Summary
This study evaluated the outcome of partial exposure of dentin matrix to ethylenediaminetetraacetic acid (EDTA) and application of platelet‐rich fibrin (PRF) scaffold on regeneration of necrotic immature permanent teeth in a dog model. The present study was carried out on 216 permanent immature roots in nine mongrel dogs aged 6‐9 months. Pulp necrosis and periapical pathosis were induced in 180 roots. These roots were divided into five equal groups (36 roots each) according to the treatment protocol: group I: blood clot; group II: 17% EDTA solution and blood clot; group III: PRF; group IV: 17% EDTA solution and PRF; and group V: without treatment (positive control). The negative control group (group VI) represented 36 untouched normal roots for normal maturation. The groups were followed up for 1, 2 and 3 months (subgroups). Maturation of the roots was monitored by radiography and histopathology. All data were statistically analysed. Group IV exhibited the highest increase in root length and thickness, decrease in apical diameter, the highest score of vital tissue infiltration and least inflammatory scores. There was a significant difference regarding the increase in root length and thickness and decrease in apical diameter in all subgroups of the experimental and negative control groups (P ≤ .05). PRF has a better regenerative potential than the blood clot during treatment of immature permanent teeth with necrotic pulp. Inclusion of 17% EDTA solution as a final irrigation enhances the regenerative potential of both PRF and blood clot.
Keywords: odontoblasts, periapical pathosis, pulp tissue, regenerative endodontics, revascularization, root maturation
1. INTRODUCTION
Immature permanent teeth with necrotic pulp and peri‐apical tissues constitute a challenging endodontic problem due to difficult root canal debridement and obturation as well as high risk of tooth fracture. The conventional treatment of such cases is apexification by calcium hydroxide, which results in acceptable endodontic outcomes. Apexification has several disadvantages such as multiple visits and alteration of the mechanical properties of dentin. 1
One form of recent treatment option is the one‐step creation of an artificial apical barrier using mineral trioxide aggregate (MTA). 2 MTA is an ideal apical barrier due to its good sealing capacity, biocompatibility, induction of hard tissue and setting in the presence of moisture. 3 The main disadvantage of both traditional apexification and artificial apical barriers is lack of root development. 4
Another recent alternative approach is called regenerative endodontic treatment. The main goal of this treatment is to create a suitable environment in the root canal by elimination of infection, provision of a good scaffold and a tight apical seal ‐ this combination should enhance pulp regeneration and root maturation. 5 Revascularization of the necrotic immature permanent teeth after disinfection of the root canal is the most applicable method for regenerative endodontics. 6 , 7 , 8 The revascularization method delivers the stem cells from periradicular tissue to the root canal system and provides a natural scaffold (fibrin clot) as well as growth factors in the blood platelets. 9 In the revascularization method, the type and concentration of cells trapped in the fibrin clot are unpredictable. Therefore, a recent biomedical technology has been introduced to the regenerative endodontics to provide more appropriate condition for tissue regeneration with several biomaterials. 10
Platelet‐rich fibrin (PRF) constitutes a suitable scaffold for regenerative endodontics. Unlike other platelet concentrates, neither anti‐coagulants nor bovine thrombin or jellifying agents are required. PRF provides an autologous fibrin matrix with high numbers of blood platelets and growth factors. 11
Another key factor in this field of tissue engineering is the role of growth factors. One of the most important growth factors is transforming growth factor ß (TGF‐ß) family, which is essential for odontoblast differentiation and enhancement of dentin matrix production. These growth factors are produced by odontoblasts and stored inside the dentin matrix in an active form. 12 Ethylenediaminetetraacetic acid (EDTA) is a very effective material for the release of the growth factors from dentin. 13 , 14
This study assesses the outcomes of partial exposure of dentin matrix to EDTA solution and application of PRF scaffold on regeneration of necrotic immature permanent teeth in the dog model.
2. MATERIALS AND METHODS
2.1. Animal model
A total of nine mongrel dogs aged 6‐9 months were selected for the study. The selected dogs were of both sexes and clinically normal. Animals were quarantined in separate cages and kept under observation for 2 weeks before the study. Three maxillary premolars (two double‐rooted teeth and one single‐rooted tooth: n = 5 roots) and four mandibular premolars (three double‐rooted teeth and one single‐rooted tooth: n = 7 roots) in each quadrant were included in the study. In summary 216 roots from 126 teeth (14 teeth with 24 roots/dog) were examined for the study.
2.2. Classification of the roots
The roots were divided into six equal groups (36 roots each) according to the treatment protocol. Group (I): blood clot; group (II): 17% EDTA and blood clot; group (III): PRF; group (IV): 17% EDTA and PRF; group (V): positive control (no treatment); and group (VI): negative control (normal untouched teeth for normal maturation).
Each experimental group was further subdivided into three subgroups according to the post‐treatment evaluation period: subgroup A: 1 month; subgroup B: 2 months; and subgroup C: 3 months (12 roots each). All groups were represented in each dog in a randomized manner, and each individual root was taken as the unit of measure.
2.3. Induction of periapical pathosis
General anaesthesia was administrated after fasting the dogs for 12 hours. The dogs were premedicated by 0.05 mg/kg weight atropine sulphate (atropine sulphate 1%®, ADWIA, Egypt) injected subcutaneously and 1 mg/kg weight xylazine HCl (Xylaject 2%®, ADWIA) injected intramuscularly. The anaesthesia was induced by using ketamine HCl (ketamine hydrochloride®, Rotexmedica Co.) injected intravenously through a cannula in the cephalic vein at a dose of 5 mg/kg by weight. The anaesthesia was maintained by 2.5% thiopental sodium (thiopental sodium®; Sandoz) at a dose of 25 mg/kg weight solution injected intravenously.
All teeth were examined radiographically to confirm incomplete root formation and to establish baseline working length. Endodontic access cavity was done in all experimental and positive control teeth. Exposing the pulp chamber was done using #2 diamond stone with conventional speed handpiece mounted on an electric micromotor. A sterile file #40 was used to disrupt the pulp. A piece of cotton was inserted into the entrance of each canal, and the access was left open for 4 weeks. Carprofen tablets were given orally for 15 days at a dose of 4.4 mg/kg once daily as postoperative analgesia.
2.4. Root canal disinfection
The triple antibiotic paste (TAP) was prepared by mixing metronidazole 500 mg tablets, ciprofloxacin 250 mg tablets and doxycycline 100 mg capsule with macrogol and propylene glycol as vehicle. 6
After the infection period, dogs were re‐anaesthetized. All surfaces were dressed with povidone iodine. The experimental teeth were re‐entered under complete aseptic conditions and cotton roll isolation. Minimal instrumentation was done with file #40 to disrupt the biofilm on the canal walls and remove bacteria in the dentinal surface. The canals were irrigated with 20 mL of 1.5% NaOCl for 5 minutes, then irrigated with 20 mL of 0.9% saline solution for 5 minutes and dried by paper points. The root canals were filled with 1‐2 mL of the prepared TAP using a sterile plastic syringe with 20‐gauge needle. The access cavity was sealed using temporary restoration for 3 weeks.
2.5. Treatment protocols
After the disinfection period and under general anaesthesia and strict aseptic conditions, the teeth were re‐entered, and the antibiotic paste was removed by copious irrigation using 20 mL of 1.5% NaOCl solution for 5 minutes and then irrigated with 20 mL of 0.9% saline solution for 5 minutes. Root canals were dried and treated according to the group as follows:
2.5.1. Group I (Blood clot)
Hand file size #40 was inserted past to the canal terminus until bleeding was induced to fill up to the coronal 2‐3 mm of the canal space. MTA was mixed according to the manufacturer's instructions and inserted into the canal using Micro‐Apical Placement system (MAP system) to form MTA orifice plug. Teeth were radiographed to check the MTA orifice plug. A sterile cotton pellet was then placed in the access cavity to absorb excess moisture from MTA for 10 minutes. 6 The cotton was then removed, and glass ionomer filling was used to seal the remaining part of access cavity.
2.5.2. Group II (17% EDTA solution and blood clot)
After disinfection period, 20 mL of 1.5% NaOCl was used for irrigation followed by 5 mL of 17% EDTA solution to treat each canal for 5 minutes. 13 A subsequent rinse with 20 mL of 0.9% saline for 5 minutes was performed to stop the effect of EDTA. The procedure was then continued as group I for blood clot formation, MTA application and coronal restoration.
2.5.3. Group III (PRF)
A 20 mL sample of whole venous blood was drawn from the dog's right cephalic vein. The blood sample was transferred into a test tube without anti‐coagulant and centrifuged immediately using a tabletop centrifuge (REMI Laboratories) at 3000 revolutions per minute (RPM) for 10 minutes. Three distinct layers were formed in the tube: platelet‐poor plasma at the top, platelet‐rich fibrin clot (PRF) at the middle and red blood cells at the bottom. Sterile tweezers were inserted into the tube to gently remove the PRF clot. The PRF clot was squeezed between sterile dry gauze. The freshly prepared PRF membrane was cut, and the fragments were placed incrementally in the canal using a finger plugger up to the level of the cemento‐enamel junction (CEJ). Orifices were plugged using MTA, and the access cavities were sealed by glass ionomer filling.
2.5.4. Group IV (17% EDTA and PRF)
Each disinfected canal was irrigated with 20 mL of 1.5% NaOCl and then by 5 mL of 17% EDTA for 5 min. A subsequent rinse with 20 mL of 0.9% saline solution for 5 minutes was performed to stop the effect of EDTA. The procedure was then continued as group III for preparation and application of PRF, MTA application and coronal restoration.
2.5.5. Group V (Positive control)
The teeth were left open without treatment after induction of peri‐apical pathosis.
2.5.6. Group VI (Negative control)
The teeth were left untouched for tooth maturation.
2.6. Radiographic evaluation
Perii‐apical radiographs were taken after induction of the periapical lesion and compared with follow‐up radiographs taken for all subgroups in each group. Perii‐apical radiographs were digitized using a transparency scanner. Digital image files were converted to 32‐bitt TIFF files using ImageJ analysis software. TurboReg plug‐in was used to transform non‐standardized radiographs into standardized images. 6 The following criteria were assessed:
2.6.1. Increase in root length
Root lengths (mm) were measured as a straight line from the CEJ to the radiographic apex of the tooth. The difference in root length and percentage of increase in length were calculated. 6
2.6.2. Increase in root thickness
The level of the apical third was determined and fixed from the cement‐enamel junction. The root thickness and pulp space width were measured at this level in millimetres. Dentin thickness was measured by subtraction of pulp space from whole root thickness. The difference in thickness and percentage of increase in dentin thickness were calculated. 6
2.6.3. Decrease in apical diameter
Measurements were done prei‐operatively to the level of the apex and postoperatively at the upper border of the apical barrier. The difference in apical diameter and percentage of apical closure were calculated. 13
2.7. Histopathologic evaluation
The animals were sacrificed according to the subgroups using anaesthetic overdose (thiopental sodium). Bone segments including the experimental and control teeth were resected. Blocks were fixed in 10% buffered formalin solution with ratio 1:50 for 2 weeks and then decalcified using 17% EDTA solution for about 120 days.
The decalcified specimens were prepared as usual for histopathology and sectioned in bucco‐lingual sections of 6µm thickness. Sections were stained by haematoxylin and eosin dye. The following parameters were assessed:
2.7.1. Presence or absence of vital tissues inside the pulp space
Scores 0, 1, 2 and 3 represented no tissue in‐growth into the canal space and evidence of tissue in‐growth extending to the apical third, middle third and cervical third of root canal respectively. 13
2.7.2. Presence or absence of new hard tissue
Scores 0, 1 and 2 represented no, partial and complete new hard tissue formation respectively. 13
2.7.3. Apical closure
Scores 0 and 1 represented no apical closure and apical closure. 13
2.7.4. Intensity of inflammatory cell infiltrates
Scores 0, 1, 2 and 3 represented absence or very few cells, <10 cells, 10‐25 cells and >25 cells on average respectively.
2.8. Statistical analysis
Data were statistically described as mean, standard deviation (SD), frequency (N) and percentage (%) when appropriate. Data were explored for normality using Kolmogorov‐Smirnov test. Kruskal‐Wallis test was used to compare between different groups and subgroups for non‐parametric data. For parametric data, one‐way ANOVA was used to compare between the groups and subgroups. Significant level was set at P < .05. Statistical analysis was performed with IBM® SPSS® (SPSS Inc, IBM Corporation) Statistics version 24 for Windows.
3. RESULTS
3.1. Radiographic findings
The positive control group had no increase in root length and thickness and no signs of apical closure among all subgroups, whereas the negative control group showed the significant highest increase (P ≤ .05) in root length and thickness with signs of apical closure among all subgroups (Figure 1). The four experimental groups showed different degrees of changes in root length and thickness and apical closure (Figures 2 and 3).
FIGURE 1.
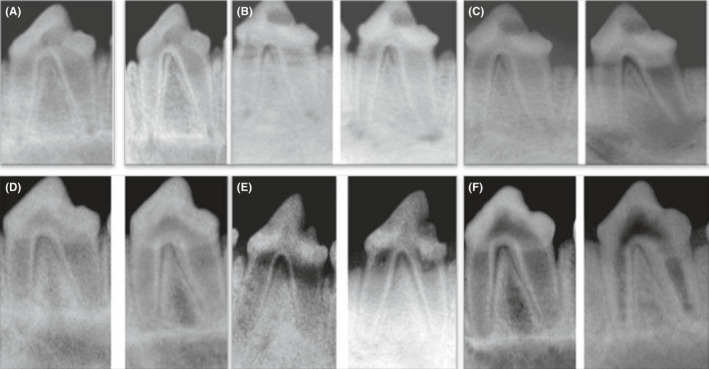
Representative prei‐operative (left side) and postoperative (right side) radiographs of the positive control group showing no changes in root length and thickness and apical diameter after 1 mo (A), 2 mo (B) and 3 mo (c). Representative (left side) and postoperative (right side) radiographs of the negative control group showing marked increase in root length and thickness and decrease in apical diameter after 1 mo (D), 2 mo (E) and 3 mo (F)
FIGURE 2.
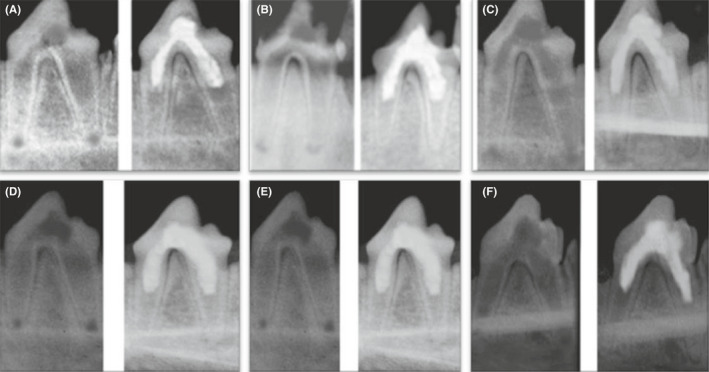
Representative prei‐operative (left side) and postoperative (right side) radiographs of group I after 1 mo (A), 2 mo (B) and 3 mo (C) and group II after 1 mo (D), 2 mo (E) and 3 mo (F) showing increase in the root length and thickness and decrease in apical diameter
FIGURE 3.
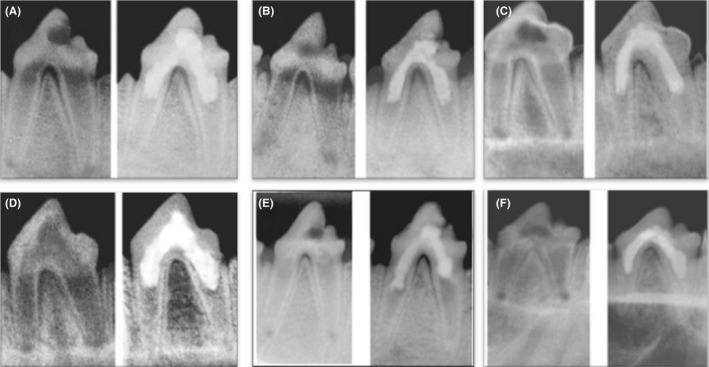
Representative prei‐operative (left side) and postoperative (right side) radiographs of group III after 1 mo (A), 2 mo (B) and 3 mo (C) and group IV after 1 mo (D), 2 mo (E) and 3 mo (F) showing increase in the root length and thickness and decrease in apical diameter
Tables 1, 2, 3 show the increase in root length and thickness and decrease in apical diameter in all groups and subgroups. Regarding the experimental groups, group IV (17% EDTA + PRF) showed the highest increase in root length and thickness and decrease in apical diameter, whereas group I (blood clot) showed the least measurements among the experimental groups. There was a significant difference between groups I and II (17% EDTA + blood clot) compared to groups III (PRF) and IV (P ≤ .05). However, there was no significant difference between groups III and IV (P > .05).
TABLE 1.
Mean value and standard deviation (SD) of increase in root length (mm) and the percentage of increase in all groups
| Groups |
Subgroup A (1 mo) |
Subgroup B (2 mo) |
Subgroup C (3 mo) |
P‐value | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
|
Group I (blood clot) |
0.98aA (9.7%) |
0.08 |
1.21bA (12.8%) |
0.04 |
1.88cA (17.3%) |
0.10 | ≤.001* |
|
Group II (EDTA + blood clot) |
1.09aB (10.1%) |
0.06 |
1.34bB (14.1%) |
0.04 |
1.90cAB (18.2%) |
0.12 | ≤.001* |
|
Group III (PRF) |
1.20aC (11.3%) |
0.03 |
1.50bC (15.8%) |
0.06 |
1.95cAB (18.6%) |
0.07 | ≤.001* |
|
Group IV (EDTA + PRF) |
1.21aC (11.4%) |
0.07 |
1.58bD (16.6%) |
0.07 |
1.97cB (18.9%) |
0.08 | ≤.001* |
|
Group V (positive control) |
0.00 ( 0%) |
0.00 (0%) |
0.00 (0%) |
||||
|
Group VI (negative control) |
1.31aD (13.2%) |
0.08 |
1.77bE (17.8%) |
0.12 |
2.10cC (20.1%) |
0.12 | ≤.001* |
| P‐value | ≤.001* | ≤.001* | ≤.001* | ||||
The same upper‐case letters within each column indicate insignificant difference.
The same lower‐case letters within each row indicate insignificant difference.
Abbreviation: NS, non‐significant.
Significant at P ≤ .05.
TABLE 2.
Mean value and standard deviation (SD) of increase in root thickness (mm) and the percentage of increase in all groups
| Groups |
Subgroup A (1 mo) |
Subgroup B (2 mo) |
Subgroup C (3 mo) |
P‐value | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
|
Group I (blood clot) |
0.26aA (8%) |
0.07 |
0.31bA (9.5%) |
0.05 |
0.35cA (10.7%) |
0.06 | ≤.001* |
|
Group II (EDTA + blood clot) |
0.30aAB (9.2%) |
0.07 |
0.34bAB (10.4%) |
0.06 |
0.37cA (11.35%) |
0.08 | ≤.001* |
|
Group III (PRF) |
0.32aAB (9.6%) |
0.04 |
0.38bBC (12.16%) |
0.07 |
0.44cB (14.1%) |
0.06 | ≤.001* |
|
Group IV (EDTA + PRF) |
0.34aB (10.1%) |
0.05 |
0.43bC (12.7%) |
0.09 |
0.50cC (14.9%) |
0.08 | ≤.001* |
|
Group V (positive control) |
0.00 | 0.00 | 0.00 | ||||
| Group VI (negative control) |
0.43aC (12.7%) |
0.08 |
0.51bD (15.7%) |
0.08 |
0.58cD (17.96%) |
0.05 | .003* |
| P‐value | ≤0.001* | ≤0.001* | ≤0.001* | ||||
The same upper‐case letters within each column indicate differences that were not significant.
The same lower‐case letters within each row indicate differences that were not significants.
Abbreviation: NS, not significant.
Significant at P ≤ .05.
TABLE 3.
Mean value and standard deviation (SD) of decrease in apical diameter (mm) and the percentage of increase in all groups
| Groups |
Subgroup A (1 mo) |
Subgroup B (2 mo) |
Subgroup C (3 mo) |
P‐value | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
|
Group I (blood clot) |
0.37aA (6.5%) |
0.02 |
0.50bA (9.1%) |
0.07 |
0.82cA (27.8%) |
0.11 | ≤.001* |
|
Group II (EDTA + blood clot) |
0.39aAB (7.1%) |
0.03 |
0.57bA (10.4%) |
0.10 |
0.91cB (29.1%) |
0.09 | ≤.001* |
|
Group III (PRF) |
0.40aAB (8%) |
0.06 |
0.68bB (14.1%) |
0.08 |
0.98cB (35.1%) |
0.11 | ≤.001* |
|
Group IV (EDTA + PRF) |
0.42aB (8.1%) |
0.05 |
0.72bB (14.9%) |
0.12 |
1.20cC (36.3%) |
0.10 | ≤.001* |
|
Group V (positive control) |
0.00 | 0.00 | 0.00 | ||||
|
Group VI (negative control) |
0.83aC (13.2%) |
0.09 |
1.20bC (22.1%) |
0.11 |
1.40cD (38.2%) |
0.05 | ≤.001* |
| P‐value | ≤0.001* | ≤0.001* | ≤0.001* | ||||
The same upper‐case letters within each column indicate differences that were not significant.
The same lower‐case letters within each row indicate differences that were not significant.
Abbreviation: NS, not significant.
Significant at P ≤ .05.
There was a significant difference regarding the increase in root length and thickness and decrease in apical diameter in all subgroups of the experimental and negative control groups (P ≤ .05).
3.2. Histopathological findings
3.2.1. Group I (blood clot)
In subgroup A (1 month), the absence of vital tissue infiltration and hard tissue formation, open apex, moderate inflammation and migrating well‐differentiated epithelial cells towards the root apex were observed (Figure 4A).
FIGURE 4.
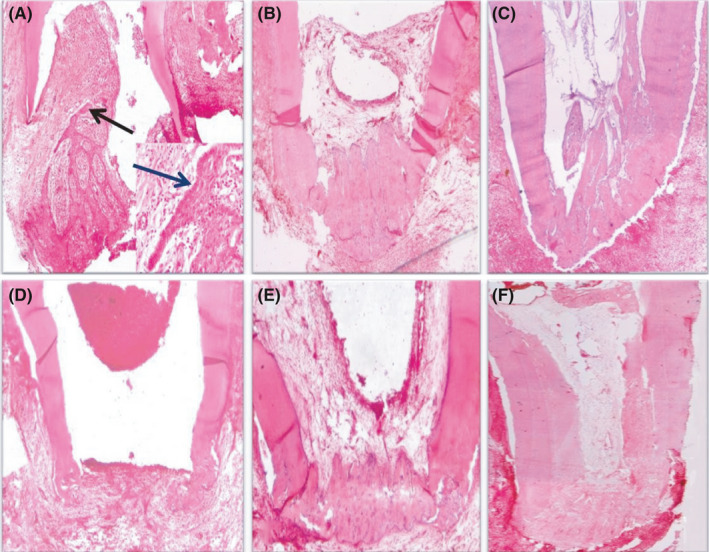
(A) Photomicrograph of the apical third of a root in group I (blood clot) after 1 month showing differentiation of the epithelial cells (black arrow), no vital tissue infiltration, no sign of hard tissue formation, open apex and moderate inflammatory cell infiltration (H&E, ×40). Notice the well‐differentiated epithelial cells migrating towards the root apex (blue arrow) as seen in the small box (H&E, ×200). (B) Photomicrograph of the apical third of a root in group I after 2 months showing vital tissue infiltration towards the apical third, partial hard tissue formation, signs of apical closure and mild inflammatory cell infiltration (H&E, ×40). (C) Photomicrograph of the apical two‐thirds of a root in group I after 3 months showing vital tissue infiltration along the apical two‐thirds of the pulp space, fibrous tissue formation and calcified structures (black arrow) inside the pulp space, partial hard tissue formation, signs of apical closure and mild inflammation (H&E, ×40). (D) Photomicrograph of the apical third of a root in group II (17% EDTA + blood clot) after 1 month showing no vital tissue infiltration, signs of partial hard tissue formation, open apex and mild inflammation (H&E, ×40). (E) Photomicrograph of the apical third of a root in group II after 2 months showing vital tissue infiltration at the apical third, partial hard tissue formation, signs of apical closure and mild inflammation (H&E, ×40). (F) Photomicrograph of the apical two‐thirds of a root in group II after 3 months showing vital tissue infiltration along the pulp space, fibrous tissue formation inside the pulp space, signs of complete hard tissue formation, apical closure and mild inflammation (H&E, ×40)
In subgroup B (2 months), vital tissue infiltration towards the apical third, partial hard tissue formation, signs of apical closure and mild inflammatory cell infiltration were seen (Figure 4B).
In subgroup C there was (3 months), vital tissue infiltration along the apical two‐thirds of the pulp space, fibrous tissues and calcified structures inside the pulp space, partial hard tissue formation, signs of apical closure and mild inflammation (Figure 4C).
3.2.2. Group II (17% EDTA + blood clot)
In subgroup A, no vital tissue infiltration, signs of partial hard tissue formation, open apex and mild inflammation were observed (Figure 4D). In subgroup B, vital tissue infiltration at the apical third, partial hard tissue formation, signs of apical closure and mild inflammatory cell infiltration were seen (Figure 4E). In subgroup C there was, vital tissue infiltration along the pulp space, fibrous tissue formation inside the pulp space, signs of complete hard tissue formation, apical closure and mild inflammatory cell infiltration (Figure 4F).
3.2.3. Group III (PRF)
In subgroup A, vital tissue infiltration in the apical third, signs of partial hard tissue formation, open apex and mild inflammatory cell infiltration were seen (Figure 5A). In subgroup B, vital tissue infiltration at the apical third, partial hard tissue formation, signs of apical closure and no sign of inflammation were noticed (Figure 5B). In subgroup, vital tissue infiltration along the pulp space, odontoblast‐like cells with increased vascularity inside the pulp space, complete hard tissue formation, signs of apical closure and no signs of inflammation were observed (Figure 5C).
FIGURE 5.
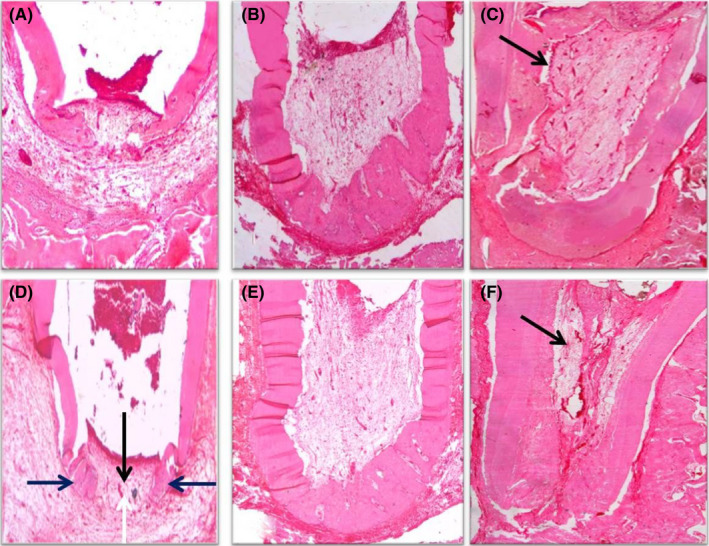
(A) Photomicrograph of the apical third of a root in group III (PRF) after 1 month showing vital tissue infiltration into the apical third, signs of partial hard tissue formation, open apex, mild inflammation and a line of demarcation separating the newly formed hard tissue from the old one (black arrow) (H&E, ×40). (B) Photomicrograph of the apical half of a root in group III after 2 months showing vital tissue infiltration at the apical third, partial hard tissue formation, signs of apical closure and no sign of inflammation (H&E, ×40). (C) Photomicrograph of the apical two‐thirds of a root in group III after 3 months showing vital tissue infiltration along the pulp space, odontoblast‐like cells (black arrow), increased vascularity inside the pulp space, complete hard tissue formation, signs of apical closure and no inflammation (H&E, ×40). (D) Photomicrograph of the apical third of a root in group VI (17% EDTA + PRF) after 1 month showing vital tissue infiltration at the apical third (black arrow), signs of partial hard tissue formation (blue arrows), open apex (white arrow) and mild inflammation (H&E, ×40). (E) Photomicrograph of the apical half of a root in group IV after 2 months showing vital tissue infiltration towards the middle third, partial hard tissue formation, signs of apical closure and no sign of inflammation (H&E, ×40). (F) Photomicrograph of the apical two‐thirds of a root in group IV after 3 months showing vital tissue infiltration along the pulp space, odontoblast‐like cells undergoing differentiation (black arrow), increased vascularity inside the pulp space, complete hard tissue formation, signs of apical closure and no sign of inflammation (H&E, ×40)
3.2.4. Group IV (17% EDTA + PRF)
In subgroup A, vital tissue infiltration in the apical third, signs of partial hard tissue formation, open apex and mild inflammatory cell infiltration were seen (Figure 5D). In subgroup B, vital tissue infiltration towards the middle third, partial hard tissue formation, signs of apical closure and no signs of inflammation were observed (Figure 5E). In subgroup C, vital tissue infiltration along the pulp space, dontoblast‐like cells undergoing differentiation with increased vascularity inside the pulp space, complete hard tissue formation, signs of apical closure and no signs of inflammation were noticed (Figure 5F).
3.2.5. Group V (positive control)
In subgroup A, no vital tissue infiltration, no sign of hard tissue formation, open apex, severe inflammatory cell infiltration and granulation tissue formation were observed (Figure 6A). In subgroup B, similar findings of subgroup A with increased granulation tissue formation were observed (Figure 6B). In subgroup C, similar findings of subgroup B with abscess formation were seen (Figure 6C).
FIGURE 6.
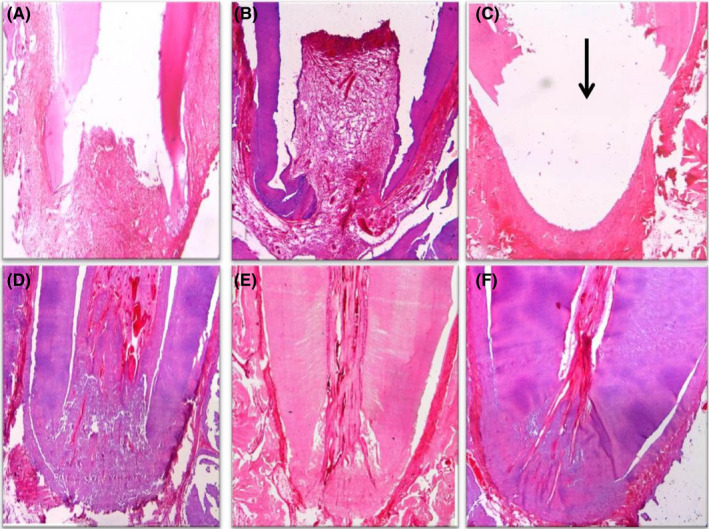
(A) Photomicrograph of the apical third of a root in group V (positive control) after 1 month showing no vital tissue infiltration, no signs of hard tissue formation, open apex, severe inflammation and granulation tissue formation (H&E, ×40). (B) Photomicrograph of the apical half of a root in group V after 2 months showing the same findings after 1 month with increased granulation tissue (H&E, ×40). (C) Photomicrograph of the apical third of a root in group V after 3 months showing the same findings after 1 month with abscess cavity formation (black arrow) (H&E, ×40). Photomicrographs of the apical third of a root in group VI (negative control) after 1 month (D), 2 months (E) and 3 months (F) showing normal architecture of pulpal tissue, signs of apical closure and no inflammation (H&E, ×40)
3.2.6. Group VI (negative control)
Normal architecture of pulpal tissue, signs of apical closure and no inflammatory cell infiltration were seen in all subgroups (Figure 6D‐F).
Statistical analysis of the histopathologic findings revealed the following:
3.2.7. Presence or absence of vital tissues in the pulp space (Table 4)
Table 4 shows that connective tissue in‐growth was seen in the pulp space in many samples. The nature of this tissue resembled periodontal connective tissue with variable amounts of inflammatory cell infiltration and noticeable angiogenesis. In groups III and IV some samples showed the formation of odontoblast‐like cells with various degrees of differentiation.
TABLE 4.
Mean values and standard deviation (SD) of tissue infiltration score in all groups
| Groups |
Subgroup A (1 mo) |
Subgroup B (2 mo) |
Subgroup C (3 mo) |
P‐value | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| Group I (blood clot) | 0.33aA | 0.47 | 1.25aB | 0.43 | 2.08aC | 0.28 | ≤.001* |
| Group II (EDT + blood clot) | 0.50bA | 0.50 | 1.50aB | 0.50 | 2.33bC | 0.47 | ≤.001* |
| Group III (PRF) | 0.67cA | 0.47 | 2.00 bB | 0.00 | 2.50 bC | 0.50 | ≤.001* |
| Group IV (EDTA + PRF) | 1.42dA | 0.49 | 2.17bB | 0.37 | 2.67cC | 0.50 | ≤.001* |
| Group V (positive control) | 0.50bA | 0.50 | 0.58cA | 0.49 | 0.58dA | 0.49 | 1.00 NS |
| P‐value | ≤0.001* | ≤0.001* | ≤0.001* | ||||
The same upper‐case letters within each row indicate differences that were not significant.
The same lower‐case letters within each column indicate differences that were not significant.
Abbreviation: NS, not significant.
Significant at P ≤ .05.
In all subgroups, group IV showed the highest score of vital tissue infiltration, whereas group I showed the least score among the experimental groups. Group V showed the least vital tissue infiltration score among all groups. A significant difference was seen among all experimental groups in all subgroups (P ≤ .05). However, there was no significant difference between all subgroups in the positive control group (P > .05).
3.2.8. Presence or absence of new hard tissue
Hard tissue formation of different thicknesses and shapes was observed. The newly formed hard tissue resembled osteodentin‐ or bony‐like structure or cementum. Some samples showed various calcified islands in the root canal.
In all subgroups, group IV showed the highest mineralization score, whereas group I showed the least mineralization score among the experimental groups. However, groups III and IV showed equal high mineralization score in subgroup B. Group V showed the least mineralization score among all subgroups.
A significant difference was reported between all experimental groups among all subgroups (P ≤ .05). However, there was no significant difference between all subgroups in the positive control group (P > .05).
3.2.9. Presence or absence of apical closure
Hard tissue formation of different thicknesses and shapes forming the apical barrier was seen. The newly formed hard tissue appeared to be osteodentin‐ or cementum‐like or bony‐like structure.
The negative control group showed the highest score of apical closure in all subgroups. The positive control group showed no signs of apical closure in all subgroups. There was no significant difference among all subgroups for the positive and negative control groups (P ≤ .05).
There was a significant difference between groups I, II, III, IV and V compared to the negative control group (P ≤ .05). There was no significant difference among groups I, II, III, IV and V in subgroup A (P > .05).
In subgroup B, there was a significant difference between the experimental and control groups (P ≤ .05). There was a significant difference between groups I and II compared to groups III and IV (P ≤ .05). There was no significant difference, neither between groups I and II (P > .05) nor between groups III and IV (P > .05).
In subgroup C, there was a significant difference between groups I, II, III, IV and VI compared to group V (P ≤ .05). There was no significant difference between groups I, II, III, IV and VI (P > .05).
3.2.10. Inflammatory score
Different degrees of chronic inflammatory cells were seen in groups I‐V. Among experimental groups, group II showed the highest inflammatory score, whereas group III showed the least inflammatory score among all subgroups. Group VI showed the least inflammatory score, whereas group V showed the highest inflammatory score among all groups.
In subgroup A, a significant difference was recorded between the experimental and control groups (P ≤ .05). There was a significant difference between groups I, II and IV compared to group III (P ≤ .05). However, there was no significant difference between groups I, II and IV (P > .05).
In subgroup B, a significant difference was reported between the experimental and control groups (P ≤ .05). There was a significant difference between groups I and II compared to group III (P ≤ .05). However, there was no significant difference between groups I and II (P > .05) and between groups I, II and III compared to group IV (P > .05).
In subgroup C, a significant difference between the experimental and control groups was seen (P ≤ .05). There was a significant difference between groups I and II compared to groups III and IV (P ≤ .05). However, there was no significant difference between groups I and II (P > .05) and between groups III and IV (P > .05).
4. DISCUSSION
Therapy of immature permanent teeth with pulp necrosis is a big challenge to clinicians. Several studies have been conducted on regenerative potential of these necrotic pulps in both animal models and humans with great success in the form of continuation of normal root development. 6 , 7 , 8 , 13
The choice of dog as an animal model for this experiment was because dogs have similar apical repair compared with that of humans but of short duration. 15 Premolars were selected due to their good accessibility, high number of roots for a reliable statistical analysis and wide root canals for endodontic treatment. Age of the selected dogs was 6‐9 months because the premolars are permanent and immature at this age and the dogs can withstand repeated general anaesthesia. 6 In the present study, the evaluation periods were 1, 2 and 3 months, which are equivalent to 6, 12 and 18 months, respectively, in the human analogue. 16
To simulate the clinical cases, peri‐apical pathosis was induced by creation of access cavities and leaving them open for 4 weeks. The peri‐apical pathosis was evident radiographically and histologically. Similar findings were recorded before. 6 , 8 To avoid generation of a smear layer that could occlude the dentinal walls or tubules, minimal instrumentation was used in this study as recorded before. 17
The choice of 1.5% NaOCl solution as an irrigant was based on its potent antimicrobial activity and ability to preserve viability of stem cells, which have a crucial role in revascularization as mentioned before. 18 , 19 PRF was chosen as a treatment protocol because it is autologous, has a three‐dimensional architecture and includes bio‐active molecules as recorded previously. 20
The choice of 17% EDTA solution for conditioning was based on its ability to demineralize the superficial dentin layer, exposure of growth factors entrapped in dentin matrix and removal of loosely attached smear layer. 12 , 21 TAP was applied to kill all types of bacteria inside the root canal. Application of TAP for 3 weeks was sufficient time for canal disinfection as recorded by several studies. 6 , 7 , 8 , 22 , 23
Outcome of the tested treatment protocols was assessed by summing up both radiographic and histological findings to obtain a comprehensive conclusion. Radiographic evaluation was standardized using ImageJ software including TurboReg plug‐in. This computer software is based on mathematical alignment of source and target pictures using multiple identical points on the two pictures. 24
After 1 month, the PRF group showed higher score of vital tissues inside the pulp than the blood clot group because PRF provides an ideal scaffold, prolonged source of growth factors and better cell organization and diffusion than those of the blood clot. This was supported by other studies, which concluded that PRF is a fibrin biomaterial and optimal matrix for migration of endothelial cells and fibroblasts as well as rapid angiogenesis. 20 , 25 The tissue infiltration score in groups II and IV showed higher scores with a statistical significant difference compared to groups I and III respectively (P ≤ .05). This may be attributed to the chelating effect of EDTA on the dentin surface, which leads to the exposure of collagen fibrils and growth factors from the dentin matrix. 18 , 26 Group VI showed the highest degree of tissue infiltration among all subgroups due to the synergistic effect of PRF and EDTA on tissue infiltration. Continuation of tissue infiltration in group I after 2‐ and 3‐month evaluation periods may be attributed to controllable biodegradation and incorporation of growth factors in the blood clot, which was supported by other studies. 6 , 13 , 27
There was a significant difference between groups I and IV regarding hard tissue formation after 2 and 3 months due to the synergistic effect of PRF and EDTA on the root maturation.
The radiographic findings regarding increase in the root length and thickness were consistent with the histologic findings of hard tissue formation. This was in agreement with other studies. 6 , 28 In contrast, another study recorded that radiographic findings were not accurate regarding actual increase in root length or thickness due to different angulations and image resolution. 29 This could be avoided in our study by standardization of radiographs using computer software.
After 2‐month follow‐up evaluation period, groups III and IV showed higher score of hard tissue formation with a significant difference compared to groups I and II. This may be attributed to the positive effect of PRF on mineralization due to its properties. The newly formed hard tissue deposited was osteodentin‐ or cementum‐like or bony‐like structure, possibly due to the presence of growth factors as reported before. 30 In the present study, the newly formed hard tissue was comparable to that obtained with previous studies. 29 , 31
After 2 months, there was a significant difference in the percentage of apical closure between groups I and III and also between groups II and IV (P ≤ .05). These results could be related to the positive effect of PRF and EDTA on the continuation of root development. On the other hand, there was no significant difference in the apical closure among all experimental groups after 3 months (P > .05). This could be explained by hard tissue deposition and consequently apical closure. These findings are in agreement with findings of other studies. 29 , 32
Regarding the inflammatory scores, all the experimental groups showed a significant difference compared to the negative control group after 1 month. These findings could be attributed to the inflammatory reaction of periradicular tissues to the performed treatment protocols superimposed by the immunological reaction against the previously induced infection. There was a significant difference in the mean inflammatory score between groups I and III, where group I showed higher scores. This might be attributed to the traumatic insult of periapical tissues through overinstrumentation to induce bleeding. Similar findings were recorded in a previous study. 30 Moreover, the use of 17% EDTA and PRF treatment resulted in higher mean inflammatory score than that of group III (PRF). This could be attributed to the mild inflammatory effect of EDTA on the periapical tissues.
All experimental groups exhibited lower mean inflammatory scores in subgroups B and C than subgroup A. This indicates a progressive healing of the periapical lesion due to eradication of the aetiological factors. In contrast, the positive control group had a significant highest inflammatory reaction due to progression of the induced infection that resulted from lack of treatment.
5. CONCLUSION
PRF has a better regenerative potential than blood clot during treatment of immature permanent teeth with necrotic pulp. Inclusion of 17% EDTA solution as a final irrigation enhances the regenerative potential of both PRF and blood clot.
ETHICAL APPROVAL
This study was approved by the Ethical Committee at the Faculty of Dental Medicine for Girls, Al‐Azhar University, Cairo, Egypt (15‐04‐2014‐Endo).
CONFLICT OF INTEREST
The authors declare no conflict of interests.
Authors' Contributions
All authors contributed significantly and have read and approved this article.
ACKNOWLEDGEMENT
This research did not receive funding from any agency.
El Halaby HM, Abu‐Seida AM, Fawzy MI, Farid MH, Bastawy HA. Evaluation of the regenerative potential of dentin conditioning and naturally derived scaffold for necrotic immature permanent teeth in a dog model. Int. J. Exp. Path. 2020;101:264–276. 10.1111/iep.12372
REFERENCES
- 1. Andreasen J, Farik B, Munksgaard E. Long‐term calcium hydroxide as a root canal dressing may increase risk of root fracture. Dent Traumatol. 2002;18:134‐137. [DOI] [PubMed] [Google Scholar]
- 2. Giuliani V, Baccetti T, Pace R. The use of MTA in teeth with necrotic pulp and open apices. Dent Traumatol. 2002;18:217‐221. [DOI] [PubMed] [Google Scholar]
- 3. Shabahang S, Torabinejad M, Boyne P, Abedi H, McMillan P. A comparative tudy of root‐end induction using osteogenic protein‐1, calcium hydroxide, and mineral trioxide aggregate in dogs. J Endod. 1999;25:1‐5. [DOI] [PubMed] [Google Scholar]
- 4. Petrino J, Boda K, Shambarger S, Bowles W, McClanahan S. Challenges in regenerative endodontics: a case series. J Endod. 2010;36:536‐541. [DOI] [PubMed] [Google Scholar]
- 5. Hargreaves K, Geisler T, Henry M, Wang Y. Regeneration potential of the young permanent tooth: what does the future hold? J Endod. 2008;34:551‐556. [DOI] [PubMed] [Google Scholar]
- 6. Tawfik H, Abu‐Seida AM, Hashem AA, Nagy MM. Regenerative potential following revascularization of immature permanent teeth with necrotic pulps. Int Endod J. 2013;46:910‐922. [DOI] [PubMed] [Google Scholar]
- 7. El‐Tayeb MM, Abu‐Seida AM, El Ashry SH, El‐Hady S. Evaluation of antibacterial activity of propolis on regenerative potential of necrotic immature permanent teeth in dogs. BMC Oral Health. 2019;19:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nagy MM, Tawfik HE, Hashem AA, Abu‐Seida AM. Regenerative potential of immature permanent teeth with necrotic pulps after different regenerative protocols. J Endod. 2014;40:192‐198. [DOI] [PubMed] [Google Scholar]
- 9. Banchs F, Trope M. Revascularization of immature permanent teeth with apical periodontitis: new treatment protocol? J Endod. 2004;30:196‐200. [DOI] [PubMed] [Google Scholar]
- 10. Nakashima M, Akamine A. The application of tissue engineering to regeneration of pulp and dentin in endodontics. J Endod. 2005;31:711‐718. [DOI] [PubMed] [Google Scholar]
- 11. Choukroun J, Diss A, Simonpieri A, Girard M, Schoeffler C, Dohan S. Platelet‐rich fibrin (PRF): a second‐generation platelet concentrate. Part IV: clinical effects on tissue healing. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e56‐e60. [DOI] [PubMed] [Google Scholar]
- 12. Roberts‐Clark D, Smith A. Angiogenic growth factors in human dentine matrix. Arch Oral Biol. 2000;45:1013‐1016. [DOI] [PubMed] [Google Scholar]
- 13. El Ashry SH, Abu‐Seida AM, Bayoumi AA, Hashem AA. Regenerative potential of immature permanent non‐vital teeth following different dentin surface treatments. Exp Toxicol Pathol. 2016;68:181‐190. [DOI] [PubMed] [Google Scholar]
- 14. Huang G, Shagramanova K, Chan S. Formation of odontoblast‐like cells from cultured human dental pulp cells on dentin in vitro. J Endod. 2006;32:1066‐1073. [DOI] [PubMed] [Google Scholar]
- 15. El Ashry S, Abu‐Seida AM, Al‐Boghdady H, Abdel‐Fattah M. The effect of different formulations of calcium hydroxide on healing of intentionally induced periapical lesions in dogs. Pak Vet J. 2013;3:48‐52. [Google Scholar]
- 16. Citrome GP, Kaminski EJ, Heuer M. A comparative study of tooth apexification in the dog. J Endod. 1979;5:290‐297. [DOI] [PubMed] [Google Scholar]
- 17. Lin L, Shimizu E, Gibbs J, Loghin S, Ricucci D. Histologic and histibacteriolgic observations of failed revascularization/revitalization therapy: a case report. J Endod. 2014;40:291‐295. [DOI] [PubMed] [Google Scholar]
- 18. Martin D, Flavio J, Henery M, et al. Concentration‐dependent effect of sodium hypochlorite on stem cells of apical papilla survival and differentiation. J Endod. 2014;40:51‐55. [DOI] [PubMed] [Google Scholar]
- 19. Trevino E, Amol N, Patwardhan A, Henry M. Effect of irrigants on the survival of human stem cells of the apical papilla in a platelet‐rich plasma scaffold in human root tips. J Endod. 2011;37:1109‐1115. [DOI] [PubMed] [Google Scholar]
- 20. Huang F, Yang S, Zhao J, Chang Y. Platelet‐rich fibrin increases proliferation and differentiation of human dental pulp cells. J Endod. 2010;36:1628‐1632. [DOI] [PubMed] [Google Scholar]
- 21. Verdelis K, Eliades G, Oviir T, Margelos J. Effect of chelating agents on the molecular composition and extent of decalcification at cervical, middle and apical root dentin locations. Endod Dent Traumatol. 1999;15:164‐170. [DOI] [PubMed] [Google Scholar]
- 22. Hoshino E, Kurihara‐Ando N, Sato I, et al. In‐vitro antibacterial susceptibility of bacteria taken from infected root dentine to a mixture of ciprofloxacin, metronidazole and minocycline. Int Endod J. 1996;29:125‐130. [DOI] [PubMed] [Google Scholar]
- 23. Sato I, Ando‐Kurihara N, Kota K, Iwaku M, Hoshino E. Sterilization of infected root‐ canal dentine by topical application of a mixture of ciprofloxacin, metronidazole and minocycline in situ. Int Endod J. 1996;29:118‐124. [DOI] [PubMed] [Google Scholar]
- 24. Bose R, Nummikoski P, Hargreaves K. A retrospective evaluation of radiographic outcomes in immature teeth with necrotic root canal systems treated with regenerative endodontic procedures. J Endod. 2009;35:1343‐1349. [DOI] [PubMed] [Google Scholar]
- 25. Choukroun J, Dohan D, Diss A, Dohan S, Dohan A, Mouhyi J. Platelet‐rich fibrin (PRF): a second‐generation platelet concentrate. Part I: technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e37‐e44. [DOI] [PubMed] [Google Scholar]
- 26. Heino J, Kapyla J. Cellular receptors of extracellular matrix molecules. Curr Pharm Des. 2009;15:1309‐1317. [DOI] [PubMed] [Google Scholar]
- 27. Galler K, D’Souza R, Hartgerink J, Schmalz G. Scaffolds for dental pulp tissue engineering. Adv Dent Res. 2011;23:333. [DOI] [PubMed] [Google Scholar]
- 28. Thibodeau B, Teixeira F, Yamauchi M, Caplan D, Trope M. Pulp revascularization of immature dog teeth with apical periodontitis. J Endod. 2007;33:680‐689. [DOI] [PubMed] [Google Scholar]
- 29. Wang X, Thibodeau B, Trope M, Lin L, Huang G. Histologic characterization of regenerated tissues in canal space after the revitalization/revascularization procedure of immature dog teeth with apical periodontitis. J Endod. 2010;36:56‐63. [DOI] [PubMed] [Google Scholar]
- 30. Gronthos S, Brahim J, Li W, Fisher L, Cherman N, Boyde A. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81:531‐535. [DOI] [PubMed] [Google Scholar]
- 31. Meschi N, Hilkens P, Lambrichts I, et al. Regenerative endodontic procedure of an infected immature permanent human tooth: an immunohistological study. Clin Oral Investig. 2016;20:807‐814. [DOI] [PubMed] [Google Scholar]
- 32. Da Silva L, Nelson‐Filho P, Da Silva R, et al. Revascularization and periapical repair after endodontic treatment using apical negative pressure irrigation versus conventional irrigation plus triantibiotic intracanal dressing in dogs, teeth with apical periodontitis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:779‐787. [DOI] [PubMed] [Google Scholar]


