Abstract
Objectives
This study investigated the effects of physically damaged and resin-contaminated tips on radiant emittance, comparing them with new undamaged, non-contaminated tips using 3 pieces of spectrophotometric laboratory equipment.
Materials and Methods
Nine tips with damage and/or resin contaminants from actual clinical situations were compared with a new tip without damage or contamination (control group). The radiant emittance was recorded using 3 spectrophotometric methods: a laboratory-grade thermopile, a laboratory-grade integrating sphere, and a portable light collector (checkMARC).
Results
A significant difference between the laboratory-grade thermopile and the laboratory-grade integrating sphere was found when the radiant emittance values of the control or damaged/contaminated tips were investigated (p < 0.05), but both methods were comparable to checkMARC (p > 0.05). Regardless of the method used to quantify the light output, the mean radiant emittance values of the damaged/contaminated tips were significantly lower than those of the control (p < 0.05). The beam profile of the damaged/contaminated tips was less homogeneous than that of the control.
Conclusions
Damaged/contaminated tips can reduce the radiant emittance output and the homogeneity of the beam, which may affect the energy delivered to composite restorations. The checkMARC spectrophotometer device can be used in dental offices, as it provided values close to those produced by a laboratory-grade integrated sphere spectrophotometer. Dentists should assess the radiant emittance of their light-curing units to ensure optimal curing in photoactivated, resin-based materials.
Keywords: Dental, Dental materials, Light curing, Operative, Polymerization, Resin composite
INTRODUCTION
The use of resin composite restorations has remarkably increased due to significant improvements in material properties and appearance in the last 2 decades [1]. Despite the advancement of resin composite materials, the replacement rate in clinical practice is a prominent issue [2]. Restoration replacement accounts for 60% of the procedures performed in dental practice [3]. The replacement of restorations can lead to the additional removal of the tooth structure and may cause more stress to the pulp [4]. Several factors can have a significant influence on the longevity and clinical performance of resin composites, including the efficiency and quality of light-curing units [5].
Light-curing units should generate a proper amount of radiant emittance, in a specific wavelength range, to excite the photoinitiators responsible for the polymerization reaction [6]. Resin composites that receive adequate radiant exposure may provide long-term clinical service [7,8,9]. The amount of radiant exposure energy received by resin composites depends on several factors related to the light-curing unit itself, the operator, and the materials used [9,10,11]. Resin composite restorations that receive low radiant exposure might have inferior mechanical properties, low dimensional stability, increased biofilm formation, and color change over time, which may lead to the premature failure of such restorations [12].
Light-curing units may differ in their characteristics and capacity of radiant emittance they generate [8]. Various types of equipment can be used to check the performance of light-curing unit output on a regular basis [13]. Dental hand-held radiometers are generally the first choice commercially available for dentists. Dental radiometers can quantify an electric response that varies with incident light. Previous studies over the last 2 decades have identified factors associated with the inaccuracy and lack of full characterization of measurements of the energy delivered at the end of the tip (radiant emittance) [10,14]. For this reason, considerable efforts have been made in recent years to develop more accurate methods based on spectrophotometers to measure the spectral radiant emittance of light-curing units. These methods identify variations in the distribution of beam intensity across the light-curing tip that dentists use. A reliable spectrophotometry-based method can help clinicians to recognize light-curing units with low performance, and then to fix or replace them to assure adequate energy delivery when placing light-cured restorations. Among the spectrophotometers that are currently available on the market, the checkMARC light collector is portable, facilitating its use in dental settings. A comparative assessment of this portable spectrophotometer with other spectrophotometric devices would help us to understand the quality of its outcomes.
Furthermore, in the hectic routine of daily practice, where very often more 60% of procedures require light-curing, abrasions, striations, and chipping of the light-curing tip may lead to the formation of surface microcracks and sometimes craters on the surface tip [15]. Another route through which tips are damaged is the presence of contaminants and chemical deterioration by disinfectants on the tip surface. Generally speaking, contamination is a removable defect on the tip surface or on the barriers used over the tip surface, such as remaining dental materials and aerosols [16]. Most commonly, composite is the main contaminant [17,18,19,20,21]. However, dental bonding and other translucent materials are omnipresent and usually are not visible to the naked eye. Residual composite is opaque and cannot transmit light. Even small opaque residues obstruct the light transmitted by the tip, just as the moon blocks the sun during a solar eclipse [22,23,24,25]. These defects and contaminants affect the optical quality of the tip surface, regardless of the type of materials that it is made of, and may lead to a drop in the optical transmission [26,27,28]. These factors underscore the importance of developing a suitable method of recording the radiant emittance values of damaged/contaminated light-curing tips.
Thus, the purpose of this study was to investigate the effects of physically damaged and resin-contaminated tips on radiant emittance using 3 pieces of accurate laboratory equipment based on spectrophotometers. The null hypotheses tested were (I) that the radiant emittance values would not be influenced by the spectrophotometric methods used, and (II) that the radiant emittance values would not be influenced by the presence of damage and contaminants on the tip surface.
MATERIALS AND METHODS
Study design
Nine tips with damage or resin contaminants with the same tip design and diameter (ø = 11 mm) from different light-curing units (Demetron VCL Optilux 401 Dental Curing Light, Kerr, Brea, CA, USA) used for clinical service were selected for this study.
To avoid the influence of the clinical age of the donor units, the light tips were removed from their respective light-curing units and assembled with a new unit (Demetron VCL Optilux 401 Dental Curing Light, Kerr; power input: 160 VA, lamp: 12 V, 80 W) before assessment.
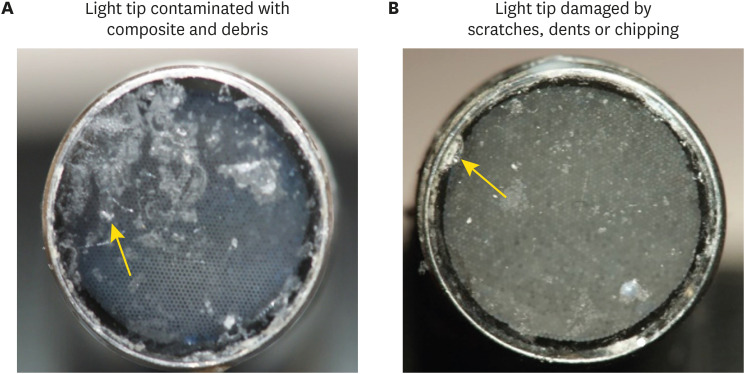
Figure 1 illustrates the appearance of the tip surface with damage or contamination. Light-curing units of the corresponding design without damage that were free of contamination were used as a control group. The radiant emittance values were recorded via a laboratory-grade integrating sphere, laboratory-grade thermopile, and portable light collector (checkMARC). Three measures were performed for each sample using the 3 different devices. Figure 2 illustrates the 3 devices used in this study. Damaged/contaminated light tips of light-curing units (experimental) and light-curing units with no damage or contamination (control) were assessed.
Figure 1. Contaminated and damaged light tips. (A) Light tip contaminated with resin composite and debris. (B) Damaged light tip by scratches, dents, and chipping.
Figure 2. The radiant emittance values were recorded via spectrophotometric methods. (A) A laboratory-grade thermopile system. (B) A laboratory-grade integrating sphere spectrophotometer. (C) An in-office checkMARC portable spectrophotometer.
Laboratory-grade thermopile system
A laboratory grade thermopile with a power of 10 W (S310C, Thor Labs, Newton, NJ, USA) was used to quantify the amount of radiant emittance produced by each light-curing unit. Radiant emittance power between 400 and 490 nm is expected to be measured by this device with an accuracy of ± 5% [29]. The distance between the tip and the sensor was minimized to the extent possible. The amount of radiant emittance was then calculated as the power divided by the cross-sectional surface area (cm2) of the tips [29].
Laboratory-grade integrating sphere spectrophotometer
The output of each light-curing unit was analyzed using a 6-inch integrating sphere (Labsphere, North Sutton, NH, USA) attached to a fiberoptic spectrophotometer (USB 4000, Ocean Optics, Dunedin, FL, USA) [30]. The spectrophotometer was calibrated using a National Institute of Standards and Technology (Gaithersburg, MD, USA) traceable light source (Labsphere). Each light-curing unit tip was placed at the entrance of the sphere to calculate the light-curing unit output using Spectrasuite v2.0.162 software (Ocean Optics). The radiant emittance values were obtained from the power output emitted between 340 nm and 550 nm [31].
Portable light collector (checkMARC)
The portable spectrophotometer analyzed in this study (Managing Accurate Resin Curing; checkMARC, BlueLight Analytics, Halifax, NS, Canada) was a system connected to a laboratory-grade spectroradiometer (USB 4000, Ocean Optics). The sensor is connected to a spectrophotometer with a bifurcated fiber optic cable. A pre-configured laptop computer with custom software (MARC Light Collector) was used to collect and export the data. Based on the light-curing unit selected in the software, checkMARC can quantify the radiant emittance of the light-curing unit [32,33].
Beam profile
A laser beam profiler camera with a 50-mm focal length lens (USB-L070, Ophir-Spiricon, Logan, UT, USA) was used to capture the radiant emittance distribution across the light-curing tip. The same distance between the camera and the light was maintained and focused onto the frosted surface plane of a translucent, ground-glass target (DG2X2-1500, Thor Labs) as previously described [29]. After 10 seconds of light emission by the light-curing unit, the light distribution was captured using the beam analyzer software (BeamGage v5.11, Ophir-Spiricon), taking into consideration the mean radiant emittance values produced by the laboratory-grade integrating sphere spectrophotometer [34,35]. Graphics software (Origin Pro v9.0, OriginLab, Northampton, MA, USA) was used to export the numerical data of each image. The software was used to generate an average radiant emittance value for each square from the approximately 3,200 individual pixels in the square. The areas with high or low radiant emittance were then recorded [36].
Statistical analysis
Data normality was evaluated via the Shapiro-Wilk test (SigmaPlot, version 12.0, Systat Software, Inc., San Jose, CA, USA). Analysis of variance for ranks and the Dunn test were used to compare the radiant emittance values generated by the 3 spectrophotometric methods. The Student's t-test was used to compare the radiant emittance from the tip without damage and/or resin contaminants (control) to that of the tips with damage and/or resin contaminants within the laboratory-grade thermopile group and the laboratory-grade integrating sphere group. The Mann-Whitney U test was used to compare the radiant emittance from the tip without damage and/or resin contaminants (control) to that of the tips with damage and/or resin contaminants within the checkMARC group. A p value < 0.05 was considered to indicate statistical significance.
RESULTS
Radiant emittance by spectrophotometric methods
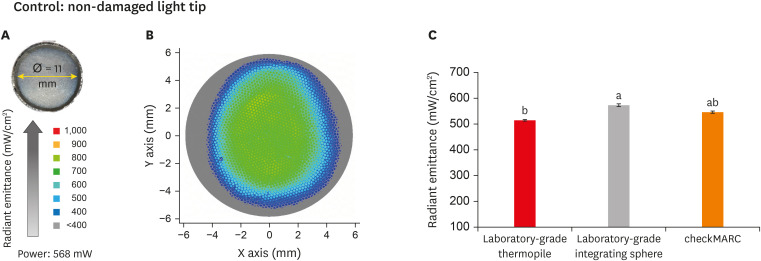
Figure 3A displays the appearance of the control tip and the measured active tip diameter. Figure 3B shows a representative image of the light beam radiant emittance profile for the control tip. The color bar on the right shows the visual representation of radiant emittance expressed in mW/cm2 corresponding to the colors seen. The profile presents an abundance of the green color, representative of the range of 600–700 mW/cm2, with scattered dots in a hot color (yellow-orange) representing radiance with emissions in the range of 800–900 mW/cm2. The profile beam image also shows peripheral areas in blue and gray colors representative of radiance at 400 and below 400 mW/cm2, respectively. In Figure 3C, the radiant emittance assessment of the non-damaged and non-contaminated tips by the 3 spectrophotometric methods are shown. The radiant emittance assessed by the thermopile for the control tip was significantly (p < 0.05) lower than the values for the laboratory-grade sphere. No significant difference was found between the checkMARC spectrophotometer and the other methods (p > 0.05).
Figure 3. Radiant emittance levels of the control tip. (A) An appearance of the control tip and the measured active tip diameter. (B) Representative image of the light beam radiant emittance profile for the control tip. The color bar on the right shows the visual representation of radiant emittance expressed in mW per cm2 corresponding to the colors seen. The profile presents an abundance of the green color representative of the range of 600–700 mW/cm2 with scattered dots in a hot color (yellow-orange) representing radiance with emission in the range of 800–900 mW/cm2. The profile beam image also shows peripheral areas in blue and gray colors, representative of radiance at 400 and below 400 mW/cm2, respectively. (C) Radiant emittance assessment of non-damaged and non-contaminated tips by the 3 spectrophotometric methods. No significant difference was found between the checkMARC spectrophotometer and the other methods (p < 0.05). Values with different letters are significantly different (p < 0.05).
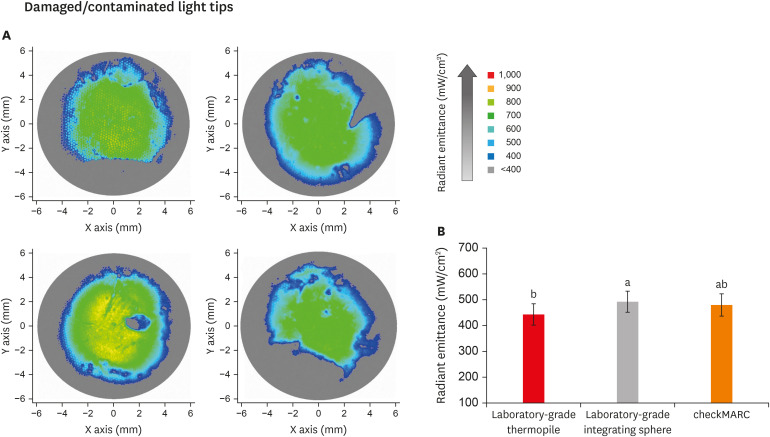
Figure 4A displays representative images of the beam profile of the damaged/contaminated light tips. The colors in the beam profile represent the amount of radiant emittance captured at each area across the light-curing unit tip. The higher the normalized radiant emittance value, the better performance of the light-curing unit (high-value areas: light green). The color bar on the right shows the visual representation of radiant emittance, expressed in mW/cm2, corresponding to the colors seen. The profile beam images for damaged and contaminated tips show an increased abundance of peripheral and central areas in blue and gray colors, representative of radiance at 400 and below 400 mW/cm2, respectively. The surface area of the radiant emittance value of > 700 mW/cm2 across the tips is smaller for the damaged/contaminated light tips compared to the control. The surface area with a lower radiant emittance (400–534 mW/cm2) is more prominent and can be seen more extensively in the peripheries and as small dots in the middle of the tip.
Figure 4. Radiant emittance levels of the damaged/contaminated tips. (A) Representative images of the beam profile of damaged/contaminated light tips. The colors in the beam profile represent the amount of radiant emittance captured at each area across the light-curing unit tip. The higher the normalized radiant emittance value, the better the performance of the light-curing unit (high-value areas: light green). The color bar on the right shows the visual representation of radiant emittance expressed in mW per cm2 corresponding to the colors seen. The profile beam images for damaged/contaminated tips show an increased abundance of peripheral and central areas in blue and gray colors representative of radiance at 400 and below 400 mW/cm2, respectively. In B, the radiant emittance assessments of damaged/contaminated tips (n = 9) by the 3 spectrophotometric methods are shown. The radiant emittance assessed by the thermopile was significantly (p < 0.05) lower than the values for the laboratory-grade sphere. No significant differences were found between the checkMARC spectrophotometer and the other methods (p < 0.05). Values with different letters are significantly different (p < 0.05).
In Figure 4B, the radiant emittance assessment of the damaged/ contaminated tips by the 3 spectrophotometric methods is shown. The mean radiant emittance value measured using the thermopile (456.1 ± 39.8 mW/cm2) was significantly (p < 0.05) lower than measured using the laboratory-grade sphere (502.0 ± 45.8 mW/cm2). No significant difference was found between checkMARC (488.9 ± 40.5 mW/cm2) and the other spectrophotometric methods. The lowest reported values in a damaged/contaminated tip were 376, 407, and 412 mW/cm2 for thermopile, laboratory-grade sphere, and checkMARC, respectively, using the same tip. The highest reported values were 489, 538, and 520 mW/cm2 when the thermopile, laboratory-grade sphere, and checkMARC, respectively, were used.
Effect of damaged/contaminated light-curing unit tips on radiant emittance
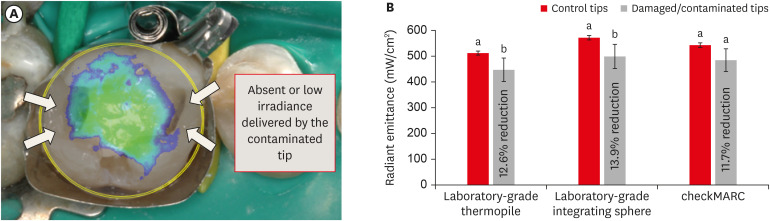
Figure 5A presents a representative illustration of a clinical restorative scenario where a mesio-distal occlusal composite restoration is placed and cured by a damaged tip. The overlapping image of the non-uniform beam profile over the composite restoration should be noted. A lack of homogeneity in the produced radiant emittance profile could compromise the optimal polymerization of the restoration. The black arrows indicate the areas that may not receive appropriate radiant emittance.
Figure 5. Impact of damage/contamination on radiant emittance. (A) Representative illustration of a clinical restorative scenario where a mesio-distal occlusal composite restoration is placed and cured by a damaged tip. The overlapping image of the non-uniform beam profile over the composite restoration should be noted. The lack of homogeneity in the produced radiant emittance profile could compromise the optimal polymerization of the restoration. The black arrows indicate areas that may not receive the radiant emittance appropriately. (B) Radiant emittance results and percentage reduction of radiance relative to the control. On the first y-axis, the bar plot shows radiant emittance results expressed in mW/cm2. Each method was used to capture the radiant emittance values from damaged/contaminated tips and compared to those with no damage or contamination (control). The difference in the power output between the damaged/contaminated and control tips was significant when the thermopile and the laboratory-grade sphere spectrophotometer were used. Damaged/contaminated tips significantly demonstrated lower radiant emittance values than the control (p < 0.05). In this plot, values with different letters are significantly different (p < 0.05).
In Figure 5B, the radiant emittance results of each spectrophotometric method in both damaged/contaminated and control tips are shown. Each method was used to capture the radiant emittance values from damaged/contaminated tips and compared to those of the non-damaged/non-contaminated tips (control). The difference in the power output between the damaged/contaminated and control tips was significant when the thermopile and laboratory-grade sphere spectrophotometer were used. The damaged/contaminated tips significantly demonstrated lower radiant emittance values than the control (p < 0.05). In this plot, values with dissimilar letters are significantly different (p < 0.05). However, using the checkMARC portable device, no significant difference was found between the damaged/contaminated and control tips (p = 0.05). The amount of reduction observed with the thermopile and laboratory-grade sphere spectrophotometer was 12.6% and 13.9%, respectively. For the checkMARC portable collector, the reduction in the percentage was 11.7% in relation to the control.
DISCUSSION
Optimal radiant emittance is necessary to transfer the required energy both to the top of a resin composite restoration and throughout its bulk. Restorations with low radiant exposure are associated with compromised mechanical properties, placing them at high risk of failure [8,9,10]. This study is the first to report possible differences between different spectrophotometric methods in capturing radiant emittance from damaged/contaminated tips. This study also found that damaged/contaminated tips had lower radiant emittance than their non-damaged and non-contaminated counterparts. Several studies have reported variations in radiant emittance when evaluating light-curing units at institutional and private dental clinics. In one study, contaminated light-curing tips were significantly associated with reduced irradiance [19], which is similar to what was found in our study. El-Mowafy et al. [21] reported that around 12% of the light-curing units in 100 dental offices had low radiant emittance. Another study found that approximately 46.1% of the screened light-curing units in 295 dental offices had radiant emittance of less than 300 mW/cm2 [16]. Reduced power output was found in 48% [18] and 30% [17] of the screened light-curing units in other investigations.
In this study, less beam uniformity was observed from damaged/contaminated light tips than from the control. The beam profile is a qualitative assessment of the light beam produced by a light-curing unit. Analyzing the beam profile enables reporting of the radiant emittance recorded by each pixel on the captured image [29]. A homogeneous beam profile is critical for ensuring an equal distribution of the energy across the tip [9]. An inhomogeneous beam profile is associated with unevenly distributed radiant energy exposure to the intended surface, which may compromise the integrity of restorations in some areas [9]. Furthermore, it is critical to realize that the proper radiant emittance output does not always accompany an appropriate beam profile, which may affect the polymerization of such restorations in certain areas over the resin composite surface [37,38]. However, the consequences of an inhomogeneous beam profile could be overestimated as the transmitted light penetrates the resin composite, and the amount of scattering could vary depending on the type of light and material [39]. In response to this limitation, microhardness has been suggested as a more clinically relevant method to assess homogeneity [29].
Several methods have been suggested to measure the amount of radiant emittance in dental clinics. The use of a radiometer was found to be unreliable [26,27,28]. Moreover, the light-curing unit tips cannot fit into the narrow apertures of a radiometer's detector. As the light is not uniform, the radiant emittance is influenced by the position of the tip [28]. Thus, it is necessary to develop another method to quantify the power output. In this study, radiant emittance was quantified using 3 different methods. Spectrophotometric methods are more accurate than radiometers, as spectrophotometers have internal sensors that can instantaneously measure the light and divide the incoming signal across a detector array, which measures the signal in small bands or individual wavelengths based on the resolution of the system (e.g., with 1 nm, 5 nm, or 10 nm resolution). A laboratory-grade thermopile was used, following the current ISO standard 10650 [40], while the laboratory-grade integrating sphere was used as the gold standard [25]. The checkMARC device was recently introduced as a spectrophotometric method to assess the amount of radiant emittance and radiant exposure. This spectrophotometer tool has a light collector chamber to receive the transmitted light. The chamber is linked to a fiber optic connector to the built-in spectrophotometer.
Among the spectrophotometric methods, we included a portable spectrophotometer (the checkMARC light collector). The main advantage of using checkMARC is its ability to measure the radiant emittance of most available light-curing units while being convenient to keep and use inside a dental office. In this study, no significant differences in radiant emittance values were found between the checkMARC and the laboratory-grade integrating sphere. In contrast, the difference between the laboratory-grade integrating sphere and the thermopile was substantial. These results are in agreement with another study where the checkMARC spectrophotometer was more comparable than a thermopile to a laboratory-grade integrating sphere for 8 commercially available light-curing units [25].
Our results underscore the importance of investigating possible differences in the methods that are used. Moreover, all of the spectrophotometer methods used in this study to evaluate the light-curing tips could be applicable to monitor the quality of the light-curing tips once all light-curing units become portable. However, we understand that not all dentists may have access to research labs or direct access to laboratory-level devices. For this reason, we compared the performance of a portable spectrophotometer, aiming indirectly to highlight a possible translation of accurate lab devices to the clinical setting.
The rationale for using laboratory-level spectrophotometric devices is based on a comparative assessment of their outcomes regarding the radiant emittance of damaged/contaminated tips using a more reliable methodology.
It is essential to provide information about the possible differences among these methods, not only for clinicians, but also to assist in the standardization of protocols for laboratory research. The comparative assessment of this portable spectrophotometer with other spectrophotometric devices helps to understand the quality of its outcomes. Although the ISO standard recommends using a thermopile as a reference, this method presents some limitations, including its low response in recording short-time radiation and the lack of quantification of radiant exposure energy and spectral characteristics [25].
Furthermore, the current ISO standard can only estimate the radiant emittance between 400 and 515 nm. As a result, radiant emittance below 400 nm cannot be recorded, and subsequently, the protocol cannot be used to assess poly-wave light-curing units [25]. Based on these findings, the use of checkMARC or a laboratory-grade integrating sphere spectrophotometer seems more reliable and effective for monitoring the performance of light-curing units than using a thermopile.
To ensure the best use of light-curing units, dentists should monitor the output of their devices frequently, and carry out the optimum infection control protocols recommended by the manufacturer to keep the device clean and non-contaminated [41,42]. Studies in the literature contain divergent opinions concerning disposable barriers for infection control. A previous study suggested a reduction in radiant emittance of approximately 10% [43]. In contrast, others suggested that there was no marked detrimental effect on polymerization [44]. Using disinfectants to clean the light tips also reduces the reflector efficiency of the tips [23]. Therefore, the use of autoclavable light tips has been recommended as a gold standard to ensure optimum cleaning [23]. Additionally, dentists should follow manufacturers' guidelines to disinfect light-curing tips appropriately.
Future studies may consider light-curing tips and expand the groups to include as many commercially available brands and different types of damaged tips as possible. It should also be noted that this study was performed at only a single institution. Studies conducted at different institutions or in private practice may yield more insights into the effect of light tip damage and contamination on radiant emittance.
CONCLUSIONS
Damaged/contaminated tips of light-curing units produced reduced radiant emittance and a non-uniform beam profile. Dentists should optimally and frequently clean their light-curing tips and ensure proper handling to prevent damaging the tips. In light of the findings of this study regarding possible differences between different spectrophotometer-based methods, the checkMARC portable spectrophotometer device can be used in dental offices to monitor the output of light-curing units, as it provided values close to those produced by a laboratory-grade integrated sphere spectrophotometer. In contrast, the thermopile readings differed significantly compared to those of a laboratory-grade integrated sphere spectrophotometer. Therefore, dentists may consider using the checkMARC portable device to assess the radiant emittance of their light-curing units to ensure optimal curing in photoactivated, resin-based materials.
ACKNOWLEDGEMENT
The authors thank Blue Light Analytics for supplying the devices used in this work. AA acknowledges a scholarship from the Imam AbdulRahman bin Faisal University, Dammam, Saudi Arabia. IMG recognizes a scholarship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-Brasil (CAPES)-Finance Code 001-scholarship.
Footnotes
Funding: This work was supported by a University of Maryland Baltimore seed grant (MM), and the University of Maryland School of Dentistry departmental fund (MM).
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: Strassler H, Melo MA.
- Data curation: Ganesh N, Alkabashi Q.
- Formal analysis: Garcia I, Collares F.
- Funding acquisition: Strassler H, Melo MA.
- Methodology: Balhaddad AA, Garcia I.
- Project administration: Strassler H, Melo MA.
- Resources: Strassler H, Melo MA.
- Software: Alkabashi Q.
- Supervision: Massei W.
- Validation: Massei W.
- Visualization: Felix CM, Ganesh N, Alkabashi Q.
- Writing - original draft: Balhaddad AA, Garcia I.
- Writing - review & editing: Strassler H, Melo MA.
References
- 1.Chan KHS, Mai Y, Kim H, Tong KCT, Ng D, Hsiao JCM. Review: resin composite filling. Materials (Basel) 2010;3:1228–1243. [Google Scholar]
- 2.Balhaddad AA, Kansara AA, Hidan D, Weir MD, Xu HHK, Melo MAS. Toward dental caries: exploring nanoparticle-based platforms and calcium phosphate compounds for dental restorative materials. Bioact Mater. 2018;4:43–55. doi: 10.1016/j.bioactmat.2018.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Oral health surveys: basic methods. 3rd ed. Geneva: World Health Organization; 1987. [Google Scholar]
- 4.Gordan VV, Riley JL, 3rd, Rindal DB, Qvist V, Fellows JL, Dilbone DA, Brotman SG, Gilbert GH National Dental Practice-Based Research Network Collaborative Group. Repair or replacement of restorations: a prospective cohort study by dentists in the National Dental Practice-Based Research Network. J Am Dent Assoc. 2015;146:895–903. doi: 10.1016/j.adaj.2015.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.David JR, Gomes OM, Gomes JC, Loguercio AD, Reis A. Effect of exposure time on curing efficiency of polymerizing units equipped with light-emitting diodes. J Oral Sci. 2007;49:19–24. doi: 10.2334/josnusd.49.19. [DOI] [PubMed] [Google Scholar]
- 6.Santini A, Gallegos IT, Felix CM. Photoinitiators in dentistry: a review. Prim Dent J. 2013;2:30–33. doi: 10.1308/205016814809859563. [DOI] [PubMed] [Google Scholar]
- 7.Samaha S, Bhatt S, Finkelman M, Papathanasiou A, Perry R, Strassler H, Kugel G, Garcia-Godoy F, Price R. Effect of instruction, light curing unit, and location in the mouth on the energy delivered to simulated restorations. Am J Dent. 2017;30:343–349. [PubMed] [Google Scholar]
- 8.Price RB, Ferracane JL, Shortall AC. Light-curing units: a review of what we need to know. J Dent Res. 2015;94:1179–1186. doi: 10.1177/0022034515594786. [DOI] [PubMed] [Google Scholar]
- 9.Maktabi H, Balhaddad AA, Alkhubaizi Q, Strassler H, Melo MAS. Factors influencing success of radiant exposure in light-curing posterior dental composite in the clinical setting. Am J Dent. 2018;31:320–328. [PubMed] [Google Scholar]
- 10.AlShaafi MM. Factors affecting polymerization of resin-based composites: a literature review. Saudi Dent J. 2017;29:48–58. doi: 10.1016/j.sdentj.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rueggeberg FA, Giannini M, Arrais CAG, Price RBT. Light curing in dentistry and clinical implications: a literature review. Braz Oral Res. 2017;31:e61. doi: 10.1590/1807-3107BOR-2017.vol31.0061. [DOI] [PubMed] [Google Scholar]
- 12.Maktabi H, Ibrahim M, Alkhubaizi Q, Weir M, Xu H, Strassler H, Fugolin APP, Pfeifer CS, Melo MAS. Underperforming light curing procedures trigger detrimental irradiance-dependent biofilm response on incrementally placed dental composites. J Dent. 2019;88:103110. doi: 10.1016/j.jdent.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 13.Alshaafi MM. Evaluation of light-curing units in rural and urban areas. Saudi Dent J. 2012;24:163–167. doi: 10.1016/j.sdentj.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Price RBT, Ehrnford L, Andreou P, Felix CA. Comparison of quartz-tungsten-halogen, light-emitting diode, and plasma arc curing lights. J Adhes Dent. 2003;5:193–207. [PubMed] [Google Scholar]
- 15.Vandenbulcke JDE, Marks LAM, Martens LC, Verbeeck RMH. Comparison of curing depth of a colored polyacid-modified composite resin with different light-curing units. Quintessence Int. 2010;41:787–794. [PubMed] [Google Scholar]
- 16.Maghaireh GA, Alzraikat H, Taha NA. Assessing the irradiance delivered from light-curing units in private dental offices in Jordan. J Am Dent Assoc. 2013;144:922–927. doi: 10.14219/jada.archive.2013.0210. [DOI] [PubMed] [Google Scholar]
- 17.Barghi N, Fischer DE, Pham T. Revisiting the intensity output of curing lights in private dental offices. Compend Contin Educ Dent. 2007;28:380–384. [PubMed] [Google Scholar]
- 18.Santos GC, Jr, Santos MJMC, El-Mowafy O, El-Badrawy W. Intensity of quartz-tungsten-halogen light polymerization units used in dental offices in Brazil. Int J Prosthodont. 2005;18:434–435. [PubMed] [Google Scholar]
- 19.Nassar HM, Ajaj R, Hasanain F. Efficiency of light curing units in a government dental school. J Oral Sci. 2018;60:142–146. doi: 10.2334/josnusd.17-0071. [DOI] [PubMed] [Google Scholar]
- 20.Al Shaafi M, Maawadh A, Al Qahtani M. Evaluation of light intensity output of QTH and LED curing devices in various governmental health institutions. Oper Dent. 2011;36:356–361. doi: 10.2341/10-247-O. [DOI] [PubMed] [Google Scholar]
- 21.El-Mowafy O, El-Badrawy W, Lewis DW, Shokati B, Kermalli J, Soliman O, Encioiu A, Zawi R, Rajwani F, Rajwani F. Intensity of quartz-tungsten-halogen light-curing units used in private practice in Toronto. J Am Dent Assoc. 2005;136:766–773. doi: 10.14219/jada.archive.2005.0260. [DOI] [PubMed] [Google Scholar]
- 22.Jandt KD, Mills RW, Blackwell GB, Ashworth SH. Depth of cure and compressive strength of dental composites cured with blue light emitting diodes (LEDs) Dent Mater. 2000;16:41–47. doi: 10.1016/s0109-5641(99)00083-4. [DOI] [PubMed] [Google Scholar]
- 23.Shortall AC, Price RB, MacKenzie L, Burke FJT. Guidelines for the selection, use, and maintenance of LED light-curing units - part II. Br Dent J. 2016;221:551–554. doi: 10.1038/sj.bdj.2016.814. [DOI] [PubMed] [Google Scholar]
- 24.International Organization for Standardization. ISO 10650-2. Dentistry—powered polymerization activators—part 2: light-emitting diode (LED) lamps. Geneva: International Organization for Standardization; 2007. [Google Scholar]
- 25.Shortall AC, Felix CJ, Watts DC. Robust spectrometer-based methods for characterizing radiant exitance of dental LED light curing units. Dent Mater. 2015;31:339–350. doi: 10.1016/j.dental.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 26.Marović D, Matić S, Kelić K, Klarić E, Rakić M, Tarle Z. Time dependent accuracy of dental radiometers. Acta Clin Croat. 2013;52:173–180. [PubMed] [Google Scholar]
- 27.Kameyama A, Haruyama A, Asami M, Takahashi T. Effect of emitted wavelength and light guide type on irradiance discrepancies in hand-held dental curing radiometers. ScientificWorldJournal. 2013;2013:647941. doi: 10.1155/2013/647941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Price RB, Labrie D, Kazmi S, Fahey J, Felix CM. Intra- and inter-brand accuracy of four dental radiometers. Clin Oral Investig. 2012;16:707–717. doi: 10.1007/s00784-011-0562-7. [DOI] [PubMed] [Google Scholar]
- 29.Michaud PL, Price RBT, Labrie D, Rueggeberg FA, Sullivan B. Localised irradiance distribution found in dental light curing units. J Dent. 2014;42:129–139. doi: 10.1016/j.jdent.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 30.Harlow JE, Sullivan B, Shortall AC, Labrie D, Price RB. Characterizing the output settings of dental curing lights. J Dent. 2016;44:20–26. doi: 10.1016/j.jdent.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 31.Shimokawa CAK, Harlow JE, Turbino ML, Price RB. Ability of four dental radiometers to measure the light output from nine curing lights. J Dent. 2016;54:48–55. doi: 10.1016/j.jdent.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 32.Konerding KL, Heyder M, Kranz S, Guellmar A, Voelpel A, Watts DC, Jandt KD, Sigusch BW. Study of energy transfer by different light curing units into a class III restoration as a function of tilt angle and distance, using a MARC patient simulator (PS) Dent Mater. 2016;32:676–686. doi: 10.1016/j.dental.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 33.Soares CJ, Rodrigues MP, Oliveira LRS, Braga SSL, Barcelos LM, Silva GR, Giannini M, Price RB. An evaluation of the light output from 22 contemporary light curing units. Braz Dent J. 2017;28:362–371. doi: 10.1590/0103-6440201601466. [DOI] [PubMed] [Google Scholar]
- 34.Price RBT, Labrie D, Rueggeberg FA, Felix CM. Irradiance differences in the violet (405 nm) and blue (460 nm) spectral ranges among dental light-curing units. J Esthet Restor Dent. 2010;22:363–377. doi: 10.1111/j.1708-8240.2010.00368.x. [DOI] [PubMed] [Google Scholar]
- 35.Price RBT, Rueggeberg FA, Labrie D, Felix CM. Irradiance uniformity and distribution from dental light curing units. J Esthet Restor Dent. 2010;22:86–101. doi: 10.1111/j.1708-8240.2010.00318.x. [DOI] [PubMed] [Google Scholar]
- 36.Rueggeberg FA, Caughman WF, Curtis JW., Jr Effect of light intensity and exposure duration on cure of resin composite. Oper Dent. 1994;19:26–32. [PubMed] [Google Scholar]
- 37.Al-Zain AO, Eckert GJ, Lukic H, Megremis S, Platt JA. Polymerization pattern characterization within a resin-based composite cured using different curing units at two distances. Clin Oral Investig. 2019;23:3995–4010. doi: 10.1007/s00784-019-02831-1. [DOI] [PubMed] [Google Scholar]
- 38.Eshmawi YT, Al-Zain AO, Eckert GJ, Platt JA. Variation in composite degree of conversion and microflexural strength for different curing lights and surface locations. J Am Dent Assoc. 2018;149:893–902. doi: 10.1016/j.adaj.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 39.Yeh CL, Miyagawa Y, Powers JM. Optical properties of composites of selected shades. J Dent Res. 1982;61:797–801. doi: 10.1177/00220345820610062901. [DOI] [PubMed] [Google Scholar]
- 40.International Organization for Standardization. ISO 10650-1. Dentistry—powered polymerization activators—part 1: quartz tungsten halogen lamps. Geneva: International Organization for Standardization; 2004. [Google Scholar]
- 41.Arikawa H, Kanie T, Fujii K, Takahashi H, Ban S. Effect of inhomogeneity of light from light curing units on the surface hardness of composite resin. Dent Mater J. 2008;27:21–28. doi: 10.4012/dmj.27.21. [DOI] [PubMed] [Google Scholar]
- 42.Rueggeberg FA. State-of-the-art: dental photocuring--a review. Dent Mater. 2011;27:39–52. doi: 10.1016/j.dental.2010.10.021. [DOI] [PubMed] [Google Scholar]
- 43.Price RB, Shortall AC, Palin WM. Contemporary issues in light curing. Oper Dent. 2014;39:4–14. doi: 10.2341/13-067-LIT. [DOI] [PubMed] [Google Scholar]
- 44.Khode RT, Shenoi PR, Kubde RR, Makade CS, Wadekar KD, Khode PT. Evaluation of effect of different disposable infection control barriers on light intensity of light-curing unit and microhardness of composite - an in vitro study. J Conserv Dent. 2017;20:180–184. doi: 10.4103/JCD.JCD_171_16. [DOI] [PMC free article] [PubMed] [Google Scholar]