Visual Abstract
Key Words: acellular, angiogenesis, extracellular vesicles, regeneration, senescence
Abbreviations and Acronyms: BM, bone marrow; CVD, cardiovascular disease; EC, endothelial cell; EPC, endothelial progenitor cell; EV, extracellular vesicle; FBS, fetal bovine serum; lin− BMC, lineage negative bone marrow cell; MEM, minimum essential medium; MI, myocardial infarction; miR, microRNA; MSC, mesenchymal stromal cell; NTA, nanotracking analysis; PBS, phosphate-buffered saline; qPCR, quantitative transcription polymerase chain reaction; TEV, tailored extracellular vesicle; VEGF, vascular endothelial growth factor
Highlights
-
•
EVs derived from young, but not aged, MSCs rejuvenate senescent EPCs in vitro, recapitulating the effect of MSC transplantation.
-
•
Aged MSCs can be genetically modified to produce tailored EVs with increased EPC rejuvenation capacity in vitro and increased angiogenesis capacity following ischemic event in vivo.
-
•
EVs represent a promising platform to develop an acellular therapeutic approach in regenerative medicine for cardiovascular diseases.
Summary
Mesenchymal stromal cell (MSC) transplantation is a form of the stem-cell therapy that has shown beneficial effects for many diseases. The use of stem-cell therapy, including MSC transplantation, however, has limitations such as the tumorigenic potential of stem cells and the lack of efficacy of aged autologous cells. An ideal therapeutic approach would keep the beneficial effects of MSC transplantation while circumventing the limitations associated with the use of intact stem cells. This study provides proof-of-concept evidence that MSC-derived extracellular vesicles represent a promising platform to develop an acellular therapeutic approach that would just do that. Extracellular vesicles are membranous vesicles secreted by MSCs and contain bioactive molecules to mediate communication between different cells. Extracellular vesicles can be taken up by recipient cells, and once inside the recipient cells, the bioactive molecules are released to exert the beneficial effects on the recipient cells. This study, for the first time to our knowledge, shows that extracellular vesicles secreted by MSCs recapitulate the beneficial effects of MSCs on vascular repair and promote blood vessel regeneration after ischemic events. Furthermore, MSCs from aged donors can be engineered to produce extracellular vesicles with improved regenerative potential, comparable to MSCs from young donors, thus eliminating the need for allogenic young donors for elderly patients.
Atherosclerosis is a chronic inflammatory process and the underlying cause of ischemic cardiovascular disease (CVD) (1). Although the hallmark of atherosclerosis is accumulation of lipid-laden macrophages in the subendothelial area of the arterial wall, the process is proceeded with imbalance between endothelial injury and repair (2). Endothelial progenitor cells (EPCs) have the capability of self-renewal and differentiation into mature endothelial cells (ECs). Upon endothelial injury, EPCs are mobilized from bone marrow and migrate to sites of vascular injury. Through proliferation and differentiation into mature ECs, as well as activating resident ECs via the secretion of angiogenic growth factors (3, 4, 5, 6, 7, 8), EPCs halt the inflammatory process and prevent atherosclerosis (9, 10, 11, 12, 13). In addition, EPCs play a crucial role in promoting angiogenesis following ischemic events (5,7,9,10). Aging-associated EPC senescence leads to insufficient endothelial repair and angiogenesis, increasing the risk of developing ischemic events and poorer prognosis after ischemia events (14). As such, rejuvenating endogenous senescent EPCs is of substantial interest in regenerative medicine for the prevention and treatment of CVD.
Mesenchymal stromal cells (MSCs) are adult stem cells that have multilineage differential potentials. MSC transplantation has been evaluated as a new and promising therapeutic approach for many diseases (15). Indeed, there are over 600 clinical trials using MSCs listed at www.clinicaltrials.gov. Initially, MSCs were thought to exert their regenerative effects by engrafting and differentiating into desired cells at the damaged tissues. Recent studies, however, have suggested that the beneficial effect of MSCs are mainly attributable to their paracrine signaling actions (16, 17, 18, 19, 20). Consistent with the current dogma that MSCs ameliorate diseases through secreting bioactive factors to boost endogenous repair pathways, such as EPC-mediated vascular repair and angiogenesis, it has been observed that MSC transplantation improved circulating EPC functionality (21). Notably, the beneficial effects were only observed with MSCs from young allogeneic bone marrow (BM), but not aged autologous BM (22).
In recent years, extracellular vesicles (EVs) have been gaining increasing interest as a promising platform to mediate the regenerative potential of MSCs’ secretome (23). EVs are membranous vesicles secreted by most cell types, containing many bioactive molecules such as mRNAs, microRNAs (miRs), proteins, and lipids. The cargo of EVs reflects the pathophysiological state of their cells of origin (24). EVs can be taken up by recipient cells through receptor-mediated endocytosis or fusion of membranes. Once inside the recipient cells, EV miRs are released to regulate gene expression in the recipient cells (25). With their ability to transport bioactive molecules between cells, EVs mediate intercellular communication between cells in both analogous and disparate tissues (26,27). Recent studies support the notion that EVs play a pivotal role in stem cell plasticity and tissue regeneration, promoting angiogenesis and improving blood supply in targeted organs (28, 29, 30). However, no study has examined the role of MSC-secreted EVs, particularly tailored EVs (TEVs) produced by genetic modification of MSCs, in the context of improving EPC-mediated vascular repair and angiogenesis. Herein, we sought to characterize the effect of MSC-derived EVs/TEVs on rejuvenating senescent EPCs.
Methods
Animals
Wild-type C57BL/6J mice were purchased from The Jackson Laboratory (Bar Harbor, Maine). Aged C57BL/6J mice were raised and maintained in the University of Miami Animal Facility. Aged Balb/c mice (>26 months) were obtained from The National Institute of Aging (Bethesda, Maryland). Animals were housed in sterile microisolator cages in which they received autoclaved food and autoclaved acidified drinking water in a specific pathogen-free facility. Pups were weaned at 3 weeks of age and fed regular chow diet as described before (31). All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Miami.
Isolation of EPCs and MSCs
To isolate young and aged cells, wild-type C57BL/6J mice were euthanized at the age of 1 month or 2 years. At the time of euthanasia, whole BM cells were collected. Briefly, femur and tibia bones were dissected, cut and flushed with Minimum Essential Medium (MEM) medium (Thermo Fisher Scientific, Waltham, Massachusetts) until translucent. The flushed material was passed through a 100-μm strainer into a 50-ml sterile plastic tube, which was centrifuged at 300 × g for 7 min. One milliliter of RBC Lysis Buffer (BioLegend, San Diego, California) was added to the tube and incubated for 10 min at room temperature. The reaction was deactivated after the incubation time by the addition of MEM medium (10 ml). The material was centrifuged at 300 × g for 7 min, and the pelleted cells were washed with MEM medium once more. Mouse EPCs/lineage negative bone marrow cells (lin− BMCs) were isolated by magnetic separation using mouse Lineage Cell Depletion Kit (Miltenyi Biotec, Bergisch Gladbach, Germany) following the manufacturer’s protocol. In brief, BM cells were magnetically labeled with a cocktail of biotinylated antibodies against a panel of lineage+ antigens (CD5, CD45R [B220], CD11b, anti–Gr-1 [Ly-6G/C], 7-4, and Ter-119 antibodies) and anti-biotin microbeads. The lineage+ cells were depleted by retaining them on a MACS Column (Miltenyi Biotec) in the magnetic field of a MACS separator (Miltenyi Biotec), while the unlabeled lineage− cells (lin− BMCs) passed through the column. Isolated lin− BMCs/EPCs were cultured on plates coated with mouse plasma fibronectin (Sigma-Aldrich, St. Louis, Missouri) and endothelial basal medium-2 (Lonza, Basel, Switzerland) supplemented with EGM-2MV single aliquots (Lonza) containing vascular endothelial growth factor (VEGF), fibroblast growth factor-2, epidermal growth factor, insulin-like growth factor, ascorbic acid, hydrocortisone, gentamycin, amphotericin-B, and 20% fetal bovine serum (FBS). For MSC isolation, BM cells were cultured in complete MEM medium supplemented with 20% FBS and 1% penicillin-streptomycin until the third passage before downstream applications (32). This was done to prevent culture contamination with other cell types, and only MSCs were selected (32). After the third passage, cells were expanded for EV extraction. For stable transduction experiments, MSCs were used within 5 passages after the stable line was established. The pace of cell growth and morphology of aged MSCs was consistently different from young MSCs. Aged MSCs, for all passages, grew slower, had less cytoplasmic prolongments, and were overall rounder, with flattened cytoplasm compared with young MSCs.
Coculture of EPCs and MSCs
EPCs were seeded onto a 6-well plate at a density of 2 × 105/well. MSCs were seeded onto 6-well Transwell inserts (Corning, Corning, New York) at the same density. Once the MSCs were attached, the Transwell inserts were transferred to the top to the EPC culture. MSCs and EPC were cocultured for 5 days before downstream analysis.
Isolation and characterization of EVs
For EV extraction, MSCs were seeded at a density of 1 × 106 cells/10-cm plate and cultured until 80% confluence. Cell plates were then washed with phosphate-buffered saline (PBS) and 10 ml of medium without FBS was added to each plate. After 24 h of incubation, the medium was collected, and EVs were isolated as previously described (33). Briefly, culture medium was centrifuged for 10 min at 300 × g. The supernatant was centrifuged at 2,000 × g for 10 min, followed by another supernatant centrifugation at 10,000 × g for 30 min. The supernatant was then centrifuged at 100,000 × g for 70 min. The pellet (containing EVs) was resuspended with PBS solution and was centrifuged again for 70 min at 100,000 × g. EV pellets isolated from each 10 ml of medium were resuspended in 200 μl of PBS for downstream analysis. Protein concentration of EV samples was determined using the Pierce BCA Protein Assay Kit (Thermo Fisher Scientific). Nanotracking analysis (NTA) was used to examine the size and particle number of EVs. Briefly, the isolated EVs were diluted 100 times and were analyzed using the Nanosight NS300 system with Nanosight NTA 2.3 Analytical Software (Malvern Instruments, Malvern United Kingdom). The isolated EVs were purified using CD63-coated Dynabeads magnetic beads (Invitrogen, Carlsbad, California). The bead-bound EVs were incubated with anti-CD63, anti-CD9, and anti-CD81 rabbit anti-mouse antibody (1:500 dilution each, ExoAb Antibody Kit, System Biosciences, Palo Alto, California) for 1 h at room temperature, washed with PBS containing 0.1% bovine serum albumin, and then incubated with Alexa Fluor 488 goat anti-rabbit IgG (1:1,000 dilution, Invitrogen) in the dark for 1 h. The expression of these EV markers was analyzed by flow cytometry (CytoFlex Flow Cytometer, Beckman Coulter, San Jose, California).
Generation of TEVs
HEK 293 cells were used to package lentivirus particles with a plasmid encoding hsa-miR-126 using Lipofectamine 2000 (Thermo Fisher Scientific) transfection reagent according to the manufacturer’s instructions. Virus particles were harvested and then concentrated using a Lenti-X Concentrator (Clontech, Thermo Fisher Scientific) according to the manufacturer’s protocol. Aged MSCs were seeded on a 6-well plate (2 0078 105 cells/well). Cells were selected for the presence of the plasmid by treatment with puromycin (10 μg/ml) for 2 days. Surviving cells expressing green fluorescent protein (also present in transduced plasmid) were later expanded and constituted the stable new cell line.
Exposure of aged EPCs to EVs
Aged EPCs were seeded onto 6-well plates (1 × 105 cells/well). For EV/TEV exposure treatment, serum-free medium was supplemented with EVs collected from cultured aged MSCs, young MSCs, aged MSCs transduced with lentivirus vector encoding miR-126 (TEVs), or control miR (miR-Ctrl). The final concentration of EVs/TEVs (by total protein quantification) in the medium was 100 μg/ml. Cells were incubated for ∼2 to 4 days before downstream analysis. For EV/TEV tracking analysis, isolated EVs/TEVs were incubated with 50 nmol/l 1'-dioctadecyl-3,3,3',3'-tetramethylindocarbocyanine perchlorate (DiI) in methanol for 1 hour in the dark at room temperature with gentle agitation. Stained EVs/TEVs were added to serum-free EPC culture medium. Twenty-four hours later, cells were fixed and stained with wheat germ agglutinin for membranes, and DAPI (4′,6-diamidino-2-phenylindole) for nuclei. Digital images were acquired using a Nikon Eclipse TE2000-U fluorescent microscope (Nikon, Melville, New York).
Cellular and molecular assays
Senescence-associated β-galactosidase staining
Cells were washed with PBS and stained with β-galactosidase (β-gal) staining kit (Sigma-Aldrich). Images of the staining were acquired in 5 to 10 random microscopic fields/sample at ×200 magnification. Percentage of the positive cells was calculated by counting ≥600 cells for each sample.
Western blotting
Cells were lysed in cell lysis buffer (Cell Signaling Technology, Danvers, Massachusetts) with protease inhibitor cocktail (Sigma-Aldrich). The protein concentration was determined using the Pierce BCA Protein Assay Kit (Thermo Fisher Scientific). Equal amounts of protein (60 μg) were loaded onto 4% to 20% Tris-Glycine Gels (Invitrogen) and transferred to polyvinylidene difluoride membranes (Millipore, Burlington, Massachusetts). The membranes were blocked in Tris-buffered saline with 0.05% Tween-20 and 5% milk, incubated with primary (rabbit polyclonal anti-p16INK4a, anti-p19ARF, and anti-GAPDH [glyceraldehyde 3-phosphate dehydrogenase]) and secondary antibodies (horseradish peroxidase–labeled anti-rabbit), and washed according to standard procedures. Antibodies were purchased from Santa Cruz Biotechnology (Dallas, Texas) or Sigma-Aldrich. Protein bands were visualized with SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Fisher Scientific) and quantified with Quantity One System (Bio-Rad Laboratories, Hercules, California).
Quantitative reverse transcription polymerase chain reaction
Total RNAs, including small RNAs, were extracted from cells and EVs using the miRNeasy Mini Kit (Qiagen, Hilden, Germany). For miRs, reverse transcription quantitative transcription polymerase chain reaction (qPCR) was performed using specific primers and probes supplied in TaqMan MicroRNA Assay kits (Thermo Fisher Scientific). The U6 small nucleolar RNA was used as the housekeeping small RNA reference. For mRNAs, reverse transcription qPCR was performed using the High Capacity RNA-to-cDNA kit (Applied Biosystems, Foster City, California) and TaqMan gene Expression Assay for spred-1, normalized to the 18S ribosome housekeeping gene. qPCR was performed on the ABI PRISM 7300 system (Applied Biosystems), and the relative expression was calculated using the 2−ΔΔCt method.
Tube formation assay
EPCs were seeded onto a Corning Matrigel Matrix-coated 96-well plate (2 × 104 cells/well) and cultured with fresh medium supplemented with 10 ng/ml VEGF (Thermo Fisher Scientific). After 16 h, images of tubes were acquired in 10 random microscopic fields per sample at ×100 magnification, tube formation was defined as a tube-like structure exhibiting a length 4 times its width, and the cumulative tube lengths were measured by ImageJ software version 1.45 (NIH, Bethesda, Maryland).
MTT assay for cell proliferation
EPCs were seeded onto a 96-well plate (2 × 104 cells/well and were exposed to MTT solution for 3 h. The absorbance was read at 570 nm in a plate reader.
Hindlimb ischemia model/in vivo angiogenesis assay
The mouse model of hindlimb ischemia was generated by ligating the right proximal femoral artery and vein of 26- to 27-month-old Balb/c mice. EVs/TEVs were delivered by intramuscular injection at 3 different sites (gastrocnemius, gracilis, and quadriceps muscles, respectively, 200 μg/200 μl per injection) of the ischemic leg the following day. To evaluate limb perfusion ratio (ischemic limb [right]/normal limb [left]), laser Doppler perfusion imaging (Moor Instruments, Devon, United Kingdom) was performed at days 0 and 7 post-ischemia as previously described (31). After 7 days, muscle tissues were collected, fixed with 4% paraformaldehyde, and then embedded in optimal cutting temperature compound as described (34). Tissue sections,10-μm, were made with a cryotome. Immunohistochemistry was performed using anti-CD31/platelet and endothelial cell adhesion molecule-1 (PECAM-1) antibody (Novus Biologicals, Centennial, Colorado) to stain ECs. Large and small blood vessels, as well as single ECs, were included in the angiogenesis evaluation. Five random microscopic fields per sample were photographed at ×200 magnification. The percentage of the area that was positively stained with anti-CD31/PECAM-1 antibody was determined using ImageJ analysis software.
Statistical analyses
Results are expressed as mean ± SEM. Shapiro-Wilks normality test was used to check the distribution of continuous variable. For normally distributed variables, Tukey’s post test following analysis of variance was used. For variables that are not normally distributed, nonparametric Kruskal-Wallis test followed by Dunn’s multiple comparisons was used. Statistical analysis was performed using SPSS 16.0 software (SPSS, Chicago, Illinois). A value of p < 0.05 was considered statistically significant.
Results
The secretome of young MSCs, but not aged MSCs, rejuvenates aged EPCs
MSCs have been shown to promote angiogenesis in vivo via secreting paracrine factors. We reasoned that MSCs, especially young MSCs, could rejuvenate senescent EPCs through their secreted factors. To that end, we cocultured MSCs with aged EPCs in a Transwell system, which prevents direct MSC–EPC contact. Coculture with MSCs from young mice rejuvenated aged EPCs, as evidenced by decreased β-gal staining (72.1 ± 2.9% vs. 19.4 ± 2.2%, for aged EPCs vs. aged EPCs cocultured with young MSCs; p < 0.05), a well-established marker for cellular senescence. In addition, the protein level of p16INK4a and the mRNA level of p19ARF decreased, further supporting the rejuvenating effects demonstrated by β-gal staining (Figure 1). Coculture with MSCs from aged mice, however, did not show the rejuvenating effects on EPCs. These data indicate that paracrine factors secreted by young MSCs mediate the cellular effects of MSCs on the rejuvenation of aged EPCs.
Figure 1.
Coculture of Aged EPCs With Young MSCs Rejuvenates Aged EPCs
Molecular markers for cell senescence, including senescence-associated β-galactosidase (β-gal) (A and B), p16Ink4a (C and D), and p19Arf (E), were increased in aged EPCs compared with young EPCs. These EPC senescence markers were down-regulated when aged EPCs were cocultured with young MSCs, but not with aged MSCs. ∗p < 0.05 compared with young EPCs. All experiments were performed in triplicates. Scale bar: 100 μm. EPC = endothelial progenitor cell; MSC = mesenchymal stromal cell.
MSC-derived EVs recapitulate the effects of MSCs on rejuvenating EPCs
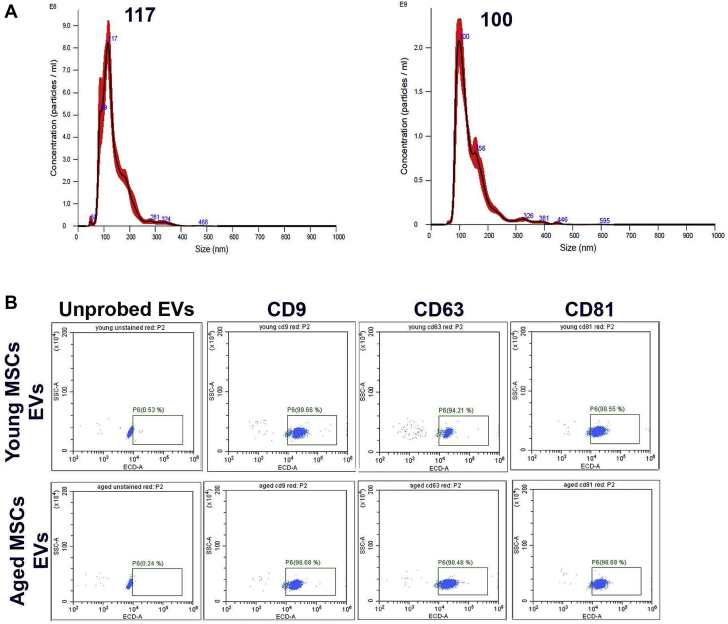
Because EVs serve as vehicles to transport bioactive molecules, such as miRs, between cells, we asked whether MSC-derived EVs would mediate MSCs’ paracrine effects on rejuvenating senescent EPCs. EVs were isolated from the medium of cultured MSCs. Purified EVs were first analyzed by NTA for size distribution and number of particles. There were no significant differences in size distributions, number of particles, and protein concentrations of EVs isolated from the culture medium of young versus aged MSCs (Table 1, Figure 2A). Purified EVs were then analyzed by flow cytometry for the presence of EV markers CD63, CD9, and CD81. This analysis demonstrated that EV-specific markers were expressed in ∼98% of EVs isolated from the culture medium of both young and aged MSCs (Figure 2B).
Table 1.
Concentration of MSC-Derived EVs
| EV Samples | Concentration |
|
|---|---|---|
| Particles/Frame | Total Protein, mg/mL | |
| Young MSC EVs | 30.0 ± 0.9 | 22.1± 3.9 |
| Aged MSC EVs | 27.8 ± 1.4 | 24.1 ± 4.6 |
EV = extracellular vesicle; MSC = mesenchymal stromal cell.
Figure 2.
Characterization of MSC-Derived EVs
Extracellular vesicles (EVs) derived from young and aged MSCs were analyzed by nanoparticle tracking analysis (NTA) for particle size and number. There was no difference in average particle diameter size between EVs from young and aged MSCs (A). EVs from young and aged MSCs were stained with antibodies against EV-specific markers (CD9, CD63, and CD81) and analyzed by flow cytometry. These EV markers were expressed in ∼98% of EVs isolated from both young and aged MSCs cultures, and there was no difference between these 2 populations (B). MSC = mesenchymal stromal cell.
To test the rejuvenating potentials of MSC-derived EVs, senescent EPCs were cultured in the presence of MSC-derived EVs for 4 days. EVs isolated from young MSCs significantly rejuvenated aged EPCs, as evidenced by both decreased levels of β-gal staining (70.1 ± 2.0% vs. 18.5 ± 1.4% for aged MSCs vs. aged MSCs treated with EVs from young MSCs; p < 0.05) and p16INK4a expression, to a degree similar to that observed with the young MSC-aged EPC coculture experiments. By contrast, EVs from aged MSCs had no detectable effects (Figure 3). These data indicate that EVs recapitulate the effects of young MSCs on rejuvenating aged EPCs as observed in the coculture experiment, suggesting that young MSC-derived EVs carry the bioactive molecules responsible for EPC rejuvenation.
Figure 3.
EVs Recapitulate the Effects of MSCs on the Rejuvenation of EPCs in Vitro
Molecular markers for cell senescence-associated β-gal (A and B) and p16Ink4a(C and D) were down-regulated when aged EPCs were cultured with EVs isolated from young MSCs, but not aged MSCs. All experiments were conducted in triplicate. Scale bar: 100 μm. ∗p < 0.05 compared with young EPCs. Abbreviations as in Figures 1 and 2.
EVs derived from young and aged MSCs show a miR expression pattern similar to that of their parent cells
On the basis of microarray analyses and detailed functional studies, we have previously shown that increased expression of miR-146a, miR-10A∗, and miR-21 was associated with EPC senescence (31,35). Furthermore, our genomic profiling data and the work of others have supported the role of increased expression of miR-29c and decreased expression of miR-126 in EPC aging (36). Therefore, we examined the expression of these miRs in young and aged MSCs, as well as in EVs derived from them, respectively. Interestingly, aged MSCs have higher levels of miR-146a, miR-10A∗, miR-21, and miR-29c, and a lower level of miR-126—an expression pattern characteristic of aged EPCs (Figure 4), suggesting that a same set of miRs may regulate both MSC and EPC senescence. Furthermore, EVs isolated from young and aged MSCs showed an expression pattern of these miRs in the same direction as their parent cells (Figure 4). These findings are consistent with the notion that the cargo of EVs reflects the pathophysiological/aging state of the parental cells of the EV origin.
Figure 4.
EV miRNAs and Intracellular miRNAs Show Similar Expression Patterns During Aging
Intracellular miRNAs that have been associated with cellular senescence in EPCs show similar patterns in young and aged MSCs (A) and their respective EVs (B). ∗p < 0.05 aged versus young MSCs. miRNA = microRNA; other abbreviations as in Figures 1 and 2.
Genetic modification of aged MSCs enables the production of TEVs loaded with miR-126
Previous studies have shown that miR-126 is an essential player in maintaining EC functionality and promoting angiogenesis (37, 38, 39, 40, 41). The expression levels of miR-126 in aged MSCs and EVs derived from them are significantly down-regulated (Figure 4). We examined whether transduction of miR-126–overexpressing lentiviral vector in aged MSCs could result in the production of TEVs that would carry the increased amount of miR-126. Indeed, TEVs isolated from miR-126–transduced aged MSCs contained 20-fold higher levels of miR-126, as compared with EVs isolated from scrambled control (miR-Ctrl)-transduced cells (Figure 5A). Overexpression of miR-126 in aged MSCs did not significantly change the expression levels of other EPC senescence-related miRs, such as miR-21, miR-10A∗, miR-146a, and miR-29c, in the TEVs (Figure 5A). Importantly, when incubated with aged EPCs, these miR-126–overexpressing TEVs were readily taken up by the aged EPCs (Figure 5B). Consistently, the intracellular expression of spred-1 (a key target gene of miR-126 and an endogenous inhibitor of VEGF signaling) was suppressed in aged EPCs treated with the TEVs (Figure 5C), which was accompanied with increased intracellular level of miR-126 (Figure 5D). These data indicate that MSCs can be engineered to produce TEVs with desired cargo, and the cargo can then be delivered into recipient cells to modify gene expression.
Figure 5.
miR-126–Transduced Aged MSCs Enable the Production of TEVs
Lentiviral-mediated miR-126 transduction in aged MSCs results in the production of TEVs that contain increased levels of miR-126, but with no changes in other senescence-associated miRs, except for miR-10a (A). TEVs were stained with DiI before being added to aged EPC culture. Twenty-four hours later, cells were fixed and stained with wheat germ agglutinin (green) for cell membrane and DAPI (blue) for nuclei. TEVs from MSCs overexpressing miR-126 or miR-Ctrl were readily taken up by aged EPCs as shown by DiI (red) staining (B). Intracellular expression of spred-1, a target gene of miR-126, was suppressed in aged EPCs after incubation with TEVs from aged MSCs transduced with miR-126, compared with EVs from aged MSCs transduced with miR-Ctrl (C). Intracellular expression of miR-126, was up-regulated in aged EPCs after incubation with TEVs from aged MSCs transduced with miR-126, compared with EVs from aged MSCs transduced with miR-Ctrl (D). ∗p < 0.05; ∗∗p < 0.01. miR = microRNA; TEV = tailored extracellular vesicle; other abbreviations as in Figures 1 and 2.
TEVs rejuvenate aged EPCs in vitro
We then determined whether TEVs would rejuvenate aged EPCs in a similar fashion as EVs derived from young MSCs. Indeed, these miR-126–overexpressing TEVs, but not EVs from miR-Ctrl–transduced aged MSCs, rejuvenated aged EPCs, as evidenced by decreased senescence-associated β-gal expression. In addition, 2 functional assays—tube formation and proliferation—were carried out to evaluate the functionality of rejuvenated aged EPCs. Consistent with decreased senescence-associated β-gal expression, increased tube formation capacity (102.0 ± 4.4 nmol/l vs. 232.2 ± 17.3 nmol/l for aged EPCs treated with EVs from aged MSCs vs. miR-126-transduced aged MSCs; p < 0.001) and increased proliferation rate were observed in rejuvenated aged EPCs (Figure 6). These findings indicate that TEVs derived from genetically engineered aged MSCs have the potential to restore EPC functions in vitro.
Figure 6.
TEVs From Genetically Engineered MSCs Improved Aged EPC Functions in Vitro
Incubation with TEVs isolated from aged MSCs overexpressing miR-126 substantially reduced senescence-associated β-gal expression in aged EPCs, compared with the same cells exposed to EVs from aged MSCs transduced with miR-Ctrl (A and B). Similarly, TEVs from aged MSCs transduced with miR-126, but not with EVs from aged MSCs transduced with miR-Ctrl, significantly improved EPC proliferation as measured by MTT assay compared with the aged EPCs cocultured with aged MSCs (C). Aged EPCs had poor tube formation, which was significantly improved by treatment with TEVs from aged MSCs transduced with miR-126, but not with EVs from aged MSCs transduced with miR-Ctrl (D and E). Aged EPCs treated with EVs from aged MSCs served as the reference group, and aged EPCs treated with EVs from young MSCs served as the positive control (∗p < 0.05, ∗∗∗p < 0.001, compared with the cells treated with aged MSC EVs). All experiments were conducted in triplicate. Scale bar: 100 μm. Abbreviations as in Figures 1, 2, and 5.
TEVs promote angiogenesis in vivo
Next, we tested whether TEVs could improve the functionality of endogenous EPCs, resulting in improved angiogenesis in vivo. We used TEVs isolated from miR-126–transduced aged MSCs to treat ischemia in an aged mouse hindlimb ischemia model (age >2 years, n = 6 in each group). Local injection of miR-126–loaded TEVs substantially improved blood flow in the ischemic legs as shown by laser Doppler imagine analysis with the average of perfusion ratios being 8.3 ± 0.3% in aged mice treated with PBS compared with 33.9 ± 3.5% in mice treated with aged MSCs + miR-126 EVs (p < 0.001) (Figures 7A and 7B). The effect was similar to that of EVs isolated from young MSCs, but not EVs from aged MSCs. Moreover, consistent with their capability to rejuvenate aged EPCs in vitro, these TEVs stimulated blood vessel development, as shown by the increased number of blood vessels and ECs (CD31 staining) (Figures 7C and 7D). These data support the notion that aged MSCs can be engineered to produce TEVs, which, in turn, promote angiogenesis and vascular repair in vivo.
Figure 7.
TEVs Are Effective in Treating Hind-Limb Ischemia
Aged male Balb/c mice, 26 to 27 months old, were anesthetized, and the external iliac artery was ligated. The mice were divided into 4 groups, and each group had 4 mice. Each group was injected with saline (PBS), EVs derived from young MSCs, aged MSCs, and aged MSCs transduced with miR-126 overexpressing vectors. Intramuscular injection at 3 different sites (gastrocnemius, gracilis, and quadriceps muscles, respectively, 200 μg/200 μl per injection) of the ischemic leg was done daily after ischemia overnight. At post-operative days 0 and 7, mouse limbs were scanned with a laser Doppler. The median perfusion of each limb was determined, and the ratio between the ischemia and the non-operated limb (relative perfusion) was calculated. EVs from young MSCs and TEVs from miR-26–transduced aged MSCs improved blood supply in the ischemic limb (A and B). Ischemic limb tissues were collected, fixed, and stained with DAPI (blue) for nuclei and CD31 antibody (orange) for blood vessel observation. Red arrows point to the examples of CD31 staining for blood vessel. EVs from young MSCs and TEVs from miR-126–transduced aged MSCs significantly increased angiogenesis in the ischemic limb (C and D). (∗p < 0.05 compared with the PBS group). Scale bar: 100 μm. PBS = phosphate-buffered saline; other abbreviations as in Figures 1, 2, and 5.
Discussion
MSCs have been shown to have regenerative and therapeutic effects for many diseases. The functional mechanisms for the therapeutic effects, however, are not completely understood. Accumulating evidence suggests that the beneficial effects of MSCs are mainly attributable to the bioactive factors secreted by MSCs. Furthermore, the efficacy of the MSCs is associated with the age of the donors. We have recently demonstrated that myocardial injection of young allogenic MSCs, but not aged autologous MSCs, resulted in improved cardiac function in patients with dilated cardiomyopathy. Remarkably, dilated cardiomyopathy patients had reduced baseline levels of EPCs, as measured by colony forming units (21). Three months after MSC injection, a significant increase in EPC colony forming units was observed. This increase of EPC levels was associated with cardiac functional improvement and was caused by the paracrine activity of the MSCs (21). Several classes of molecules are present in the complex mixture that constitutes the secretome (paracrine/autocrine activity) of MSCs. These include cytokines, growth factors, and miRs. Because RNA molecules are vulnerable to degradation in the extracellular environment, they are encapsulated in EVs to mediate cell–cell communications (16, 17, 18, 19, 20,42). Thus, EVs serve as vehicles to transport miRs from parent cells, including MSCs, to analogous and disparate tissues. As such, it is logical to investigate whether EVs would mediate the effects of MSCs. In this report, we present data showing that EVs derived from MSCs recapitulate their parent cells’ rejuvenation effects (young MSCs) or lack thereof (aged MSCs), on aged EPCs. Importantly, we demonstrate that genetic modifications of aged MSCs enable them to produce TEVs that are capable of rejuvenating aged EPCs and improving angiogenesis, supporting the notion that therapeutic oligos can be “transmitted” from parent cells to EVs and delivered to recipient cells, result in regulation of gene expression and functional changes in the recipient cells (25).
The focus on studying the mechanisms underlying the therapeutic effects of MSCs has shifted from MSCs’ ability to differentiate into functional cells and engraft at the site of injury to the paracrine actions of MSCs to stimulate endogenous repair pathways. As such, MSC-EVs have been attracting attention from researchers in the field of regenerative medicine. Indeed, an early study showed that MSC-EVs reduced infarct size in a mouse model of myocardial infarction (MI) (43). Other studies have also shown that the conditioned medium of MSCs protects ischemic hearts and MI (44,45). Importantly, administration of the EV-enriched, but not the EV-depleted, fraction of conditioned medium of MSCs reduced MI size (43). Subsequently, many studies have reported that MSC-EVs were as effective as their parental cells in promoting tissue regeneration in many organs/tissues, including kidney, liver, and muscle (46, 47, 48, 49). Recent studies have supported that MSC-EVs play a pivotal role promoting angiogenesis and improving blood supply in targeted organs (29,30). Our study is the first to our knowledge to demonstrate that EVs from young MSCs can effectively rejuvenate senescent EPCs. Given that functional EPCs promote angiogenesis and vascular repair, our data provide a potential mechanism for MSC-EV–stimulated tissue regeneration and angiogenesis.
The use of MSCs as a therapeutic approach is associated with several limitations. These include:1) therapeutic cells may have mutated or damaged DNAs with tumor forming potential; 2) whole cells may be too large to circulate easily through capillaries, and many cells may not get beyond the first-pass pulmonary capillary bed if delivered systemically; 3) the dose of infused cells can quickly diminish post-transplantation; 4) only a fraction of MSCs survive beyond a few days after injection (50); and 5) cell-based therapy requires stringently monitored manufacturing and storage to ensure optimal cell viability and vitality resulting in significant costs and challenges. In addition, we and others have demonstrated that aged MSCs and their EVs, lack the therapeutic efficacy observed with young MSCs and young MSC-EVs. This poses a critical challenge in clinical applications. Our report shows that aged MSCs can be programmed to produce TEVs with efficacy similar to that of young MSCs. This will not only avoid the limitations associated with MSCs, but also obviate the need to recruit young donors, which may represent a clinical bottleneck for timely treatment, and eliminate any concern for an alloimmune reaction.
Using genomic approaches and functional studies, we have characterized several miR–mRNA axes that regulate EPC senescence. In agreement with our genomic profiling findings, others have also shown that miR-126 is an essential player in maintaining endothelial cell functionality and promoting angiogenesis (37, 38, 39, 40, 41). It is up-regulated in plasma EVs in response to acute MI (51). It has been speculated that after MI, miR-126 is required to enhance vascular integrity and angiogenesis. Interestingly, we have demonstrated that the expression level of miR-126 in aged MSCs and aged MSC-EVs is significantly lower than young MSCs and young MSC-EVs, in addition to the increased expression levels of miR-21, miR-10A∗, miR-29c, and miR-146a—miRs associated with EPC senescence (31,35,36). This shows that MSCs and EPCs share the same set of miR regulators in their aging processes, and the decreased miR-126 levels in aged MSC-EVs may be partially responsible for their poor rejuvenation effects on EPCs. Indeed, when we overexpressed miR-126 in aged MSCs, these cells were able to produce TEVs that were loaded with miR-126. Importantly, the miR-126–loaded TEVs can be taken up by the EPCs and suppressed intracellular expression of spred-1, a key target gene of miR-126 and an endogenous inhibitor of VEGF signaling, in EPCs. Furthermore, miR-126–loaded TEVs were capable of rejuvenating aged EPCs in vitro and improving angiogenesis in vivo. Thus, miR-126 can be an effective molecular target used for generating MSC-TEVs with improved efficacy for angiogenesis.
We and others have reported that the number and functionality of EPCs decrease with age, leading to compromised EC integrity, increased risk for atherosclerosis, and diminished angiogenesis following ischemic events (52, 53, 54, 55, 56, 57, 58). Indeed, the number and functionality of circulating EPCs have been negatively correlated with Framingham risk score and is a strong predictor of CVD mortality (59,60). We have shown that EPCs from young donors or genetically modified, rejuvenated EPCs from aged donors promote neovascularization in a hindlimb ischemia mouse model (31,35). Consistently, several preclinical and clinical studies have suggested that EPCs from young donors can restore tissue vascularization after ischemic events in multiple organs and tissues, such as limb, ophthalmic retina, and myocardium (61, 62, 63, 64).
Study limitations
In the current study, we tested and demonstrated the efficacy of using EVs as an acellular approach to rejuvenate endogenous senescent EPCs and improve angiogenesis following ischemic events. Our study, however, has limitations. First, EVs contain many bioactive molecules including miRNAs, mRNAs and proteins. To fully understand the complex mechanisms for EV-mediated biological effects, extensive genomic, proteomic and functional studies are required to profile and characterize the bioactive content of EVs. For example, it is possible that other beneficial factors, in addition to miR-126, were induced in the aged MSCs transduced with miR-126, and were packaged within the TEVs. An RNA-seq and proteomic analysis of TEVs derived from MSCs transduced with miR-126 and miR-Ctrl would identify additional rejuvenating factors in the TEVs and guide future studies aiming at further improving the efficacy of TEVs. Second, although our data demonstrated that local injection of miR-126–overexpressing TEVs improved angiogenesis in aged mice with hindlimb ischemia in vivo and rejuvenated aged EPCs in vitro, we could not rule out, however, the contribution of activation, migration, and rejuvenation of adjacent ECs to the angiogenic process. In a mechanical denudation injury model where a segment of the aortic endothelium was removed, the endothelial regeneration seemed to be mainly driven by activation and proliferation of ECs adjacent to the injury site (65). Considering that femoral artery–induced ischemia affects most, if not all, ECs locally, the contribution of adjacent ECs to the angiogenesis in vivo in our model is likely to be minimal. Nonetheless, future studies, such as evaluating the proliferation of CD31+ ECs and lineage tracing of EC cells in the hindlimb ischemia model, are needed to determine the source of new ECs during angiogenesis upon TEV treatment. Finally, a recent study showed that in an ischemia–reperfusion heart injury model, stem-cell therapy improved heart function mainly through an acute immune response (66). It would be interesting to investigate whether EVs promote regenerations via triggering immune responses as well. Future studies are needed to fully elucidate the mechanisms underlying the beneficial effects of TEVs and further improve the efficacy of this acellular approach.
Conclusions
In summary, we report an acellular approach involving genetic modification of autologous aged MSCs to produce miR-126–overexpressing TEVs to rejuvenate senescent EPCs and promote angiogenesis. Compared with the EPC or MSC-based cellular approach, MSC-TEVs do not involve whole-cell transplantation and do not need to find young donors and are easier to isolate, manage, safer to use, and without the concern for alloimmunity.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Restoring efficient blood supply is critical in the treatment of coronary artery disease, including acute coronary syndrome, peripheral artery disease, and heart failure. Biological treatments including cell therapy using EPCs and MSCs, as well as gene therapy, may serve as alternatives to traditional medications with promise in promoting angiogenesis. However, the use of EPCs and MSCs is restricted by: 1) autologous cells are usually senescent and functionally impaired; 2) allogeneic cells can cause alloimmune reactions; and 3) intact cells have tumorigenic and arrhythmogenic potential, and are expensive to process. The use of gene therapy has been plagued by the lack of efficient and safe delivery vectors.
TRANSLATIONAL OUTLOOK: Our data indicate that MSCs can be genetically programmed to generate TEVs that carry target-miR, in our case miR-126—a potent angiogenic miR. These TEVs serve as an efficient gene-delivery vehicle to rejuvenate aged EPCs in vitro and promote angiogenesis in the mouse hindlimb ischemia model. Our work provides proof-of-concept evidence that generating TEVs can be a promising therapeutic strategy for CVD and perhaps other diseases. Future studies are warranted to evaluate the safety of EV injection, especially in the long term, and to fully understand the mechanisms underlying the therapeutic potentials of MSC-derived EVs. Furthermore, until approaches that allow targeted delivery of EVs in specific organs are developed, the use of EVs is likely limited by local injection.
Author Disclosures
This work is supported by National Institute of Allergy and Infectious Diseases grant 5R21AI136898-02 and the Miami Heart Research Institute grant to Dr. Dong. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors would like to thank the National Institutes of Health and the Miami Heart Research Institute for their support of this work.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
References
- 1.Gisterå A., Klement M.L., Polyzos K.A. Low-density lipoprotein-reactive t cells regulate plasma cholesterol levels and development of atherosclerosis in humanized hypercholesterolemic mice. Circulation. 2018;138:2513–2526. doi: 10.1161/CIRCULATIONAHA.118.034076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmidt-Lucke C., Rössig L., Fichtlscherer S. Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: proof of concept for the clinical importance of endogenous vascular repair. Circulation. 2005;111:2981–2987. doi: 10.1161/CIRCULATIONAHA.104.504340. [DOI] [PubMed] [Google Scholar]
- 3.Bianconi V., Fallarino F., Mannarino M.R. Autologous cell therapy for vascular regeneration: the role of proangiogenic cells. Curr Med Chem. 2018;25:4518–4534. doi: 10.2174/0929867324666171012111603. [DOI] [PubMed] [Google Scholar]
- 4.Attar A., Monabati A., Parsanezhad M.E. Endothelial progenitor cell subsets and preeclampsia: findings and controversies. J Chin Med Assoc. 2017;80:615–622. doi: 10.1016/j.jcma.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 5.Rehman J., Li J., Orschell C.M., March K.L. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003;107:1164–1169. doi: 10.1161/01.cir.0000058702.69484.a0. [DOI] [PubMed] [Google Scholar]
- 6.Abe Y., Ozaki Y., Kasuya J. Endothelial progenitor cells promote directional three-dimensional endothelial network formation by secreting vascular endothelial growth factor. PloS One. 2013;8 doi: 10.1371/journal.pone.0082085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanzler I., Tuchscheerer N., Steffens G. Differential roles of angiogenic chemokines in endothelial progenitor cell-induced angiogenesis. Basic Res Cardiol. 2013;108:310. doi: 10.1007/s00395-012-0310-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liao S., Luo C., Cao B. Endothelial progenitor cells for ischemic stroke: update on basic research and application. Stem Cells Int. 2017;2017:2193432. doi: 10.1155/2017/2193432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Masuda H., Asahara T. Post-natal endothelial progenitor cells for neovascularization in tissue regeneration. Cardiovasc Res. 2003;58:390–398. doi: 10.1016/s0008-6363(02)00785-x. [DOI] [PubMed] [Google Scholar]
- 10.Jujo K., Ii M., Losordo D.W. Endothelial progenitor cells in neovascularization of infarcted myocardium. J Mol Cell Cardiol. 2008;45:530–544. doi: 10.1016/j.yjmcc.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi Q., Rafii S., Wu M.H. Evidence for circulating bone marrow-derived endothelial cells. Blood. 1998;92:362–367. [PubMed] [Google Scholar]
- 12.Lapidot T., Petit I. Current understanding of stem cell mobilization: the roles of chemokines, proteolytic enzymes, adhesion molecules, cytokines, and stromal cells. Exp Hematol. 2002;30:973–981. doi: 10.1016/s0301-472x(02)00883-4. [DOI] [PubMed] [Google Scholar]
- 13.Cottler-Fox M.H., Lapidot T., Petit I. Stem cell mobilization. Hematology Am Soc Hematol Educ Program. 2003:419–437. doi: 10.1182/asheducation-2003.1.419. [DOI] [PubMed] [Google Scholar]
- 14.Imanishi T., Tsujioka H., Akasaka T. Endothelial progenitor cells dysfunction and senescence: contribution to oxidative stress. Curr Cardiol Rev. 2008;4:275–286. doi: 10.2174/157340308786349435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahla R.S. Stem cells applications in regenerative medicine and disease therapeutics. Int J Cell Biol. 2016;2016:6940283. doi: 10.1155/2016/6940283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meirelles Lda S., Fontes A.M., Covas D.T., Caplan A.I. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 2009;20:419–427. doi: 10.1016/j.cytogfr.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Gnecchi M., He H., Liang O.D. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11:367–368. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 18.Zheng G., Huang R., Qiu G. Mesenchymal stromal cell-derived extracellular vesicles: regenerative and immunomodulatory effects and potential applications in sepsis. Cell Tissue Res. 2018;374:1–15. doi: 10.1007/s00441-018-2871-5. [DOI] [PubMed] [Google Scholar]
- 19.Aghajani Nargesi A., Lerman L.O., Eirin A. Mesenchymal stem cell-derived extracellular vesicles for kidney repair: current status and looming challenges. Stem Cell Res Ther. 2017;8:273. doi: 10.1186/s13287-017-0727-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo Q., Zhang B., Kuang D., Song G. Role of stromal-derived factor-1 in mesenchymal stem cell paracrine-mediated tissue repair. Curr Stem Cell Res Ther. 2016;11:585–592. doi: 10.2174/1574888x11666160614102629. [DOI] [PubMed] [Google Scholar]
- 21.Premer C., Blum A., Bellio M.A. Allogeneic mesenchymal stem cells restore endothelial function in heart failure by stimulating endothelial progenitor cells. EBioMedicine. 2015;2:467–475. doi: 10.1016/j.ebiom.2015.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Premer C., Wanschel A., Porras V. Mesenchymal stem cell secretion of SDF-1α modulates endothelial function in dilated cardiomyopathy. Front Physiol. 2019;10:1182. doi: 10.3389/fphys.2019.01182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Niel G., D'Angelo G., Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19:213–228. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 24.Yáñez-Mó M., Siljander P.R., Andreu Z. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066. doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patton J.G., Franklin J.L., Weaver A.M. Biogenesis, delivery, and function of extracellular RNA. J Extracell Vesicles. 2015;4:27494. doi: 10.3402/jev.v4.27494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valadi H., Ekström K., Bossios A., Sjöstrand M., Lee J.J., Lötvall J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 27.Cai J., Han Y., Ren H. Extracellular vesicle-mediated transfer of donor genomic DNA to recipient cells is a novel mechanism for genetic influence between cells. J Mol Cell Biol. 2013;5:227–238. doi: 10.1093/jmcb/mjt011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harting M.T., Srivastava A.K., Zhaorigetu S. Inflammation-stimulated mesenchymal stromal cell-derived extracellular vesicles attenuate inflammation. Stem Cells. 2018;36:79–90. doi: 10.1002/stem.2730. [DOI] [PubMed] [Google Scholar]
- 29.Anderson J.D., Johansson H.J., Graham C.S. Comprehensive proteomic analysis of mesenchymal stem cell exosomes reveals modulation of angiogenesis via nuclear factor-kappaB signaling. Stem Cells. 2016;34:601–613. doi: 10.1002/stem.2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McBride J.D., Rodriguez-Menocal L., Guzman W., Candanedo A., Garcia-Contreras M., Badiavas E.V. Bone marrow mesenchymal stem cell-derived CD63+ exosomes transport Wnt3a exteriorly and enhance dermal fibroblast proliferation, migration, and angiogenesis in vitro. Stem Cells Dev. 2017;26:1384–1398. doi: 10.1089/scd.2017.0087. [DOI] [PubMed] [Google Scholar]
- 31.Deng S., Wang H., Jia C. MicroRNA-146a induces lineage-negative bone marrow cell apoptosis and senescence by targeting polo-like kinase 2 expression. Arterioscler Thromb Vasc Biol. 2017;37:280–290. doi: 10.1161/ATVBAHA.116.308378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gomes S.A., Rangel E.B., Premer C. S-nitrosoglutathione reductase (GSNOR) enhances vasculogenesis by mesenchymal stem cells. Proc Natl Acad Sci U S A. 2013;110:2834–2839. doi: 10.1073/pnas.1220185110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Théry C., Amigorena S., Raposo G., Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006;Chapter 3 doi: 10.1002/0471143030.cb0322s30. Unit 3.22. [DOI] [PubMed] [Google Scholar]
- 34.Fischer A.H., Jacobson K.A., Rose J., Zeller R. Hematoxylin and eosin staining of tissue and cell sections. CSH Protoc. 2008;2008 doi: 10.1101/pdb.prot4986. pdb.prot4986. [DOI] [PubMed] [Google Scholar]
- 35.Zhu S., Deng S., Ma Q. MicroRNA-10A∗ and microRNA-21 modulate endothelial progenitor cell senescence via suppressing high-mobility group A2. Circ Res. 2013;112:152–164. doi: 10.1161/CIRCRESAHA.112.280016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lyu G., Guan Y., Zhang C. TGF-β signaling alters H4K20me3 status via miR-29 and contributes to cellular senescence and cardiac aging. Nat Commun. 2018;9:2560. doi: 10.1038/s41467-018-04994-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ji J.S., Xu M., Song J.J. Inhibition of microRNA-126 promotes the expression of Spred1 to inhibit angiogenesis in hepatocellular carcinoma after transcatheter arterial chemoembolization: in vivo study. Onco Targets Ther. 2016;9:4357–4367. doi: 10.2147/OTT.S106513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou Q., Anderson C., Hanus J. Strand and cell type-specific function of microRNA-126 in angiogenesis. Mol Ther. 2016;10:1823–1835. doi: 10.1038/mt.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meng S., Cao J.T., Zhang B., Zhou Q., Shen C.X., Wang C.Q. Downregulation of microRNA- 126 in endothelial progenitor cells from diabetes patients, impairs their functional properties, via target gene Spred-1. J Mol Cell Cardiol. 2012;1:64–72. doi: 10.1016/j.yjmcc.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 40.Fish J.E., Santoro M.M., Morton S.U. miR-126 regulates angiogenic signaling and vascular integrity. Dev Cell. 2008;15:272–284. doi: 10.1016/j.devcel.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang S., Aurora A.B., Johnson B.A. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev Cell. 2008;15:261–271. doi: 10.1016/j.devcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Timmers L., Lim S.K., Arslan F. Reduction of myocardial infarct size by human mesenchymal stem cell conditioned medium. Stem Cell Res. 2007;1:129–139. doi: 10.1016/j.scr.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 43.Lai R.C., Arslan F., Lee M.M. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4:214–222. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 44.Yamaguchi S., Shibata R., Yamamoto N. Dental pulp-derived stem cell conditioned medium reduces cardiac injury following ischemia-reperfusion. Sci Rep. 2015;5:16295. doi: 10.1038/srep16295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Timmers L., Lim S.K., Hoefer I.E. Human mesenchymal stem cell-conditioned medium improves cardiac function following myocardial infarction. Stem Cell Res. 2011;6:206–214. doi: 10.1016/j.scr.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 46.He J., Wang Y., Sun S. Bone marrow stem cells-derived microvesicles protect against renal injury in the mouse remnant kidney model. Nephrology (Carlton) 2012;17:493–500. doi: 10.1111/j.1440-1797.2012.01589.x. [DOI] [PubMed] [Google Scholar]
- 47.Kilpinen L., Impola U., Sankkila L. Extracellular membrane vesicles from umbilical cord blood-derived MSC protect against ischemic acute kidney injury, a feature that is lost after inflammatory conditioning. J Extracell Vesicles. 2013;10:2. doi: 10.3402/jev.v2i0.21927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li T., Yan Y., Wang B. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev. 2013;15(22):845–854. doi: 10.1089/scd.2012.0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakamura Y., Miyaki S., Ishitobi H. Mesenchymal-stem-cell-derived exosomes accelerate skeletal muscle regeneration. FEBS Lett. 2015;8(589):1257–1265. doi: 10.1016/j.febslet.2015.03.031. [DOI] [PubMed] [Google Scholar]
- 50.Eggenhofer E., Luk F., Dahlke M.H., Hoogduijn M.J. The life and fate of mesenchymal stem cells. Front Immunol. 2014;5:148. doi: 10.3389/fimmu.2014.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Akbar N., Digby J.E., Cahill T.J. Endothelium-derived extracellular vesicles promote splenic monocyte mobilization in myocardial infarction. JCI Insight. 2017;2 doi: 10.1172/jci.insight.93344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rauscher F.M., Goldschmidt-Clermont P.J., Davis B.H. Aging, progenitor cell exhaustion, and atherosclerosis. Circulation. 2003;108:457–463. doi: 10.1161/01.CIR.0000082924.75945.48. [DOI] [PubMed] [Google Scholar]
- 53.Dimmeler S., Zeiher A.M. Vascular repair by circulating endothelial progenitor cells: the missing link in atherosclerosis? J Mol Med (Berl) 2004;82:671–677. doi: 10.1007/s00109-004-0580-x. [DOI] [PubMed] [Google Scholar]
- 54.Laufs U., Werner N., Link A. Physical training increases endothelial progenitor cells, inhibits neointima formation, and enhances angiogenesis. Circulation. 2004;109:220–226. doi: 10.1161/01.CIR.0000109141.48980.37. [DOI] [PubMed] [Google Scholar]
- 55.Imanishi T., Kobayashi K., Hano T., Nishio I. Effect of estrogen on differentiation and senescence in endothelial progenitor cells derived from bone marrow in spontaneously hypertensive rats. Hypertens Res. 2005;28:763–772. doi: 10.1291/hypres.28.763. [DOI] [PubMed] [Google Scholar]
- 56.Chang E.I., Loh S.A., Ceradini D.J. Age decreases endothelial progenitor cell recruitment through decreases in hypoxia-inducible factor 1alpha stabilization during ischemia. Circulation. 2007;116:2818–2829. doi: 10.1161/CIRCULATIONAHA.107.715847. [DOI] [PubMed] [Google Scholar]
- 57.Li W., Du D.Y., Liu Y., Jiang F., Zhang P., Li Y.T. Long-term nicotine exposure induces dysfunction of mouse endothelial progenitor cells. Exp Ther Med. 2017;13:85–90. doi: 10.3892/etm.2016.3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Echeverría P., Gómez-Mora E., Roura S. Variable endothelial cell function restoration after initiation of 2 antiretroviral regimens in HIV-infected individuals. J Antimicrob Chemother. 2017;72:2049–2054. doi: 10.1093/jac/dkx074. [DOI] [PubMed] [Google Scholar]
- 59.Hill J.M., Zalos G., Halcox J.P. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 60.Werner N., Kosiol S., Schiegl T. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353:999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- 61.Fujita Y., Kawamoto A. Stem cell-based peripheral vascular regeneration. Adv Drug Deliv Rev. 2017;120:25–40. doi: 10.1016/j.addr.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 62.Scotti F., Maestroni A., Palini A. Endothelial progenitor cells and response to ranibizumab in age-related macular degeneration. Retina. 2014;34:1802–1810. doi: 10.1097/IAE.0000000000000147. [DOI] [PubMed] [Google Scholar]
- 63.Geng J., Wang L., Qu M. Endothelial progenitor cells transplantation attenuated blood- brain barrier damage after ischemia in diabetic mice via HIF-1α. Stem Cell Res Ther. 2017;8:163. doi: 10.1186/s13287-017-0605-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Morrone D., Felice F., Scatena C. Role of circulating endothelial progenitor cells in the reparative mechanisms of stable ischemic myocardium. Int J Cardiol. 2018;257:243–246. doi: 10.1016/j.ijcard.2017.05.070. [DOI] [PubMed] [Google Scholar]
- 65.McDonald A.I., Shirali A.S., Aragón R. Endothelial regeneration of large vessels is a biphasic process driven by local cells with distinct proliferative capacities. Cell Stem Cell. 2018;23:210–225. doi: 10.1016/j.stem.2018.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vagnozzi R.J., Maillet M., Sargent M.A. An acute immune response underlies the benefit of cardiac stem cell therapy. Nature. 2020;577:405–409. doi: 10.1038/s41586-019-1802-2. [DOI] [PMC free article] [PubMed] [Google Scholar]