Highlights
-
•
The role of miRNAs in PAH is fast expanding, and it is increasingly difficult to identify which molecules have the highest translational potential.
-
•
This review discusses the challenges in miRNA analysis and interpretation in PAH and highlights 4 promising miRNAs in this field.
-
•
Additional pre-clinical studies and clinical trials are urgently needed to bring miRNAs from the bench to the bedside soon.
Key Words: animal model, microarray, micro-RNA, pulmonary arterial hypertension
Abbreviations and Acronyms: BMPR2, bone morphogenetic protein receptor type 2; EPC, endothelial progenitor cell; HIF, hypoxia-inducible factor; HPAH, hereditary pulmonary arterial hypertension; lncRNA, long noncoding RNA; MCT, monocrotaline; miRNA, micro-RNA; mRNA, messenger RNA; ncRNAs, noncoding RNAs; PAAF, pulmonary arterial adventitial fibroblast; PAEC, pulmonary artery endothelial cell; PAH, pulmonary arterial hypertension; PASMC, pulmonary artery smooth muscle cells; PH, pulmonary hypertension; RV, right ventricle; SU/Hx/Nx, association of Sugen 5416 with chronic hypoxia followed by normoxia; WHO, World Health Organization
Summary
Pulmonary arterial hypertension (PAH) is a rare, chronic disease of the pulmonary vasculature that is associated with poor outcomes. Its pathogenesis is multifactorial and includes micro-RNA (miRNA) deregulation. The understanding of the role of miRNAs in PAH is expanding quickly, and it is increasingly difficult to identify which miRNAs have the highest translational potential. This review summarizes the current knowledge of miRNA expression in PAH, discusses the challenges in miRNA analysis and interpretation, and highlights 4 promising miRNAs in this field (miR-29, miR-124, miR-140, and miR-204).
Central Illustration
Pulmonary arterial hypertension (PAH) is a rare multifactorial condition, hemodynamically defined as an increase in the mean pulmonary arterial pressure (>20 mm Hg, according to the recently revised 6th World Symposium on Pulmonary Hypertension definition), with normal left ventricular filling pressures and elevated pulmonary vascular resistance (1,2). It is characterized by structural and functional changes in the pulmonary arterial vasculature that evolve from isolated medial hypertrophy to end-stage plexiform fibrosis, originating an increase in pulmonary vascular resistance (3). The progressive increase in right ventricular (RV) afterload leads to RV hypertrophy and, ultimately, RV failure and death (4).
Despite considerable progress in the understanding of the epidemiology, pathophysiology, and management of PAH, the prognosis remains poor, especially in patients with severe disease (i.e., World Health Organization [WHO] functional class IV) (1,5). Patients in WHO class IV have higher right atrial pressure and pulmonary vascular resistance, lower exercise capacity and peak oxygen consumption, and a 3-year survival rate of 38%, compared with >60% observed for patients in WHO classes I to III (5, 6, 7). Current PAH therapies primarily target 3 vasomotor pathways: the nitric oxide, the endothelin-1, and the prostacyclin pathways (1). However, these interventions might not accurately target the intricate molecular primum movens of PAH, because genetic susceptibility, hypoxia, inflammation, DNA damage, viral infection, and shear stress are all involved in various amounts within the pathogenic events that contribute to the patient-specific phenotype of disease manifestation and progression (8). PAH involves a single organ, the lungs, but multiple pulmonary arterial cell types—pulmonary artery endothelial cells (PAECs), pulmonary artery smooth muscle cells (PASMCs), and pulmonary arterial adventitial fibroblasts (PAAFs) (9). The inability to fully understand this complex interplay of cells and soluble mediators has hampered the development of new drugs. In the last 15 years, no new drug aimed at novel pathological pathways has been approved.
One of the players of this complex network of stimuli are micro-RNAs (miRNAs). Most of our genome is not directly implicated in protein synthesis but, rather, in the production of noncoding RNAs (ncRNAs) with regulatory functions (10). Among the several RNA species, 2 varieties of ncRNAs, loosely defined as long and small RNA, have been described recently. Among the small RNAs, miRNAs are short length, noncoding endogenous RNA molecules that consist of approximately 22-nucleotide-long sequences profusely expressed in all human cells and are believed to control approximately 50% of all protein-coding messenger RNA (mRNA) (11).
The biogenesis of miRNAs is classified into 2 pathways: the canonical (the dominant pathway by which miRNAs are processed), and non-canonical pathways. In the canonical pathway, transcription of miRNAs genes produces primary miRNAs that are hairpin-like and can be >1,000 nucleotides long; this transcription is dependent on polymerase II and is regulated by transcription factors (12). Primary miRNAs are cleaved in the nucleus to form precursor miRNAs, which are a shorter hairpin approximately 70 nucleotides long, by the Microprocessor complex, which consists of an RNA-binding protein DiGeorge Syndrome Critical Region 8 and a ribonuclease III enzyme, Drosha. Precursor miRNAs are then exported from the nucleus to the cytoplasm by exportin-5. In the cytoplasm, precursor miRNAs are processed into short, double-stranded, immature miRNA by another ribonuclease, Dicer. In the following step, the 2 strands are separated, and 2 of these strands (mature miRNA) are recruited by Argonaute proteins to incorporate a multiprotein complex known as the RNA-induced silencing complex (13). In addition to the canonical miRNA biogenesis pathway, multiple non-canonical pathways have been revealed by using different combinations of the proteins involved in the canonical pathway. In general, they can be classified as Drosha- and Dicer-independent pathways (13).
MiRNAs are able to modify protein expression by binding to complementary sequences on mRNA, mainly in the 3′-untranslated region of the target mRNA transcripts, thereby promoting translational inhibition and degradation by altering the stability of target mRNAs (14). The mature miRNA is integrated into the RNA-induced silencing complex and then binds to its mRNA target. Once bound to an mRNA, the miRNA−RNA-induced silencing complex promotes the downregulation of the protein that the mRNA encodes, mainly through direct mRNA degradation (15). A specific miRNA can target multiple mRNAs (the so-called divergent pathway), and a mRNA can have multiple binding sites for several miRNAs. In addition, various related miRNAs can affect a pathway at different levels, termed a convergent pathway (14). The interactome of miRNAs with mRNA is a multidimensional network and plays a crucial role in regulating cellular pathways and biological functions in health and disease (16). During pathological processes, such as pulmonary vascular processes involved in PAH, the dysregulation of specific and distinct miRNAs has profound consequences in cell function due to changes in protein synthesis (14). Therefore, miRNAs have been recognized as promising biomarkers and therapeutic options for many cardiovascular diseases (14,17). However, one of the issues regarding the study of miRNA in PAH resides in the large heterogeneity of models and cells analyzed (18,19). miRNA regulation is not conserved between the various pulmonary hypertension (PH) animal models. These differences may have important implications in how preclinical miRNA variations should be interpreted as potential biomarkers and therapeutic targets and consequently translated to patients with PAH (19).
Among the various techniques available to study miRNAs regulation in cell lines, animal models, and patients, microarray analysis is of great use, because it allows the detection of genome-wide gene expression (20). The development of these powerful, effective, and high-throughput data methodologies enables the unbiased screening of mechanisms altered in a specific dataset and the search for differentially expressed genes, according to the experimental design (e.g., healthy control subjects vs. patients with disease). They have been broadly applied to investigate the pathophysiology of diverse cardiovascular diseases (14,17).
Recently, the role of long non-coding RNAs (lncRNA) in the pathobiology of PAH has also been explored. LncRNAs are longer than 200 nucleotides and are abundantly present in the genome (21). Based on their subcellular localization, lncRNAs are able to regulate gene expression through different mechanisms. In contrast to miRNA, lncRNA can yield their regulatory effects, both at the transcriptional and post-transcriptional levels, through interactions with DNA, chromatin, and other species of RNA. In addition, they can act as epigenetic modulators (22). Emerging evidence suggests that these molecules play an important role in PAH pathogenesis as fundamental drivers and gatekeepers in the regulation of key cellular and molecular trafficking in PASMC and PAEC dysregulation (21).
In this review, we summarize the current knowledge on miRNA expression in experimental models of PH and patients with PAH, focusing our analysis on those miRNAs that have been found to have a conserved regulation across animal models and human PAH samples using microarray analysis (Central Illustration). Finally, we highlight future perspectives and potential challenges concerning miRNA application in the clinical arena, namely, in the development of novel diagnostic and therapeutic tools for PAH.
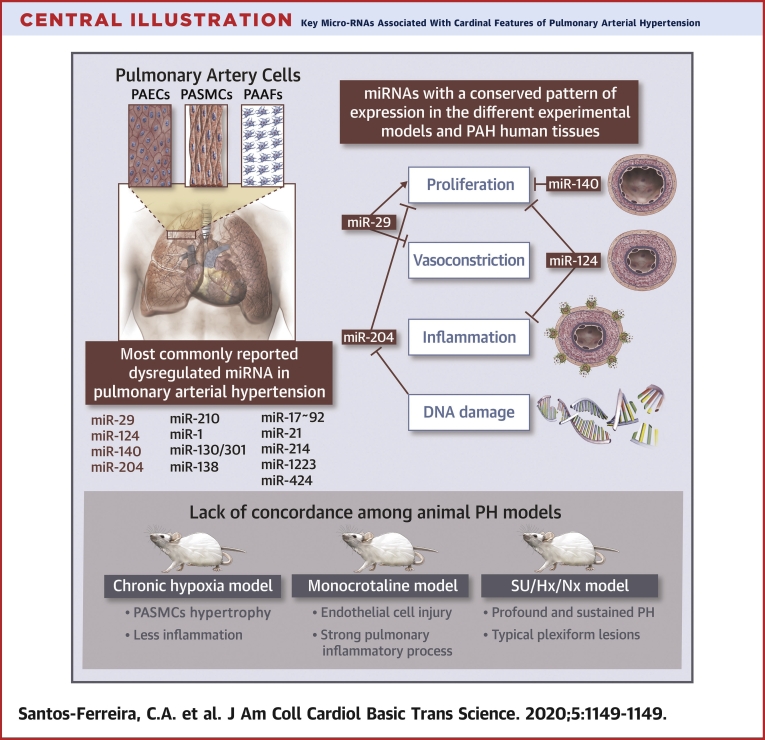
Central Illustration.
Key Micro-RNAs Associated With Cardinal Features of Pulmonary Arterial Hypertension
The miRNA dysregulation plays an important role in the hyperproliferative and apoptosis-resistant phenotype of pulmonary vascular cells in PAH, including PAECs, PASMCs, and PAAFs. This figure illustrates the lack of concordance in the pattern of expression of most miRNAs between human PAH and different animal PH models and highlights 4 miRNAs that might ultimately represent a greater potential of translating into the clinical arena. miRNA = microRNA; PAAF = pulmonary arterial adventitial fibroblasts; PAEC = pulmonary artery endothelial cell; PAH = pulmonary arterial hypertension; PASMC = pulmonary artery smooth muscle cell; PH = pulmonary hypertension; SU/Hx/Nx = association of Sugen 5416 with chronic hypoxia followed by normoxia.
Challenges in Micro-RNA Analysis and Interpretation in PAH
The first evidence that suggested that miRNAs contribute to the pathogenesis of PAH resulted from the observation that bone morphogenetic protein receptor type 2 (BMPR2) protein, but not mRNA levels, was reduced in animal models of PH (23). Since then, miRNAs have been implicated in a wide range of pulmonary vascular processes involved in PAH (14). However, many challenges limit the validation of miRNAs discovered in experimental models.
Lack of concordance among animal PH models
First, although all PH animal models are characterized by an increase in RV afterload and progression to RV failure, the trigger is substantially different between them (19,24). Therefore, there is a lack of concordance in the pattern of miRNA expression among different experimental models of PAH (19,24). For example, administration of monocrotaline (MCT) to rats mostly targets the pulmonary vascular endothelium and elicits a strong pulmonary inflammation process, especially monocyte recruitment, which plays an important role in human idiopathic PAH (24). Conversely, in the chronic hypoxia murine model, persistent hypoxia causes vascular structural remodeling in all 3 layers of the pulmonary arteriolar wall, with hypertrophy of PASMCs being a major histological finding and having less inflammation compared with the MCT model (19,25). The model induced by the association of vascular endothelial growth factor receptor antagonist, Sugen 5416, with chronic hypoxia followed by normoxia (SU/Hx/Nx) causes profound and sustained PH, along with inflammation and angio-obliteration, culminating in RV remodeling and failure, in both rats and mice (24). The subjacent mechanism is believed to involve death of PAECs, with subsequent proliferation of an apoptosis-resistant cell type. The restitution of normoxia results in the development of neointimal lesions extremely similar to human plexogenic arteriopathy (24). Although the SU/Hx/Nx model has been suggested as a more relevant model of human PAH (19,26), miRNAs have predominantly been studied in the MCT or chronic hypoxia models. Despite reproducing important characteristics of human PAH, these models generally fail to reproduce the severe pulmonary arteriopathy usually present in patients with PAH (19). Other models have been developed to better reproduce the natural history of human PAH. Recently, a rat model with a monoallelic deletion of 71 bp in exon 1 in the Bmpr2 gene was developed. This model showed not only some of the pivotal cellular and molecular dysfunctions described in human PAH, but also the gradual phenotype seen in humans. It also showed myocardial abnormalities, supporting the hypothesis that the RV might also be affected in PAH besides the increased afterload imposed by pulmonary vascular disease (27).
Considering these pathophysiological differences, it is not surprising there is divergence in miRNA regulation among the different animal models reported in the literature (18,19). Schlosser et al. (19) explored these disparities in the pattern of expression of a set of miRNAs causally implicated in PAH in the plasma, lung, and RV of different animal models and in plasma from patients with PAH (19). They found that most of the miRNA investigated had discordant patterns across the different tissues and models (19). Moreover, after analyzing the mRNA targets of miRNAs, they also found discordant regulation in the different models, which suggested that in these scenarios miRNA might not be the only or the most significant regulation mechanism (19).
Heterogeneity in regulation among different human PAH etiologies
Second, phenotypic variability between predominantly hypoxic and nonhypoxic human forms of PAH regarding miRNA levels might therefore be recapitulated by what is found in the animal models, which suggests different pathological mechanisms of disease. As in animal models, the different forms of human PAH are unlikely to be equivalent, and it remains unclear whether the various types of PAH share the same pathophysiology (26). Although they have similar histopathological reflections in the pulmonary artery, there are different forms of PAH within the same classification; therefore, differential miRNA profiles may reflect different mechanisms of disease (18). We have previously reported that in patients in whom the hypoxic phenotype is predominant (e.g., Eisenmenger syndrome, lung-disease related PH, or advanced right heart failure, in which low cardiac output leads to hypoxia at a peripheral tissue level), the upregulation of miR-424(322) promotes hypoxia-inducible factor (HIF)-1α accumulation, which, in turn, contributes to increased miR-424(322) levels (28). Although in this hypoxic phenotype—exposed to high HIF-1α levels—miR-424(322) likely promotes some amount of pulmonary vascular proliferation, it is also necessary to maintain the life-saving compensatory mechanisms that mitigate the pathophysiological manifestations of chronic hypoxia (e.g., erythrocytosis). This might explain why lower levels of miR-424(322) were associated with a poorer prognosis in patients with Eisenmenger syndrome in our cohort, because they might be a cause (or a consequence) of a detrimental lower global activation of HIF-1α compensatory pathways (28). For example, it is well recognized that relative anemia due to iron deficiency is a marker of poor prognosis in Eisenmenger syndrome (29,30).
Conversely, in other patients with PAH, such as those with idiopathic PAH, hereditary PAH, drug-induced PAH, or connective tissue disease−associated PAH, the hypoxic component is likely not as relevant to the mechanism of disease. For instance, in patients with hereditary PAH, the BMPR2 pathway can be downregulated by mutations in several genes (BMPR2, ACVRL1, ENG, SMAD1, SMAD4, SMAD9) (31). In addition, even if there are no identified mutations, the pathway can be downregulated in patients with idiopathic PAH (32). PAECs subjected to hypoxia conditions show downregulation of miR-424(322) levels, whereas, in PASMCs, no differences have been found compared with control cells (28). Paradoxically, we (28) and others (19) found that circulating miR-424(322)/503 levels were elevated in patients with PAH and patients with chronic thromboembolic PH. However, those patients were themselves in various phases of the disease at the time of analysis. Once again, the timepoint of miR-424(322) assessment and organ-specific differences (lung, heart) might have an important role in these apparently contradictory results (19,28). Together, these findings suggest that the balance of hypoxic and inflammatory and/or genetic stimuli in each patient will determine the specific patient-level deregulation of miRNAs, again adding complexity to translational approaches.
Different miRNA expression profile between RV hypertrophy and RV failure
Finally, there are differences in the miRNA expression profile between RV hypertrophy and RV failure, which suggests specific changes in the signature of miRNAs during PAH progression (33,34). The comparison of the available data on miRNA expression in the RV reveals few overlaps among the studies (35, 36, 37). The different approaches to induce RV hypertrophy and failure (pulmonary artery banding vs. chronic hypoxia), different surgical interventions leading to different banding gradients, and the use of different species (mouse, rat, ovine) are likely reasons (34). The mechanisms underlying the transition from compensated RV hypertrophy to RV failure remain unclear, and further investigations are needed to gain a better understanding of miRNA expression patterns in RV pathological remodeling (34,36).
Microarray Technology and Data Analysis
Genome-wide studies, as microarrays and RNA sequencing, deliver screening technologies that can rapidly identify genes, clusters of co-regulated genes, or pathways that are engaged in pathological processes. Microarray technology has been extensively applied in the study of complex disorders, including cancer, diabetes, and cardiovascular diseases (38). The intricate pathogenesis of PAH is a suitable target for microarray technology, which provides an additional tool to further explore the expression of key genes and regulatory networks (18). In addition, high-throughput screening is useful in the detection of novel molecular targets for diagnosis, prognosis, and treatment. Therefore, in addition to the identification of coding RNAs, the expression of miRNAs can also be analyzed by microarray technologies. These technologies rely on nucleic acid hybridization target miRNAs and their corresponding complementary probes (39). First, complementary DNA is synthesized from isolated miRNAs by reverse transcription, labeled with a fluorescent dye, and then hybridized to the microarray. After eliminating any unbound complementary DNAs with a series of washing steps, the microarray is scanned to measure the fluorescence intensity on each probe spot, which is translated into the relative amount of each target miRNA from the original sample (39).
MiRNA arrays have experienced substantial technological progress in recent years, with innovations in probe design, immobilization techniques, sample labeling, and signal detection methods (40). Although genome-wide miRNA microarray platforms convey an inexpensive way to analyze a large number of simultaneous measurements, they are unable to identify new miRNAs (41), which is only possible with RNA sequencing (42). Additional disadvantages of these platforms are related to miRNA intrinsic characteristics, such as a small margin to optimize the hybridization conditions secondary to their short length. Another disadvantage is the limited specificity for miRNAs that have similar sequences and may differ by as little as a single nucleotide, which decreases the sensitivity and specificity of microarrays. Another pitfall of the microarray is the inability to quantify absolute miRNA abundance (39). Despite all the limitations, microarrays are still the best available tool for comparing the relative abundance of pre-defined miRNAs between 2 conditions (e.g., patients with disease and healthy control subjects) (39). Regarding PAH, the information on expression, function, and role of miRNA in human tissues is still limited. Overall, the array studies provide a solid characterization of end-stage damage in PAH (18). Although the current goal of microarray analysis in PAH has been to explore patterns of expression that identify novel biomarkers or help to understand the pathophysiology behind the disease and the effects of drugs, they are also useful at confirming the relevance of possible etiologies unveiled in experimental models in humans (18).
Considering this, we performed an analysis of human multitissue microarray datasets (Table 1). These datasets included endothelial cells derived from circulating endothelial progenitor (EPCs) (GSE73674), lung (GSE113439), PASMCs (GSE108707), and peripheral blood mononuclear cell (GSE131793) samples of both patients with PAH and healthy control subjects. We used GEOquery library to extract GEO datasets. Differentially expressed genes were determined by using the limma package (43). A design matrix was created for the 2 groups of samples (control subjects and patients with PAH), and a linear model was fitted to the log-transformed expression values. The differential expression was achieved after an empirical Bayes adjustment.
Table 1.
Human Microarray Studies in PAH
| Patient Population | Tissue | Microarray Platform | Analysis Method | Observations | Dataset |
|---|---|---|---|---|---|
| 6 patients with PAH-SSc, 20 SSc patients without PAH and 9 healthy control subjects | EPCs | Affymetrix Human Exon 1.0 ST Array | A linear model was fitted to the log-transformed expression values using the limma package | 2,175 genes were found differentially expressed between control and SSc PAH | GSE73674 |
| 15 patients with PAH (6 IPAH, 4 PAH CTD-PAH, 4 PAH secondary to CHD and 1 CTEPH) and 11 normal subjects | Lung | Affymetrix Human Gene 1.0 ST Array | A linear model was fitted to the log-transformed expression values using the limma package | 12,834 genes were found differentially expressed in the PAH cohort compared with control subjects | GSE113439 |
| 6 patients with PAH and 3 healthy control subjects | PASMCs | Affymetrix Multispecies miRNA-4 Array | A linear model was fitted to the log-transformed expression values using the limma package | — | GSE108707 |
| 14 patients with PAH and 14 sex-/age- matching control subjects | PBMCs | Affymetrix GeneChip Human Gene 1.0 ST Array | A linear model was fitted to the log-transformed expression values using the limma package | 2,624 genes were found differentially expressed between control subjects and patients with PAH | GSE131793 |
CHD = congenital heart disease; CTD-PAH = connective tissue disease-associated pulmonary arterial hypertension; CTEPH= chronic thromboembolic pulmonary hypertension; EPC = endothelial cells derived from circulating endothelial progenitor; IPAH = idiopathic pulmonary arterial hypertension; miRNA = micro-RNA; PASMC = pulmonary artery smooth muscle cell; PAH = pulmonary arterial hypertension; PBMC = peripheral blood mononuclear cell; SSc = systemic sclerosis.
MiRNA with similar patterns of expression in animal PH models and human PAH
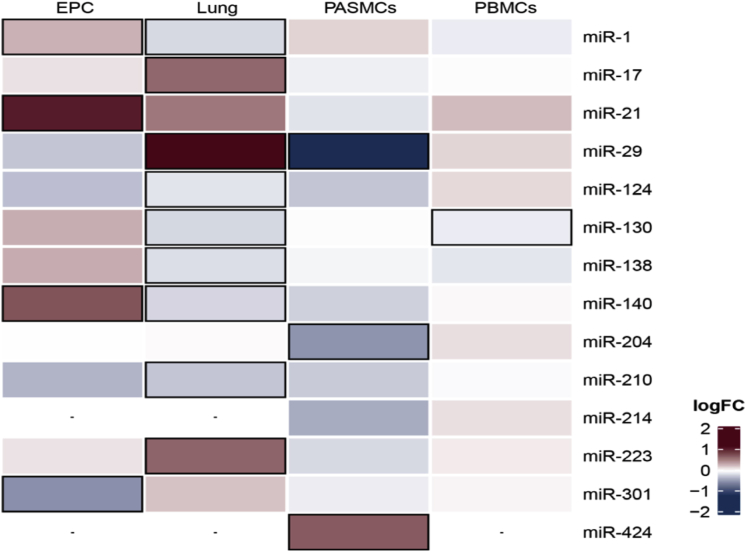
Over the years, mounting evidence has explored the role of dysregulated miRNAs in the hyperproliferative and apoptosis-resistant phenotype of pulmonary vascular cells, including PAECs, PASMCs, and PAAFs, in the different PH rodent models and human PAH tissues. Because of the exhaustive list of such molecules (Table 2), we initially focused on key miRNAs with a well-established role in PAH pathogenesis (Table 3, Supplemental Figure 1). We then constructed a heatmap that showed the log-fold change expression of these miRNAs between patients with PAH and healthy control subjects in different tissues (Figure 1). As depicted in the heatmap, miRNAs could be distinctly expressed between the different tissues. Although miR-29, miR-17, and miR-223 were highly expressed in human PAH lung samples, miR-1, miR-124, miR-130, miR-138, miR-140, and miR-210 were downregulated. Regarding PASMCs, miR-204 and miR-29 were downregulated; conversely, miR-424 was upregulated. In EPCs, miR-1, miR-140, and miR-21 were upregulated, and miR-301 showed lower levels of expression compared with those of healthy control subjects. Finally, miR-130, the only miRNA with differential expression in patients with PAH versus healthy control subjects in peripheral blood mononuclear cells, was downregulated. In addition, in line with previous reports in the literature (19), we observed a lack of concordance in the pattern of expression of most miRNAs between human PAH (Figure 1) and different animal PH models (Table 3). This suggested that different miRNA-dependent mechanisms might contribute to experimental PH and human PAH, impairing potential diagnostic and therapeutic applications. Among all this heterogeneity, we highlighted 4 miRNAs, that due to their similar expression pattern among the different models, might ultimately represent a greater potential of translating into the clinical arena, namely, miR-29, miR-124, miR-140, and miR-204 (Figure 2).
Table 2.
Dysregulated miRNAs in Pulmonary Hypertension Organized by Their Mechanistic Role
| Mechanism | miRNA |
|---|---|
| Proliferation | miR-210; miR-204; miR-424; miR-503; miR-130/301; miR-193; miR-17∼92; miR-145; miR-21; miR-124; miR-140; miR-500b-3p; miR-206; miR-200; miR-338-3p; miR-141-3p; miR-133a-3p: miR-29a; miR-214; miR-223; miR-100; miR-222; miR-25; miR-138; miR-1; miR-497, miR-1268, miR-665; miR-98; miR-191; miR-30a-5p; miR-593-5p; miR-203; miR-429; miR-371b-5p; miR-760; miR-1181; miR-143; miR-92b-3p; miR-135a-5p; miR-23a; miR-1281; miR-361-5p; miR-195-5p; miR-150; miR-4632; miR-221-3p; miR-125; miR-34; miR-103/107 miR-322: miR-199b-5p; let-7a-5p; miR-20a; miR-328 |
| Vasoconstriction | miR-130/301; miR-328; miR-190; miR-29b; miR-1; miR-543; miR-27b; mR-328 |
| DNA damage | miR-223; miR-204 |
| Estrogen signaling and sex-specific | miR-96; miR-29 |
| Angiogenesis | miR-208; miR-126; miR-495 miR-206 |
| Inflammation | miR-181a/b-5p; miR-146b; miR-135; miR-124 |
Abbreviation as in Table 1.
Table 3.
A List of Well-Established Dysregulated miRNAs in PAH
| miRNA | Changes in PH | Sample type | Targets | Function | (Ref. #) |
|---|---|---|---|---|---|
| miR-29 | ↓ |
Hypoxic PH rat PAAFs | α-SMA, extracellular matrix collagen | Fibrosis | (44) |
| MCT PH rat PASMCs | Collagen I | (45) | |||
| (in adaptative RV hypertrophy) ↑ |
Fetally-implanted aortopulmonary shunt ovine | Collagen A1, Collagen 3A1 | (37) | ||
| miR-124 | ↓ | Human PAH PASMCs | NFAT | PASMC proliferation | (46) |
| Human PAH PAAFs and human PAH PAECs | PTPB1, MCP-1 | Proliferation | (47, 48, 49) | ||
| miR-140 | ↓ ↑ |
MCT PH rat PASMCs | TNF-α | PASMC proliferation | (50) |
| SU/Hx/Nx and MCT PH rat PASMCs | SMURF1 | PASMC proliferation | (51) | ||
| Human hypoxia PASMCs | SOD2 | PASMC proliferation | (52) | ||
| SU/Hx/Nx PH rat RV | MFN1 | Apoptosis of cardiac myocytes | (53) | ||
| miR-204 | ↓ | Human PAH PASMCs | HIF-1α | PASMC proliferation | (54) |
| Human PAH PASMCs | RUNX2 | PASMC proliferation | (55) | ||
| Human PAH and MCT PH rats PASMCs | SHP2, Src kinase, NFAT | PASMC proliferation | (56) | ||
| Human PAH and hypoxia rat PAECs | ATG7 | Autophagy | (57) | ||
| miR-210 | ↑ | Hypoxic PH rat PASMCs | ISCU | PASMC proliferation | (58) |
| E2F3 | PASMC proliferation | (59) | |||
| MKP-1 | PASMC proliferation | (60) | |||
| miR-1 | ↑ ↓ |
Plasma human PAH and Su/Hx/Nx PH rat lungs | SOD1, Cx43, CAV2, KLF4 | Endothelial dysfunction | (61) |
| SU/Hx/Nx PH rat lungs | KCNA5 | PASMC hypertrophy | (62) | ||
| Human PAH and hypoxic PH rat PASMCs | SphK1 | PASMC proliferation | (63) | ||
| MCT PH rat RV | TGFβ | RV hypertrophy | (64) | ||
| miR-130/301 | ↑ | SU/Hx/Nx mouse model | EDN1 | Vasoconstriction | (65) |
| MCT PH mouse PAECs | BMPR2 | Lung vascular remodeling | (66) | ||
| Pulmonary fibrosis mouse lung | PPARγ-APOE-LRP8 | Fibrosis | (67) | ||
| miR-138 | ↑ | Hypoxic PH rat PASMCs | Mst1 | PASMC proliferation | (68) |
| Human PAH PASMCs | MCUC | PASMC proliferation | (69) | ||
| Human PAH PASMCs | TASK-1 | PASMC proliferation | (70) | ||
| miR-17∼92 | ↑ | Human PAH PAECs | BMPR2 | PAEC survival | (71) |
| Hypoxic PH mouse and MCT PH rat lungs | p21 | PASMC proliferation | (72) | ||
| Human PAH PASMCs | PDLIM5 | PASMC dedifferentiation | (73) | ||
| Hypoxic PH mouse PASMCs | PHD2 | PASMC proliferation | (74) | ||
| miR-21 | ↑ | Human PAH PAECs | BMPR2, RhoB | Decrease angiogenesis and vasodilation | (75) |
| Human PAH PASMCs | PTEN | PASMC proliferation | (76,77) | ||
| Human PAH lung tissue | DDAH1 | PASMC proliferation | (78) | ||
| Hypoxia PH mouse lung | BMPR2 | PASMC proliferation | (79) | ||
| miR-214 | ↑ | Human PAH PASMCs | PTEN, CCNL2 | PASMC proliferation | (80,81) |
| Hypoxia PASMCs | ARHGEF12 | PASMC proliferation | (82) | ||
| Human PAH PASMCs | MEF2C, MYOCD, SMC | PASMC proliferation | (83) | ||
| miR-223 | ↓ | Human PAH PASMCs | PARP-1 | PASMC proliferation | (84) |
| Hypoxia PH mouse and rat lung and hypoxia PASMCs | RhoB, MYPT1, MLC2 | PASMC proliferation | (85) | ||
| MCT PH rat PASMCs | ITGB3 | PASMC proliferation | (86) | ||
| miR-424 | ↑ | Human plasma and MCT PH rat PAECs | SMURF1, BMPR2 | RV hypertrophy | (28) |
| ↓ | SU/Hx/Nx, MCT PH rat and human PAH PAECs | FGF2, FGFR1 | PASMC proliferation | (87) |
MCT = monocrotaline; PAAF = pulmonary arterial adventitial fibroblasts; PAEC = pulmonary artery endothelial cell; PASMC = pulmonary artery smooth muscle cell; PH = pulmonary hypertension; RV = right ventricle; SU/Hx/Nx = association of Sugen 5416 with chronic hypoxia followed by normoxia; other abbreviation as in Table 1.
Figure 1.
Heatmap Showing logFC Expression of the miRNAs Between Patients With PAH and Healthy Control Subjects in Different Tissues
The highlighted boxes represent p values <0.05. EPC = endothelial cells derived from circulating endothelial progenitor; logFC = log-fold change; miR = micro-RNA; PAH = pulmonary arterial hypertension; PASMC = pulmonary artery smooth muscle cell; PBMC = peripheral blood mononuclear cell.
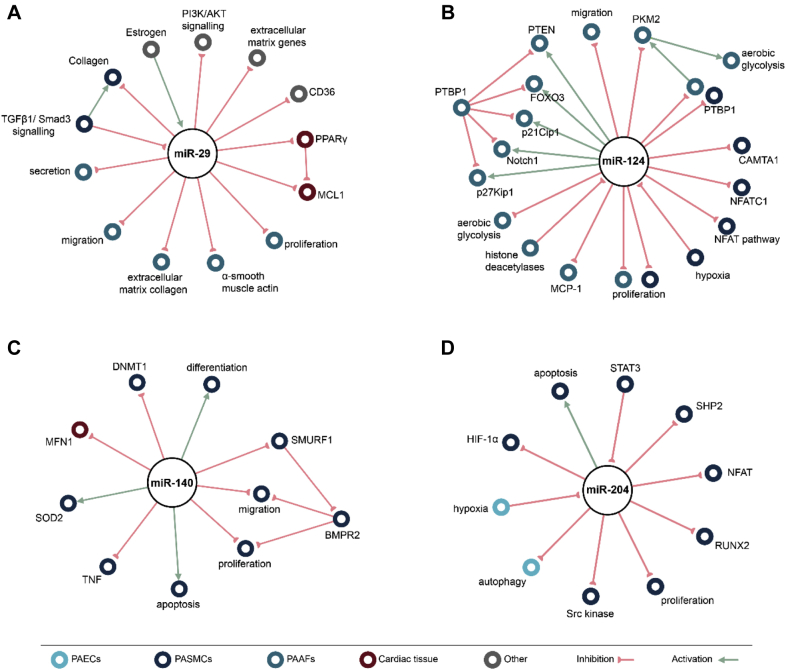
Figure 2.
Networks Representing Known Interactions
Networks representing known interactions involving (A) miR-29, (B) miR-124, (C) miR-140, and (D) miR-204 related to PAH. The green arrows depict activation, whereas the red lines show inhibition. PAAF = pulmonary arterial adventitial fibroblasts; PAEC = pulmonary artery endothelial cell; other abbreviations as in Figure 1.
MiR-29
The hypoxamiR miR-29 (88) is a pro-apoptotic miRNA family that targets myeloid cell leukemia 1 (MCL1), which is a B-cell lymphoma 2 (BCL2) family apoptosis regulator that can be suppressed by peroxisome proliferator-activated receptor gamma (Pparg) agonists in rat hearts (89). Likewise, the miR-29 family directly targets >16 extracellular matrix genes, providing solid evidence for antifibrotic effects in different organs, including the lungs and heart (90). In human PAH, miR-29 is significantly upregulated in the lung, but downregulated in PASMCs and has shown a trend to lower levels in EPCs and peripheral blood mononuclear cells (Figure 1). Chen et al. (91) found an elevation of miR-29 in lung tissue among patients with hereditary PAH, with a sex-specific interaction of miR-29 with estrogen metabolism. Estrogen exposure led to significant reductions in PPARg and CD36 via the upregulation of miR-29 in Bmpr2 transgenic mice lungs (91), which suggested a correlation between energy metabolism and female hormone signaling, with a predisposition to PAH. Regarding PASMCs, TGFb1/ SMAD3 signaling negatively regulated the expression of miR-29b and promoted collagen synthesis in an MCT rat model (45). MiR-29b treatment suppressed collagen synthesis by directly targeting collagen I and blocking PI3K/AKT signaling (45). Activation of PAAFs also played a role in the pulmonary vascular remodeling of a chronic hypoxia rat model through a drastic decrease of miR-29a, and it induced the expression of α-smooth muscle actin (α-SMA) and extracellular matrix collagen. In contrast, a miR-29a mimic repressed the proliferation, migration, and secretion of PAAFs induced by hypoxia and ameliorated pulmonary vascular remodeling (44). miR-29 seemed to be upregulated in adaptative RV hypertrophy (37) but was decreased in maladaptive RV hypertrophy, which revealed a specific miRNA signature in RV failure (35,37). Finally, it was reported that humans with moderate to severe PAH had significant downregulation of plasma levels of circulatory miR-29 (92).
MiR-124
MiR-124 is significantly downregulated in the lungs of patients with PAH, and there is a trend for downregulation in PASMCs and EPCs (Figure 1). Nevertheless, the first reports of miR-124 dysregulation in PH were associated with PAAFs. In vitro loss- and gain-of-function experiments showed that decreased miR-124 in PAAFs from patients with PAH and experimental PH models led to a highly proliferative and migratory phenotype through upregulation of polypyrimidine tract–binding protein 1 (PTBP1) (47,48). MiR-124 inhibited the expression of monocyte chemotactic protein-1 (MCP-1) and PTBP1, which regulated NOTCH1, PTEN/FOXO3/p21CIP1, P27KIP1 signaling, and PAAF proliferation and migration (47). The PTBP1 expression regulation by miR-124 controlled alternative splicing of the 2 major pyruvate kinase muscle isoforms (PKM1 and PKM2), which resulted in an increased PKM2/PKM1 ratio and enhanced glycolysis and PAAFs proliferation. Furthermore, histone deacetylases inhibited miR-124, and miR-124 expression was restored by histone deacetylase inhibitors, which suppressed fibroblast proliferation and suggested a potential therapeutic role for histone deacetylase inhibitors (47,48).
Recently, and consistent with our microarray findings (Figure 1), miR-124 was reported to be downregulated by hypoxia in human PASMCs and mouse lungs with chronic hypoxia (46). MiR-124 exerted its effects by targeting nuclear factor of activated T cell 1 (NFATC1), a known component of the nuclear factor of activated T cell pathway, and calmodulin-binding transcription activator 1 (CAMTA1) and PTBP1 (46), 2 regulators of nuclear factor of activated T cell signaling. Moreover, the overexpression of miR-124 repressed the nuclear factor of activated T cell pathway, inhibited PASMC proliferation, and maintained its differentiated phenotype (46). Finally, the metabolic shift from oxidative phosphorylation to aerobic glycolysis seen in PAAFs was also reproduced in PAECs from patients with PAH through the reduced expression of miR-124, and consequently, upregulation of PTBP1 and PKM2/PKM1 (49).
MiR-140
MiR-140 is a tumor-suppressor miRNA that modulates cell proliferation and migration. Reduced levels of this miRNA have been reported in multiple cancers (93). In human PAH lung and EPCs, miR-140 is significantly downregulated (Figure 1). A similar pattern of results was described in the literature (50). Zhang et al. (52) found downregulation of miR-140-5p in human PAH tissues and PASMCs in hypoxia. This downregulation led to DNA methyltransferase 1 (DNMT1) upregulation and downregulation of superoxide dismutase 2 (SOD2) expression (52). A miR-140-5p mimic, via the regulation of DNMT1, could suppress proliferation and induce apoptosis and differentiation of PASMCs in vitro (52). In addition, downregulation of miR-140 was involved in the modulation of Smad ubiquitination regulatory factor 1 (SMURF1), an E3-ubiquitin protein ligase 1, which, in turn, inhibited BMPR2 signaling and promoted PASMC proliferation and migration in vitro (51). The administration of a miR-140-5p mimic prevented the development and progression of PH (51). A negative association was found between miR-140-5p and tumor necrosis factor-α in PASMCs. Also, the overexpression of miR-140-5p inhibited PAH pathogenesis by suppressing the proliferation, migration, and phenotypic variation of PASMCs (50). Moreover, miR-140 expression was increased in hypertrophic RVs from SU/Hx/Nx rats, and it was demonstrated that miR-140 seemed to play a role in the development of RV dysfunction associated with PAH through the downregulation of its target protein, mitofusin-1 (53).
MiR-204
MiR-204 is encoded inside the intronic region of transient receptor potential melastatin (TRPM3), shares an overlapping regulatory mechanism with TRPM3, and is suppressed by the signal transducer and activator of transcription 3 (STAT3) (56). miR-204 is predominantly expressed in PASMCs, and its expression levels are downregulated in both chronic hypoxia and MCT-induced PH in rats and in human PAH (42), which corroborated our findings of reduced levels of miR-204 in PAH PASMCs (Figure 1). Reduced expression of miR-204 leads to Src homology region 2 (SHP2) up-regulation, activates the Src kinase, NFAT (54,56), HIF-1α (54), and RUNX2 (55), all of which contribute to PASMC proliferation and higher resistance to apoptosis. Interestingly, DNA damage induced by inflammation is accountable for miR-204 downregulation in PAH (54). The pivotal role of miR-204 in PH was also strengthened by experiments in PH animal models, in which restitution of miR-204 expression significantly reduced PH severity (55,56).
In contrast, Liu et al. (57) reported that the downregulation of miR-204 by hypoxia in the chronic hypoxia rat model and human PAECs had a dichotomic role in enhancing autophagy and attenuating endothelial-mesenchymal transition, ultimately ameliorating hypoxia-induced PH.
Future Perspectives and Challenges
There is little doubt that miRNAs yield tremendous potential to become clinically useful diagnostic and prognostic biomarkers, as well as novel therapeutic targets. However, it is generally accepted that several obstacles limit widespread translation to the PAH clinical arena. Although our knowledge of the roles of miRNA in the pathophysiology of PAH has considerably improved, further research is fundamental to identify and to validate miRNAs to their full potential.
Circulating miRNA as diagnostic and prognostic biomarkers
A major concern in circulating miRNAs biomarker discovery and subsequent validation for many diseases, including PAH, is the underlying biological complexity of the disease pathogenesis. In addition, there can be conflicting observations regarding changes in miRNA levels. These disparities can be attributed to differences in sample size, time of sampling, miRNA quantification methods, and miRNA normalization parameters (94). Another challenge is a direct consequence of noncardiac conditions, such as cancer, infection, and drug use, affecting miRNA expression (94). Thus, if miRNAs are to be successfully used as biomarkers, it is important to define standard operating procedures for blood collection, processing, and storage, as well as miRNA analysis, to ensure accurate quantitation (95). Distinct methods of detection (high-throughput sequencing, real-time polymerase chain reaction, microarrays) can be used to measure miRNA levels, and their application for specific indications should be determined to minimize differences between studies (96) The establishment of standardized protocols for miRNA quantification reduces experimental variability and allows the combination of the results from multiple studies, resolving the issue of sample number (17). Finally, acquiring data from multiple time points and detailed medical histories is essential to better identify the associations between miRNAs and PAH and to control for potential confounders (94), as demonstrated previously. Several groups observed time-dependent dynamic changes in miRNA expression (33,35). We found that in the MCT model of PH, miR-424(322) increased 1 week after the insult and decreased afterward until the installation of overt heart failure (28).
miRNA as therapeutic targets
MiRNAs might have a promising role as PAH therapies due to their short length, highly conserved sequence, and pre-clinical evidence of their efficacy in human disease. miRNA-based therapy focuses on 2 different approaches: 1) specific artificial elevation by miRNA mimics; or 2) inhibition of selected miRNA levels, which can be achieved by antagomiRs (anti-miRNA antisense oligomers), masking, sponges, and erasers (96). Although some miRNA-based drugs are currently in clinical trials, none have yet reached the status to be considered a pharmacological breakthrough (97).
Regarding PAH, the therapeutic deployment of miRNA is still in the pre-clinical stage. Although numerous studies in experimental PH have shown promising results for future clinical application, including local administration to the lungs by aerosolization, robust large-animal studies and phase I and/or II clinical trials are lacking (14). To make miRNA-based therapies come true in a near future, some challenges need to be overcome, such as the development of effective animal models that closely resemble the human PAH phenotype, and fine tuning of miRNA mimics and antagonists that take into consideration different comorbidities and age- and sex-related issues (97). Moreover, the stability, delivery, and off-target effects of the molecules are still 3 main issues (97,98). First, RNA molecules are fairly unstable because of their 2′-OH chemical group (99) and require chemical modification to reduce their high reactivity. Second, the delivery of the miRNA to the desired site of action is still a hurdle. When the treatment is administered intravenously, the delivery strategies are either passive or active (97). The passive strategy takes advantage of the propensity of several organs, namely, the liver, the spleen, and the lymph nodes, to internalize accumulated particles. In contrast, the active strategies combine the miRNA with a specific molecule that binds to the cells of interest and mediates its endocytosis (98). Finally, the off-target effects of miRNAs result mainly from the lack of target specificity due to the short seed sequence, the existence of several targets for a single miRNA, and toxicity from miRNA-induced immune responses (42).
Ultimately, the challenge is to select which miRNA to target in PAH. Besides being responsible for off-target effects, the intrinsic pleiotropy of several miRNAs implicated in PAH may become a disadvantage due to their overlapping and redundant biology (100). A notion that “miRNAs regulators” may exist, which control multiple, apparently independent, molecular pathways, as well as, “fine tuners” rather than binary “on/off” switches, is emerging and endeavors to unravel this myriad of functional hierarchies (100). Moreover, it is unlikely that a single miRNA is sufficient in preventing, improving, or regressing PAH. Perhaps targeting a combination of convergent miRNAs may be a more effective therapeutic approach (100).
Despite the various challenges, pre-clinical studies provide a promising outlook for the clinical application of miRNA therapeutics in the future.
Conclusions
MiRNAs yield tremendous potential to serve as clinically applicable diagnostic and prognostic biomarkers, as well as novel therapeutic targets in PAH. However, their variable regulation across models and cell types must be taken into consideration when assessing their clinical translation potential. Confirming and validating the role of key miRNAs in humans, with a well-established role in PAH pathogenesis, may help to identify the most promising miRNA for clinical practice. Four miRNAs, miR-29, miR-124, miR-140, and miR-204, may merit special attention, because they have shown a conserved pattern of expression in different experimental models and PAH human tissues. Additional pre-clinical studies and clinical trials are urgently needed to bring these small RNAs from the bench to the bedside in the near future.
Author Disclosures
Dr. Baptista was supported by grants from Fundação para Ciência e Tecnologia (POCI-01-0145-FEDER-032414). Dr. Girão was supported by the European Regional Development Fund through the Operational Program for Competitiveness Factors (POCI-01-0145-FEDER-016385, CENTRO-01-0145-FEDER-000012-N2323, POCI-01-0145-FEDER-007440, CENTRO-01-0145-FEDER-032179, POCI-01-0145-FEDER-022122, UID/NEU/04539/2019, UIDB/04539/2020, and UIDP/04539/2020). All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
Appendix
For a supplemental figure, please see the online version of this paper.
Appendix
References
- 1.Galiè N., Humbert M., Vachiery J.L. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Heart J. 2016;37:67–119. doi: 10.1093/eurheartj/ehv317. [DOI] [PubMed] [Google Scholar]
- 2.Simonneau G., Montani D., Celermajer D.S. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53:1801913. doi: 10.1183/13993003.01913-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heath D., Edwards J.E. The pathology of hypertensive pulmonary vascular disease; a description of six grades of structural changes in the pulmonary arteries with special reference to congenital cardiac septal defects. Circulation. 1958;18:533–547. doi: 10.1161/01.cir.18.4.533. [DOI] [PubMed] [Google Scholar]
- 4.Badano L.P., Ginghina C., Easaw J. Right ventricle in pulmonary arterial hypertension: haemodynamics, structural changes, imaging, and proposal of a study protocol aimed to assess remodelling and treatment effects. Eur J Echocardiogr. 2010;11:27–37. doi: 10.1093/ejechocard/jep152. [DOI] [PubMed] [Google Scholar]
- 5.Corris P., Degano B. Severe pulmonary arterial hypertension: treatment options and the bridge to transplantation. Eur Respir Rev. 2014;23:488–497. doi: 10.1183/09059180.00007214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Humbert M., Sitbon O., Chaouat A. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med. 2006;173:1023–1030. doi: 10.1164/rccm.200510-1668OC. [DOI] [PubMed] [Google Scholar]
- 7.Hsu C.H., Glassner C., Foreman A.J. Treadmill testing improves survival prediction models in pulmonary arterial hypertension. Am Heart J. 2011;162:1011–1017. doi: 10.1016/j.ahj.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 8.Chun H.J., Bonnet S., Chan S.Y. Translational advances in the field of pulmonary hypertension. Translating microRNA biology in pulmonary hypertension. It will take more than "miR" words. Am J Respir Crit Care Med. 2017;195:167–178. doi: 10.1164/rccm.201604-0886PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boucherat O., Potus F., Bonnet S. MicroRNA and pulmonary hypertension. Adv Exp Med Biol. 2015;888:237–252. doi: 10.1007/978-3-319-22671-2_12. [DOI] [PubMed] [Google Scholar]
- 10.Pachnis V., Brannan C.I., Tilghman S.M. The structure and expression of a novel gene activated in early mouse embryogenesis. EMBO J. 1988;7:673–681. doi: 10.1002/j.1460-2075.1988.tb02862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Afonso-Grunz F., Müller S. Principles of miRNA-mRNA interactions: beyond sequence complementarity. Cell Mol Life Sci. 2015;72:3127–3141. doi: 10.1007/s00018-015-1922-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silva D.C.P.D., Carneiro F.D., Almeida K.C., Fernandes-Santos C. Role of miRNAs on the pathophysiology of cardiovascular diseases. Arq Bras Cardiol. 2018;111:738–746. doi: 10.5935/abc.20180215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Brien J., Hayder H., Zayed Y., Peng C. Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front Endocrinol (Lausanne) 2018;9:402. doi: 10.3389/fendo.2018.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Condorelli G., Latronico M.V., Cavarretta E. MicroRNAs in cardiovascular diseases: current knowledge and the road ahead. J Am Coll Cardiol. 2014;63:2177–2187. doi: 10.1016/j.jacc.2014.01.050. [DOI] [PubMed] [Google Scholar]
- 15.Romaine S.P., Tomaszewski M., Condorelli G., Samani N.J. MicroRNAs in cardiovascular disease: an introduction for clinicians. Heart. 2015;101:921–928. doi: 10.1136/heartjnl-2013-305402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dangwal S., Thum T. MicroRNA therapeutics in cardiovascular disease models. Annu Rev Pharmacol Toxicol. 2014;54:185–203. doi: 10.1146/annurev-pharmtox-011613-135957. [DOI] [PubMed] [Google Scholar]
- 17.Zhou S.S., Jin J.P., Wang J.Q. miRNAS in cardiovascular diseases: potential biomarkers, therapeutic targets and challenges. Acta Pharmacol Sin. 2018;39:1073–1084. doi: 10.1038/aps.2018.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Menon S., Fessel J., West J. Microarray studies in pulmonary arterial hypertension. Int J Clin Pract Suppl. 2011:19–28. doi: 10.1111/j.1742-1241.2010.02604.x. [DOI] [PubMed] [Google Scholar]
- 19.Schlosser K., Taha M., Deng Y., Jiang B., Stewart D.J. Discordant regulation of microRNA between multiple experimental models and human pulmonary hypertension. Chest. 2015;148:481–490. doi: 10.1378/chest.14-2169. [DOI] [PubMed] [Google Scholar]
- 20.Hoffmann J., Wilhelm J., Olschewski A., Kwapiszewska G. Microarray analysis in pulmonary hypertension. Eur Respir J. 2016;48:229–241. doi: 10.1183/13993003.02030-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zahid K.R., Raza U., Chen J., Raj J.U., Gou D. Pathobiology of pulmonary artery hypertension: role of lncRNAs. Cardiovasc Res. 2020 doi: 10.1093/cvr/cvaa050. cvaa050. [DOI] [PubMed] [Google Scholar]
- 22.Guttman M., Rinn J.L. Modular regulatory principles of large non-coding RNAs. Nature. 2012;482:339–346. doi: 10.1038/nature10887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sessa R., Hata A. Role of microRNAs in lung development and pulmonary diseases. Pulm Circ. 2013;3:315–328. doi: 10.4103/2045-8932.114758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Batkai S., Bär C., Thum T. MicroRNAs in right ventricular remodelling. Cardiovasc Res. 2017;113:1433–1440. doi: 10.1093/cvr/cvx153. [DOI] [PubMed] [Google Scholar]
- 25.Abe K., Toba M., Alzoubi A. Formation of plexiform lesions in experimental severe pulmonary arterial hypertension. Circulation. 2010;121:2747–2754. doi: 10.1161/CIRCULATIONAHA.109.927681. [DOI] [PubMed] [Google Scholar]
- 26.Paulin R., Michelakis E.D. The metabolic theory of pulmonary arterial hypertension. Circ Res. 2014;115:148–164. doi: 10.1161/CIRCRESAHA.115.301130. [DOI] [PubMed] [Google Scholar]
- 27.Hautefort A., Mendes-Ferreira P., Sabourin J. Bmpr2 mutant rats develop pulmonary and cardiac characteristics of pulmonary arterial hypertension. Circulation. 2019;139:932–948. doi: 10.1161/CIRCULATIONAHA.118.033744. [DOI] [PubMed] [Google Scholar]
- 28.Baptista R., Marques C., Catarino S. MicroRNA-424(322) as a new marker of disease progression in pulmonary arterial hypertension and its role in right ventricular hypertrophy by targeting SMURF1. Cardiovasc Res. 2018;114:53–64. doi: 10.1093/cvr/cvx187. [DOI] [PubMed] [Google Scholar]
- 29.Van De Bruaene A., Delcroix M., Pasquet A. Iron deficiency is associated with adverse outcome in Eisenmenger patients. Eur Heart J. 2011;32:2790–2799. doi: 10.1093/eurheartj/ehr130. [DOI] [PubMed] [Google Scholar]
- 30.Oechslin E., Mebus S., Schulze-Neick I. The adult patient with Eisenmenger syndrome: a medical update after Dana Point Part III: specific management and surgical aspects. Curr Cardiol Rev. 2010;6:363–372. doi: 10.2174/157340310793566127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morrell N.W., Aldred M.A., Chung W.K. Genetics and genomics of pulmonary arterial hypertension. Eur Respir J. 2019;53:1801899. doi: 10.1183/13993003.01899-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Atkinson C., Stewart S., Upton P.D. Primary pulmonary hypertension is associated with reduced pulmonary vascular expression of type II bone morphogenetic protein receptor. Circulation. 2002;105:1672–1678. doi: 10.1161/01.cir.0000012754.72951.3d. [DOI] [PubMed] [Google Scholar]
- 33.Caruso P., MacLean M.R., Khanin R. Dynamic changes in lung microRNA profiles during the development of pulmonary hypertension due to chronic hypoxia and monocrotaline. Arterioscler Thromb Vasc Biol. 2010;30:716–723. doi: 10.1161/ATVBAHA.109.202028. [DOI] [PubMed] [Google Scholar]
- 34.Thum T., Batkai S. MicroRNAs in right ventricular (dys)function (2013 Grover Conference series) Pulm Circ. 2014;4:185–190. doi: 10.1086/675981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reddy S., Zhao M., Hu D.Q. Dynamic microRNA expression during the transition from right ventricular hypertrophy to failure. Physiol Genomics. 2012;44:562–575. doi: 10.1152/physiolgenomics.00163.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Drake J.I., Bogaard H.J., Mizuno S. Molecular signature of a right heart failure program in chronic severe pulmonary hypertension. Am J Respir Cell Mol Biol. 2011;45:1239–1247. doi: 10.1165/rcmb.2010-0412OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kameny R.J., He Y., Zhu T. Analysis of the microRNA signature driving adaptive right ventricular hypertrophy in an ovine model of congenital heart disease. Am J Physiol Heart Circ Physiol. 2018;315:H847–H854. doi: 10.1152/ajpheart.00057.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiao T., Xie L., Huang M., Shen J. Differential expression of microRNA in the lungs of rats with pulmonary arterial hypertension. Mol Med Rep. 2017;15:591–596. doi: 10.3892/mmr.2016.6043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pritchard C.C., Cheng H.H., Tewari M. MicroRNA profiling: approaches and considerations. Nat Rev Genet. 2012;13:358–369. doi: 10.1038/nrg3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu C.G., Calin G.A., Meloon B. Na oligonucleotide microchip for genome-wide microRNA profiling in human and mouse tissues. Proc Natl Acad Sci U S A. 2004;10:9740–9744. doi: 10.1073/pnas.0403293101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shalaby T., Fiaschetti G., Baumgartner M., Grotzer M.A. MicroRNA signatures as biomarkers and therapeutic target for CNS embryonal tumors: the pros and the cons. Int J Mol Sci. 2014;15:21554–21586. doi: 10.3390/ijms151121554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou G., Chen T., Raj J.U. MicroRNAs in pulmonary arterial hypertension. Am J Respir Cell Mol Biol. 2015;52:139–151. doi: 10.1165/rcmb.2014-0166TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ritchie M.E., Phipson B., Wu D. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Research. 2015;43 doi: 10.1093/nar/gkv007. e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luo Y., Dong H.Y., Zhang B. miR-29a-3p attenuates hypoxic pulmonary hypertension by inhibiting pulmonary adventitial fibroblast activation. Hypertension. 2015;65:414–420. doi: 10.1161/HYPERTENSIONAHA.114.04600. [DOI] [PubMed] [Google Scholar]
- 45.Wang T., Li Y., Chen J., Xie L., Xiao T. TGF-β1/Smad3 signaling promotes collagen synthesis in pulmonary artery smooth muscle by down-regulating miR-29b. Int J Clin Exp Pathol. 2018;11:5592–5601. [PMC free article] [PubMed] [Google Scholar]
- 46.Kang K., Peng X., Zhang X. MicroRNA-124 suppresses the transactivation of nuclear factor of activated T cells by targeting multiple genes and inhibits the proliferation of pulmonary artery smooth muscle cells. J Biol Chem. 2013;288:25414–25427. doi: 10.1074/jbc.M113.460287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang D., Zhang H., Li M. MicroRNA-124 controls the proliferative, migratory, and inflammatory phenotype of pulmonary vascular fibroblasts. Circ Res. 2014;114:67–78. doi: 10.1161/CIRCRESAHA.114.301633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang H., Wang D., Li M. Metabolic and proliferative state of vascular adventitial fibroblasts in pulmonary hypertension is regulated through a microRNA-124/PTBP1 (polypyrimidine tract binding protein 1)/pyruvate kinase muscle axis. Circulation. 2017;136:2468–2485. doi: 10.1161/CIRCULATIONAHA.117.028069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Caruso P., Dunmore B.J., Schlosser K. Identification of microRNA-124 as a major regulator of enhanced endothelial cell glycolysis in pulmonary arterial hypertension via PTBP1 (polypyrimidine tract binding protein) and pyruvate kinase M2. Circulation. 2017;136:2451–2467. doi: 10.1161/CIRCULATIONAHA.117.028034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu T.T., Zhang W.F., Yin Y.L. MicroRNA-140-5p targeting tumor necrosis factor-α prevents pulmonary arterial hypertension. J Cell Physiol. 2019;234:9535–9550. doi: 10.1002/jcp.27642. [DOI] [PubMed] [Google Scholar]
- 51.Rothman A.M., Arnold N.D., Pickworth J.A. MicroRNA-140-5p and SMURF1 regulate pulmonary arterial hypertension. J Clin Invest. 2016;126:2495–2508. doi: 10.1172/JCI83361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Y., Xu J. miR-140-5p regulates hypoxia-mediated human pulmonary artery smooth muscle cell proliferation, apoptosis and differentiation by targeting Dnmt1 and promoting SOD2 expression. Biochem Biophys Res Commun. 2016;473:342–348. doi: 10.1016/j.bbrc.2016.03.116. [DOI] [PubMed] [Google Scholar]
- 53.Joshi S.R., Dhagia V., Gairhe S., Edwards J.G., McMurtry I.F., Gupte S.A. MicroRNA-140 is elevated and mitofusin-1 is downregulated in the right ventricle of the Sugen5416/hypoxia/normoxia model of pulmonary arterial hypertension. Am J Physiol Heart Circ Physiol. 2016;311:H689–H698. doi: 10.1152/ajpheart.00264.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meloche J., Pflieger A., Vaillancourt M. Role for DNA damage signaling in pulmonary arterial hypertension. Circulation. 2014;129:786–797. doi: 10.1161/CIRCULATIONAHA.113.006167. [DOI] [PubMed] [Google Scholar]
- 55.Ruffenach G., Chabot S., Tanguay V.F. Role for Runt-related transcription factor 2 in proliferative and calcified vascular lesions in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2016;194:1273–1285. doi: 10.1164/rccm.201512-2380OC. [DOI] [PubMed] [Google Scholar]
- 56.Courboulin A., Paulin R., Giguère N.J. Role for miR-204 in human pulmonary arterial hypertension. J Exp Med. 2011;208:535–548. doi: 10.1084/jem.20101812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu T., Zou X.Z., Huang N. Down-regulation of miR-204 attenuates endothelial-mesenchymal transition by enhancing autophagy in hypoxia-induced pulmonary hypertension. Eur J Pharmacol. 2019;863:172673. doi: 10.1016/j.ejphar.2019.172673. [DOI] [PubMed] [Google Scholar]
- 58.Lu Y., Huang J., Geng S. MitoKATP regulating HIF/miR210/ISCU signaling axis and formation of a positive feedback loop in chronic hypoxia-induced PAH rat model. Exp Ther Med. 2017;13:1697–1701. doi: 10.3892/etm.2017.4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gou D., Ramchandran R., Peng X. miR-210 has an antiapoptotic effect in pulmonary artery smooth muscle cells during hypoxia. Am J Physiol Lung Cell Mol Physiol. 2012;303:L682–L691. doi: 10.1152/ajplung.00344.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jin Y., Pang T., Nelin L.D. MKP-1 is a target of miR-210 and mediate the negative regulation of miR-210 inhibitor on hypoxic hPASMC proliferation. Cell Biol Int. 2015;39:113–120. doi: 10.1002/cbin.10339. [DOI] [PubMed] [Google Scholar]
- 61.Mondejar-Parreño G., Callejo M., Barreira B. miR-1 induces endothelial dysfunction in rat pulmonary arteries. J Physiol Biochem. 2019;75:519–529. doi: 10.1007/s13105-019-00696-2. [DOI] [PubMed] [Google Scholar]
- 62.Mondejar-Parreño G., Callejo M., Barreira B. miR-1 is increased in pulmonary hypertension and downregulates Kv1.5 channels in rat pulmonary arteries. J Physiol. 2019;597:1185–1197. doi: 10.1113/JP276054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sysol J.R., Chen J., Singla S. Micro-RNA-1 is decreased by hypoxia and contributes to the development of pulmonary vascular remodeling via regulation of sphingosine kinase 1. Am J Physiol Lung Cell Mol Physiol. 2018;314:L461–L472. doi: 10.1152/ajplung.00057.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Connolly M., Garfield B.E., Crosby A., Morrell N.W., Wort S.J., Kemp P.R. miR-1-5p targets TGF-βR1 and is suppressed in the hypertrophying hearts of rats with pulmonary arterial hypertension. PLoS One. 2020;15 doi: 10.1371/journal.pone.0229409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bertero T., Cottrill K., Krauszman A. The microRNA-130/301 family controls vasoconstriction in pulmonary hypertension. J Biol Chem. 2015;290:2069–2085. doi: 10.1074/jbc.M114.617845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li L., Kim I.K., Chiasson V., Chatterjee P., Gupta S. NF-κB mediated miR-130a modulation in lung microvascular cell remodeling: implication in pulmonary hypertension. Exp Cell Res. 2017;359:235–242. doi: 10.1016/j.yexcr.2017.07.024. [DOI] [PubMed] [Google Scholar]
- 67.Bertero T., Cottrill K.A., Annis S. A YAP/TAZ-miR-130/301 molecular circuit exerts systems-level control of fibrosis in a network of human diseases and physiologic conditions. Sci Rep. 2015;5:18277. doi: 10.1038/srep18277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li S., Ran Y., Zhang D., Chen J., Li S., Zhu D. MicroRNA-138 plays a role in hypoxic pulmonary vascular remodelling by targeting Mst1. Biochem J. 2013;452:281–291. doi: 10.1042/BJ20120680. [DOI] [PubMed] [Google Scholar]
- 69.Hong Z., Chen K.H., DasGupta A. MicroRNA-138 and microRNA-25 down-regulate mitochondrial calcium uniporter, causing the pulmonary arterial hypertension cancer phenotype. Am J Respir Crit Care Med. 2017;195:515–529. doi: 10.1164/rccm.201604-0814OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu J.J., Zhang H., Xing F. MicroRNA-138 promotes proliferation and suppresses mitochondrial depolarization in human pulmonary artery smooth muscle cells through targeting TASK-1. Mol Med Rep. 2018;17:3021–3027. doi: 10.3892/mmr.2017.8200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brock M., Trenkmann M., Gay R.E. Interleukin-6 modulates the expression of the bone morphogenic protein receptor type II through a novel STAT3-microRNA cluster 17/92 pathway. Circ Res. 2009;104:1184–1191. doi: 10.1161/CIRCRESAHA.109.197491. [DOI] [PubMed] [Google Scholar]
- 72.Pullamsetti S.S., Doebele C., Fischer A. Inhibition of microRNA-17 improves lung and heart function in experimental pulmonary hypertension. Am J Respir Crit Care Med. 2012;185:409–419. doi: 10.1164/rccm.201106-1093OC. [DOI] [PubMed] [Google Scholar]
- 73.Chen T., Zhou G., Zhou Q. Loss of microRNA-17∼92 in smooth muscle cells attenuates experimental pulmonary hypertension via induction of PDZ and LIM domain 5. Am J Respir Crit Care Med. 2015;191:678–692. doi: 10.1164/rccm.201405-0941OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen T., Zhou Q., Tang H. miR-17/20 controls prolyl hydroxylase 2 (PHD2)/hypoxia-inducible factor 1 (HIF1) to regulate pulmonary artery smooth muscle cell proliferation. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.116.004510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Parikh V.N., Jin R.C., Rabello S. MicroRNA-21 integrates pathogenic signaling to control pulmonary hypertension: results of a network bioinformatics approach. Circulation. 2012;125:1520–1532. doi: 10.1161/CIRCULATIONAHA.111.060269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Green D.E., Murphy T.C., Kang B.Y. PPARγ ligands attenuate hypoxia-induced proliferation in human pulmonary artery smooth muscle cells through modulation of microRNA-21. PLoS One. 2015;10 doi: 10.1371/journal.pone.0133391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhu B., Gong Y., Yan G. Down-regulation of lncRNA MEG3 promotes hypoxia-induced human pulmonary artery smooth muscle cell proliferation and migration via repressing PTEN by sponging miR-21. Biochem Biophys Res Commun. 2018;495:2125–2132. doi: 10.1016/j.bbrc.2017.11.185. [DOI] [PubMed] [Google Scholar]
- 78.Iannone L., Zhao L., Dubois O. miR-21/DDAH1 pathway regulates pulmonary vascular responses to hypoxia. Biochem J. 2014;462:103–112. doi: 10.1042/BJ20140486. [DOI] [PubMed] [Google Scholar]
- 79.Yang S., Banerjee S., Freitas Ad. miR-21 regulates chronic hypoxia-induced pulmonary vascular remodeling. Am J Physiol Lung Cell Mol Physiol. 2012;302:L521−9. doi: 10.1152/ajplung.00316.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu H., Yin T., Yan W. Dysregulation of microRNA-214 and PTEN contributes to the pathogenesis of hypoxic pulmonary hypertension. Int J Chron Obstruct Pulmon Dis. 2017;12:1781–1791. doi: 10.2147/COPD.S104627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu H., Tao Y., Chen M. Upregulation of microRNA-214 contributes to the development of vascular remodeling in hypoxia-induced pulmonary hypertension via targeting CCNL2. Sci Rep. 2016;6:24661. doi: 10.1038/srep24661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xing X.Q., Li B., Xu S.L., Liu J., Zhang C.F., Yang J. MicroRNA-214-3p regulates hypoxia-mediated pulmonary artery smooth muscle cell proliferation and migration by targeting ARHGEF12. Med Sci Monit. 2019;25:5738–5746. doi: 10.12659/MSM.915709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sahoo S., Meijles D.N., Al Ghouleh I. MEF2C-MYOCD and Leiomodin1 suppression by miRNA-214 promotes smooth muscle cell phenotype switching in pulmonary arterial hypertension. PLoS One. 2016;11 doi: 10.1371/journal.pone.0153780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Meloche J., Le Guen M., Potus F. miR-223 reverses experimental pulmonary arterial hypertension. Am J Physiol Cell Physiol. 2015;309:C363−72. doi: 10.1152/ajpcell.00149.2015. [DOI] [PubMed] [Google Scholar]
- 85.Zeng Y., Zhang X., Kang K. MicroRNA-223 attenuates hypoxia-induced vascular remodeling by targeting RhoB/MLC2 in pulmonary arterial smooth muscle cells. Sci Rep. 2016;6:24900. doi: 10.1038/srep24900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu A., Liu Y., Li B., Yang M., Liu Y., Su J. Role of miR-223-3p in pulmonary arterial hypertension via targeting ITGB3 in the ECM pathway. Cell Prolif. 2019;52 doi: 10.1111/cpr.12550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim J., Kang Y., Kojima Y. An endothelial apelin-FGF link mediated by miR-424 and miR-503 is disrupted in pulmonary arterial hypertension. Nat Med. 2013;19:74–82. doi: 10.1038/nm.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hale A.E., White K., Chan S.Y. Hypoxamirs in pulmonary hypertension: breathing new life into pulmonary vascular research. Cardiovasc Diagn Ther. 2012;2:200–212. doi: 10.3978/j.issn.2223-3652.2012.08.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ye Y., Hu Z., Lin Y., Zhang C., Perez-Polo J.R. Downregulation of microRNA-29 by antisense inhibitors and a PPAR-gamma agonist protects against myocardial ischaemia-reperfusion injury. Cardiovasc Res. 2010;87:535–544. doi: 10.1093/cvr/cvq053. [DOI] [PubMed] [Google Scholar]
- 90.Kriegel A.J., Liu Y., Fang Y., Ding X., Liang M. The miR-29 family: genomics, cell biology, and relevance to renal and cardiovascular injury. Physiol Genomics. 2012;44:237–244. doi: 10.1152/physiolgenomics.00141.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen X., Talati M., Fessel J.P. Estrogen metabolite 16α-hydroxyestrone exacerbates bone morphogenetic protein receptor type II-associated pulmonary arterial hypertension through microRNA-29-mediated modulation of cellular metabolism. Circulation. 2016;133:82–97. doi: 10.1161/CIRCULATIONAHA.115.016133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wei C., Henderson H., Spradley C. Circulating miRNAs as potential marker for pulmonary hypertension. PLoS One. 2013;8 doi: 10.1371/journal.pone.0064396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kapodistrias N., Bobori C., Theocharopoulou G. miR-140-3p downregulation in association with PDL-1 Overexpression in many cancers: a review from the literature using predictive bioinformatics tools. Adv Exp Med Biol. 2017;988:225–233. doi: 10.1007/978-3-319-56246-9_18. [DOI] [PubMed] [Google Scholar]
- 94.Navickas R., Gal D., Laucevičius A., Taparauskaitė A., Zdanytė M., Holvoet P. Identifying circulating microRNAs as biomarkers of cardiovascular disease: a systematic review. Cardiovasc Res. 2016;111:322–337. doi: 10.1093/cvr/cvw174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Felekkis K., Papaneophytou C. Challenges in using circulating micro-RNAs as biomarkers for cardiovascular diseases. Int J Mol Sci. 2020;21:561. doi: 10.3390/ijms21020561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schulte C., Karakas M., Zeller T. MicroRNAs in cardiovascular disease - clinical application. Clin Chem Lab Med. 2017;55:687–704. doi: 10.1515/cclm-2016-0576. [DOI] [PubMed] [Google Scholar]
- 97.Bonneau E., Neveu B., Kostantin E., Tsongalis G.J., De Guire V. How close are miRNAs from clinical practice? A perspective on the diagnostic and therapeutic market. EJIFCC. 2019;30:114–127. [PMC free article] [PubMed] [Google Scholar]
- 98.Burnett J.C., Rossi J.J. RNA-based therapeutics: current progress and future prospects. Chem Biol. 2012;19:60–71. doi: 10.1016/j.chembiol.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Haussecker D. Current issues of RNAi therapeutics delivery and development. J Control Release. 2014;195:49–54. doi: 10.1016/j.jconrel.2014.07.056. [DOI] [PubMed] [Google Scholar]
- 100.Negi V., Chan S.Y. Discerning functional hierarchies of microRNAs in pulmonary hypertension. JCI Insight. 2017;2 doi: 10.1172/jci.insight.91327. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.