastric cancer cells, but also change the sensitivity to radiotherapy and chemotherapy by regulating different tumour‐related target genes. 10 , 11 , 12 Therefore, in‐depth study on the function of miRNAs and their target genes is helpful to elucidate the mechanisms of gastric cancer development and provide potential therapeutic targets.
miR‐484 is located on chr6 and has been shown to have low expression low‐expressed in many cancers. Previous studies have demonstrated that miR‐484 is involved many different functions. For example, miR‐484 showed an anti‐cancer role in cervical cancer cells via targeting ZEB1 and SMAD2. 13 Downregulation of miR‐484 suppressed the apoptosis of non–small‐cell lung cancer (NSCLC) through targeting Apaf‐1. 14 Further, the expression of miR‐484 has been closely related to metastatic renal cell carcinoma treated with sunitinib. 15 However, until now, the role and mechanisms of miR‐484 in the development and progression of gastric cancer are not clear.
CCL‐18 is a CC‐type chemokine, also known as pulmonary and activation‐regulated chemokine (PARC) located at 17q11.2 and is a secretory protein, mainly produced by monocytes/macrophages and dendritic cells. 16 In recent years, the further study of CCL‐18 on tumours at home and abroad has confirmed its role in the occurrence of tumours and its long‐term significances as a potential biomarker for assisting diagnosis. CCL‐18 has been shown to have proߚcancer effects in breast cancer, ovarian cancer, bladder cancer, prostate cancer and pancreatic cancer, but the prognosis of patients with high expression of CCL‐18 in gastric and colon cancer is improved. 17 , 18 Nevertheless, it is unclear whether CCL‐18 could be regulated by miR‐484 in gastric cancer. Therefore, this study was designed to explore whether miR‐484 exerted its functional role in the growth and metastasis of gastric cancer through targeting CCL‐18.
2. MATERIALS AND METHODS
2.1. Gastric cancer tissue
Approximately 50 mm3 pieces of gastric carcinoma tissue and non‐dysplastic background mucosa were removed from each patient’s operative specimen at the same time and before there had been any therapeutic intervention. All the operations were performed in the Suqian First Hospital and the specimens handled according to a standard protocol. Fresh tissues were stored at −80°C for further experiments.
2.2. Cell lines and cell culture
The gastric cancer cell lines MGC‐803, BGC‐823, SGC‐7901, MKN‐45 and MKN‐7 and gastric mucosal epithelial cell GES‐1 were obtained from American Type Tissue Culture Collection (Manassas, VA) and routinely cultured in Dulbecco's modified Eagle's medium (DMEM, Invitrogen) supplemented with 10% foetal bovine serum (FBS, Invitrogen) and 1% penicillin‐streptomycin (Sigma‐Aldrich) in a humidified condition with 5% CO2 at 37°C.
2.3. Cell transfection
miR‐484 mimics and control oligonucleotides, empty vector and pcDNA3.1 cloned with CCL‐18 were synthesized in GenePharma Company. Lipofectamine 2000 (Invitrogen) was used for transfection based on the manufacturer’s specification. After 24 hours, relative expressions of miR‐484 and CCL‐18 were determined by qRT‐PCR assay.
2.4. CCK‐8 assay
Viability of gastric cancer cells after transfection was assessed using CCK‐8 assay. Cells at a density of 104/well were cultured in 96‐well plates, and after incubation for 0, 24, 48 and 72 hours respectively, cells were incubated with 10 μL of CCK‐8 reagent for another 4 hours at 37°C. The absorbance was detected at the wavelength of 490 nm (Multiskan MK3, Thermo Scientific).
2.5. Cell cycle analysis
Cell cycle was analysed by propidium iodide (Nanjing, China) (PI) staining. Briefly, transfected gastric cancer cells were cultured in a 6‐well plate at a density of 105 cells/well and cultured for 24 hours. After fixation with 70% ethanol at 4°C for 2 hours and PI staining, cells were analysed by FACStar flow cytometry (BD Biosciences).
2.6. Apoptosis
Apoptosis was evaluated using apoptosis and necrosis assay kit (Oncogene Research Products). Briefly, transfected gastric cancer cells were cultured in a 6‐well plate at a density of 105 cells/well and cultivated for 24 hours. Afterwards, cells were suspended in binding buffer and incubated at room temperature in the dark for 15 minutes. Annexin‐V‐fluorescein isothiocyanate and propidium iodide were added to cell suspension for 15 minutes in the dark at room temperature according to the protocol of the manufacturer. Stained cells were analysed on a flow cytometer (Beckman Coulter).
2.7. Wound healing assay
Cell motility of gastric cancer cells after transfection was evaluated by wound healing assay. Cells at a density of 105/mL were seeded in a 6‐well plate and carefully wounded using a yellow pipette tip. The cellular debris was removed by washing with DMEM. The dimensions of the wound acorss width each well was photographed under an Olympus IX‐71 microscope (Japan) at 0, 12 and 24 hours, respectively.
2.8. Transwell invasion assays
Transwell chambers (Corning, New York, USA) were used to evaluate the invasion of gastric cancer cells after transfection. Briefly, the cells (1 × 105) cells per well were added to the upper chamber of the inserts with matrix gel, and 600 μL of medium with 50% FBS was added to the lower chamber. The invading cells were stained by crystal violet and imaged after 30 hours of culture.
2.9. RNA extraction and qRT‐PCR analysis
Total RNA was extracted from human gastric cancer tissues and gastric cancer cell lines according to the manufacturer's protocol. Then, total RNA was reverse transcribed into cDNA using RNeasy plus micro kit, which was used as starting materials for qRT‐PCR on the Step One System (Life Technologies Corp). Primer sequences used were designed as follows: miR‐484 forward, 5′‐AGCAGATAGTTCGACTGGACAGGC‐3′, miR‐484 reverse, 5′‐CGCTCGATGAGTGTAGTGGG CT‐3′, U6 forward, 5′‐CTCGCTTCGGCAGCACA‐3′, U6 reverse, 5′‐AACGCTTCACGAATTTGCG T‐3′. PI3K forward, 5′‐GTGTGCATGTCACCGAGGAG‐3′, PI3K reverse, 5′‐AGGAATTTGGTCAG CTCGCA‐3′, AKT forward, 5′‐TACGAGATGATGTGCGGTCG‐3′, AKT reverse, 5′‐CGAGTAGGA GAACTGGGGGA‐3′, MMP‐2 forward, 5′‐GTGCTGAAGGACACACTAAAGAAGA‐3′, MMP‐2 reverse, 5′‐TTGCCTACCTTCTCAAAGTTGTAGG‐3′, MMP‐9 forward, 5′‐TCCCTGGAGACCTGA GAACC‐3′, MMP‐9 reverse, 5′‐ATGTCTGCGTCCCGGCTGT‐3′, GAPDH forward, 5′‐AGAAGGC TGGGGCTCATTTG‐3′, GAPDH reverse, 5′‐AGGGGCCATCCACAGTCTTC‐3′. Conditions for qRT‐PCR were used as follows: 95°C for 10 minutes, 40 cycles of 95°C for 15 seconds and 60°C for 1 minutes. All target gene transcripts were normalized to U6 or GAPDH using the 2−ΔΔCT method.
2.10. Western blot analysis
The protein of transfected gastric cancer cells was lysed in RIPA lysis buffer. After 12 000 g centrifugation for 15 minutes at 4°C, the total protein concentrations were determined by BCA protein assay kit (Beyotime, Haimen, China). Equivalent samples were separated using 10% SDS‐PAGE and then transferred onto a PVDF membrane for 2 hours, and then blocked with 5% non‐fat skim milk in Tris‐Buffered Saline and Tween‐20 (TBS‐T) buffer at room temperature for 1 hour and incubated with the following primary antibodies at 4°C overnight: MMP‐2 (ab97779), MMP‐9 (ab38898), p‐PI3K (ab139307), PI3K (ab70912), p‐Akt (ab38449), Akt (ab179463), CCL‐18 (ab104867), GAPDH (ab181602). After washing 3 times with TBS‐T, the second antibody was added and incubated for 2 hours at room temperature. Enhanced Chemiluminescence Detection System was carried out to evaluate the protein expressions. GAPDH and U6 were used as the loading controls. Antibodies were purchased from Abcam.
2.11. Dual‐luciferase 3′‐UTR reporter assay
The wild‐type and mutant CCL‐18 3′‐UTR Dual‐Luciferase reporter vectors were constructed by subcloning the human CCL‐18 mRNA 3′‐UTR and mutant 3′‐UTR sequences into the pcDNA3.1 Dual‐Luciferase Reporter Vectors (Promega). Cells were transfected with 80 ng luciferase reporter vectors and miR‐484 mimics using the Lipofectamine 2000 (Invitrogen). After 24 hours, luciferase activities were measured using Dual‐Luciferase Reporter System (Berthold) according to the manufacturer's instructions.
2.12. Xenograft model
100 µL of 2 × 106 MGC‐803 cells stably expressing control, miR‐NC, miR‐484 mimics, miR‐484 + Vector and miR‐484 + CCL‐18 were injected into the axilla of the male Balb/c nude mice (6 weeks old, 18‐22 g), which were obtained from. The experimental mice were monitored routinely and sacrificed on day 30. The tumours were removed and their length and width were measured manually using a Vernier caliper.
2.13. Data analysis
All experiments were carried out at least three times, and the data were presented as the mean ± standard deviation and analysed by GraphPad Prism 5.0 (La Jolla, CA, USA). The Student's t test or one‐way ANOVA was used to measured statistical significance of differences between two groups or multiple groups, respectively. A P value < .05 was considered statistically significant.
3. RESULTS
3.1. miR‐484 and CCL‐18 was downregulated in clinical gastric cancer tissues and cell lines
To investigate the effects and possible mechanisms of miR‐484 and CCL‐18 on the development and progression of gastric cancer, firstly, qRT‐PCR was adapted to evaluate the expression of miR‐484 in gastric cancer tissues. As shown in Figure 1A,B, the miR‐484 was expressed less in clinical gastric cancer tissues compared with that in nondysplastic background tissues. However, higher levels of CCL‐18 were expressed in clinical gastric cancer tissues compared with that seen in nondysplastic background. Further, miR‐484 expression in gastric cancer cells including MGC‐803, BGC‐823, SGC‐7901, MKN‐45 and MKN‐7 was also assessed with qRT‐PCR assay, and ‐ as expected ‐ miR‐484 expression in gastric cancer cell lines was significantly downregulated, and CCL‐18 expression in gastric cancer cell lines was significantly upregulated relative to that seen in gastric mucosal epithelial cell GES‐1 shown in Figure 1C,D. These data suggested that miR‐484 might act as a tumour suppressor in development and progression of gastric cancer, and CCL‐18 might act as a tumour promoter.
Figure 1.
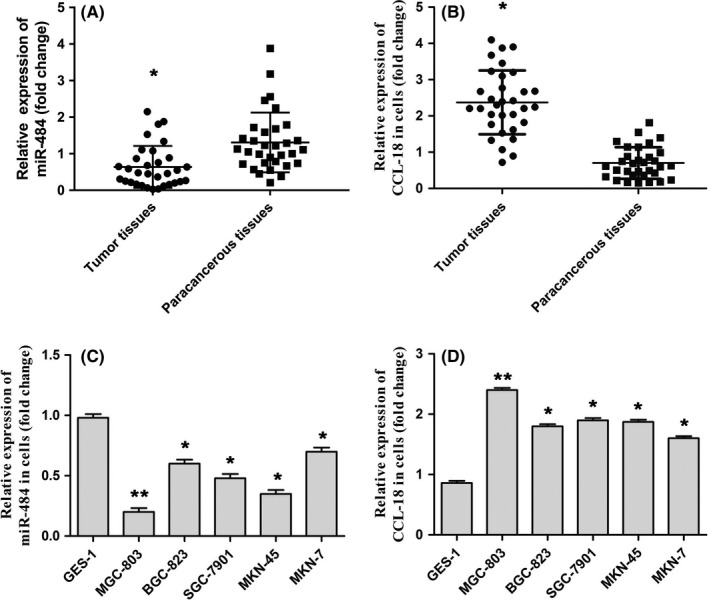
miR‐484 was downregulated in human clinical gastric cancer tissues and cell lines. A, The expression of miR‐484 in gastric cancer tissues and paracancerous tissues was evaluated by qRT‐PCR assay. B, miR‐484 expression of miR‐484 in gastric cancer cells and human gastric mucosal epithelial cell line was determined by qRT‐PCR assay. * P < .05 compared with that of paracancerous tissues; * P < .05, ** P < .01 compared with that of control
3.2. CCL‐18 was a direct target of miR‐484
In order to study the possible target genes of miR‐484 involved in the occurrence and development of gastric cancer, TargetScan (http://www.targetscan.org/vert_72/) was carried out to check that CCL‐18 was a potential candidate of miR‐484 (Figure 2A). In addition, dual‐luciferase reporter gene analysis was performed to verify whether miR‐484 directly regulated CCL‐18. The results of Figure 2B indicated that co‐expression with miR‐484 mimics significantly inhibited the activity of a firefly luciferase reporter containing wild‐type CCL‐18 3′‐UTR, while this was not detected on a reporter with a mutated CCL‐18 3′‐UTR. The data suggested that CCL‐18 was a direct target of miR‐484. Our results in Western blot demonstrated that miR‐484 mimics could decrease the expression of CCL‐18 and upregulated of CCL‐18 could restore the effect of miR‐484 mimics on CCL‐18 (Figure 2C).
Figure 2.
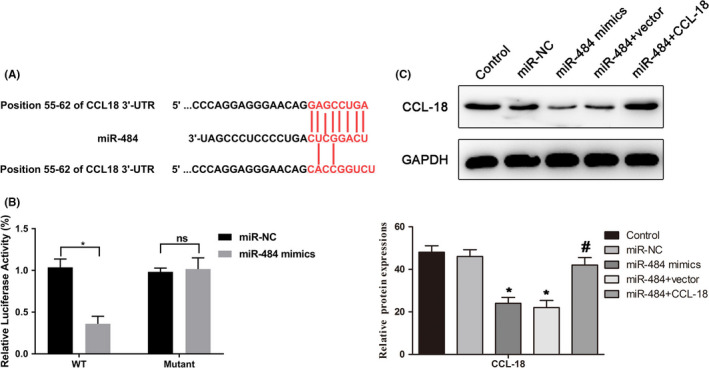
CCL‐18 was a direct target of miR‐484. A, The prediction of the binding between miR‐484 and CCL‐18 by TargetScan. B, Luciferase reporter assays were performed to verify the binding of miR‐484 in 3′‐UTR of CCL‐18. * P < .05 compared with that of miR‐NC. C, Western blot was used to demonstrate weather miR‐484 target to regulate CCL‐18. * P < .05 compared with that of control; #P < .05 compared with that of miR‐484 + vector
3.3. miR‐484 suppressed the proliferation of gastric cancer cells via targeting CCL‐18
As shown in Figure 1B, miR‐484 was least expressed in MGC‐803 cells; thus, MGC‐803 cells were selected for further investigation. Firstly, miR‐484 mimics and CCL‐18‐plasmid were transfected into MGC‐803 cells to upregulate the expression levels of miR‐484 and CCL‐18, respectively. As indicated in Figure 3A,B, the data demonstrated that miR‐484 mimics could notably promote the expression of miR‐484 and CCL‐18‐plasmid could remarkably increase the expression of CCL‐18.
Figure 3.
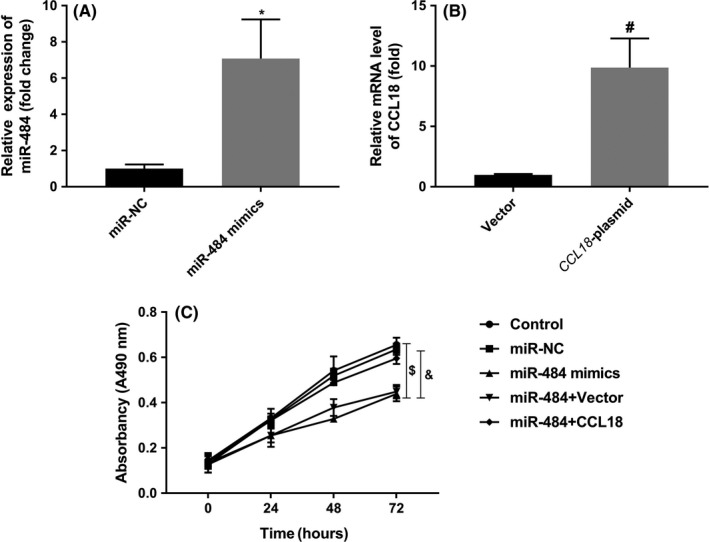
miR‐484 suppressed the proliferation of gastric cancer cells via targeting CCL‐18. A, Transfection efficiency was assessed by qRT‐PCR. B, The proliferation of gastric cancer cells after transfection was assayed by CCK‐8 assay. * P < .05 compared with that of miR‐NC; #P < .05 compared with that of vector; $P < .05 compared with that of control; &P < .05 compared with that of miR484 + vector
Furthermore, the CCK‐8 assay was conducted to assess the effects of miR‐484 on the proliferation of MGC‐803 cells. As shown in Figure 3C, over‐expression of miR‐484 significantly inhibited proliferation of MGC‐803 cells, while CCL‐18‐plasmid could reverse the effects of miR‐484 on the proliferation of MGC‐803 cells. These data suggested that miR‐484 could suppress the proliferation of MGC‐803 cells via targeting CCL‐18.
3.4. miR‐484 promoted apoptosis and induced G1 phase arrest of gastric cancer cells via targeting CCL‐18
In addition, the effects of miR‐484 on cell apoptosis were evaluated by flow cytometry analysis. The results shown in Figure 4A demonstrated that the number of apoptotic cells were increased statistically by miR‐484 mimics, while over‐expression of CCL‐18 dramatically decreased the effects of miR‐484 on the apoptosis of MGC‐803 cells.
Figure 4.
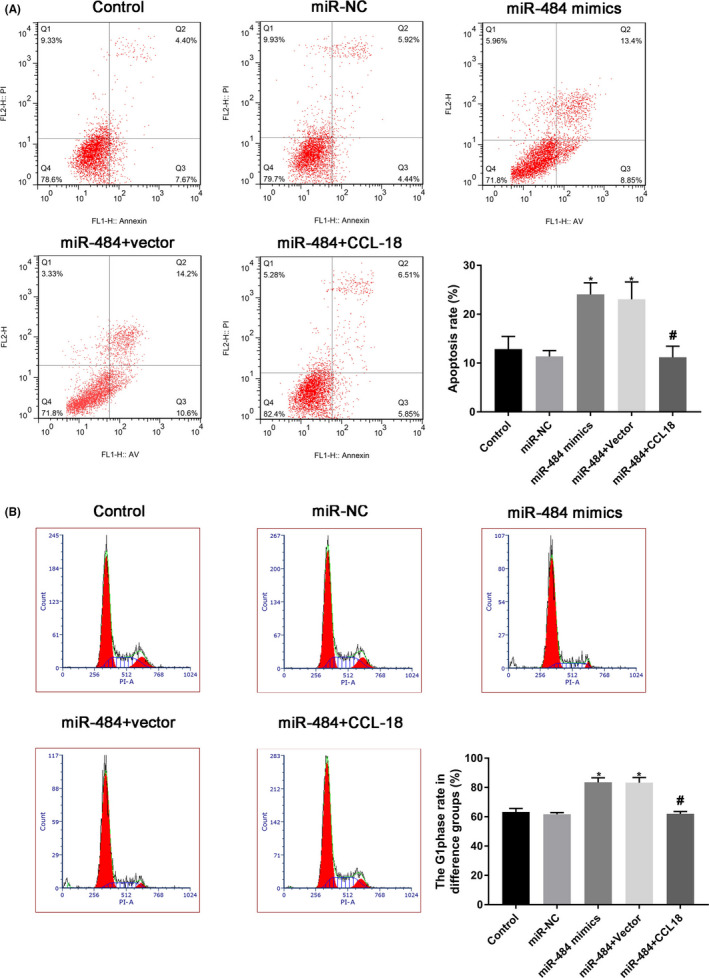
miR‐484 promoted apoptosis and induced G1 phase arrest of gastric cancer cells via targeting CCL‐18. Gastric cancer cells after transfection were harvested for 24 h, and flow cytometry analysis was performed to evaluate the apoptosis and cell cycle distribution. * P < .05 compared with that of control; #P < .05 compared with that of miR‐484 + vectoir
Moreover, the effects of miR‐484 on cell cycle distribution were determined by flow cytometry analysis, and the results shown in Figure 4B demonstrated that miR‐484 mimics could obviously induce MGC‐803 cell arrest in G1 phase, while over‐expression of CCL‐18 dramatically restore the effects of miR‐484 on the cell cycle distribution of MGC‐803 cells. These data suggested that miR‐484 could promote apoptosis and induce G1 phase arrest of MGC‐803 cells via targeting CCL‐18.
3.5. miR‐484 inhibited migration and invasion of gastric cancer cells via targeting CCL‐18
The wound healing assay was carried out to explore the effects of miR‐484 on the MGC‐803 cell migration, and the results as shown in Figure 5A indicated that MGC‐803 cells treated with miR‐484 mimics suppressed the migration of MGC‐803 cells at a slower rate, while over‐expression of CCL‐18 dramatically restore the effects of miR‐484 on migration of MGC‐803 cells. Moreover, an invasion assay was performed to measure the effects of miR‐484 on MGC‐803 cell invasion, As indicated in Figure 5B, the number of invasion cells was obviously reduced after transfection with miR‐484 mimics in MGC‐803 cells; however, over‐expression of CCL‐18 rescue the effects of miR‐484 on invasion of MGC‐803 cells. To further determine the effects of miR‐484 on MGC‐803 migration and invasion, Western blotting assays were adapted to determine the MMP‐2 and MMP‐9 levels. With the upregulation of miR‐484, expression of MMP‐2 and MMP‐9 was obviously downregulated in MGC‐803 cells, and over‐expression of CCL‐18 restores the effects of miR‐484 on the expressions of MMP‐2 and MMP‐9 in MGC‐803 cells (Figure 5C). Overall, these findings indicated that miR‐484 inhibited migration and invasion of gastric cancer cells via targeting CCL‐18.
Figure 5.
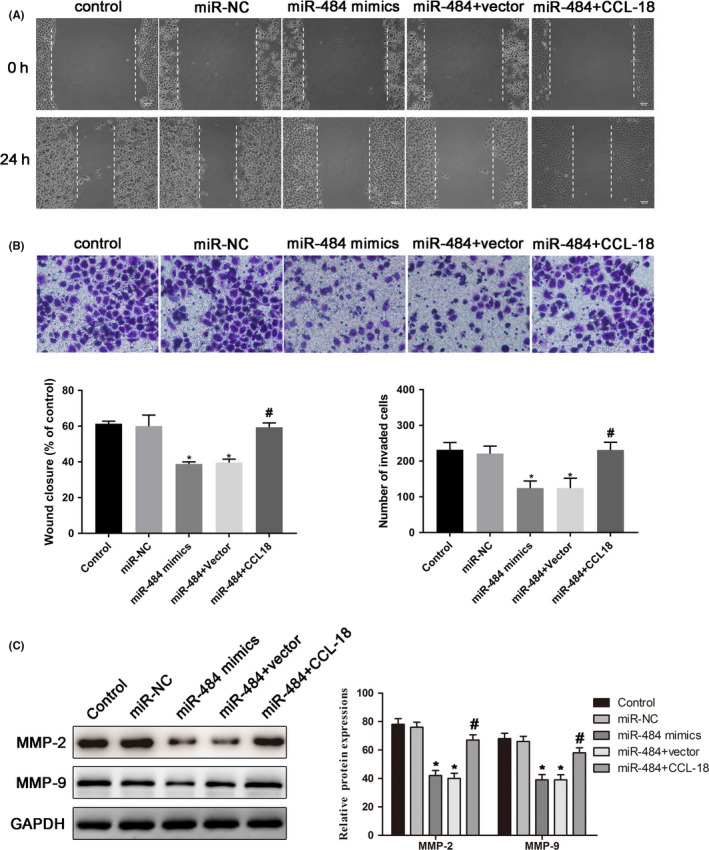
miR‐484 inhibited migration and invasion of gastric cancer cells via targeting CCL‐18. A, The non‐directional migration abilities of gastric cancer cells with transfection for 24 h were examined by wound healing assay. B, The abilities of invasion in gastric cancer cells after transfection for 24 h were evaluated by transwell invasion assay. C, Expressions of the MMP‐2 and MMP‐9 proteins were determined in gastric cancer cells after transfection for 24 h. Then, the band intensity was quantified by ImageJ software. * P < .05 compared with that of control; #P < .05 compared with that of miR‐484 + vector
3.6. miR‐484 suppressed the PI3K/AKT signalling pathway of gastric cancer cells via targeting CCL‐18
To further investigate the mechanisms of miR‐484 in MGC‐803 cells, the expressions of PI3K/AKT signalling pathway were determined by qRT‐PCR and Western blot assays. The results of qRT‐PCR (Figure 6A) demonstrated that upregulation of miR‐484 significantly inhibited the mRNA expressions of PI3K and AKT, and over‐expression of CCL‐18 notably promoted the expression of PI3K and AKT, which reversed the effects of miR‐484. In addition, the results of Western blot (Figure 6B) revealed that miR‐484 mimics inhibited the phosphorylation of PI3K and AKT and over‐expression of CCL‐18 notably promoted the phosphorylation of PI3K and AKT. The data suggested that miR‐484 suppressed the PI3K/AKT signalling pathway of gastric cancer cells via targeting CCL‐18.
Figure 6.
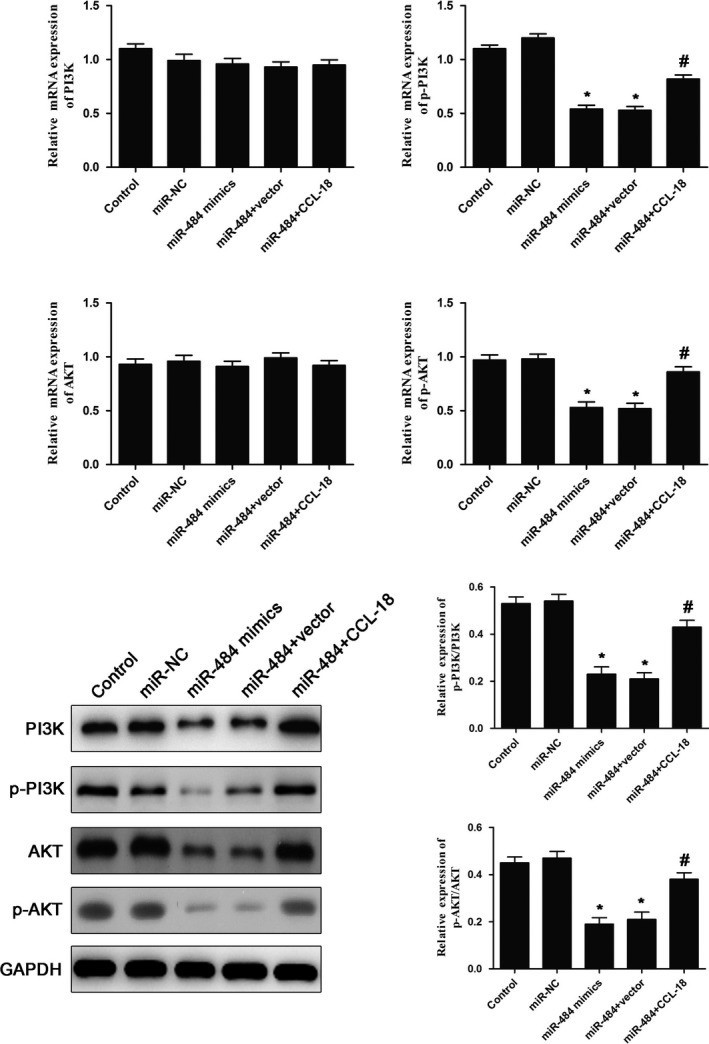
miR‐484 suppressed the PI3K/AKT signalling pathway of gastric cancer cells via targeting CCL‐18. The expression of PI3K/AKT signalling pathway was evaluated by qRT‐PCR (A) and Western blot (B) assays. * P < .05 compared with that of control; #P < .05 compared with that of miR‐484 + vector
3.7. miR‐484 restrained the tumour growth in vivo via targeting CCL‐18
To further test and verify the role of miR‐484 in gastric cancer tumour growth in vivo, MGC‐803 cells after transfection were injected into nude mice. Compared with control groups, over‐expression of miR‐484 decreased the tumour volume and tumour weight, while over‐expression of CCL‐18 dramatically restored the effects of miR‐484 on tumour growth in vivo (Figure 7A‐D). These results suggested that miR‐484 could significantly inhibit tumour growth in vivo via targeting CCL‐18.
Figure 7.
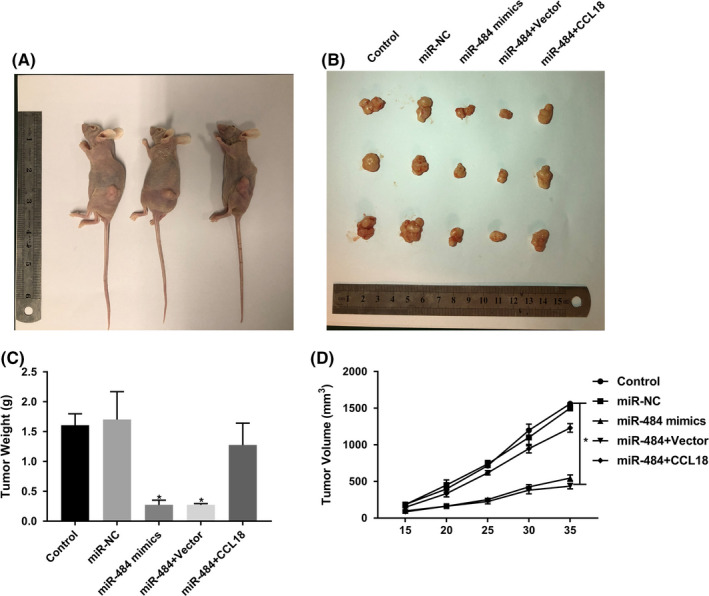
miR‐484 restrained the tumour growth in vivo via targeting CCL‐18. Transfected gastric cancer cells were injected subcutaneously into the right axilla of the nude mice. The representative pictures of mice (A) and tumour tissues (B) were shown. (C) The weight and (D) the volumes of tumours from each group were determined at the end. * P < .05 compared with that of control; #P < .05 compared with that of miR‐484 + vector
4. DISCUSSION
The correlation between microRNA and cancer originated from Calin's research on chronic lymphoblastic leukemia. They found that there were some genes encoding miR‐15a and miR‐16‐1 in the HD region of chromosome 13q14, and more than 65% of chronic lymphoblastic leukemia patients had allele deletions in this region. Thereafter there was an upsurge in the research into the role of miRNAs in the field of cancer. 19 More and more studies have found that the expression of miRNAs was closely related to the occurrence of tumours, including gastric cancer. 20 , 21 , 22 Previous studies have shown that miR‐484 played a key role in various malignant tumours and could be used as a marker for diagnosis of malignant tumours. 13 , 14 , 15 However, there are few reports on the expression and function of miR‐484 in gastric cancer. To further understand the possible role of miR‐484 in the development of gastric cancer, qRT‐PCR was performed to evaluate the expression of miR‐484 in human gastric cancer tissues and human gastric cancer cell lines. The data showed that miR‐484 was significantly decreased in gastric cancer tissues and cell lines. The data suggested that miR‐484 might act as tumour suppressor in the development of gastric cancer.
Early diagnosis of gastric cancer has always been a difficult clinical problem. At present, some tumour markers, such as CEA, CA‐724, CA‐125 and AFP, can be used to assist in diagnosis. However, these tumour markers are not sensitive and specific enough to meet the needs of early diagnosis of gastric cancer. Some studies have found that some certain miRNAs can escape the digestive effects of ribonuclease and exist stably. 23 Moreover, the expression of miRNAs in the serum of some cancer patients was significantly different from that found in people with no known cancer, so they can be used as a specific tumour marker. For example, the expression of miR‐155, miR‐210 and miR‐21 in the serum of patients with B‐cell lymphoma was significantly higher than that of normal controls. 24 Chell et al found that serum miR‐141 was highly expressed in 25 patients with prostate cancer compared with the control group. The sensitivity and specificity of miR‐141 as a diagnostic marker were 60% and 100%, respectively and was correlated with the expression of prostate‐specific antigen (PSA). Therefore, it has been suggested that the application of miRNAs as tumour markers in disease diagnosis has great potential. 25 It has been found that miRNAs play an important role in many different cellular mechanisms including differentiation, proliferation, apoptosis, invasion, and metastasis. 26 In the normal physiological state miRNAs are mainly involved in the differentiation of hematopoietic cells, skeletal muscles, myocardium and nervous system. Malpeli et al found that eight miRNAs, including miR‐323, miR‐138, miR‐9, miR‐211, miR‐149, were involved in the late differentiation of B cells. 27 With the further study of the relationship between miRNAs and tumours, more and more evidence has shown that abnormal expression of miRNAs in tumours can act the important molecules regulating the proliferation and apoptosis of cancer cells. Most of these miRNAs inhibited cell proliferation or induced cell apoptosis to play the role of anti‐oncogenes, while a few promoted cell proliferation and suppressed cell apoptosis to play the role of oncogenes. 28 , 29 , 30 Thus, miRNAs may influence the biological behaviour of cancer cells by regulating the expression of related transcription factors and related proteins of cell biological behaviour, thus promoting or inhibiting the occurrence and development of cancer. So far, there are relatively few studies on the correlation between miR‐484 and tumours, so the functional study of miR‐484 in tumours is still in its infancy. Therefore we conducted in vitro experiments on proliferation, apoptosis, cell cycle, migration and invasion of gastric cancer cells, and found that over‐expression of miR‐484 inhibited the proliferation, migration and invasion of gastric cancer cells and induced apoptosis and G1 phase arrest of gastric cancer cells. This further suggested that miR‐484 may play an anti‐oncogene role in the occurrence and development of gastric cancer.
At present, the most frequently reported action of miRNAs is to promote the degradation of mRNA or inhibit its translation through the combination of miRISC and 3′‐UTR sequence of target genes, so as to suppress the expression of target genes. 31 In tumours, the expression of some miRNAs was inhibited, and the binding of miRISC to the 3′‐UTR of target genes was reduced, resulting in the release of translation inhibition of downstream target genes. It has been reported that miR‐484 can target the expression of Apaf‐1, ZEB1, SMAD2 and TUSC5, 13 , 14 , 15 , 32 thus affecting the occurrence and development of tumours. Bioinformatics software is an important tool for predicting target genes of miRNAs. In order to further explore the molecular mechanism of the growth and metastasis of miR‐484 in gastric cancer, Targetscan was selected to predict the target gene of miR‐484, and CCL‐18 was selected as our research objective. To explore this we used the dual‐luciferase reporter gene assay to confirm that CCL‐18 was a direct target gene of miR‐484. CCL‐18, as a chemokine, plays an important role in proliferation, differentiation and development in the process of immunity. Further, CCL‐18 was closely associated with the proliferation, differentiation, metastasis and angiogenesis properties of cancer cells, such as breast cancer, non–small‐cell lung cancer, and prostate cancer 33 , 34 , 35 which suggested that CCL‐18 may play an oncogene role in human tumours. The data from the present study confirmed the effects of CCL‐18 on the growth and metastasis of gastric cancer cells and revealed that CCL‐18 could reverse the role of miR‐484 in growth and metastasis by gastric cancer cells.
Previous studies have shown that miRNAs can inhibit the growth and metastasis of cancer cells through the PI3K/AKT pathway. For example, Yu et al showed that miR‐345 suppressed the invasion and metastasis of hepatocellular carcinoma by inhibiting the PI3K/AKT pathway related to IRF‐1. 36 In addition, miR‐193a‐3p and ‐5p can inhibit the invasion and metastasis of non–small‐cell lung cancer through PI3K/AKT pathway. 37 To further explore the effects of miR‐484 on PI3K/AKT pathway, we detected the expression of PI3K/AKT pathway‐related mRNAs and proteins after upregulation of miR‐484 by qRT‐PCR and Western blot analysis. The results showed miR‐484 mimics could significantly suppress the PI3K/AKT signalling pathway. CCL‐18, as the upstream gene of PI3K/AKT, 38 , 39 , 40 could obviously reverse the effects of miR‐484 on the expression of PI3K/AKT signalling pathway. Therefore, we speculated that miR‐484 may suppress the growth and metastasis and induce apoptosis of gastric cancer cells through regulation of CCL‐18/PI3K/AKT pathway.
Finally, we used animal models to observe the effects of miR‐484 targeting CCL‐18 on tumorigenicity in nude mice. Our results showed that over‐expression of miR‐484 inhibited the subcutaneous tumorigenicity of gastric cancer cells, and the inhibition was blocked after over‐expression of CCL‐18, suggesting that miR‐484 could suppress the proliferation of gastric cancer via targeting CCL‐18 and thus inhibiting the proliferation of gastric cancer in vivo.
In conclusion, miR‐484 was downregulated in gastric cancer tissues and cells and played an anti‐cancer role in the occurrence and development of gastric cancer, which may be achieved by inhibiting the expression of transcription factor CCL‐18 and blocking the PI3K/AKT pathway.
ETHICAL APPROVAL
This study was approved by the ethics committee of Suqian First Hospital and written informed consent was obtained from 32 patients. Animal welfare and experimental procedures were performed according to National Guidelines and were approved by the Suqian First Hospital.
CONFLICT OF INTEREST
There were no conflicts of interest.
Liu J, Li SM. MiR‐484 suppressed proliferation, migration, invasion and induced apoptosis of gastric cancer via targeting CCL‐18. Int. J. Exp. Path. 2020;101:203–214. 10.1111/iep.12366
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394‐424. [DOI] [PubMed] [Google Scholar]
- 2. Zong L, Abe M, Seto Y, , Ji J. The challenge of screening for early gastric cancer in China. Lancet. 2016;388(10060):2606. [DOI] [PubMed] [Google Scholar]
- 3. Pasechnikov V, Chukov S, Fedorov E, et al. Gastric cancer: prevention, screening and early diagnosis. World J Gastroenterol. 2014;20(38):13842‐13862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brungs D, Aghmesheh M, Vine KL, Becker TM, Carolan MG, Ranson M. Gastric cancer stem cells: evidence, potential markers, and clinical implications. J Gastroenterol. 2016;51(4):313‐326. [DOI] [PubMed] [Google Scholar]
- 5. Liu HS, Xiao HS. MicroRNAs as potential biomarkers for gastric cancer. World J Gastroenterol. 2014;20(34):12007‐12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Al‐Qatati A, Akrong C, Stevic I, et al. Plasma microRNA signature is associated with risk stratification in prostate cancer patients. Int J Cancer. 2017;141(6):1231‐1239. [DOI] [PubMed] [Google Scholar]
- 7. Haendeler J, Mlynek A, Büchner N, et al. Two isoforms of Sister‐Of‐Mammalian Grainyhead have opposing functions in endothelial cellsand in vivo. Arterioscler Thromb Vasc Biol. 2013;33(7):1639‐1646. [DOI] [PubMed] [Google Scholar]
- 8. Falcone G, Felsani A, D’Agnano I. Signaling by exosomal microRNAs in cancer. J Exp Clin Cancer Res. 2015;34:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mirzaei H, Khataminfar S, Mohammadparast S, et al. Circulating microRNAs as potential diagnostic biomarkers and therapeutic targets in gastric cancer: current status and future perspectives. Curr Med Chem. 2016;23(36):4135‐4150. [DOI] [PubMed] [Google Scholar]
- 10. Yang T‐S, Yang X‐H, Wang X‐D, Wang Y‐L, Zhou B, Song Z‐S. MiR‐214 regulate gastric cancer cell proliferation, migration and invasion by targeting PTEN. Cancer Cell Int. 2013;13(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang F, Li T, Zhang B, et al. MicroRNA‐19a/b regulates multidrug resistance in human gastric cancer cells by targeting PTEN. Biochem Biophys Res Commun. 2013;434(3):688‐694. [DOI] [PubMed] [Google Scholar]
- 12. Hsu K‐W, Wang A‐M, Ping Y‐H, et al. Downregulation of tumor suppressor MBP‐1 by microRNA‐363 in gastric carcinogenesis. Carcinogenesis. 2014;35(1):208‐217. [DOI] [PubMed] [Google Scholar]
- 13. Hu Y, Xie H, Liu Y, Liu W, Liu M, Tang H. miR‐484 suppresses proliferation and epithelial‐mesenchymal transition by targeting ZEB1 and SMAD2 in cervical cancer cells. Cancer Cell Int. 2017;17:36. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14. Li T, Ding Z‐L, Zheng Y‐L, Wang W. MiR‐484 promotes non‐small‐cell lung cancer (NSCLC) progression through inhibiting Apaf‐1 associated with the suppression of apoptosis. Biomed Pharmacother. 2017;96:153‐164. [DOI] [PubMed] [Google Scholar]
- 15. Merhautova J, Hezova R, Poprach A, et al. miR‐155 and miR‐484 are associated with time to progression in metastatic renal cell carcinoma treated with sunitinib. Biomed Res Int. 2015;2015:941980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schutyser E, Richmond A, Van Damme J. Involvement of CC chemokine ligand 18 (CCL18) in normal and pathological processes. J Leukoc Biol. 2005;78(1):14‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang QI, Tang Y, Yu H, et al. CCL18 from tumor‐cells promotes epithelial ovarian cancer metastasis via mTOR signaling pathway. Mol Carcinog. 2016;55(11):1688‐1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Leung SY, Yuen ST, Chu K‐M, et al. Expression profiling identifies chemokine (C‐C motif) ligand 18 as an independent prognosticindicator in gastric cancer. Gastroenterology. 2004;127(2):457‐469. [DOI] [PubMed] [Google Scholar]
- 19. Calin GA, Ferracin M, Cimmino A, et al. A MicroRNA signature associated with prognosis and progression in chronic lymphocytic leukemia. N Engl J Med. 2005;353(17):1793‐1801. [DOI] [PubMed] [Google Scholar]
- 20. Cao W, Wei W, Zhan Z, Xie D, Xie Y, Xiao Q. Role of miR‐647 in human gastric cancer suppression. Oncol Rep. 2017;37(3):1401‐1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kang BW, Choi YongHun, Kwon OK, et al. High level of viral microRNA‐BART20‐5p expression is associated with worse survival of patients with Epstein‐Barr virus‐associated gastric cancer. Oncotarget. 2017;8(9):14988‐14994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li H, Wu Q, Li T, et al. The miR‐17‐92 cluster as a potential biomarker for the early diagnosis of gastric cancer: evidence and literature review. Oncotarget. 2017;8(28):45060‐45071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen XI, Ba YI, Ma L, et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancerand other diseases. Cell Res. 2008;18(10):997‐1006. [DOI] [PubMed] [Google Scholar]
- 24. Lawrie CH, Gal S, Dunlop HM, et al. Detection of elevated levels of tumour‐associated microRNAs in serum of patients with diffuselarge B‐cell lymphoma. Br J Haematol. 2008;141(5):672‐675. [DOI] [PubMed] [Google Scholar]
- 25. Mitchell PS, Parkin RK, Kroh EM, et al. Circulating microRNAs as stable blood‐based markers for cancer detection. Proc Natl Acad Sci U S A. 2008;105(30):10513‐10518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hwang HW, Mendell JT. MicroRNAs in cell proliferation, cell death, and tumorigenesis. Br J Cancer. 2006;94(6):776‐780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Malpeli G, Barbi S, Zupo S, et al. Identification of microRNAs implicated in the late differentiation stages of normal B cells suggests a central role for miRNA targets ZEB1 and TP53. Oncotarget. 2017;8(7):11809‐11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang M, Liu C, Su Y, et al. miRNA‐34c inhibits myoblasts proliferation by targeting YY1. Cell Cycle. 2017;16(18):1661‐1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yu B, Chen X, Li J, et al. microRNA‐29c inhibits cell proliferation by targeting NASP in human gastric cancer. BMC Cancer. 2017;17(1):109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ma Z, Luo Y, Qiu M. miR‐143 induces the apoptosis of prostate cancer LNCap cells by suppressing Bcl‐2 expression. Med Sci Monit. 2017;23:359‐365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wu E, Thivierge C, Flamand M, et al. Pervasive and cooperative deadenylation of 3'UTRs by embryonic microRNA families. Mol Cell. 2010;40(4):558‐570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang S, Wang W, Han X, Wang Y, Ge Y, Tan Z. Dysregulation of miR484‐TUSC5 axis takes part in the progression of hepatocellular carcinoma. J Biochem. 2019;166(3):271‐279. [DOI] [PubMed] [Google Scholar]
- 33. Little AC, Pathanjeli P, Wu Z, et al. IL‐4/IL‐13 stimulated macrophages enhance breast cancer invasion via rho‐GTPase regulation of synergistic VEGF/CCL‐18 signaling. Front Oncol. 2019;9:456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Huang H, Li J, Hu W‐J, et al. The serum level of CC chemokine ligand 18 correlates with the prognosis of non‐small cell lung cancer. Int J Biol Markers. 2019;34(2):156‐162. [DOI] [PubMed] [Google Scholar]
- 35. Xu Y, Zhang L, Sun S‐K, Zhang X. CC chemokine ligand 18 and IGF‐binding protein 6 as potential serum biomarkers for prostate cancer. Tohoku J Exp Med. 2014;233(1):25‐31. [DOI] [PubMed] [Google Scholar]
- 36. Yu M, Xue H, Wang Y, et al. miR‐345 inhibits tumor metastasis and EMT by targeting IRF1‐mediated mTOR/STAT3/AKT pathway in hepatocellular carcinoma. Int J Oncol. 2017;50(3):975‐983. [DOI] [PubMed] [Google Scholar]
- 37. Yu T, Li J, Yan M, et al. MicroRNA‐193a‐3p and ‐5p suppress the metastasis of human non‐small‐cell lung cancer by downregulating the ERBB4/PIK3R3/mTOR/S6K2 signaling pathway. Oncogene. 2015;34(4):413‐423. [DOI] [PubMed] [Google Scholar]
- 38. Zhou Z, Peng Y, Wu X, et al. CCL18 secreted from M2 macrophages promotes migration and invasion via the PI3K/Akt pathway in gallbladder cancer. Cell Oncol (Dordr). 2019;42(1):81‐92. [DOI] [PubMed] [Google Scholar]
- 39. Bo S, Donghao S, Guangqi K, Ye T. CC Chemokine Ligand 18 Promotes Cell Proliferation and Metastasis of Urothelial Carcinoma via Activating PI3K/mTOR Signaling in Patient with Renal Transplantation. Urol Int. 2018;101(4):450‐458. [DOI] [PubMed] [Google Scholar]
- 40. Zhang B, Yin C, Li H, et al. Nir1 promotes invasion of breast cancer cells by binding to chemokine (C‐C motif) ligand 18 through the PI3K/Akt/GSK3β/Snail signalling pathway. Eur J Cancer. 2013;49(18):3900‐3913. [DOI] [PubMed] [Google Scholar]


