Abstract
Events in fetal life impact long-term health outcomes. The placenta is the first organ to form and is the site of juxtaposition between the maternal and fetal circulations. Most diseases of pregnancy are caused by, impact, or are reflected in the placenta. The purpose of this review is to describe the main inflammatory processes in the placenta, discuss their immunology, and relate their short- and long-term disease associations. Acute placental inflammation (API), including maternal and fetal inflammatory responses corresponds to the clinical diagnosis of chorioamnionitis and is associated with respiratory and neurodevelopmental diseases. The chronic placental inflammatory pathologies (CPI), include chronic villitis of unknown etiology, chronic deciduitis, chronic chorionitis, eosinophilic T-cell vasculitis, and chronic histiocytic intervillositis. These diseases are less-well studied, but have complex immunology and show mechanistic impacts on the fetal immune system. Overall, much work remains to be done in describing the long-term impacts of placental inflammation on offspring health.
Keywords: maternal-fetal inflammation, placenta, DOHaD, chorioamnionitis, chronic villitis, asthma, neurodevelopmental outcomes
Introduction
The developmental origins of health and disease (DOHaD) theory, in which in utero or early life events can have a significant impact on adult outcomes, has become the organizing principle of fetal and perinatal biology (1–3). Extensive research has focused on maternal nutritional status and later metabolic disease in offspring, but some of the most striking DOHaD findings come from examination of the long term impact of exposure to inflammation. In utero exposure to the 1918 (Spanish) influenza pandemic has been associated with increased hospitalizations, heart disease, and cancer in middle age and older survivors (4, 5). In the last decade, the placenta has become a new focus within DOHaD research (6). A recent paper described the placenta as the “center of the chronic disease universe” (7). While the U-shaped relationship between birthweight and risk of heart disease has been reported across numerous studies and populations, less recognized is the similar U-shaped relationship between the ratio of placental weight to birthweight and coronary heart disease (8, 9). Placental inflammation is a sub-focus in the study of chronic disease risk, particularly within the context of the global obesity epidemic and low-level, chronic inflammation that is present in pregnant women with a high BMI. Rigorous characterization of inflammation in the placenta is a longstanding component of pathological examination, yet diagnoses are complex and poorly understood outside of perinatal pathology (10). The purpose of this review is to first examine the inflammatory lesions in the placenta and describe their characteristics. For each lesion, we then describe the associations with long-term outcomes and relate studies relevant to potential or known mechanisms.
Acute Placental Inflammation (API)
Acute placental inflammation (API) is the microscopic equivalent to the clinical diagnosis of chorioamnionitis (11, 12). The term histologic chorioamnionitis has been used and is still used as a stage of maternal inflammatory response (which is a subcategory of API, discussed below). The difference in terminology reflects that, while API is strongly associated with clinical chorioamnionitis, it can be seen without symptoms and signs of clinical chorioamnionitis (13, 14). Significantly, low-stage API can be seen in up to 50% of uncomplicated vaginal deliveries following uncomplicated pregnancies (15).
API, Acute Inflammation and Infection
The relationship between API and other forms of inflammation and infection is complex, hence the retirement of prior terminology including amniotic fluid infection (AFI), intrauterine infection (IUI), and ascending infection (11). Presumed pathogenic bacteria are identified in 72% (16), 89% (17), 38% (18), 61% (14), and 4% (19) of cases, depending on the clinical circumstances and methodology. In general, bacteria are more frequently identified in preterm delivery and when API and clinical chorioamnionitis are present. Distinguishing sterile API vs. API with bacterial contaminants vs. API with bacterial bystanders vs. API with bona fide pathogenic bacteria is challenging and likely blurs our understanding of the epidemiology and long-term consequences of this lesion. For example, in a study of amniotic fluid collected before rupture of membranes, women with elevated IL-6 were likely to deliver preterm regardless of culture or PCR results (20). Does this indicate that sterile inflammation is real and problematic, or that the microbiologic results are false negatives?
If acute inflammation is not in response to infection, what is the stimulus? In vitro studies suggest the forces of labor themselves induce inflammation. Mechanical stretch induces expression of cyclooxygenase 2 (COX2), activator protein 1 (AP1), NF-κB, and connexin 43 in amnion explants (21, 22). Mechanical stretch of immortalized human myometrial cells induced expression of multiple cytokines, including IL-6 and IL-12, chemokines CXCL8 and CXCL1, and induced transendothelial migration (23). These studies support a path from sporadic contractions (i.e. Braxton-Hicks) or labor to acute inflammation. Further, maternal obesity causes low-grade inflammation that may be reflected in the placenta and associated adverse pregnancy outcomes (24, 25).
Maternal Inflammatory Response (MIR)
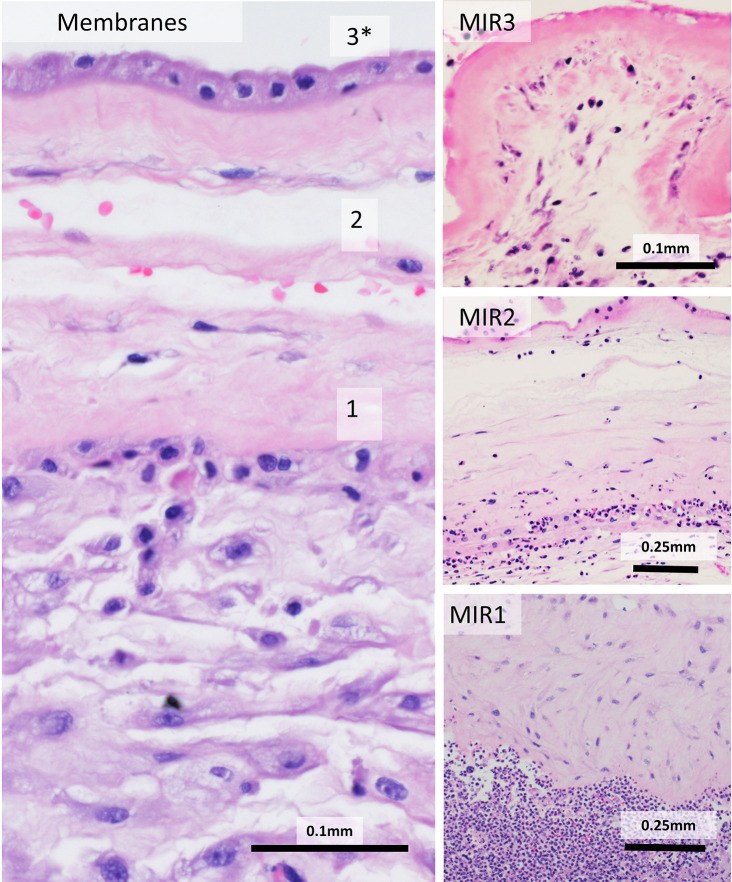
API is divided into the maternal inflammatory response (MIR) and fetal inflammatory response (FIR) depending on the source of the inflammatory response (26). MIR is staged 1 to 3, with higher stages corresponding to a longer exposure to insult. Histologically, MIR consists of extravasating maternal neutrophils which approach and then cross into the chorionic layer, move through the amnion and into the amniotic space ( Figure 1 ). MIR is staged as subchorionitis (Stage 1) when neutrophils congregate at the border between the subchorionic fibrin and chorion in the chorionic plate or between the cellular and fibrous chorion in the extraplacental membranes. Inflammation of the chorion (chorionitis) or chorion and amnion (chorioamnionitis) is Stage 2 - the gap between chorion and amnion not acting as a significant barrier to the passage of neutrophils. MIR Stage 3, so called chorioamnionitis with amnion necrosis, can be diagnosed on the basis of amniocyte necrosis, but is more reliably diagnosed by the presence of neutrophil karyorrhectic debris (11, 12). Based on rhesus models, analogy, and expert experience, Stage 1 MIR tends to occur 6 to 12 h after exposure to an inflammatory stimulus, Stage 2 MIR occurs at 12 to 36 h, and Stage 3 MIR indicates exposure of >36 h (27).
Figure 1.
Maternal inflammatory response (MIR) stages: Normal membranes (left) contain amnion (top), fibrous chorion (middle) and decidua (bottom). Maternal inflammation is staged by the location and state of neutrophils. Neutrophils lined up at the decidua/chorion border are MIR1. Once neutrophils cross into the chorion, MIR2 is reached. Neutrophilic debris, death of amnion cells, and thickened basement membrane are diagnostic of MIR3.
Fetal Inflammatory Response (FIR)
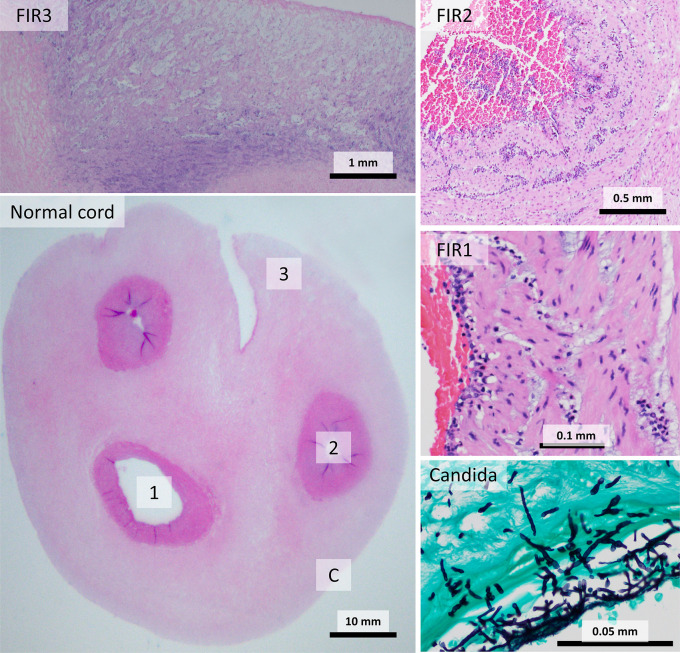
FIR consists of extravasating fetal neutrophils, which traverse fetal tissues to and move toward the amniotic space ( Figure 2 ). FIR is at Stage 1 when neutrophils are seen crossing fetal vessels in the chorionic plate (chorionic vasculitis) or involving the umbilical vein (phlebitis). Inflammation of the umbilical arteries (arteritis) indicates Stage 2, while inflammation of Wharton’s jelly with necrosis, necrotizing funisitis is Stage 3. In contrast, non-necrotizing funisitis is ambiguous. In the clinical literature, funisitis is used to mean any FIR in the umbilical cord. In the pathologic literature, funisitis is defined as neutrophilic infiltration of Wharton’s jelly, any degree of which was considered diagnostic of FIR stage 2. The significance of this finding has been down-graded in the pathology literature (11). Timing of FIR lesions is less clear than MIR, possibly reflecting the differing maturation of the fetal immune system over the course of gestation.
Figure 2.
Fetal inflammatory response (FIR) stages: A normal umbilical cord (bottom left) includes two arteries (circular vessels) and one vein (larger, ovoid vessel) surrounded by Wharton’s jelly. FIR1 consists of inflammation of the vein. Arterial inflammation is diagnostic of FIR2. Inflammation of Wharton’s jelly with necrosis is FIR3. Candida infections produce peripheral abscesses with invasive organisms (Grocott Methenamine Silver stain).
Acute Villitis
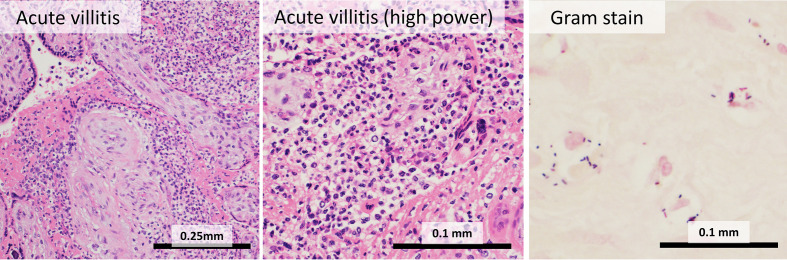
Acute villitis is an uncommon histological pattern which involves neutrophil infiltration of the chorionic villi beneath the trophoblastic membrane, and can occur with or without chorioamnionitis (28) ( Figure 3 ). Acute villitis is associated with maternal sepsis from listeriosis, and with other infections, usually bacterial, including Group B Streptococci, Klebsiella, Escherichia coli, Campylobacter, Haemophilus, tuberculosis and syphilis (12). Acute villitis is suggestive of acute fetal infection with serious fetal consequences, including fetal sepsis and death (29).
Figure 3.
Acute villitis: In acute villitis, terminal villi show dense inflammation with fibrin. Gram stain demonstrates bacterial forms.
Immunology of API
Neutrophils are the major cell type involved in API (11, 30). Outside the placenta, maternal leukocytosis is one of the criteria for clinical chorioamnionitis, while fetal complete blood count shows leukocytosis and neutrophilia (31, 32). Monocyte/macrophages are increased in amniotic fluid in clinical chorioamnionitis, indicating they also undergo migration through the placenta in response to inflammatory stimuli (33). Bacterial products, such as lipopolysaccharide (LPS) would be expected to induce a classical activation pattern (termed M1) in macrophages (34). Perhaps surprisingly resident maternal decidual macrophages, showed a switch toward an alternatively activated (M2) polarization in API, while fetal macrophages resident in the terminal villi were primarily M2 at baseline, which was unaffected by clinical chorioamnionitis (35, 36). Clinical chorioamnionitis is associated with a change in umbilical cord monocyte histone marks, suggesting reprogramming of the fetal immune system as well (37). M1/M2 (and M2-subtype) polarization has been more extensively studied in animal models compared to humans (38).
Eosinophils are an uncommon component of the acute inflammatory response in general, but are frequently encountered in the fetal inflammatory response of preterm infants (39, 40). This is presumed to be due to the immaturity of the fetal immune system. The significance of eosinophil predominant versus neutrophil predominant fetal inflammatory response has been incompletely explored. However the synergy between preterm delivery and chorioamnionitis as risk factors for asthma and wheezing (explored in more detail below) is suggestive (41).
Alterations in fetal and neonatal T-cells have been identified. Within the umbilical cord, high stage API was associated with an increased proportion of Foxp3+ cells coexpressing retinoic acid receptor-related orphan receptor gamma T (RORγT) (42). Preterm API was also associated with a shift toward the T helper 17 (Th17) phenotype, including increased numbers of progenitor and mature Th17 cells, IL-17+ Treg cells and effector memory T-cells that coexpressed Th17 antigens (43). Fetal tissues also showed altered lymphocytes. In stillbirths complicated by API, splenic Foxp3+ cells were decreased, while pulmonary CD3+ cells were increased (42).
The humoral components of clinical chorioamnionitis and API are well-studied (26). Umbilical cord and post-delivery infant blood show increased levels of IL-1, IL2R, IL-6, IL-8, TNF-α, MIP-1β, RANTES, and I-TAC (44–47). The fetus may respond directly to bacteria that enter either through the bloodstream (sepsis) or through inhalation of bacteria-laden amniotic fluid (pneumonia).
Hereditary Risk of API
A genome wide association study (GWAS) of clinical chorioamnionitis using DNA from newborn blood spots showed no genome-wide associations. However, several exonic variants in inflammation-associated genes showed nominal significance, including Fc receptor like 5 (FCRL5), interleukin 23 receptor (IL23R), phospholipase A2 receptor 1 (PLA2R1), complement C1 receptor (C1R), interleukin 10 receptor alpha (IL10RA), DNA cross-link repair 1C (DCLRE1C), TRAF3 interacting protein 1 (TRAF3IP1), and fibroblast growth factor 3 (FGFR3) (48). Variants in TRAF3IP1 and FGFR3 have been associated with changes in the forced expiratory volume in 1 s over forced vital capacity ratio (FEV1/FVC), a diagnostic feature of asthma (49, 50). Several genes show associations with a variety of infectious and autoimmune conditions. IL23R variants are associated with autoinflammatory conditions, including inflammatory bowel disease, psoriatic arthritis, and autoimmune conditions in pediatric patients (51, 52). PLA2R1 variants are associated with autoimmune membranous glomerulonephritis and inflammatory bowel disease (53, 54). IL10RA variants have been associated with pneumonia in adults (55). Interestingly, DCLRE1C variants have been associated with response to cognitive behavioral therapy in anxiety and migraine gesturing toward neurocognitive outcomes (56, 57).
A study on placental (fetal) genotype from API cases, also focusing on immune-associated genes, found an association between chorioamnionitis, a promoter variant in interleukin 6 (IL6), methylation of the IL6 promoter and IL6 gene expression (58). Significantly IL6 variants have been associated with asthma and childhood onset of asthma (59).
Clinical Associations With API
Neonatal Mortality and Morbidity
Maternal inflammatory response is associated with adverse neonatal outcomes when combined with fetal inflammatory response (60, 61) and fetal inflammatory response alone is often associated with poor outcomes (62–65). Multiple studies demonstrate an increased risk of neonatal death in the presence of FIR (60, 63, 66). Early onset sepsis is associated with FIR (62, 63) as are severe retinopathy of prematurity (61) and necrotizing enterocolitis and spontaneous intestinal perforation in the preterm (64).
Respiratory Outcomes
Bronchopulmonary dysplasia (BPD) is the most common respiratory disorder in preterm infants characterized by an interruption in pulmonary vascular and alveolar development which may originate in the antepartum, intrapartum or postpartum period (67). The role of placental inflammation and BPD is conflicting, with some studies finding an association between FIRS and BPD (63, 68) and histological chorioamnionitis and BPD (69) while other studies find either no association between placental inflammation and BPD (65) or a decreased risk of BPD with histological chorioamnionitis with fetal inflammatory response (70).
In preterm infants, API or MIR2 are risk factors for recurrent wheeze (71), asthma (41, 72), and chronic lung disease (73) but not altered lung function (71, 74). Preterm birth is an independent risk factor for both API and respiratory disease. (26, 75, 76). A series of studies from overlapping groups of authors have used causal path analysis to untangle this interdependency (73, 77, 78). In one study, MIR and FIR were directly causative of chronic lung disease of prematurity and indirectly causative through their influence on prematurity and mechanical ventilation (77). A more recent study re-demonstrated a direct effect of FIR on chronic lung disease of prematurity, which then had a risk of progression to asthma in childhood (73). These studies are valuable but include relatively few patients and are sensitive to permutations in model design.
The mechanism of the inflammation-lung outcomes association in animal chorioamnionitis models has been suggested to be related to altered metalloproteinase activation in the airway (79–81) and FOXP3 CNS 3 methylation, decreasing the balance of Treg and Th17 cells (82, 83). However, these studies used an acute endotoxin injection model which is more in keeping with API. Stillborn fetuses and liveborn infants exposed prenatally to API and chronic villitis both had Treg and Th17 marker co-expressing cells (42) which may suggest a shift in Tregs to a Th17 phenotype (43, 84). Another study also showed elevated numbers of Th17 cells in cord blood of only very preterm neonates, with a trend to lower Tregs/Th17 ratios in preterm infants who were exposed to chorioamnionitis. This same study also showed a trend to higher numbers of Tregs co-expressing the canonical IL-17 transcription factor RORγt, again suggesting a shift to Th17 type immunity in the context of histological chorioamnionitis (43). One reason these immune deviation effects are seen more in preterm infants is that there is a developmental shift to Th17 cells in preterm children (85). RNA sequencing of cord blood from a small number of infants suggests that there may be additional pathways such as changes in CCR2 and other pathways involved in T cell survival and Treg development (86). These shifts in Th17 and Treg patterns may have implications on Th2/Th17 high endotypes of asthma (87–89).
Neurocognitive and Developmental Outcomes
Intraventricular hemorrhage and periventricular leukomalacia are serious complications in preterm neonates. Intraventricular hemorrhage in preterm is the most common cause of hydrocephalus and increases the risk for poor neurodevelopment outcomes (90). Periventricular leukomalacia is a type of preterm brain injury associated with adverse neurodevelopment (90), including cerebral palsy (91, 92). Both intraventricular hemorrhage and periventricular leukomalacia are associated with FIRS (62, 63, 65).
The association between API and neurocognitive outcomes has been extensively examined with mixed results. As with asthma, the three-way association between prematurity, API, and adverse outcomes raises issues of causation. Using data from the Collaborative Perinatal Project (1959–1976), Liu et al. showed an association between FIR and low IQ scores (93). Specifically, FIR in early preterm infants (20–34 weeks) was associated with an increased risk of low IQ (<70) at 4 years, but not 7 years. FIR in term infants was associated with an increased risk of low Performance IQ (vs. Verbal IQ or Full Spectrum IQ) at 7 years, but not 4 years. These findings are compelling, but the use of multiple subgroups and measures, and the lack of consistency between ages 4 and 7 years complicate interpretation. In a recent meta-analysis of studies using the Bayley II developmental scale, MIR was associated with a lower mental development index, but a nonsignificant increase in the motor development index (94). In a case-control study of 254 children, API was associated with an increased risk of autism spectrum disorder, with a further elevated risk in FIR (95). Further complicating matters is the possible interaction of histological chorioamnionitis and clinical chorioamnionitis. An observational study of the Eunice Kennedy Shriver National Institute of Child Health and Human Development National Research Network with 2,390 extremely preterm infants found that histological chorioamnionitis alone when adjusted for gestational age was associated with lower odds of poor neurodevelopment outcomes whereas histological combined with clinical chorioamnionitis resulted in an increased risk of cognitive impairment at 18 to 22 months when compared to no chorioamnionitis (96).
Many studies have challenged this association. A study of 86 infants born prematurely in Orlando, Florida showed no difference in Bayley scale at 1 year when infants were matched for gestational age, birth weight, respiratory distress syndrome, and intraventricular hemorrhage grade (97). In a study from Western Australia, MIR2 in preterm infants born <30 weeks gestation was not associated with decreased Bayley III developmental scores at 2 years (98). At three years, a study of 2,201 children born <34 weeks gestation from Japan showed no difference in cerebral palsy, risk of developmental quotient <70, or neurodevelopmental impairment between pregnancies with and without MIR2 (99). A matched-case control study of extremely preterm infants with CP examined the role of placental pathology and found no association between histological chorioamnionitis or funisitis and CP (100).
The mechanism by which API may cause neurologic impairment is unclear. In rodents, maternal injection with lipopolysaccharide, a Gram-negative bacterial component, induces neurocognitive and behavioral abnormalities without fetal infection (101). In a small case-control study, severe FIR and severe MIR were associated with cerebral palsy (CP) in very low birthweight infants (<1,500 g) (102). However, the relationships were indirect. Using a series of logistic regressions, severe FIR was associated with CP via its association with thrombi in fetal vessels, while severe MIR was associated with CP via its association with villous edema. Thromboemboli (from FIR) or under perfusion (from MIR) would then be the immediate cause of CP. A study from Sweden using Bayley-III scales and developmental outcomes at an adjusted 2.5 years of age to diagnose CP in extremely preterm children suggests placental infarction as a contributor to CP but did not find associations with other placental pathology outcomes (103).
API Associated With Candida
Infection by Candida albicans results in a distinct pathologic appearance, most characteristically punctate abscesses on the periphery of the umbilical cord (peripheral funisitis,) (30, 104). In preterm infants, Candida is associated with cutaneous candidiasis, sepsis, pneumonia and a high rate of perinatal death, while at term it is more often an incidental finding (104–106). Foreign bodies, such as retained intrauterine device or uterine cerclage are risk factors for Candida (104, 107). Candida glabrata and Candida lusitania are associated with in vitro fertilization and are associated with high risk of adverse outcomes. API due to Candida is relatively rare. The immunologic features and long-term consequences are unknown.
Chronic Placental Inflammation (CPI)
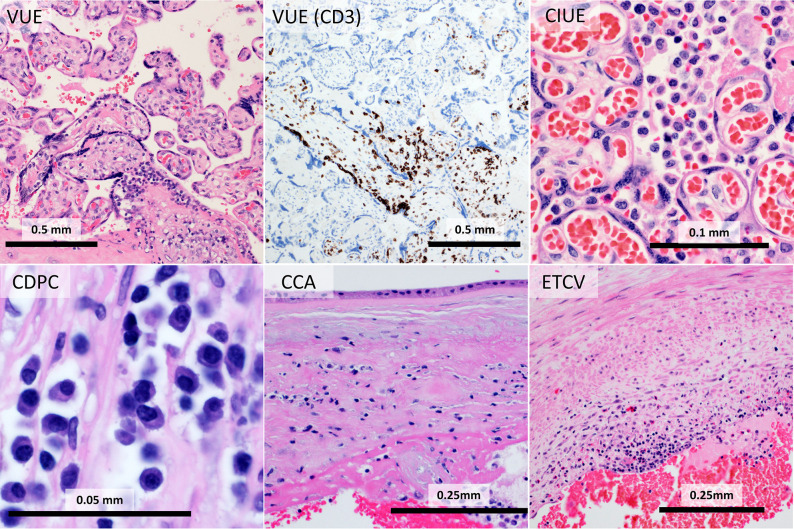
The chronic inflammatory lesions of the placenta are a group of frequently co-occurring lymphocytic, histiocytic, and plasmacytic processes distinguished by the cells present and their location in the placenta (108) ( Figure 4 ). Diseases with high rates of maternal-fetal transfer, including Toxoplasma, Treponema, rubella, cytomegalovirus (CMV), herpesvirus (HSV1 and HSV2), human immunodeficiency virus (HIV1, “TORCH” infections), are the most commonly identified in CPI (109). The effect of congenital TORCH infections has been extensively reviewed elsewhere. Therefore, this review will focus on the >95% of cases in which no etiology is identified (110). Two competing theories have arisen to explain these cases 1) That CPI results from failure of maternal tolerance to fetal antigens or 2) That unknown or untested-for infectious agents in the placenta induce a maternal response, akin to transplant rejection (111). Evidence for the alloimmune theory includes the increased frequency of CPI in egg donor pregnancies, where the fetus is fully allogeneic, rather than ½ self and ½ allogeneic (112, 113). Conversely, interbreeding of inbred mouse strains is associated with immune activation and resorption, the degree of which is strain dependent (114). Activation of the maternal immune system by lipopolysaccharide (LPS), a Gram negative bacterial component, or polyinosine:cytosine (poly-IC), a viral mimetic, increases the rate of resorption, prompting a model of immune activation in an allogeneic background (115, 116).
Figure 4.
Chronic placental inflammation (CPI): In chronic villitis of unknown etiology (VUE), CD3-positive T-cells infiltrate fetal villi. Chronic intervillositis of unknown etiology (CIUE) is characterized by intense histiocytic inflammation filling the intervillous space. Chronic deciduitis with plasma cells (CDPC) shows plasmacytic inflammation in the decidua. Chronic chorionitis (CCA) consists of maternal T-cells in the chorion. In eosinophilic T-cell vasculitis (ETCV), fetal T-cells and eosinophils inflame fetal vessels.
Chronic Villitis (VUE)
Chronic villitis of unknown etiology (VUE) is a process involving infiltration of placental villi by lymphocytes, histiocytes, and rarely plasma cells (117). VUE most commonly affects terminal villi, the sites of gas and nutrient exchange, closest to the maternal surface (“basal villitis”), however it frequently is present in other sites and, more rarely, is diffuse (12). In addition to lymphocytic infiltration, VUE is characterized by aggregation of terminal villi, destruction of villous capillaries (resulting in “avascular villi”) and stem villous vessels (“stem villous obliteration”). The antigen is unclear, however the destruction of endothelium and sparing of syncytiotrophoblast is suggestive. The prevalence of VUE in studies is estimated at 5% to 15% of placentas submitted for pathologic examination (118). However, the diagnosis is frequently missed by inexperienced or nonspecialized pathologists, doubtless impairing research (119, 120). The lymphocytes in chronic villitis are maternal in origin based on human leukocyte antigen (HLA) mismatch (121). The lymphocytes are primarily CD8, but include T-regulatory (Treg) cells and retinoic acid receptor-related orphan receptor gamma (RORγT) cells (42, 122). The mechanism by which T-cells pass the maternal-fetal barrier is unknown. Lymphocytes and histiocytes in VUE express inflammatory cell adhesion molecule ICAM1, supporting a model similar to typical leukocyte extravasation (108). Alternatively, maternal inflammatory cells may pass through disruption of the trophoblastic barrier. Lymphocytes and histiocytes also show expression of nuclear factor kappa B (NFκB) (108). Histiocytes express HLA-DR and histiocytes and syncytiotrophoblast show phosphorylated Signal Transducer and Activator of Transcription 1 (STAT1), indicating activation of the JAK-STAT pathway.
Chronic villitis is associated with increased maternal and fetal plasma chemokines CXCL9, CXCL10, and CXCL11 (123). Within the placenta, there is increased expression of chemokine mRNAs for CXCL9, CXCL10, CXCL11, CXCL13, CCL4, and CCL5 and chemokine receptor mRNA for CXCR3 and CCR5 (123). Chronic villitis is also associated with deposition of the complement component C4d on villous surfaces or fetal vascular endothelium, indicating a potential role for complement-mediated processes (124).
It is unclear whether maternal cells in VUE fully cross from the placenta to seed the developing fetal immune system. T-cells of presumed maternal origin have been identified in cord blood, children and adults based on XY fluorescence in-situ hybridization (FISH) or HLA testing (125, 126). In cord blood, the rate of this maternal microchimerism has been reported at 23% (127). The significance of these maternal cells and their interaction with autoimmune conditions is complicated (125). For example, maternal T-cells are more common in blood from patients with juvenile dermatomyositis (an autoimmune condition) than those with muscular dystrophy (a genetic condition) (128). However, in biopsies of injured muscle, the situation is reversed and there are more maternal T-cells in muscular dystrophy (129). Unfortunately, studies on T-cells of maternal origin have not cross-referenced examination of the placenta.
Chronic Deciduitis With Plasma Cells (CDPC)
This lesion is characterized by a lymphocyte and plasma cell infiltrate in the decidua (12, 130). CDPC is histologically similar to chronic endometritis - plasmacytic inflammation of the uterus in the absence of pregnancy (110). The antigen is unknown, but major histocompatibility complex/human leukocyte antigen mismatch appears to play a role (131). Maternal immunoglobulin gamma (IgG) is actively transported across the placenta, representing a straightforward mechanism for effects on the fetus (132, 133). Transplacental passage of IgG is the mechanism for hemolytic disease of the newborn, neonatal alloimmune thrombocytopenia, and congenital heart block among others (134–136). However, these diseases are not characterized by CDPC, raising the question of whether the plasma cells in CDPC are acting in a paracrine fashion.
Chronic Chorionitis/Chronic Chorioamnionitis (CCA)
Chronic chorionitis (CCA) is defined by lymphocytic or lymphoplasmacytic infiltration of the chorion or chorion and amnion (117). CCA is frequently associated with VUE (137, 138). The lymphocytes are primarily CD8 T-cells, with few CD4; B-cells and NK-cells are uncommon (138). Amniotic fluid concentrations of the chemokines CXCL9 and CXCL10, along with their receptor, CXCR3, are elevated in CCA (139). CXCL9, -10, and -11 mRNA are upregulated in placental membranes with CCA (137).
Chronic Intervillositis of Unknown Etiology (CIUE)
Chronic intervillositis is an uncommon condition in which maternal histiocytes and to a lesser extent lymphocytes fill the intervillous space (140, 141). As in other CPI conditions, it can be seen in association with infectious causes particularly malaria and cytomegalovirus, however this review will focus on the idiopathic chronic intervillositis of unknown etiology (CIUE) (142, 143). CIUE may occur in association with chronic villitis, or as a purely isolated finding. Controlled trials have not been performed, but the successful treatments support an alloimmune or prothrombotic mechanism for CIUE (144).
In CIUE, the intervillous histiocytes are M2-polarized with overexpression of complement receptor 4 (CD11c/CD18) and toll-like receptor 1 (TLR1) (142, 143, 145, 146). Unlike VUE, the T-cells in CIUE are a mixture of CD4 and CD8-cells, with admixed Tregs (147).
Eosinophilic T-Cell Vasculitis (ETCV)
Eosinophilic T-cell vasculitis (ETCV) is an uncommon chronic inflammatory condition first described in 2002 with an incidence of 0.2 to 0.7% of pregnancies (111). It consists of fetal eosinophils, histiocytes, and T-cells present in the wall and lumen of large fetal vessels (111, 148). It most commonly presents with involvement of a single chorionic plate vessel, often with an associated thrombus (149). ETCV occurs more often than chance with VUE of thrombotic conditions (149, 150). However, ETCV is frequently an isolated finding, reinforcing its place as an independent diagnosis. ETCV is differentiated from API by the absence of maternal inflammation, orientation of inflammatory cells toward the placental disc rather than toward the amnion, and the different inflammatory populations.
The infiltrate in ETCV is poorly characterized, however some facts are known. In contrast to VUE, the T-cells of ETCV are of fetal origin (148). The T-cells are a mixture of CD25+, FOXP3+ Tregs and other T cells (150). Long term outcomes have not been well described, likely related to the low incidence, recent description, and frequent co-occurrence of other pathologies.
Clinical Associations With CPI
Relative to API, fewer studies have examined CPI. Outcomes sometimes associated with CPI include: pregnancy loss; preterm delivery; growth restriction; a possible association with neonatal alloimmune thrombocytopenia; and neurocognitive and developmental outcomes. Additionally, the risk of recurrence is high with many chronic inflammatory lesions.
Pregnancy Loss
Villitis of unknown etiology is associated with fetal death and recurrent loss (110, 117, 151). In one study focused on stillbirth, placentas with VUE were analyzed and it was found that a Th1-type immune response predominated (151). Fetal demise is seen in chronic deciduitis with plasma cells though fewer studies have evaluated this pathology (151). Chronic chorioamnionitis is also associated with fetal death (117, 152). Chronic intervillositis of unknown etiology is a strongly associated with miscarriage, intrauterine fetal demise and a very high risk of recurrence (141, 153–155). Women with a history of recurrent CIUE have gone on to successful live birth after treatment with aspirin and low molecular weight heparin (LMWH), aspirin and corticosteroids, aspirin, LMWH, and steroids, or aspirin, prednisone, LMWH and hydroxychloroquine (147, 156, 157).
Preterm Delivery
Although VUE is sometimes associated with preterm labor (117), chronic chorioamnionitis is most frequently associated with late spontaneous preterm birth (117, 158). A study of 1206 preterm births found that chronic chorioamnionitis was most frequently associated with late preterm birth designated as 34 to 37 weeks while acute chorioamnionitis was most commonly associated with very early preterm birth designated as less than 28 weeks (158). Chronic deciduitis with plasma cells is also associated with preterm labor, but has not been established as an independent risk factor for long-term outcomes (158, 159).
Growth Restriction
Villitis of unknown etiology is associated with fetal growth restriction, low birth weight and small for gestational age (110, 117, 160). Chronic intervillositis of unknown etiology is the other chronic inflammatory pathology frequently associated with disorders of fetal growth (155, 161–163). Multiple studies demonstrate outcomes of fetal growth restriction with rates of 70% or higher when CIUE is present (155, 161, 162).
Neonatal Alloimmune Thrombocytopenia
Neonatal alloimmune thrombocytopenia (NAIT) is a rare pregnancy complication characterized by otherwise unexplained severe thrombocytopenia in a neonate (164). Analogous with immune hydrops, NAIT is caused by maternal alloimmunization against fetal antigens. An association between VUE and neonatal alloimmune thrombocytopenia (NAIT) has been reported (165). This study examined histopathology from 14 placentas of pregnancies affected by NAIT and found that chronic villitis was observed in untreated pregnancies compared with intravenous immunoglobulin treated pregnancies. This one small study links the histological observation of VUE to placentas affected by NAIT. As NAIT is driven by alloimmunization, the association with VUE provides further evidence that VUE is an alloimmune process.
Neurocognitive and Developmental Outcomes
Among patients with intrauterine growth restriction, VUE was associated with an increased risk of low developmental index at 2 years of age (166). In another study, VUE with stem villous obliteration was associated with an increased risk of cerebral palsy or other abnormal neurodevelopmental findings in term infants (167). The limitation to VUE with stem villous obliteration in this work was for comparison to other conditions causing stem villous anomalies and there is no evidence to suggest VUE without stem villous obliteration will have a different impact. Another study with term infants with hypoxic-ischemic encephalopathy found that chronic villitis was associated with injury in the basal ganglia and thalamus (168). Chronic chorioamnionitis has also been associated with white matter injury in newborns, but this increased risk was seen in newborns with chronic chorioamnionitis and funisitis while neither condition alone was associated with white matter injury (169). This study suggests that the interaction of insults rather than one clear etiology may be responsible for initial neurocognitive insults.
Null Associations
Many studies examining associations between inflammatory lesions and specific short- and long-term outcomes have not found meaningful relationships. Long term outcomes of isolated CCA have not been described. n a systematic review of associations with stillbirth, neonatal morbidity, and neurologic outcomes, null findings formed the bulk of those reported (159).
Conclusion
Maternal-fetal inflammation frequently involves the placenta, broadly grouped into API and CPI. Each has numerous subtypes and degrees of inflammation. Both present an inflammatory shock to the fetus, driven by maladaptation in the placenta and have been associated with long-term adverse outcomes, including asthma, cerebral palsy, abnormal neurodevelopment, and autism spectrum disorder ( Figure 5 ). Other than the classical API response to presumed ascending infection, the long-term outcomes of these diseases are poorly studied and additional associations are likely to be identified with focused research. Potential differences in outcomes by placental/fetal sex are also needed. While the NIH Human Placenta Project was established to drive discoveries in real-time placental function in utero, there has been an overall recognition of how little we know about the placenta’s relationship to the health of humans. Additional studies of placental pathology, particularly inflammatory lesions, could contribute greatly to the DOHaD field.
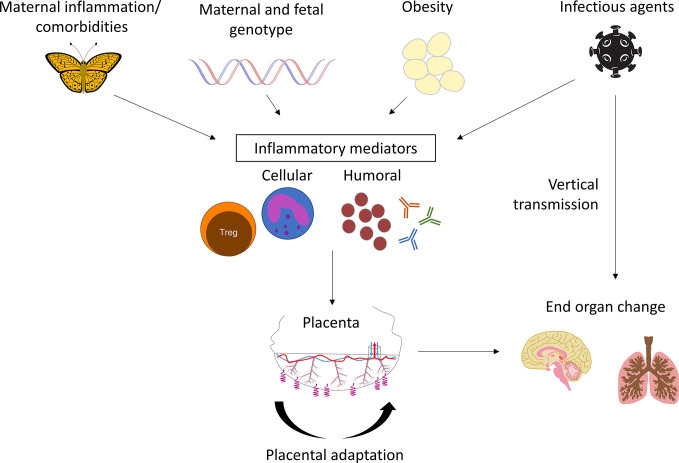
Figure 5.
Model of placental inflammation in pregnancy: Maternal inflammation integrates maternal autoimmune diseases, genetic risk factors, obesity, and infectious organisms. Cellular and humoral mediators induce placental adaptation and maladaptation. The final results are lifelong abnormalities in end organ function.
Author Contributions
All authors contributed to the article and approved the submitted version. JG created the microphotographs. Figure 5 was drafted by CB.
Funding
JG is supported by NIBIB K08 EB030120.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Dr. Elisheva Shanes provided the case of CIUE.
References
- 1. Wadhwa PD, Buss C, Entringer S, Swanson JM. Developmental origins of health and disease: brief history of the approach and current focus on epigenetic mechanisms. Semin Reprod Med (2009) 27:358–68. 10.1055/s-0029-1237424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Norris SA, Daar A, Balasubramanian D, Byass P, Kimani-Murage E, Macnab A, et al. Understanding and acting on the developmental origins of health and disease in Africa would improve health across generations. Glob Health Action (2017) 10:1334985. 10.1080/16549716.2017.1334985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goldstein JA, Norris SA, Aronoff DM. DOHaD at the intersection of maternal immune activation and maternal metabolic stress: a scoping review. J Dev Orig Health Dis (2017) 8:273–83. 10.1017/S2040174417000010 [DOI] [PubMed] [Google Scholar]
- 4. Helgertz J, Bengtsson T. The Long-Lasting Influenza: The Impact of Fetal Stress During the 1918 Influenza Pandemic on Socioeconomic Attainment and Health in Sweden, 1968-2012. Demography (2019) 56:1389–425. 10.1007/s13524-019-00799-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Acquah JK, Dahal R, Sloan FA. 1918 Influenza Pandemic: In Utero Exposure in the United States and Long-Term Impact on Hospitalizations. Am J Public Health (2017) 107:1477–83. 10.2105/AJPH.2017.303887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Burton GJ, Fowden AL, Thornburg KL. Placental Origins of Chronic Disease. Physiol Rev (2016) 96:1509–65. 10.1152/physrev.00029.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thornburg KL, Marshall N. The placenta is the center of the chronic disease universe. Am J Obstet Gynecol (2015) 213:S14–20. 10.1016/j.ajog.2015.08.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Eriksson JG, Kajantie E, Thornburg KL, Osmond C, Barker DJP. Mother’s body size and placental size predict coronary heart disease in men. Eur Heart J (2011) 32:2297–303. 10.1093/eurheartj/ehr147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Martyn CN, Barker DJ, Osmond C. Mothers’ pelvic size, fetal growth, and death from stroke and coronary heart disease in men in the UK. Lancet Lond Engl (1996) 348:1264–8. 10.1016/s0140-6736(96)04257-2 [DOI] [PubMed] [Google Scholar]
- 10. Odibo I, Gehlot A, Ounpraseuth ST, Magann EF. Pathologic examination of the placenta and its clinical utility: a survey of obstetrics and gynecology providers. J Matern Fetal Neonatal Med Off J Eur Assoc Perinat Med Fed Asia Ocean Perinat Soc Int Soc Perinat Obstet (2016) 29:197–201. 10.3109/14767058.2014.998192 [DOI] [PubMed] [Google Scholar]
- 11. Khong TY, Mooney EE, Ariel I, Balmus NCM, Boyd TK, Brundler M-A, et al. Sampling and Definitions of Placental Lesions: Amsterdam Placental Workshop Group Consensus Statement. Arch Pathol Lab Med (2016) 140:698–713. 10.5858/arpa.2015-0225-CC [DOI] [PubMed] [Google Scholar]
- 12. Yee Khong T, Mooney EE, Nikkels PGJ, Morgan TK, Gordijn SJ. Pathology of the placenta: a practical guide. New York, NY: Springer Berlin Heidelberg; (2018). [Google Scholar]
- 13. Smulian JC, Shen-Schwarz S, Vintzileos AM, Lake MF, Ananth CV. Clinical chorioamnionitis and histologic placental inflammation. Obstet Gynecol (1999) 94:1000–5. 10.1016/s0029-7844(99)00416-0 [DOI] [PubMed] [Google Scholar]
- 14. Roberts DJ, Celi AC, Riley LE, Onderdonk AB, Boyd TK, Johnson LC, et al. Acute histologic chorioamnionitis at term: nearly always noninfectious. PloS One (2012) 7:e31819. 10.1371/journal.pone.0031819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Romero R, Kim YM, Pacora P, Kim CJ, Benshalom-Tirosh N, Jaiman S, et al. The frequency and type of placental histologic lesions in term pregnancies with normal outcome. J Perinat Med (2018) 46:613–30. 10.1515/jpm-2018-0055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pankuch GA, Appelbaum PC, Lorenz RP, Botti JJ, Schachter J, Naeye RL. Placental microbiology and histology and the pathogenesis of chorioamnionitis. Obstet Gynecol (1984) 64:802–6. [PubMed] [Google Scholar]
- 17. Romero R, Salafia CM, Athanassiadis AP, Hanaoka S, Mazor M, Sepulveda W, et al. The relationship between acute inflammatory lesions of the preterm placenta and amniotic fluid microbiology. Am J Obstet Gynecol (1992) 166:1382–8. 10.1016/0002-9378(92)91609-e [DOI] [PubMed] [Google Scholar]
- 18. Ovalle A, Martínez MA, Kakarieka E, Gómez R, Torres J, Fuentes A, et al. [Placental histopathology in premature rupture of membranes. Its relationship with microbiological findings, maternal, and neonatal outcome]. Rev Med Chil (1998) 126:930–42. [PubMed] [Google Scholar]
- 19. Romero R, Miranda J, Kusanovic JP, Chaiworapongsa T, Chaemsaithong P, Martinez A, et al. Clinical chorioamnionitis at term I: microbiology of the amniotic cavity using cultivation and molecular techniques. J Perinat Med (2015) 43:19–36. 10.1515/jpm-2014-0249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Romero R, Miranda J, Chaiworapongsa T, Korzeniewski SJ, Chaemsaithong P, Gotsch F, et al. Prevalence and clinical significance of sterile intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Reprod Immunol N Y N 1989 (2014) 72:458–74. 10.1111/aji.12296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mohan AR, Sooranna SR, Lindstrom TM, Johnson MR, Bennett PR. The effect of mechanical stretch on cyclooxygenase type 2 expression and activator protein-1 and nuclear factor-kappaB activity in human amnion cells. Endocrinology (2007) 148:1850–7. 10.1210/en.2006-1289 [DOI] [PubMed] [Google Scholar]
- 22. Chowdhury B, David AL, Thrasivoulou C, Becker DL, Bader DL, Chowdhury TT. Tensile strain increased COX-2 expression and PGE2 release leading to weakening of the human amniotic membrane. Placenta (2014) 35:1057–64. 10.1016/j.placenta.2014.09.006 [DOI] [PubMed] [Google Scholar]
- 23. Lee Y-H, Shynlova O, Lye SJ. Stretch-induced human myometrial cytokines enhance immune cell recruitment via endothelial activation. Cell Mol Immunol (2015) 12:231–42. 10.1038/cmi.2014.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Romero R, Espinoza J, Gonçalves LF, Kusanovic JP, Friel LA, Nien JK. Inflammation in preterm and term labour and delivery. Semin Fetal Neonatal Med (2006) 11:317–26. 10.1016/j.siny.2006.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Challier JC, Basu S, Bintein T, Minium J, Hotmire K, Catalano PM, et al. Obesity in pregnancy stimulates macrophage accumulation and inflammation in the placenta. Placenta (2008) 29:274–81. 10.1016/j.placenta.2007.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim CJ, Romero R, Chaemsaithong P, Chaiyasit N, Yoon BH, Kim YM. Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. Am J Obstet Gynecol (2015) 213:S29–52. 10.1016/j.ajog.2015.08.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Redline RW. Inflammatory responses in the placenta and umbilical cord. Semin Fetal Neonatal Med (2006) 11:296–301. 10.1016/j.siny.2006.02.011 [DOI] [PubMed] [Google Scholar]
- 28. Schubert PT, Mason D, Martines R, Deleon-Carnes M, Zaki SR, Roberts DJ. Spectrum of Changes Seen With Placental Intravascular Organisms. Pediatr Dev Pathol (2019) 22:229–35. 10.1177/1093526618801616 [DOI] [PubMed] [Google Scholar]
- 29. Bae GE, Yoon N, Choi M, Hwang S, Hwang H, Kim J-S. Acute Placental Villitis as Evidence of Fetal Sepsis: An Autopsy Case Report. Pediatr Dev Pathol (2016) 19:165–8. 10.2350/15-06-1656-CR.1 [DOI] [PubMed] [Google Scholar]
- 30. Baergen RN. Manual of pathology of the human placenta. 2nd ed. New York Dordrecht Heidelberg London: Springer; (2011). [Google Scholar]
- 31. Gibbs RS. Diagnosis of intra-amniotic infection. Semin Perinatol (1977) 1:71–7. [PubMed] [Google Scholar]
- 32. Kim EN, Kim CJ, Park JW, Yoon BH. Acute funisitis is associated with distinct changes in fetal hematologic profile. J Matern Fetal Neonatal Med Off J Eur Assoc Perinat Med Fed Asia Ocean Perinat Soc Int Soc Perinat Obstet (2015) 28:588–93. 10.3109/14767058.2014.927426 [DOI] [PubMed] [Google Scholar]
- 33. Martinez-Varea A, Romero R, Xu Y, Miller D, Ahmed AI, Chaemsaithong P, et al. Clinical chorioamnionitis at term VII: the amniotic fluid cellular immune response. J Perinat Med (2017) 45:523–38. 10.1515/jpm-2016-0225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Italiani P, Boraschi D. From Monocytes to M1/M2 Macrophages: Phenotypical vs. Functional Differentiation. Front Immunol (2014) 5:514:514. 10.3389/fimmu.2014.00514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jašić M, Štifter S, Sindičić Dessardo N, Rukavina KM, Mustać E, Belci D. The relationship between histologic chorioamnionitis and decidual macrophage polarization and their influence on outcomes of neonates born before the 32nd gestational week. J Matern Fetal Neonatal Med Off J Eur Assoc Perinat Med Fed Asia Ocean Perinat Soc Int Soc Perinat Obstet (2019) 1–10. 10.1080/14767058.2019.1638906 [DOI] [PubMed] [Google Scholar]
- 36. Joerink M, Rindsjö E, van Riel B, Alm J, Papadogiannakis N. Placental macrophage (Hofbauer cell) polarization is independent of maternal allergen-sensitization and presence of chorioamnionitis. Placenta (2011) 32:380–5. 10.1016/j.placenta.2011.02.003 [DOI] [PubMed] [Google Scholar]
- 37. Bermick J, Gallagher K, denDekker A, Kunkel S, Lukacs N, Schaller M. Chorioamnionitis exposure remodels the unique histone modification landscape of neonatal monocytes and alters the expression of immune pathway genes. FEBS J (2019) 286:82–109. 10.1111/febs.14728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brown MB, von Chamier M, Allam AB, Reyes L. M1/M2 macrophage polarity in normal and complicated pregnancy. Front Immunol (2014) 5:606:606. 10.3389/fimmu.2014.00606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hecht JL, Allred EN, Kliman HJ, Zambrano E, Doss BJ, Husain A, et al. Histological characteristics of singleton placentas delivered before the 28th week of gestation. Pathol (Phila) (2008) 40:372–6. 10.1080/00313020802035865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Redline RW. Inflammatory response in acute chorioamnionitis. Semin Fetal Neonatal Med (2012) 17:20–5. 10.1016/j.siny.2011.08.003 [DOI] [PubMed] [Google Scholar]
- 41. Kumar R, Yu Y, Story RE, Pongracic JA, Gupta R, Pearson C, et al. Prematurity, chorioamnionitis, and the development of recurrent wheezing: a prospective birth cohort study. J Allergy Clin Immunol (2008) 121:878–884.e6. 10.1016/j.jaci.2008.01.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Singh AM, Sherenian MG, Kim K-Y, Erickson KA, Yang A, Mestan K, et al. Fetal cord blood and tissue immune responses to chronic placental inflammation and chorioamnionitis. Allergy Asthma Clin Immunol Off J Can Soc Allergy Clin Immunol (2018) 14:66. 10.1186/s13223-018-0297-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rito DC, Viehl LT, Buchanan PM, Haridas S, Koenig JM. Augmented Th17-type immune responses in preterm neonates exposed to histologic chorioamnionitis. Pediatr Res (2017) 81:639–45. 10.1038/pr.2016.254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Salafia CM, Sherer DM, Spong CY, Lencki S, Eglinton GS, Parkash V, et al. Fetal but not maternal serum cytokine levels correlate with histologic acute placental inflammation. Am J Perinatol (1997) 14:419–22. 10.1055/s-2007-994172 [DOI] [PubMed] [Google Scholar]
- 45. Yoon BH, Romero R, Park JS, Kim M, Oh SY, Kim CJ, et al. The relationship among inflammatory lesions of the umbilical cord (funisitis), umbilical cord plasma interleukin 6 concentration, amniotic fluid infection, and neonatal sepsis. Am J Obstet Gynecol (2000) 183:1124–9. 10.1067/mob.2000.109035 [DOI] [PubMed] [Google Scholar]
- 46. Mestan K, Yu Y, Thorsen P, Skogstrand K, Matoba N, Liu X, et al. Cord blood biomarkers of the fetal inflammatory response. J Matern Fetal Neonatal Med Off J Eur Assoc Perinat Med Fed Asia Ocean Perinat Soc Int Soc Perinat Obstet (2009) 22:379–87. 10.1080/14767050802609759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hecht JL, Fichorova RN, Tang VF, Allred EN, McElrath TF, Leviton A. Elgan Study Investigators. Relationship Between Neonatal Blood Protein Concentrations and Placenta Histologic Characteristics in Extremely Low GA Newborns. Pediatr Res (2011) 69:68–73. 10.1203/PDR.0b013e3181fed334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Spiegel AM, Li J, Oehlert JW, Mayo JA, Quaintance CC, Girsen AI, et al. A Genome-Wide Analysis of Clinical Chorioamnionitis among Preterm Infants. Am J Perinatol (2019) 36:1453–8. 10.1055/s-0038-1677503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wain LV, Shrine N, Artigas MS, Erzurumluoglu AM, Noyvert B, Bossini-Castillo L, et al. Genome-wide association analyses for lung function and chronic obstructive pulmonary disease identify new loci and potential druggable targets. Nat Genet (2017) 49:416–25. 10.1038/ng.3787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kichaev G, Bhatia G, Loh P-R, Gazal S, Burch K, Freund MK, et al. Leveraging Polygenic Functional Enrichment to Improve GWAS Power. Am J Hum Genet (2019) 104:65–75. 10.1016/j.ajhg.2018.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Robinson PC, Claushuis TAM, Cortes A, Martin TM, Evans DM, Leo P, et al. Genetic dissection of acute anterior uveitis reveals similarities and differences in associations observed with ankylosing spondylitis. Arthritis Rheumatol Hoboken NJ (2015) 67:140–51. 10.1002/art.38873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Li YR, Li J, Zhao SD, Bradfield JP, Mentch FD, Maggadottir SM, et al. Meta-analysis of shared genetic architecture across ten pediatric autoimmune diseases. Nat Med (2015) 21:1018–27. 10.1038/nm.3933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Stanescu HC, Arcos-Burgos M, Medlar A, Bockenhauer D, Kottgen A, Dragomirescu L, et al. Risk HLA-DQA1 and PLA(2)R1 alleles in idiopathic membranous nephropathy. N Engl J Med (2011) 364:616–26. 10.1056/NEJMoa1009742 [DOI] [PubMed] [Google Scholar]
- 54. Liu JZ, van Sommeren S, Huang H, Ng SC, Alberts R, Takahashi A, et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat Genet (2015) 47:979–86. 10.1038/ng.3359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tian C, Hromatka BS, Kiefer AK, Eriksson N, Noble SM, Tung JY, et al. Genome-wide association and HLA region fine-mapping studies identify susceptibility loci for multiple common infections. Nat Commun (2017) 8:599. 10.1038/s41467-017-00257-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rayner C, Coleman JRI, Purves KL, Hodsoll J, Goldsmith K, Alpers GW, et al. A genome-wide association meta-analysis of prognostic outcomes following cognitive behavioural therapy in individuals with anxiety and depressive disorders. Transl Psychiatry (2019) 9:150. 10.1038/s41398-019-0481-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Anttila V, Winsvold BS, Gormley P, Kurth T, Bettella F, McMahon G, et al. Genome-wide meta-analysis identifies new susceptibility loci for migraine. Nat Genet (2013) 45:912–7. 10.1038/ng.2676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Konwar C, Del Gobbo GF, Terry J, Robinson WP. Association of a placental Interleukin-6 genetic variant (rs1800796) with DNA methylation, gene expression and risk of acute chorioamnionitis. BMC Med Genet (2019) 20:36. 10.1186/s12881-019-0768-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ferreira MAR, Mathur R, Vonk JM, Szwajda A, Brumpton B, Granell R, et al. Genetic Architectures of Childhood- and Adult-Onset Asthma Are Partly Distinct. Am J Hum Genet (2019) 104:665–84. 10.1016/j.ajhg.2019.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lau J, Magee F, Qiu Z, Houbé J, Von Dadelszen P, Lee SK. Chorioamnionitis with a fetal inflammatory response is associated with higher neonatal mortality, morbidity, and resource use than chorioamnionitis displaying a maternal inflammatory response only. Am J Obstet Gynecol (2005) 193:708–13. 10.1016/j.ajog.2005.01.017 [DOI] [PubMed] [Google Scholar]
- 61. Lynch AM, Berning AA, Thevarajah TS, Wagner BD, Post MD, McCourt EA, et al. The role of the maternal and fetal inflammatory response in retinopathy of prematurity. Am J Reprod Immunol N Y N 1989 (2018) 80:e12986. 10.1111/aji.12986 [DOI] [PubMed] [Google Scholar]
- 62. Musilova I, Andrys C, Drahosova M, Zednikova B, Hornychova H, Pliskova L, et al. Late preterm prelabor rupture of fetal membranes: fetal inflammatory response and neonatal outcome. Pediatr Res (2018) 83:630–7. 10.1038/pr.2017.300 [DOI] [PubMed] [Google Scholar]
- 63. Tang Q, Zhang L, Li H, Shao Y. The fetal inflammation response syndrome and adverse neonatal outcomes: a meta-analysis. J Matern Fetal Neonatal Med Off J Eur Assoc Perinat Med Fed Asia Ocean Perinat Soc Int Soc Perinat Obstet (2019) 1–13. 10.1080/14767058.2019.1702942 [DOI] [PubMed] [Google Scholar]
- 64. Ducey J, Owen A, Coombs R, Cohen M. Vasculitis as part of the fetal response to acute chorioamnionitis likely plays a role in the development of necrotizing enterocolitis and spontaneous intestinal perforation in premature neonates. Eur J Pediatr Surg Off J Austrian Assoc Pediatr Surg Al Z Kinderchir (2015) 25:284–91. 10.1055/s-0034-1373849 [DOI] [PubMed] [Google Scholar]
- 65. Liu Z, Tang Z, Li J, Yang Y. Effects of placental inflammation on neonatal outcome in preterm infants. Pediatr Neonatol (2014) 55:35–40. 10.1016/j.pedneo.2013.05.007 [DOI] [PubMed] [Google Scholar]
- 66. Francis F, Bhat V, Mondal N, Adhisivam B, Jacob S, Dorairajan G, et al. Fetal inflammatory response syndrome (FIRS) and outcome of preterm neonates - a prospective analytical study. J Matern Fetal Neonatal Med Off J Eur Assoc Perinat Med Fed Asia Ocean Perinat Soc Int Soc Perinat Obstet (2019) 32:488–92. 10.1080/14767058.2017.1384458 [DOI] [PubMed] [Google Scholar]
- 67. Kalikkot Thekkeveedu R, Guaman MC, Shivanna B. Bronchopulmonary dysplasia: A review of pathogenesis and pathophysiology. Respir Med (2017) 132:170–7. 10.1016/j.rmed.2017.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mittendorf R, Covert R, Montag AG, Elmasri W, Muraskas J, Lee K-S, et al. Special relationships between fetal inflammatory response syndrome and bronchopulmonary dysplasia in neonates. J Perinat Med (2005) 33:428–34. 10.1515/JPM.2005.076 [DOI] [PubMed] [Google Scholar]
- 69. Erdemir G, Kultursay N, Calkavur S, Zekioğlu O, Koroglu OA, Cakmak B, et al. Histological chorioamnionitis: effects on premature delivery and neonatal prognosis. Pediatr Neonatol (2013) 54:267–74. 10.1016/j.pedneo.2013.03.012 [DOI] [PubMed] [Google Scholar]
- 70. Plakkal N, Soraisham AS, Trevenen C, Freiheit EA, Sauve R. Histological chorioamnionitis and bronchopulmonary dysplasia: a retrospective cohort study. J Perinatol Off J Calif Perinat Assoc (2013) 33:441–5. 10.1038/jp.2012.154 [DOI] [PubMed] [Google Scholar]
- 71. McDowell KM, Jobe AH, Fenchel M, Hardie WD, Gisslen T, Young LR, et al. Pulmonary Morbidity in Infancy after Exposure to Chorioamnionitis in Late Preterm Infants. Ann Am Thorac Soc (2016) 13:867–76. 10.1513/AnnalsATS.201507-411OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Getahun D, Strickland D, Zeiger RS, Fassett MJ, Chen W, Rhoads GG, et al. Effect of chorioamnionitis on early childhood asthma. Arch Pediatr Adolesc Med (2010) 164:187–92. 10.1001/archpediatrics.2009.238 [DOI] [PubMed] [Google Scholar]
- 73. Sindičić Dessardo N, Mustać E, Banac S, Dessardo S. Paths of causal influence from prenatal inflammation and preterm gestation to childhood asthma symptoms. J Asthma Off J Assoc Care Asthma (2019) 56:823–32. 10.1080/02770903.2018.1493603 [DOI] [PubMed] [Google Scholar]
- 74. Prendergast M, May C, Broughton S, Pollina E, Milner AD, Rafferty GF, et al. Chorioamnionitis, lung function and bronchopulmonary dysplasia in prematurely born infants. Arch Dis Child Fetal Neonatal Ed (2011) 96:F270–274. 10.1136/adc.2010.189480 [DOI] [PubMed] [Google Scholar]
- 75. Natarajan G, Shankaran S. Short- and Long-Term Outcomes of Moderate and Late Preterm Infants. Am J Perinatol (2016) 33:305–17. 10.1055/s-0035-1571150 [DOI] [PubMed] [Google Scholar]
- 76. Patel RM. Short- and Long-Term Outcomes for Extremely Preterm Infants. Am J Perinatol (2016) 33:318–28. 10.1055/s-0035-1571202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Dessardo NS, Mustać E, Dessardo S, Banac S, Peter B, Finderle A, et al. Chorioamnionitis and chronic lung disease of prematurity: a path analysis of causality. Am J Perinatol (2012) 29:133–40. 10.1055/s-0031-1295654 [DOI] [PubMed] [Google Scholar]
- 78. Dessardo NS, Dessardo S, Mustać E, Banac S, Petrović O, Peter B. Chronic lung disease of prematurity and early childhood wheezing: is foetal inflammatory response syndrome to blame? Early Hum Dev (2014) 90:493–9. 10.1016/j.earlhumdev.2014.07.002 [DOI] [PubMed] [Google Scholar]
- 79. Sweet DG, Huggett MT, Warner JA, Moss TJM, Kloosterboer N, Halliday HL, et al. Maternal betamethasone and chorioamnionitis induce different collagenases during lung maturation in fetal sheep. Neonatology (2008) 94:79–86. 10.1159/000115949 [DOI] [PubMed] [Google Scholar]
- 80. Curley AE, Sweet DG, MacMahon KJ, O’Connor CM, Halliday HL. Chorioamnionitis increases matrix metalloproteinase-8 concentrations in bronchoalveolar lavage fluid from preterm babies. Arch Dis Child Fetal Neonatal Ed (2004) 89:F61–64. 10.1136/fn.89.1.f61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Curley AE, Sweet DG, Thornton CM, O’Hara MD, Chesshyre E, Pizzotti J, et al. Chorioamnionitis and increased neonatal lung lavage fluid matrix metalloproteinase-9 levels: implications for antenatal origins of chronic lung disease. Am J Obstet Gynecol (2003) 188:871–5. 10.1067/mob.2003.215 [DOI] [PubMed] [Google Scholar]
- 82. Kallapur SG, Presicce P, Senthamaraikannan P, Alvarez M, Tarantal AF, Miller LM, et al. Intra-amniotic IL-1β induces fetal inflammation in rhesus monkeys and alters the regulatory T cell/IL-17 balance. J Immunol Baltim Md 1950 (2013) 191:1102–9. 10.4049/jimmunol.1300270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Rueda CM, Presicce P, Jackson CM, Miller LA, Kallapur SG, Jobe AH, et al. Lipopolysaccharide-Induced Chorioamnionitis Promotes IL-1-Dependent Inflammatory FOXP3+ CD4+ T Cells in the Fetal Rhesus Macaque. J Immunol Baltim Md 1950 (2016) 196:3706–15. 10.4049/jimmunol.1502613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Jackson CM, Wells CB, Tabangin ME, Meinzen-Derr J, Jobe AH, Chougnet CA. Pro-inflammatory immune responses in leukocytes of premature infants exposed to maternal chorioamnionitis or funisitis. Pediatr Res (2017) 81:384–90. 10.1038/pr.2016.232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Black A, Bhaumik S, Kirkman RL, Weaver CT, Randolph DA. Developmental regulation of Th17-cell capacity in human neonates. Eur J Immunol (2012) 42:311–9. 10.1002/eji.201141847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Gayen Nee’ BEtal S, Murthy S, Favara M, Fong G, Chan JSY, Addya S, et al. Histological Chorioamnionitis Induces Differential Gene Expression in Human Cord Blood Mononuclear Leukocytes from Term Neonates. Sci Rep (2019) 9:5862. 10.1038/s41598-019-42205-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Liu W, Liu S, Verma M, Zafar I, Good JT, Rollins D, et al. Mechanism of TH2/TH17-predominant and neutrophilic TH2/TH17-low subtypes of asthma. J Allergy Clin Immunol (2017) 139:1548–1558.e4. 10.1016/j.jaci.2016.08.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Irvin C, Zafar I, Good J, Rollins D, Christianson C, Gorska MM, et al. Increased frequency of dual-positive TH2/TH17 cells in bronchoalveolar lavage fluid characterizes a population of patients with severe asthma. J Allergy Clin Immunol (2014) 134:1175–86.e7. 10.1016/j.jaci.2014.05.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Cosmi L, Liotta F, Annunziato F. Th17 regulating lower airway disease. Curr Opin Allergy Clin Immunol (2016) 16:1–6. 10.1097/ACI.0000000000000227 [DOI] [PubMed] [Google Scholar]
- 90. Dorner RA, Burton VJ, Allen MC, Robinson S, Soares BP. Preterm neuroimaging and neurodevelopmental outcome: a focus on intraventricular hemorrhage, post-hemorrhagic hydrocephalus, and associated brain injury. J Perinatol Off J Calif Perinat Assoc (2018) 38:1431–43. 10.1038/s41372-018-0209-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Linsell L, Malouf R, Morris J, Kurinczuk JJ, Marlow N. Prognostic factors for cerebral palsy and motor impairment in children born very preterm or very low birthweight: a systematic review. Dev Med Child Neurol (2016) 58:554–69. 10.1111/dmcn.12972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Gotardo JW, Volkmer N de FV, Stangler GP, Dornelles AD, Bohrer BB de A, Carvalho CG. Impact of peri-intraventricular haemorrhage and periventricular leukomalacia in the neurodevelopment of preterms: A systematic review and meta-analysis. PloS One (2019) 14:e0223427. 10.1371/journal.pone.0223427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Liu C, Chen Y, Zhao D, Zhang J, Zhang Y. Association Between Funisitis and Childhood Intellectual Development: A Prospective Cohort Study. Front Neurol (2019) 10:612:612. 10.3389/fneur.2019.00612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Xiao D, Zhu T, Qu Y, Gou X, Huang Q, Li X, et al. Maternal chorioamnionitis and neurodevelopmental outcomes in preterm and very preterm neonates: A meta-analysis. PloS One (2018) 13:e0208302. 10.1371/journal.pone.0208302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Straughen JK, Misra DP, Divine G, Shah R, Perez G, VanHorn S, et al. The association between placental histopathology and autism spectrum disorder. Placenta (2017) 57:183–8. 10.1016/j.placenta.2017.07.006 [DOI] [PubMed] [Google Scholar]
- 96. Pappas A, Kendrick DE, Shankaran S, Stoll BJ, Bell EF, Laptook AR, et al. Chorioamnionitis and early childhood outcomes among extremely low-gestational-age neonates. JAMA Pediatr (2014) 168:137–47. 10.1001/jamapediatrics.2013.4248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Morales WJ. The effect of chorioamnionitis on the developmental outcome of preterm infants at one year. Obstet Gynecol (1987) 70:183–6. [PubMed] [Google Scholar]
- 98. Strunk T, Campbell C, Burgner D, Charles A, French N, Sharp M, et al. Histological chorioamnionitis and developmental outcomes in very preterm infants. J Perinatol Off J Calif Perinat Assoc (2019) 39:321–30. 10.1038/s41372-018-0288-3 [DOI] [PubMed] [Google Scholar]
- 99. Miyazaki K, Furuhashi M, Ishikawa K, Tamakoshi K, Hayashi K, Kai A, et al. Impact of chorioamnionitis on short- and long-term outcomes in very low birth weight preterm infants: the Neonatal Research Network Japan. J Matern Fetal Neonatal Med Off J Eur Assoc Perinat Med Fed Asia Ocean Perinat Soc Int Soc Perinat Obstet (2016) 29:331–7. 10.3109/14767058.2014.1000852 [DOI] [PubMed] [Google Scholar]
- 100. Gray PH, Jones P, O’Callaghan MJ. Maternal antecedents for cerebral palsy in extremely preterm babies: a case-control study. Dev Med Child Neurol (2001) 43:580–5. 10.1017/s0012162201001074 [DOI] [PubMed] [Google Scholar]
- 101. Solek CM, Farooqi N, Verly M, Lim TK, Ruthazer ES. Maternal immune activation in neurodevelopmental disorders. Dev Dyn Off Publ Am Assoc Anat (2018) 247:588–619. 10.1002/dvdy.24612 [DOI] [PubMed] [Google Scholar]
- 102. Redline RW, Wilson-Costello D, Borawski E, Fanaroff AA, Hack M. The relationship between placental and other perinatal risk factors for neurologic impairment in very low birth weight children. Pediatr Res (2000) 47:721–6. 10.1203/00006450-200006000-00007 [DOI] [PubMed] [Google Scholar]
- 103. Vinnars M-T, Vollmer B, Nasiell J, Papadogiannakis N, Westgren M. Association between cerebral palsy and microscopically verified placental infarction in extremely preterm infants. Acta Obstet Gynecol Scand (2015) 94:976–82. 10.1111/aogs.12688 [DOI] [PubMed] [Google Scholar]
- 104. Qureshi F, Jacques SM, Bendon RW, Faye-Peterson OM, Heifetz SA, Redline R, et al. Candida funisitis: A clinicopathologic study of 32 cases. Pediatr Dev Pathol Off J Soc Pediatr Pathol Paediatr Pathol Soc (1998) 1:118–24. 10.1007/s100249900014 [DOI] [PubMed] [Google Scholar]
- 105. Kaufman DA, Coggins SA, Zanelli SA, Weitkamp J-H. Congenital Cutaneous Candidiasis: Prompt Systemic Treatment Is Associated With Improved Outcomes in Neonates. Clin Infect Dis Off Publ Infect Dis Soc Am (2017) 64:1387–95. 10.1093/cid/cix119 [DOI] [PubMed] [Google Scholar]
- 106. Barton M, Shen A, O’Brien K, Robinson JL, Davies HD, Simpson K, et al. Early-Onset Invasive Candidiasis in Extremely Low Birth Weight Infants: Perinatal Acquisition Predicts Poor Outcome. Clin Infect Dis Off Publ Infect Dis Soc Am (2017) 64:921–7. 10.1093/cid/cix001 [DOI] [PubMed] [Google Scholar]
- 107. Maki Y, Fujisaki M, Sato Y, Sameshima H. Candida Chorioamnionitis Leads to Preterm Birth and Adverse Fetal-Neonatal Outcome. Infect Dis Obstet Gynecol (2017) 2017:9060138. 10.1155/2017/9060138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Labarrere CA, Hardin JW, Haas DM, Kassab GS. Chronic villitis of unknown etiology and massive chronic intervillositis have similar immune cell composition. Placenta (2015) 36:681–6. 10.1016/j.placenta.2015.03.008 [DOI] [PubMed] [Google Scholar]
- 109. Chi BH, Mudenda V, Levy J, Sinkala M, Goldenberg RL, Stringer JSA. Acute and chronic chorioamnionitis and the risk of perinatal human immunodeficiency virus-1 transmission. Am J Obstet Gynecol (2006) 194:174–81. 10.1016/j.ajog.2005.06.081 [DOI] [PubMed] [Google Scholar]
- 110. Redline RW. Villitis of unknown etiology: noninfectious chronic villitis in the placenta. Hum Pathol (2007) 38:1439–46. 10.1016/j.humpath.2007.05.025 [DOI] [PubMed] [Google Scholar]
- 111. Katzman PJ. Chronic inflammatory lesions of the placenta. Semin Perinatol (2015) 39:20–6. 10.1053/j.semperi.2014.10.004 [DOI] [PubMed] [Google Scholar]
- 112. Perni SC, Predanic M, Predanik M, Cho JE, Baergen RN. Placental pathology and pregnancy outcomes in donor and non-donor oocyte in vitro fertilization pregnancies. J Perinat Med (2005) 33:27–32. 10.1515/JPM.2005.004 [DOI] [PubMed] [Google Scholar]
- 113. Gundogan F, Bianchi DW, Scherjon SA, Roberts DJ. Placental pathology in egg donor pregnancies. Fertil Steril (2010) 93:397–404. 10.1016/j.fertnstert.2008.12.144 [DOI] [PubMed] [Google Scholar]
- 114. Clark DA, McDermott MR, Szewczuk MR. Impairment of host-versus-graft reaction in pregnant mice. II. Selective suppression of cytotoxic T-cell generation correlates with soluble suppressor activity and with successful allogeneic pregnancy. Cell Immunol (1980) 52:106–18. 10.1016/0008-8749(80)90404-9 [DOI] [PubMed] [Google Scholar]
- 115. Chaouat G. Synergy of lipopolysaccharide and inflammatory cytokines in murine pregnancy: alloimmunization prevents abortion but does not affect the induction of preterm delivery. Cell Immunol (1994) 157:328–40. 10.1006/cimm.1994.1231 [DOI] [PubMed] [Google Scholar]
- 116. Duclos AJ, Pomerantz DK, Baines MG. Relationship between decidual leukocyte infiltration and spontaneous abortion in a murine model of early fetal resorption. Cell Immunol (1994) 159:184–93. 10.1006/cimm.1994.1306 [DOI] [PubMed] [Google Scholar]
- 117. Kim CJ, Romero R, Chaemsaithong P, Kim J-S. Chronic inflammation of the placenta: definition, classification, pathogenesis, and clinical significance. Am J Obstet Gynecol (2015) 213:S53–69. 10.1016/j.ajog.2015.08.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Tamblyn JA, Lissauer DM, Powell R, Cox P, Kilby MD. The immunological basis of villitis of unknown etiology - review. Placenta (2013) 34:846–55. 10.1016/j.placenta.2013.07.002 [DOI] [PubMed] [Google Scholar]
- 119. Sun C-CJ, Revell VO, Belli AJ, Viscardi RM. Discrepancy in pathologic diagnosis of placental lesions. Arch Pathol Lab Med (2002) 126:706–9. [DOI] [PubMed] [Google Scholar]
- 120. Heerema-McKenney A, De Paepe ME, Popek EJ. Diagnostic Pathology: Placenta (2019). [Google Scholar]
- 121. Labarrere CA, Faulk WP. Maternal cells in chorionic villi from placentae of normal and abnormal human pregnancies. Am J Reprod Immunol N Y N 1989 (1995) 33:54–9. 10.1111/j.1600-0897.1995.tb01138.x [DOI] [PubMed] [Google Scholar]
- 122. Katzman PJ, Murphy SP, Oble DA. Immunohistochemical analysis reveals an influx of regulatory T cells and focal trophoblastic STAT-1 phosphorylation in chronic villitis of unknown etiology. Pediatr Dev Pathol Off J Soc Pediatr Pathol Paediatr Pathol Soc (2011) 14:284–93. 10.2350/10-09-0910-OA.1 [DOI] [PubMed] [Google Scholar]
- 123. Kim MJ, Romero R, Kim CJ, Tarca AL, Chhauy S, LaJeunesse C, et al. Villitis of unknown etiology is associated with a distinct pattern of chemokine up-regulation in the feto-maternal and placental compartments: implications for conjoint maternal allograft rejection and maternal anti-fetal graft-versus-host disease. J Immunol Baltim Md 1950 (2009) 182:3919–27. 10.4049/jimmunol.0803834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Rudzinski E, Gilroy M, Newbill C, Morgan T. Positive C4d immunostaining of placental villous syncytiotrophoblasts supports host-versus-graft rejection in villitis of unknown etiology. Pediatr Dev Pathol Off J Soc Pediatr Pathol Paediatr Pathol Soc (2013) 16:7–13. 10.2350/12-05-1195-OA.1 [DOI] [PubMed] [Google Scholar]
- 125. Stevens AM. Maternal microchimerism in health and disease. Best Pract Res Clin Obstet Gynaecol (2016) 31:121–30. 10.1016/j.bpobgyn.2015.08.005 [DOI] [PubMed] [Google Scholar]
- 126. Maloney S, Smith A, Furst DE, Myerson D, Rupert K, Evans PC, et al. Microchimerism of maternal origin persists into adult life. J Clin Invest (1999) 104:41–7. 10.1172/JCI6611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Roh EY, Yoon JH, Shin S, Song EY, Chung HY, Park MH. Frequency of fetal-maternal microchimerism: an analysis of the HLA-DRB1 gene in cord blood and maternal sample pairs. J Matern Fetal Neonatal Med Off J Eur Assoc Perinat Med Fed Asia Ocean Perinat Soc Int Soc Perinat Obstet (2017) 30:2613–9. 10.1080/14767058.2016.1259308 [DOI] [PubMed] [Google Scholar]
- 128. Artlett CM, Ramos R, Jiminez SA, Patterson K, Miller FW, Rider LG. Chimeric cells of maternal origin in juvenile idiopathic inflammatory myopathies. Childhood Myositis Heterogeneity Collaborative Group. Lancet Lond Engl (2000) 356:2155–6. 10.1016/s0140-6736(00)03499-1 [DOI] [PubMed] [Google Scholar]
- 129. Artlett CM, Sassi-Gaha S, Ramos RC, Miller FW, Rider LG. Chimeric cells of maternal origin do not appear to be pathogenic in the juvenile idiopathic inflammatory myopathies or muscular dystrophy. Arthritis Res Ther (2015) 17:238. 10.1186/s13075-015-0732-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Khong TY, Bendon RW, Qureshi F, Redline RW, Gould S, Stallmach T, et al. Chronic deciduitis in the placental basal plate: definition and interobserver reliability. Hum Pathol (2000) 31:292–5. 10.1016/s0046-8177(00)80241-5 [DOI] [PubMed] [Google Scholar]
- 131. Lee J, Romero R, Xu Y, Kim J-S, Park JY, Kusanovic JP, et al. Maternal HLA panel-reactive antibodies in early gestation positively correlate with chronic chorioamnionitis: evidence in support of the chronic nature of maternal anti-fetal rejection. Am J Reprod Immunol N Y N 1989 (2011) 66:510–26. 10.1111/j.1600-0897.2011.01066.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Kohler PF, Farr RS. Elevation of cord over maternal IgG immunoglobulin: evidence for an active placental IgG transport. Nature (1966) 210:1070–1. 10.1038/2101070a0 [DOI] [PubMed] [Google Scholar]
- 133. Virella G, Silveira Nunes MA, Tamagnini G. Placental transfer of human IgG subclasses. Clin Exp Immunol (1972) 10:475–8. [PMC free article] [PubMed] [Google Scholar]
- 134. Freda VJ, Gorman JG, Pollack W, Bowe E. Prevention of Rh hemolytic disease–ten years’ clinical experience with Rh immune globulin. N Engl J Med (1975) 292:1014–6. 10.1056/NEJM197505082921906 [DOI] [PubMed] [Google Scholar]
- 135. Mueller-Eckhardt C, Kiefel V, Grubert A, Kroll H, Weisheit M, Schmidt S, et al. 348 cases of suspected neonatal alloimmune thrombocytopenia. Lancet Lond Engl (1989) 1:363–6. 10.1016/s0140-6736(89)91733-9 [DOI] [PubMed] [Google Scholar]
- 136. Dörner T, Chaoui R, Feist E, Göldner B, Yamamoto K, Hiepe F. Significantly increased maternal and fetal IgG autoantibody levels to 52 kD Ro (SS-A) and La(SS-B) in complete congenital heart block. J Autoimmun (1995) 8:675–84. 10.1006/jaut.1995.0050 [DOI] [PubMed] [Google Scholar]
- 137. Kim CJ, Romero R, Kusanovic JP, Yoo W, Dong Z, Topping V, et al. The frequency, clinical significance, and pathological features of chronic chorioamnionitis: a lesion associated with spontaneous preterm birth. Mod Pathol Off J U S Can Acad Pathol Inc (2010) 23:1000–11. 10.1038/modpathol.2010.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Jacques SM, Qureshi F. Chronic chorioamnionitis: a clinicopathologic and immunohistochemical study. Hum Pathol (1998) 29:1457–61. 10.1016/s0046-8177(98)90016-8 [DOI] [PubMed] [Google Scholar]
- 139. Maymon E, Romero R, Bhatti G, Chaemsaithong P, Gomez-Lopez N, Panaitescu B, et al. Chronic inflammatory lesions of the placenta are associated with an up-regulation of amniotic fluid CXCR3: A marker of allograft rejection. J Perinat Med (2018) 46:123–37. 10.1515/jpm-2017-0042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Boyd TK, Redline RW. Chronic histiocytic intervillositis: a placental lesion associated with recurrent reproductive loss. Hum Pathol (2000) 31:1389–96. 10.1053/hupa.2000.19454 [DOI] [PubMed] [Google Scholar]
- 141. Bos M, Nikkels PGJ, Cohen D, Schoones JW, Bloemenkamp KWM, Bruijn JA, et al. Towards standardized criteria for diagnosing chronic intervillositis of unknown etiology: A systematic review. Placenta (2018) 61:80–8. 10.1016/j.placenta.2017.11.012 [DOI] [PubMed] [Google Scholar]
- 142. Cesselin F, Antreassian J, Schaffhauser O, Lagoguey M, Desgrez P. 5 minutes incubation for the radioimmunologic determination of somatomammotrophic chorionic hormone. Ann Biol Clin (Paris) (1976) 34:41–5. [PubMed] [Google Scholar]
- 143. Taweevisit M, Sukpan K, Siriaunkgul S, Thorner PS. Chronic histiocytic intervillositis with cytomegalovirus placentitis in a case of hydrops fetalis. Fetal Pediatr Pathol (2012) 31:394–400. 10.3109/15513815.2012.659405 [DOI] [PubMed] [Google Scholar]
- 144. Mekinian A, Costedoat-Chalumeau N, Masseau A, Botta A, Chudzinski A, Theulin A, et al. Chronic histiocytic intervillositis: outcome, associated diseases and treatment in a multicenter prospective study. Autoimmunity (2015) 48:40–5. 10.3109/08916934.2014.939267 [DOI] [PubMed] [Google Scholar]
- 145. Hussein K, Stucki-Koch A, Kreipe H, Feist H. Expression of Toll-Like Receptors in Chronic Histiocytic Intervillositis of the Placenta. Fetal Pediatr Pathol (2015) 34:407–12. 10.3109/15513815.2015.1095259 [DOI] [PubMed] [Google Scholar]
- 146. Hussein K, Stucki-Koch A, Müller AM, Arnold R, Kreipe H, Feist H. Complement receptor-associated CD163+/CD18+/CD11c+/CD206-/CD209- expression profile in chronic histiocytic intervillositis of the placenta. Placenta (2019) 78:23–8. 10.1016/j.placenta.2019.02.007 [DOI] [PubMed] [Google Scholar]
- 147. Capuani C, Meggetto F, Duga I, Danjoux M, March M, Parant O, et al. Specific infiltration pattern of FOXP3+ regulatory T cells in chronic histiocytic intervillositis of unknown etiology. Placenta (2013) 34:149–54. 10.1016/j.placenta.2012.12.004 [DOI] [PubMed] [Google Scholar]
- 148. Katzman PJ, Li L, Wang N. Identification of Fetal Inflammatory Cells in Eosinophilic/T-cell Chorionic Vasculitis Using Fluorescent In Situ Hybridization. Pediatr Dev Pathol Off J Soc Pediatr Pathol Paediatr Pathol Soc (2015) 18:305–9. 10.2350/14-12-1585-OA.1 [DOI] [PubMed] [Google Scholar]
- 149. Jacques SM, Qureshi F, Kim CJ, Lee JH, Giorgadze T, Mittal P, et al. Eosinophilic/T-cell chorionic vasculitis: a clinicopathologic and immunohistochemical study of 51 cases. Pediatr Dev Pathol Off J Soc Pediatr Pathol Paediatr Pathol Soc (2011) 14:198–205. 10.2350/10-07-0867-OA.1 [DOI] [PubMed] [Google Scholar]
- 150. Katzman PJ, Oble DA. Eosinophilic/T-cell chorionic vasculitis and chronic villitis involve regulatory T cells and often occur together. Pediatr Dev Pathol Off J Soc Pediatr Pathol Paediatr Pathol Soc (2013) 16:278–91. 10.2350/12-10-1258-OA.1 [DOI] [PubMed] [Google Scholar]
- 151. Derricott H, Jones RL, Greenwood SL, Batra G, Evans MJ, Heazell AEP. Characterizing Villitis of Unknown Etiology and Inflammation in Stillbirth. Am J Pathol (2016) 186:952–61. 10.1016/j.ajpath.2015.12.010 [DOI] [PubMed] [Google Scholar]
- 152. Gervasi M-T, Romero R, Bracalente G, Erez O, Dong Z, Hassan SS, et al. Midtrimester amniotic fluid concentrations of interleukin-6 and interferon-gamma-inducible protein-10: evidence for heterogeneity of intra-amniotic inflammation and associations with spontaneous early (<32 weeks) and late (>32 weeks) preterm delivery. J Perinat Med (2012) 40:329–43. 10.1515/jpm-2012-0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Contro E, deSouza R, Bhide A. Chronic intervillositis of the placenta: a systematic review. Placenta (2010) 31:1106–10. 10.1016/j.placenta.2010.10.005 [DOI] [PubMed] [Google Scholar]
- 154. Chen A, Roberts DJ. Placental pathologic lesions with a significant recurrence risk - what not to miss! APMIS Acta Pathol Microbiol Immunol Scand (2018) 126:589–601. 10.1111/apm.12796 [DOI] [PubMed] [Google Scholar]
- 155. Mattuizzi A, Sauvestre F, André G, Poingt M, Camberlein C, Carles D, et al. Adverse perinatal outcomes of chronic intervillositis of unknown etiology: an observational retrospective study of 122 cases. Sci Rep (2020) 10:12611. 10.1038/s41598-020-69191-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Ozawa N, Yamaguchi K, Shibata M, Sugibayashi R, Yagi H, Sago H, et al. Chronic histiocytic intervillositis in three consecutive pregnancies in a single patient: Differing clinical results and pathology according to treatment used. J Obstet Gynaecol Res (2017) 43:1504–8. 10.1111/jog.13404 [DOI] [PubMed] [Google Scholar]
- 157. Vardi L, Paterson H, Hung NA. Successful pregnancy following treatment of recurrent chronic histiocytic intervillositis. BMJ Case Rep (2017) 2017. 10.1136/bcr-2016-217886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Lee J, Kim J-S, Park JW, Park C-W, Park JS, Jun JK, et al. Chronic chorioamnionitis is the most common placental lesion in late preterm birth. Placenta (2013) 34:681–9. 10.1016/j.placenta.2013.04.014 [DOI] [PubMed] [Google Scholar]
- 159. Roescher AM, Timmer A, Erwich JJHM, Bos AF. Placental pathology, perinatal death, neonatal outcome, and neurological development: a systematic review. PloS One (2014) 9:e89419. 10.1371/journal.pone.0089419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Kovo M, Ganer Herman H, Gold E, Bar J, Schreiber L. Villitis of unknown etiology - prevalence and clinical associations. J Matern Fetal Neonatal Med Off J Eur Assoc Perinat Med Fed Asia Ocean Perinat Soc Int Soc Perinat Obstet (2016) 29:3110–4. 10.3109/14767058.2015.1114090 [DOI] [PubMed] [Google Scholar]
- 161. Nowak C, Joubert M, Jossic F, Masseau A, Hamidou M, Philippe H-J, et al. Perinatal prognosis of pregnancies complicated by placental chronic villitis or intervillositis of unknown etiology and combined lesions: About a series of 178 cases. Placenta (2016) 44:104–8. 10.1016/j.placenta.2016.04.017 [DOI] [PubMed] [Google Scholar]
- 162. Parant O, Capdet J, Kessler S, Aziza J, Berrebi A. Chronic intervillositis of unknown etiology (CIUE): relation between placental lesions and perinatal outcome. Eur J Obstet Gynecol Reprod Biol (2009) 143:9–13. 10.1016/j.ejogrb.2008.06.012 [DOI] [PubMed] [Google Scholar]
- 163. Bos M, Harris-Mostert ETMS, van der Meeren LE, Baelde JJ, Williams DJ, Nikkels PGJ, et al. Clinical outcomes in chronic intervillositis of unknown etiology. Placenta (2020) 91:19–23. 10.1016/j.placenta.2020.01.001 [DOI] [PubMed] [Google Scholar]
- 164. Peterson JA, McFarland JG, Curtis BR, Aster RH. Neonatal alloimmune thrombocytopenia: pathogenesis, diagnosis and management. Br J Haematol (2013) 161:3–14. 10.1111/bjh.12235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Althaus J, Weir EG, Askin F, Kickler TS, Blakemore K. Chronic villitis in untreated neonatal alloimmune thrombocytopenia: an etiology for severe early intrauterine growth restriction and the effect of intravenous immunoglobulin therapy. Am J Obstet Gynecol (2005) 193:1100–4. 10.1016/j.ajog.2005.06.043 [DOI] [PubMed] [Google Scholar]
- 166. Torrance HL, Bloemen MCT, Mulder EJH, Nikkels PGJ, Derks JB, de Vries LS, et al. Predictors of outcome at 2 years of age after early intrauterine growth restriction. Ultrasound Obstet Gynecol Off J Int Soc Ultrasound Obstet Gynecol (2010) 36:171–7. 10.1002/uog.7627 [DOI] [PubMed] [Google Scholar]
- 167. Redline RW. Severe fetal placental vascular lesions in term infants with neurologic impairment. Am J Obstet Gynecol (2005) 192:452–7. 10.1016/j.ajog.2004.07.030 [DOI] [PubMed] [Google Scholar]
- 168. Harteman JC, Nikkels PGJ, Benders MJNL, Kwee A, Groenendaal F, de Vries LS. Placental pathology in full-term infants with hypoxic-ischemic neonatal encephalopathy and association with magnetic resonance imaging pattern of brain injury. J Pediatr (2013) 163:968–95.e2. 10.1016/j.jpeds.2013.06.010 [DOI] [PubMed] [Google Scholar]
- 169. Korzeniewski SJ, Romero R, Cortez J, Pappas A, Schwartz AG, Kim CJ, et al. A “multi-hit” model of neonatal white matter injury: cumulative contributions of chronic placental inflammation, acute fetal inflammation and postnatal inflammatory events. J Perinat Med (2014) 42:731–43. 10.1515/jpm-2014-0250 [DOI] [PMC free article] [PubMed] [Google Scholar]