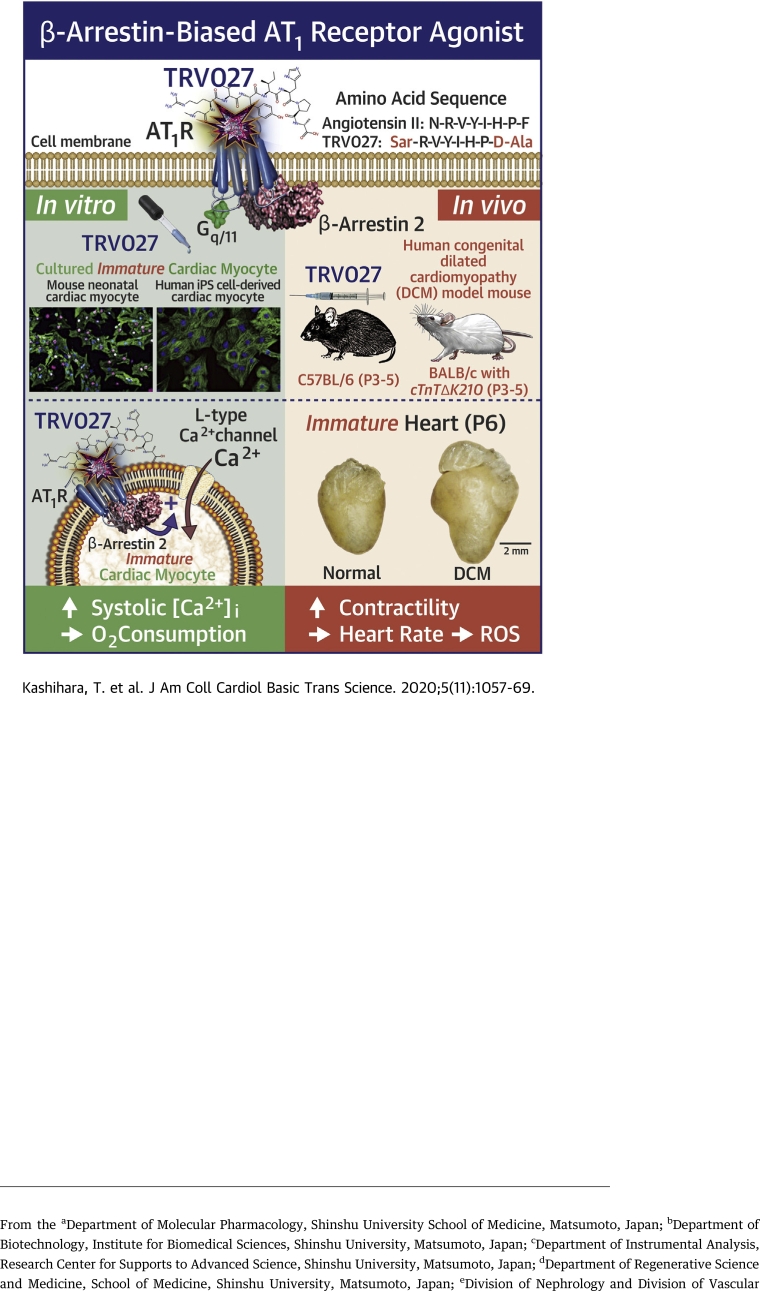
Visual Abstract
Key Words: β-arrestin–biased AT1 angiotensin receptor agonist, congenital dilated cardiomyopathy, human induced pluripotent stem cell-derived cardiac myocytes, inotropic vasodilator, neonate, pediatric heart failure, TRV027
Abbreviations and Acronyms: AngII, angiotensin II; AT1R, angiotensin type 1 receptor; BBA, β-arrestin–biased angiotensin type 1 receptor agonist; ECG, electrocardiography; GPCR, G protein–coupled receptor; hiPSC-CM, human induced pluripotent stem cell–derived cardiac myocyte; LTCC, CaV1.2 L-type Ca2+ channel; mNVCM, mouse neonatal ventricular cardiac myocyte; OCR, oxygen consumption rate; PHF, pediatric heart failure; ROS, reactive oxygen species; UCG, ultrasound cardiogram
Highlights
-
•
β-arrestin–biased AT1 agonist TRV027 causes a neonatal-specific, long-acting positive inotropic effect with minimum effect on heart rate, oxygen consumption, reactive oxygen species production, and aldosterone secretion.
-
•
Although TRV027 stimulates adrenaline secretion, it does not contribute to the inotropic effect.
-
•
TRV027 also increases twitch Ca2+ transients in human iPS cell–derived cardiac myocytes bearing immature phenotype and improves the contractility of the compromised heart of neonatal knock-in mice bearing a mutation causing human congenital dilated cardiomyopathy.
-
•
TRV027 and related peptides are also known to cause an antiapoptotic effect on the heart, dilate resistant arteries to reduce afterload, and increase Na+ diuresis to reduce preload.
-
•
Thus, TRV027 could be utilized as a valuable inotropic vasodilator specific for pediatric heart failure.
Summary
The treatment of pediatric heart failure is a long-standing unmet medical need. Angiotensin II supports mammalian perinatal circulation by activating cardiac L-type Ca2+ channels through angiotensin type 1 receptor (AT1R) and β-arrestin. TRV027, a β-arrestin–biased AT1R agonist, that has been reported to be safe but not effective for adult patients with heart failure, activates the AT1R/β-arrestin pathway. We found that TRV027 evokes a long-acting positive inotropic effect specifically on immature cardiac myocytes through the AT1R/β-arrestin/L-type Ca2+ channel pathway with minimum effect on heart rate, oxygen consumption, reactive oxygen species production, and aldosterone secretion. Thus, TRV027 could be utilized as a valuable drug specific for pediatric heart failure.
Pediatric heart failure (PHF) is an important cause of morbidity and mortality in childhood (1). However, the development of pharmacological treatments for heart failure in children significantly lags behind that of adult heart failure due to the difficulties inherent to large clinical studies, such as versatile etiologies and pathophysiologies.
The mammalian heart starts beating at a very early stage of fetal life (2). In cardiac myocytes (CMs), action potential activates sarcolemmal CaV1.2 L-type Ca2+ channels (LTCCs) and induces Ca2+ influx to evoke contraction. Mammals have to survive the great circulation changes at birth, such as sudden cessation of the placental circulation, a large increase in the pulmonary circulation, and closure of right-to-left shunts (3,4). Thus, ∼60% of children with congenital cardiac problems elicit overt heart failure in their first year (1). In the perinatal circulation, humoral factors play a crucial role in supporting immature cardiovascular system. Adrenaline from the adrenal medulla, for instance, exerts positive chronotropic and inotropic effects on the heart through a G protein–coupled receptor (GPCR); α1- and β-adrenergic receptors (ARs) coupled to Gq/11/phospholipase C-β system and Gs/adenylyl cyclase system in CMs, respectively (5); and vasoconstriction to maintain the systemic blood pressure through the α1 AR system coupled to Gq/11 in vascular smooth muscle cells (6). Orchestration of these G protein–mediated effects increases left ventricular output and blood pressure within minutes.
In the neonatal period, the kidney also differentiates to adapt to an extrauterine life by generating cortical glomeruli and elongating the loop of Henle (7). Almost all of these differentiations critically depend on angiotensin II (AngII) that is produced by an enzyme, renin, derived from the juxtaglomerular apparatus of the kidney itself (8). As a consequence, the plasma concentration of AngII sharply rises after delivery (9). AngII induces strong vasoconstriction to support the perinatal circulation through another GPCR, angiotensin type 1 receptor (AT1R) coupled with Gq/11 in vascular smooth muscle cells (9,10). In addition, we recently found that AngII directly increases the activity of LTCCs and the twitch Ca2+ transient of left ventricular cardiac myocytes specifically in neonates through their AT1Rs (11). This neonatal-specific effect of AT1R was not mediated by G proteins, but rather by β-arrestin 2, a protein that is also coupled to GPCR (12). When AngII is bound to AT1R, G proteins and β-arrestins are recruited to the receptor in that order. β-arrestins induce internalization of the receptor and initiate G protein–independent signals in an intracellular signaling platform referred to as “β-arrestin signalosome” (13). In neonatal cardiac myocytes, an AT1R/β-arrestin 2 complex evokes a signaling pathway that ultimately activates LTCCs through casein kinase 2′ (11). This effect of AngII only manifests itself in ∼1-month-old individuals in the case of mice, indicating that this pathway may have some neonatal-specific tasks. We hypothesized that this cardiac effect of AngII would also support perinatal circulation in addition to its vascular effect. Thus, AngII and AT1R seem to be physiologically very important in the perinatal period of mammals, as proposed previously (9).
Several peptidyl β-arrestin–biased AT1R agonists (BBAs) such as TRV027 were produced by Trevena Inc. (King of Prussia, Pennsylvania) (14). These BBAs activate β-arrestins and inhibit G proteins through AT1R and were reported to exert moderate but significant acute positive inotropic antiapoptotic effects in adult rodent hearts (15) and vasodilatory and Na+ diuretic effects in a canine tachypacing-induced systolic heart failure model (16,17). Because of these favorable cardiovascular effects of TRV027, a clinical trial engaging 620 adult patients suffering from acute heart failure BLAST-AHF (Biased Ligand of the Angiotensin receptor STudy in Acute Heart Failure) Trial was conducted (18). However, it did not meet either the primary or secondary endpoints in the phase IIB trial, indicating that TRV027 is safe but not effective at least in adults with acute heart failure.
Taking our own finding on the neonatal cardiac AT1R/β-arrestin/LTCC pathway, we re-examined the effect of TRV027 on the neonatal heart. We here report for the first time that TRV027 caused a unique long-acting, strong positive inotropic effect in neonatal mice lasting for at least 8 h with minimum effects on heart rate, oxygen consumption, oxidative stress, and aldosterone secretion. This effect on the neonatal heart was very different from the previously reported acute one on the adult heart (14,15) and was mediated by the AT1R/β-arrestin/LTCC pathway in mouse neonatal ventricular cardiac myocytes (mNVCMs) (11). Importantly, TRV027 also significantly increased twitch Ca2+ transients in human induced pluripotent stem cell–derived cardiac myocytes (hiPSC-CMs) exhibiting fetal to neonatal phenotype (19) and the contractility of the compromised neonatal heart of knock-in mice bearing a point mutation causing human congenital dilated cardiomyopathy (20). These results indicate that TRV027 could be utilized as a novel valuable drug specific for PHF.
Methods
In vivo studies
All mice used in this study received humane care in compliance with the Guide for the Care and Use of Laboratory Animals, published by the U.S. National Institutes of Health. This study was performed in accordance with the Guidelines for Animal Experimentation of the Shinshu University and was approved by the Committee for Animal Experimentation (Approval number: 280035, 019034, 020044, and 17-009). Prior to surgery or euthanasia, mice were deeply anesthetized with subcutaneous administration of medetomidine (0.3 mg/kg), midazolam (4.0 mg/kg), and butorphanol (5.0 mg/kg). In order to record ultrasoundcardiogram (UCG) or electrocardiography (ECG), mice were anesthetized with subcutaneous or intraperitoneal administration of 2,2,2-tribromoethanol (250 mg/kg) (21). C57BL/6 mice were purchased from Japan SLC (Hamamatsu, Japan). Knock-in mice with a BALB/c background bearing a point mutation (ΔK210) in cardiac troponin T causing human congenital dilated cardiomyopathy were produced as previously described (20,22). Homozygous mutant (cTnTΔK210) and wild-type mice were obtained by crossing heterozygous mutant mice. UCG was recorded with a Vevo2100 linear array imaging system (FUIJIFILM VisualSonics Inc., Toronto, Canada) at the appropriate time after intraperitoneal administration the drugs. ECG (lead II) was recorded with a dual-channel bioelectric amplifier (Nihon Kohden, Tokyo, Japan) and Power Lab/4SP (ADInstruments, Sydney, Australia). QTc interval was calculated as follows: QTc = QT / (RR / 100) 1/3, where QT and RR are the QT and RR intervals in milliseconds (23,24). Cardiac reactive oxygen species (ROS) production was assessed with dihydroethidium staining after drug administration (25). Adrenaline and noradrenaline levels in plasma were quantified using high-performance liquid chromatography at the Japan Institute for the Control of Aging (Nikken SEIL, Shizuoka, Japan). Aldosterone levels in serum were measured with an Accuraseed Aldosterone Chemiluminescent Enzyme Immunoassay Kit (FUJIFILM Wako Pure Chemical, Osaka, Japan) at the Department of Laboratory Medicine, Shinshu University Hospital, Matsumoto, Japan.
In vitro studies
To measure twitch Ca2+ transients in cardiac myocytes, mNVCMs were isolated as previously reported (26). hiPSC-CMs were generated from 253G1 iPSCs (Kyoto University, Kyoto, Japan) (27) by a previously reported cardiac differentiation protocol with some modifications (28, 29, 30). Twitch Ca2+ transients were measured as previously described (11). Briefly, mNVCMs and hiPSC-CMs were regularly field-stimulated at 0.33 Hz and their Ca2+ transients were monitored with Fluo 4 (6 μmol/l) or Cal-520 (5 μmol/l), respectively, using an LSM 7 LIVE laser-scanning microscope (Carl Zeiss, Jena, Germany). The sarcoplasmic reticulum Ca2+ content was estimated from caffeine-induced inward Na+-Ca2+ exchanger currents measured by the patch-clamp method, as previously reported (31). To measure the mitochondrial oxygen consumption rate (OCR), mNVCMs were isolated with Pierce Primary Cardiomyocyte Isolation Kit (Thermo Fisher Scientific, Waltham, Massachusetts). Mitochondrial OCR was assessed using the Extracellular Flux Analyzer XFp (Agilent Technologies, Tokyo, Japan).
Statistics
Continuous variables are expressed as mean ± SEM. The statistical significance of the difference between 2 means within a group or the difference between means of 2 groups was evaluated with a paired or unpaired Student’s t-test, respectively. Multiple comparison of the means was conducted with 1-way analysis of variance followed by Dunnett’s or Tukey’s test. Interaction between 2 independent variables was analyzed with 2-way analysis of variance. A p value <0.05 was considered statistically significant. All statistical analysis was conducted with SPSS 26 (IBM Corporation, Armonk, New York) and Microsoft Excel (Microsoft Corporation, Redmond, Washington) with a statistical macro (BellCurve, Tokyo, Japan).
An extended methods section is available in the Supplemental Appendix.
Results
TRV027 causes a long-acting positive inotropic effect through AT1R in a dose-dependent manner in the neonatal mouse heart
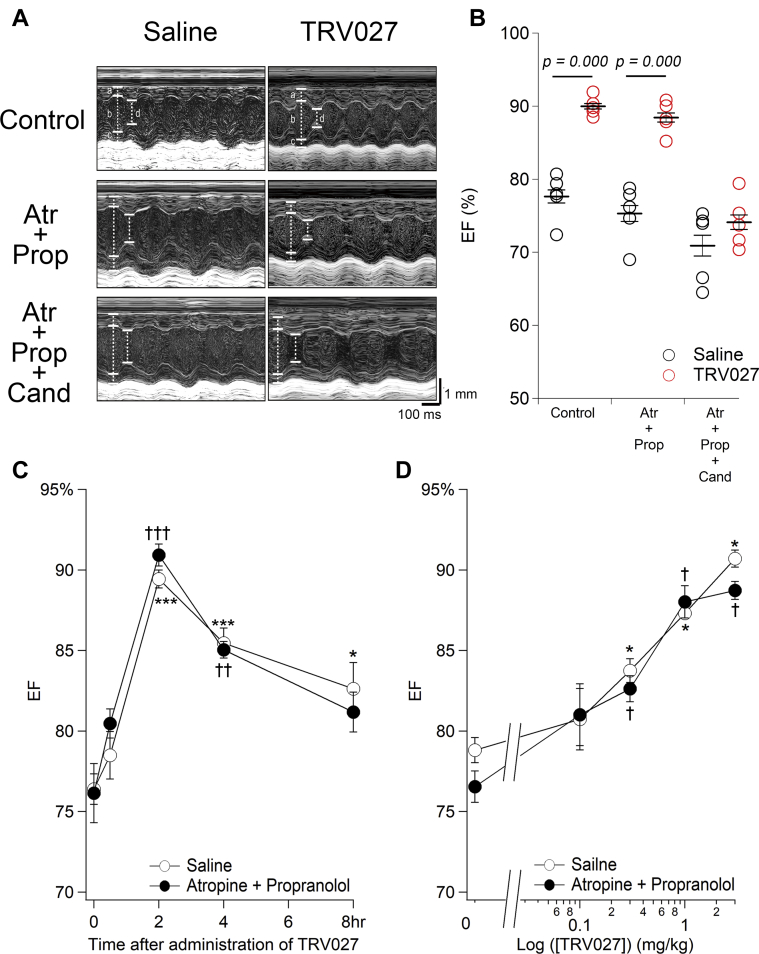
We first confirmed the physiological outcome of stimulation of our newly identified direct effect of AngII on mNVCMs in vivo. Supplemental Figure 1 shows representative M-mode recordings of UCG in the hearts of neonatal and adult mice (left- and right-hand columns, respectively) 2 h after administration of saline or AngII. AngII caused a strong inotropic effect on the neonatal (but not adult) left ventricle compared with saline. This effect was reproduced by TRV027 (Figure 1A, top row; Supplemental Figure 1). Supplemental Table 1 indicates that AngII and TRV027 reduced left ventricular end-systolic diameter without changing left ventricular end-diastolic diameter significantly in the neonatal heart. As a result, TRV027 significantly increased ejection fraction of the neonatal left ventricular (Figure 1B). The effect of TRV027 was not inhibited by a muscarinic receptor antagonist, atropine, plus a β-adrenergic receptor antagonist, propranolol (middle panel), but was inhibited significantly by an AT1R antagonist, candesartan (bottom panel, Figures 1A and 1B, Supplemental Table 2). We also confirmed that the α1-adrenergic receptor antagonist prazosin did not affect the effect of TRV027 (Supplemental Figure 2). Thus, TRV027 caused the inotropic effect directly through AT1R but not through neurohumoral factors induced via unbiased AT1R agonism involving strong Gq/11 activation.
Figure 1.
TRV027 Causes a Long-Acting Positive Inotropic Effect Through AT1R in a Dose-Dependent Manner in the Neonatal Mouse Heart
(A) Representative M-mode ultrasoundcardiogram recorded from neonatal mice treated with saline (left column) or TRV027 (right column). UCG was recorded 2 h after intraperitoneal injection of saline or TRV027 (3 mg/kg) (top row). Then, atropine (Atr) (1 mg/kg) plus propranolol (Prop) (1 mg/kg) was added, and the measurements were repeated 10 min later (middle row). Furthermore, candesartan (Cand) (3 mg/kg) was added, and the measurements were once again repeated 20 min later (bottom row). In these traces, a, b, c, and d indicate interventricular septum end-diastolic thickness, left ventricular end-diastolic diameter, posterior wall end-diastolic thickness, and end-systolic diameter, respectively. (B) The values of ejection fraction (EF) measured under each condition. Symbols indicate each data. The p value was calculated with unpaired Student’s t test. (C) Indicated time after injection of TRV027 (3 mg/kg) to neonatal mice, saline (open circles) or atropine + propranolol (1 mg/kg each) (closed circles) was added. Then, EF was measured 10 min later. Statistical significance of the time-dependent change in EF was analyzed with 1-way analysis of variance followed by Dunnett’s test. ∗†p < 0.05; ∗∗††p < 0.01; ∗∗∗†††p < 0.001 versus 0 h in the saline and Atr + Prop groups, respectively. Neither significant effect of atropine + propranolol nor interaction between time and drugs was indicated with 2-way analysis of variance. n = 5 to 6 in each group. (D) Two hours after injection of indicated doses of TRV027 to neonatal mice, saline (open circle) or Atr + Prop (closed circle) was added. Then, EF was measured 10 min later. The p value was calculated with paired Student’s t test. ∗†p < 0.05 versus 0 mg/kg in the saline and atropine + propranolol groups, respectively. n = 3 in each group.
Figure 1C shows the time-dependent effect of TRV027. The effect of TRV027 increased slowly, peaking 2 h after administration and remaining significantly active for a total of 8 h. This time-dependent effect of TRV027 is similar to that of AngII on LTCC activity in isolated mNVCMs (11) but is distinctly different from the previously reported acute one on adult mouse cardiac myocytes (in minutes) (14). The time course of the effect of TRV027 was not significantly affected by atropine + propranolol. Figure 1D shows the dose-dependent effect of TRV027 2 h after administration (Figure 1C). TRV027 caused a positive inotropic effect in a dose-dependent manner in a range of doses between 0.1 and 3 mg/kg, in both the absence and presence of atropine + propranolol (Figure 1D). These results indicate that TRV027 exerts a long-lasting, dose-dependent positive inotropic effect unique to the neonatal heart through the AT1R/β-arrestin system.
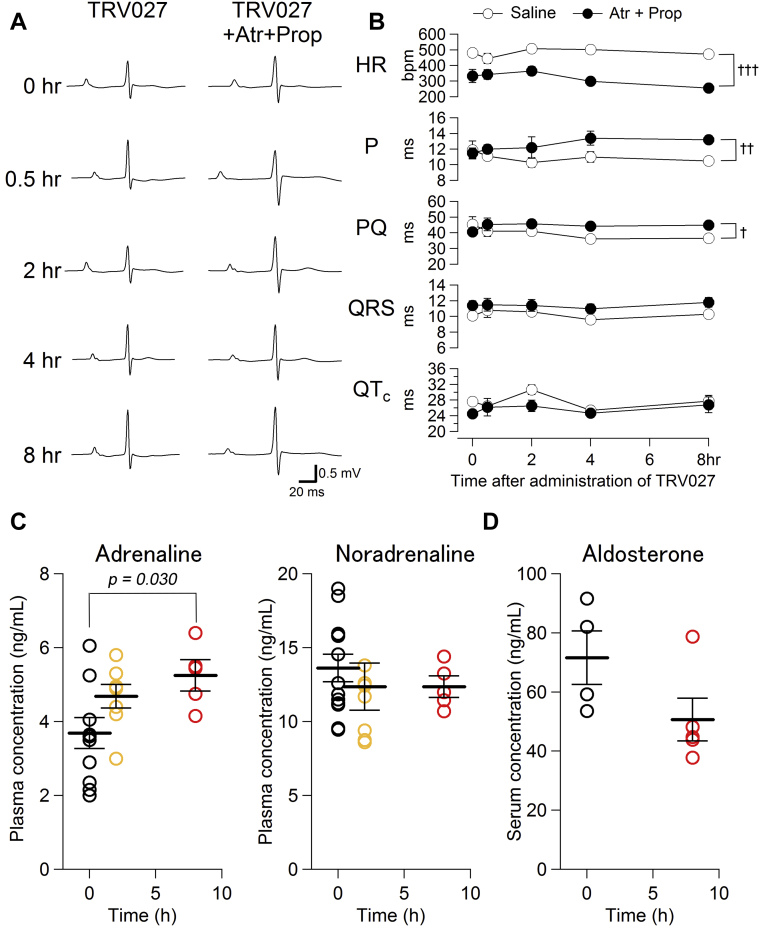
Time-dependent effect of TRV027 on ECG and humoral factors in neonatal mice
Figure 2A shows a representative ECG at the indicated time after the administration of TRV027 in the absence and presence of atropine + propranolol. No arrhythmias were observed up to 8 h after TRV027 administration. Figure 2B and Supplemental Table 3 show the time-dependent change of each parameter of ECG, revealing that TRV027 did not significantly change any of them in a time-dependent manner. However, atropine + propranolol evidently reduced heart rate at 0 h and its effect seemed to increase gradually over the following 8 h. P-wave duration also increased in that time frame, and PQ time increased toward 8 h after administration in the presence of atropine + propranolol. Thus, we measured the plasma adrenaline and noradrenaline concentrations 2 and 8 h after TRV027 administration (Figure 2C) and found that it increased adrenaline concentrations in a time-dependent manner and significantly at 8 h (but not noradrenaline), which indicates that it gradually stimulated the adrenal medulla (32). However, none of the heart rate, P, and PQ values at 8 h were significantly different from those at 0 h in the presence of atropine + propranolol (p = 0.195, 0.452, and 0.550, respectively), indicating the lack of TRV027 effects on ECG. In addition, we measured the serum aldosterone level (33) and found that TRV027 did not increase aldosterone levels in neonates at 8 h after administration (Figure 2D). These results indicate that TRV027 on its own did not change any ECG parameters, whereas the values of some parameters measured 4 or 8 h after administration may be affected by adrenaline secreted from the adrenal medulla.
Figure 2.
Time-Dependent Effect of TRV027 on ECG, Plasma Concentrations of Adrenaline and Noradrenaline, and the Serum Concentration of Aldosterone After Administration of Saline or TRV027
(A) Electrocardiography (ECG) was recorded indicated time after injection of TRV027 (3 mg/kg) to neonatal mice (left column). Then, 10 min after additional injection of atropine + propranolol, ECG was once again recorded (right column). (B) Each parameter of ECG was measured different time after injection of TRV027 (3 mg/kg) (open circles). Then, 10 min after injection of atropine + propranolol, each parameter was measured again (closed circles). No significant time-dependent change in each parameter was detected with 1-way analysis of variance (ANOVA) followed by Dunnett’s test. Two-way ANOVA indicated a significant effect of drugs in heart rate (HR), P, and PQ but no significant interaction between time and drugs. †p < 0.05; ††p < 0.01; and †††p < 0.001 between saline and atropine + propranolol groups. n = 5 in each group. (C) Plasma adrenaline and noradrenaline concentrations 2 and 8 h after TRV027 (3 mg/kg) administration. The p value was calculated with 1-way ANOVA followed by Dunnett’s test. n = 5 to 11 in each group. (D) Serum aldosterone concentration 8 h after saline or TRV027 (3 mg/kg) administration. The p value was calculated with unpaired Student’s t test. n = 5 to 6 in each group. Abbreviations as in Figure 1.
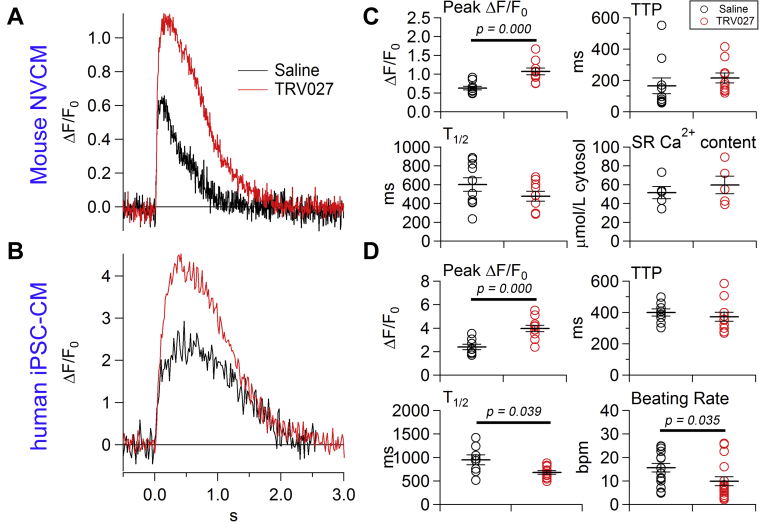
Effect of TRV027 on twitch Ca2+ transients of isolated immature mouse and human Cardiac Myocytes
Figures 3A and 3B show the effect of TRV027 on the twitch Ca2+ transient in isolated mNVCMs and hiPSC-CMs treated with saline or TRV027 for 2 h in vitro, respectively. hiPSC-CMs are known to exhibit fetal to neonatal phenotypes (19). Indeed, a half decay time of twitch Ca2+ transient which is inversely related to sarcoplasmic reticulum Ca2+ pump activity, was substantially larger than that of adult cardiac myocytes (∼200 ms) in both cell types and in the presence of saline (Figures 3C and 3D) (31). The sarcoplasmic reticulum Ca2+ content of mNVCMs was approximately half of that of adult mouse cardiac myocytes (∼100 μmol/l cytosol) (Figure 3C) (31). These data indicate that sarcoplasmic reticulum is still immature in these cells. TRV027 strongly increased the twitch Ca2+ transient in both cell types. Figure 3C shows that it significantly increased the peak amplitude of the Ca2+ transients without changing time to peak, a half decay time, or sarcoplasmic reticulum Ca2+ content in mNVCM. Thus, in the presence of TRV027, the increased Ca2+ influx may be perfectly counterbalanced by a matched increase in Ca2+ efflux via Na+/Ca2+ exchangers on a beat-by-beat basis. Figure 3D indicates that TRV027 significantly increased the peak amplitude and decreased a half decay time without changing time to peak in hiPSC-CMs. These results indicate that the AT1R/β-arrestin–mediated positive inotropic effect is preserved in human immature cardiac myocytes. It is also noteworthy that TRV027 decreased the spontaneous beating rate of hiPSC-CMs, suggesting that it could be relatively protective with respect to potential arrhythmogenic effects of excess Ca2+ loading in immature human cardiac myocytes.
Figure 3.
Effect of TRV027 on Twitch Ca2+ Transients of Isolated mNVCMs or hiPSC-CMs
Representative twitch Ca2+ transients of (A) mouse neonatal ventricular cardiac myocytes (mNVCMs) detected with Fluo-4 and digitized at 144 Hz or (B) human induced pluripotent stem cell–derived cardiac myocytes (hiPSC-CMs) detected with Cal-520 and digitized at 60 Hz 2 h after in vitro treatment with saline (black line) or TRV027 (3 μmol/l) (red line). Action potential was elicited by field stimulation at 0.33 Hz. (C) Effects of saline or TRV027 on the peak ΔF/F0, time to peak (TTP), half decay time (T1/2), and the sarcoplasmic reticulum Ca2+ content in paced mNVCMs. n = 10 in each group. (D) Effects of saline or TRV027 on the peak ΔF/F0, TTP, and a half decay time of paced hiPSC-CMs, and their spontaneous beating rate in the absence of pacing. n = 8 to 11 in each group. Statistical significance was analyzed with unpaired Student’s t test.
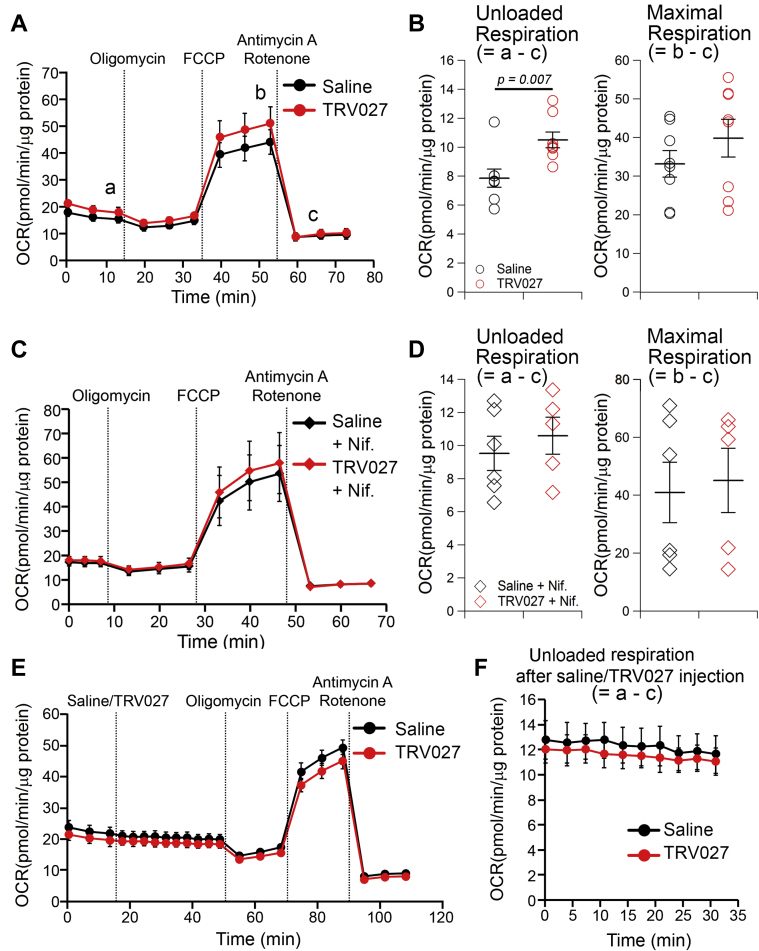
Effect of TRV027 on mitochondrial OCR in mNVCMs
Figures 4A to 4D show the effect of a 2-h in vitro treatment of mitochondrial OCR in mNVCMs with TRV027. During the analysis, mNVCMs were spontaneously beating at 37°C, both in the absence and presence of TRV027 and without conducting the external work (beating rate: 38.00 ± 2.73 min–1 vs. 38.00 ± 2.07 min–1; p = 1.00). As shown in Figure 4A, the difference between OCR before oligomycin (a) and after antimycin A plus rotenone (c) roughly corresponds to the so-called unloaded respiration in the cardiac pressure-volume loop analysis (34). This respiration supports the potential energy utilized for the basal metabolism and excitation-contraction coupling of cardiac myocytes. The difference between OCR before oligomycin (a) and after carbonyl cyanide-p-trifluoromethoxyphenylhydrazone (FCCP) (b) is a spare respiratory capacity, which can be utilized for external work in vivo. The measured value (∼20 pmol/min/μg protein) was much smaller than that of adult mouse cardiac myocytes (∼100 pmol/min/μg protein) (35), indicating that at this stage mNVCMs still bear immature mitochondria and are not ready to undergo robust physical movement. TRV027 slightly, but significantly, increased the unloaded OCR, but not the maximum OCR (Figure 4B), which might be due to the enhancement of twitch Ca2+ transients (Figures 3A and 3C) (36). We found that nifedipine- a specific LTCC blocker- completely ceased the beating (not shown) but did not change the control unloaded OCR and abolished the TRV027-induced increment (Figures 4C and 4D). Thus, the OCR for the basal excitation-contraction coupling of NVCM was undetectably low but was measurable in the presence of TRV027 in this assay system. Figures 4E and 4F show the immediate response of unloaded OCR to the wash-in of TRV027. No significant change was observed within 35 min after application, which is consistent with its slow effect on LTCCs. These results indicate that TRV027 gradually increased the unloaded OCR by 2 h after administration most likely because of its effect to increase LTCC currents (11) and enhancement of Ca transients.
Figure 4.
Effect of TRV027 on the Mitochondrial OCR in mNVCMs
(A) Oxygen consumption rate (OCR) in isolated mouse neonatal ventricular cardiac myocytes (mNVCMs) treated in vitro with saline or TRV027 (3 μmol/l) for 2 h. Oligomycin A (2 μmol/l), carbonyl cyanide-p-trifluoromethoxyphenylhydrazone (FCCP) (2 μmol/l), and antimycin A + rotenone (1 μmol/l each) were sequentially added as indicated. Letters in this panel (a, b, and c) indicate the timing at which parameters shown in B were calculated. (B) Unloaded and maximum OCRs calculated by subtracting OCR after antimycin A and rotenone (c in A) from that before oligomycin (a in A) or after FCCP (b in A), respectively. (C) Measurement of OCR in mNVCMs treated in vitro for 2 h with saline or TRV027 (3 μmol/l) and with nifedipine (10 μmol/l) for last 30 min. (D) Unloaded and maximum OCR in the presence of nifedipine. (E) Short term effect of wash-in of saline or TRV027 (3 μmol/l) on unloaded OCR in mNVCMs. Saline or TRV027 was added at indicated this time point while OCR was measured. (F) Time-dependent effect of saline or TRV027 on unloaded OCR for ∼35 min after their wash-in. Statistical significance was analyzed with unpaired Student’s t test. n = 5 to 8 in each group.
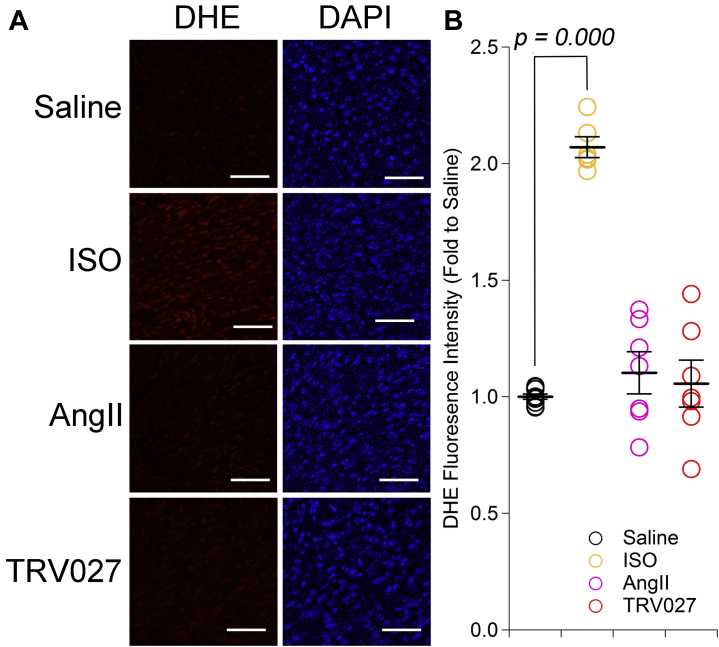
Effect of AngII and TRV027 on the production of ROS in the mouse neonatal heart
Figure 5A shows the representative dihydroethidium staining of the mouse neonatal heart 1 h after administration of isoproterenol or 2 h after administration of saline, AngII, or TRV027. Although isoproterenol significantly increased ROS as previously reported (37,38), neither AngII nor TRV027 significantly increased ROS production compared with saline in the hearts of mice of this age (Figure 5B).
Figure 5.
Effect of AngII and TRV027 on ROS in the Hearts of Neonatal Mice
(A) Representative images of dihydroethidium (DHE) and DAPI staining of the heart 1 h after administration of isoproterenol (ISO) (30 mg/kg) or 2 h after administration of saline, angiotensin II (AngII) (3 mg/kg), or TRV027 (3 mg/kg) to neonatal mice. Scale bar = 50 μm. (B) Quantification of DHE fluorescence. Statistical significance was analyzed with 1-way analysis of variance followed by Dunnett's test. n = 6 to 10 in each group.
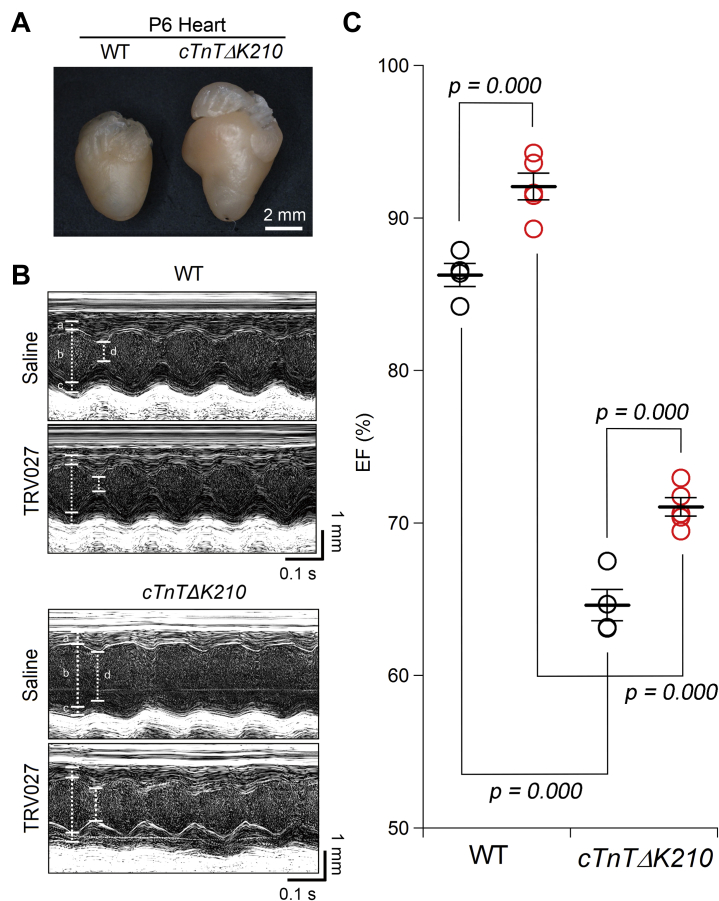
Effect of TRV027 on the contractility of the heart in a mouse model of human congenital DILATED CARDIOMYOPATHY
The cTnTΔK210 mice exhibit overt heart failure soon after birth, and ∼80% of them die by postnatal day 20 (20). We examined the effect of TRV027 on the compromised heart at postnatal days 4 to 6. cTnTΔK210 mice already had a significantly larger heart and smaller left ventricular wall motion than wild-type mice (Figures 6A and 6B) (Supplemental Table 4). However, TRV027 significantly increased ejection fraction in both wild-type and cTnTΔK210 mice (Figure 6C). Therefore, TRV027 could be utilized as a valuable drug specific for PHF.
Figure 6.
Effect of TRV027 on the Cardiac Contractility of Model Mice of Human Congenital DCM
(A) The representative hearts of wild-type (WT) mouse and model mouse of human congenital dilated cardiomyopathy (cTnTΔK210) at postnatal day 6 (P6). (B) Representative M-mode ultrasound cardiogram of wild-type and cTnTΔK210 mice 2 h after administration of saline or TRV027 (3 mg/kg). (C) Ejection fraction (EF) in each condition. Statistical significance was analyzed with 1-way analysis of variance followed by Tukey’s test. n = 5 in each group.
Discussion
Characteristic pharmacodynamics of TRV027
In this study, we for the first time showed that TRV027 increased cardiac contractility without affecting heart rate, ROS production, and adrenal aldosterone secretion in neonatal mice. Although TRV027 significantly increased adrenaline secretion at 8 h after administration, it was not involved in the inotropic effect of TRV027 and it moderately affected a few ECG parameters (Figures 1 and 2). TRV027 significantly increased the unloaded OCR of isolated cardiac myocytes by ∼30%, associated with a doubling of the peak twitch Ca2+ transient amplitude (Figures 3 and 4). This effect of TRV027 on OCR is much more modest than that of adrenaline when causing an equivalent effect (39). In vivo, cardiac O2 consumption is also affected by external work (34). The amount of external work is mainly dependent on contractility and afterload and almost linearly multiplied by heart rate. We speculate that TRV027 would not increase external work greatly because, unlike catecholamine, it reduces afterload through AT1R/Gq/11 inhibition in vascular smooth muscle cells and does not increase heart rate (Figure 2) (14). This “inotropic vasodilator effect,” without a chronotropic effect, is apparently similar to that of phosphodiesterase III inhibitors, such as milrinone, which is favored in the management of relatively severe cases of PHF (40). Because TRV027 caused inotropic effect almost solely by increasing LTCC through the AT1R/β-arrestin 2/casein kinase 2′ pathway (11), it activates Ca2+ influx into the cardiac myocytes through the physiological pathway with an exact physiological timing. This detail is important because digitalis, for instance, causes various adverse effects owing to Ca2+ overload mediated by unphysiological Ca2+ entry through Na+-Ca2+ exchangers (41). According to other reports, AT1R/β-arrestin also exerts antiapoptotic effect on the heart (42), dilates resistance arteries to reduce afterload (14), and increases Na+ diuresis to reduce preload (16). These lines of evidence support our hypothesis that the AT1R/β-arrestin pathway is the physiological pro-survival mechanism for neonatal mammals (9). This concept adds an important caveat that AT1R blocker might be harmful for neonatal patients. The clear effect of TRV027 on hiPSC-CMs (Figures 3B and 3D) indicates that this pathway is preserved in human immature cardiac myocytes and could be a valuable drug target in PHF.
Characteristic pharmacokinetics of TRV027
The slow and sustained positive inotropic effect of TRV027 (Figure 1C) is very different from its previously reported one on adult mouse dilated cardiomyopathy (14,15). In adults, TRV027 rapidly caused inotropic effects in minutes, indicating that it is based on molecular mechanisms distinct from those observed in neonates. The neonatal-specific long-lasting effect of TRV027 is very noticeable considering its short half-life in the plasma (less than a few minutes) (43). However, we ruled out the possibility that TRV027 may activate some neuronal or humoral factors in a short period after administration and that the latter factors may secondarily cause a sustained effect on the heart for the following 2 reasons: 1) AngII and TRV027 increased LTCC activity and Ca2+ transients in a similar time course in isolated mNVCMs and hiPSC-CMs in vitro (Figure 3) (11); and 2) candesartan administration 2 h after TRV027 administration promptly and almost completely inhibited the inotropic effects of TRV027 (Figures 1A and 1B). AngII also has similar protracted post-translational effects, such as regulation of Na+ absorption in the renal proximal tubules and Henle loop, despite its short half-life (10). We speculate that the slow and sustained effect of the AT1R ligands might arise from AT1R/β-arrestin complexes in the intracellular β-arrestin signalosome (13). Endocytosis of GPCR by the clathrin-coated pit is negatively regulated by PIP2 in the plasma membrane (44). Because TRV027 does not effectively degrade PIP2 through Gq/11-activated phospholipase C-β (14), the TRV027/AT1R complex might be endocytosed promptly within the time frame required for TRV027 being available in plasma. Type B GPCRs, such as AT1R, strongly interact with β-arrestin and retains endosomes longer than type A GPCRs, such as β-adrenergic receptor (45). Therefore, it could be envisaged that catecholamines have a role in regulating perinatal circulation on a minute-by-minute basis, whereas AngII and TRV027, in the order of hours.
Valuable translational aspect of TRV027
PHF is an important cause of morbidity and mortality in childhood (1). However, the development of pharmacological treatments for heart failure in children significantly lag behind that of adult heart failure due to the difficulties inherent to large clinical studies, such as versatile etiologies and pathophysiologies. Therefore, it would be crucial to find a clue to solve this issue in a bottom-up manner with a preclinical study scrutinizing perinatal circulation physiology. Importantly, we found that TRV027 was significantly effective in hiPSC-CMs (Figure 3) and the neonatal compromised heart of a mouse model of human congenital dilated cardiomyopathy (Figure 6). Its sustained effect might also make it possible to manage patients with only a few daily administrations. Thus, TRV027 could be a valuable and safe drug specific for PHF.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: There is compelling evidence that simultaneous inhibition of G protein and β-arrestin pathways coupled to AT1R with AT1R blocker is beneficial for adult patients suffering from chronic heart failure; however, little comparative evidence is available for PHF. We show that selective activation of AT1R/β-arrestin pathway by a BBA, TRV027, is supportive rather than hazardous for pre-weaning mice undergoing the circulation change. Importantly, this pathway is preserved in human immature cardiac myocytes, and ∼60% of children with congenital heart diseases or cardiomyopathy exhibit overt heart failure in their first year. Thus, this preclinical study strongly implicates that PHF should be treated with a BBA but not AT1R blocker.
TRANSLATIONAL OUTLOOK: We chose TRV027 to study its effectiveness in immature cardiac myocytes because its safety (but not efficacy) has been already proven in adult heart failure patients. If it is also safe in children, it could be used to treat PHF. Confirmation of the therapeutic potential of TRV027 requires experiments to assess its chronic effect on prognosis of cTnTΔK210 mice (in progress) and other animal models of PHF. It is important, when producing small molecular BBAs, to be aware that BBAs with different structures could affect distinct sets of intracellular signaling pathways to different extents.
Author Disclosures
This work was supported by Grants-in-Aid for Scientific Research to Dr. Kashihara (16K08546) and Dr. Yamada (18K06870), a Grant-in-Aid for Young Scientists to Dr. Kashihara (26860145) and Dr. Kawagishi (19K16488), and a Grant-in-Aid for Research Activity start-up to Dr. Kawagishi (18H06124), all from the Japan Society for the Promotion of Science; grants from the Novartis Foundation (Japan) to Dr. Kashihara and from the Shinshu Public Utility Foundation for Promotion of Medical Sciences to Dr. Kashihara and Dr. Yamada; and scholarship donations to Dr. Yamada from Astellas Pharma, Bayer Yakuhin, Bristol-Myers Squibb, Daiichi Sankyo, and Shionogi and Co. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors are grateful to Prof. Koichiro Kuwahara (Department of Cardiovascular Medicine, Shinshu University School of Medicine, Matsumoto, Japan) for critically reading our manuscript and for his suggestions, to Prof. Masamichi Hirose (Division of Molecular and Cellular Pharmacology, Department of Pathophysiology and Pharmacology, School of Pharmaceutical Sciences, Iwate Medical University, Morioka, Japan) for providing suggestions to interpret UCG data, to Ms. Reiko Sakai for secretary assistance, and to Editage (www.editage.com) for English language editing.
Footnotes
Dr. Kashihara is currently affiliated with Department of Cell Biology and Molecular Medicine, Rutgers New Jersey Medical School, Newark, New Jersey, USA.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
Appendix
For an expanded Methods and References sections as well as supplemental figures and tables, please see the online version of this paper.
Appendix
References
- 1.Hinton R.B., Ware S.M. Heart failure in pediatric patients with congenital heart disease. Circ Res. 2017;120:978–994. doi: 10.1161/CIRCRESAHA.116.308996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eisner D.A., Caldwell J.L., Kistamas K., Trafford A.W. Calcium and excitation-contraction coupling in the heart. Circ Res. 2017;121:181–195. doi: 10.1161/CIRCRESAHA.117.310230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedman A.H., Fahey J.T. The transition from fetal to neonatal circulation: normal responses and implications for infants with heart disease. Semin Perinatol. 1993;17:106–121. [PubMed] [Google Scholar]
- 4.Hong T., Shaw R.M. Cardiac T-tubule microanatomy and function. Physiol Rev. 2017;97:227–252. doi: 10.1152/physrev.00037.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robinson R.B. Autonomic receptor--effector coupling during post-natal development. Cardiovasc Res. 1996;31 Spec No:E68–E76. [PubMed] [Google Scholar]
- 6.Docherty J.R. The pharmacology of alpha1-adrenoceptor subtypes. Eur J Pharmacol. 2019;855:305–320. doi: 10.1016/j.ejphar.2019.04.047. [DOI] [PubMed] [Google Scholar]
- 7.Frolich S., Slattery P., Thomas D. Angiotensin II-AT1-receptor signaling is necessary for cyclooxygenase-2-dependent postnatal nephron generation. Kidney Int. 2017;91:818–829. doi: 10.1016/j.kint.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Stubbe J., Jensen B.L., Bachmann S., Morsing P., Skott O. Cyclooxygenase-2 contributes to elevated renin in the early postnatal period in rats. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1179–R1189. doi: 10.1152/ajpregu.00340.2002. [DOI] [PubMed] [Google Scholar]
- 9.Heymann M.A., Iwamoto H.S., Rudolph A.M. Factors affecting changes in the neonatal systemic circulation. Annu Rev Physiol. 1981;43:371–383. doi: 10.1146/annurev.ph.43.030181.002103. [DOI] [PubMed] [Google Scholar]
- 10.Forrester S.J., Booz G.W., Sigmund C.D. Angiotensin II signal transduction: an update on mechanisms of physiology and pathophysiology. Physiol Rev. 2018;98:1627–1738. doi: 10.1152/physrev.00038.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kashihara T., Nakada T., Kojima K., Takeshita T., Yamada M. Angiotensin II activates CaV 1.2 Ca(2+) channels through beta-arrestin2 and casein kinase 2 in mouse immature cardiomyocytes. J Physiol. 2017;595:4207–4225. doi: 10.1113/JP273883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shukla A.K., Xiao K., Lefkowitz R.J. Emerging paradigms of beta-arrestin-dependent seven transmembrane receptor signaling. Trends Biochem Sci. 2011;36:457–469. doi: 10.1016/j.tibs.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murphy J.E., Padilla B.E., Hasdemir B., Cottrell G.S., Bunnett N.W. Endosomes: a legitimate platform for the signaling train. Proc Natl Acad Sci U S A. 2009;106:17615–17622. doi: 10.1073/pnas.0906541106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Violin J.D., DeWire S.M., Yamashita D. Selectively engaging beta-arrestins at the angiotensin II type 1 receptor reduces blood pressure and increases cardiac performance. J Pharmacol Exp Ther. 2010;335:572–579. doi: 10.1124/jpet.110.173005. [DOI] [PubMed] [Google Scholar]
- 15.Kim K.S., Abraham D., Williams B., Violin J.D., Mao L., Rockman H.A. beta-Arrestin-biased AT1R stimulation promotes cell survival during acute cardiac injury. American journal of physiology Heart Circ Physiol. 2012;303:H1001–H1010. doi: 10.1152/ajpheart.00475.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boerrigter G., Lark M.W., Whalen E.J., Soergel D.G., Violin J.D., Burnett J.C., Jr. Cardiorenal actions of TRV120027, a novel β-arrestin-biased ligand at the angiotensin II type I receptor, in healthy and heart failure canines: a novel therapeutic strategy for acute heart failure. Circ Heart Fail. 2011;4:770–778. doi: 10.1161/CIRCHEARTFAILURE.111.962571. [DOI] [PubMed] [Google Scholar]
- 17.Boerrigter G., Soergel D.G., Violin J.D., Lark M.W., Burnett J.C., Jr. TRV120027, a novel beta-arrestin biased ligand at the angiotensin II type I receptor, unloads the heart and maintains renal function when added to furosemide in experimental heart failure. Circ Heart Fail. 2012;5:627–634. doi: 10.1161/CIRCHEARTFAILURE.112.969220. [DOI] [PubMed] [Google Scholar]
- 18.Pang P.S., Butler J., Collins S.P. Biased ligand of the angiotensin II type 1 receptor in patients with acute heart failure: a randomized, double-blind, placebo-controlled, phase IIB, dose ranging trial (BLAST-AHF) Eur Heart J. 2017;38:2364–2373. doi: 10.1093/eurheartj/ehx196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshida Y., Yamanaka S. Induced Pluripotent Stem Cells 10 Years Later For Cardiac Applications. Circ Res. 2017;120:1958–1968. doi: 10.1161/CIRCRESAHA.117.311080. [DOI] [PubMed] [Google Scholar]
- 20.Nonaka M., Morimoto S., Murayama T. Stage-dependent benefits and risks of pimobendan in mice with genetic dilated cardiomyopathy and progressive heart failure. Brit J Pharmacol. 2015;172:2369–2382. doi: 10.1111/bph.13062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pachon R.E., Scharf B.A., Vatner D.E., Vatner S.F. Best anesthetics for assessing left ventricular systolic function by echocardiography in mice. Am J Physiol Heart Circ Physiol. 2015;308:H1525–H1529. doi: 10.1152/ajpheart.00890.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Du C.K., Morimoto S., Nishii K. Knock-in mouse model of dilated cardiomyopathy caused by troponin mutation. Circ Res. 2007;101:185–194. doi: 10.1161/CIRCRESAHA.106.146670. [DOI] [PubMed] [Google Scholar]
- 23.Mitchell G.F., Jeron A., Koren G. Measurement of heart rate and Q-T interval in the conscious mouse. Am J Physiol. 1998;274:H747–H751. doi: 10.1152/ajpheart.1998.274.3.H747. [DOI] [PubMed] [Google Scholar]
- 24.Fridericia L.S. Die Systolendauer im Elektrokardiogramm bei normalen Menschen und bei Herzkranken. Acta Med Scand. 1920;53:469–486. [Google Scholar]
- 25.Wang Q., Zou M.H. Measurement of reactive oxygen species (ROS) and mitochondrial ROS in AMPK knockout mice blood vessels. Methods Mol Biol. 2018;1732:507–517. doi: 10.1007/978-1-4939-7598-3_32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ackers-Johnson M., Li P.Y., Holmes A.P., O'Brien S.M., Pavlovic D., Foo R.S. A simplified, Langendorff-free method for concomitant isolation of viable cardiac myocytes and nonmyocytes from the adult mouse heart. Circ Res. 2016;119:909–920. doi: 10.1161/CIRCRESAHA.116.309202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakagawa M., Koyanagi M., Tanabe K. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26:101–106. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- 28.Kadota S., Pabon L., Reinecke H., Murry C.E. In vivo maturation of human induced pluripotent stem cell-derived cardiomyocytes in neonatal and adult rat hearts. Stem Cell Rep. 2017;8:278–289. doi: 10.1016/j.stemcr.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ogasawara T., Okano S., Ichimura H. Impact of extracellular matrix on engraftment and maturation of pluripotent stem cell-derived cardiomyocytes in a rat myocardial infarct model. Sci Rep. 2017;7:8630. doi: 10.1038/s41598-017-09217-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shiba Y., Gomibuchi T., Seto T. Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts. Nature. 2016;538:388–391. doi: 10.1038/nature19815. [DOI] [PubMed] [Google Scholar]
- 31.Piacentino V., 3rd, Weber C.R., Chen X. Cellular basis of abnormal calcium transients of failing human ventricular myocytes. Circ Res. 2003;92:651–658. doi: 10.1161/01.RES.0000062469.83985.9B. [DOI] [PubMed] [Google Scholar]
- 32.Liu C.H., Gong Z., Liang Z.L. Arrestin-biased AT1R agonism induces acute catecholamine secretion through TRPC3 coupling. Nat Commun. 2017;8:14335. doi: 10.1038/ncomms14335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lymperopoulos A., Rengo G., Zincarelli C., Kim J., Soltys S., Koch W.J. An adrenal beta-arrestin 1-mediated signaling pathway underlies angiotensin II-induced aldosterone production in vitro and in vivo. Proc Natl Acad Sci U S A. 2009;106:5825–5830. doi: 10.1073/pnas.0811706106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suga H. Ventricular energetics. Phys Rev. 1990;70:247–277. doi: 10.1152/physrev.1990.70.2.247. [DOI] [PubMed] [Google Scholar]
- 35.Cheung J.Y., Gordon J., Wang J. Mitochondrial dysfunction in human immunodeficiency virus-1 transgenic mouse cardiac myocytes. J Cell Physiol. 2019;234:4432–4444. doi: 10.1002/jcp.27232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jafri M.S., Dudycha S.J., O'Rourke B. Cardiac energy metabolism: models of cellular respiration. Annu Rev Biomed Eng. 2001;3:57–81. doi: 10.1146/annurev.bioeng.3.1.57. [DOI] [PubMed] [Google Scholar]
- 37.Zhang J., Xiao H., Shen J., Wang N., Zhang Y. Different roles of beta-arrestin and the PKA pathway in mitochondrial ROS production induced by acute beta-adrenergic receptor stimulation in neonatal mouse cardiomyocytes. Biochem Biophys Res Commun. 2017;489:393–398. doi: 10.1016/j.bbrc.2017.05.140. [DOI] [PubMed] [Google Scholar]
- 38.Qi C., Shao Y., Liu X., Wang D., Li X. The cardioprotective effects of icariin on the isoprenaline-induced takotsubo-like rat model: Involvement of reactive oxygen species and the TLR4/NF-kappaB signaling pathway. Int Immunopharmacol. 2019;74:105733. doi: 10.1016/j.intimp.2019.105733. [DOI] [PubMed] [Google Scholar]
- 39.Suga H., Hisano R., Goto Y., Yamada O., Igarashi Y. Effect of positive inotropic agents on the relation between oxygen consumption and systolic pressure volume area in canine left ventricle. Circ Res. 1983;53:306–318. doi: 10.1161/01.res.53.3.306. [DOI] [PubMed] [Google Scholar]
- 40.Kantor P.F., Mertens L.L. Clinical practice: heart failure in children. Part I: clinical evaluation, diagnostic testing, and initial medical management. Eur J Pediatr. 2010;169:269–279. doi: 10.1007/s00431-009-1024-y. [DOI] [PubMed] [Google Scholar]
- 41.Bers D.M. Excitation-Contraction Coupling and Cardiac Contractile Force. Springer; Dordrecht, the Netherlands: 2008. Cardiac inotropy and Ca mismanagement; pp. 273–331. [Google Scholar]
- 42.Ahn S., Kim J., Hara M.R., Ren X.R., Lefkowitz R.J. {beta}-Arrestin-2 mediates anti-apoptotic signaling through regulation of BAD phosphorylation. J Biol Chem. 2009;284:8855–8865. doi: 10.1074/jbc.M808463200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soergel D.G., Subach R.A., Cowan C.L., Violin J.D., Lark M.W. First clinical experience with TRV027: pharmacokinetics and pharmacodynamics in healthy volunteers. J Clin Pharmacol. 2013;53:892–899. doi: 10.1002/jcph.111. [DOI] [PubMed] [Google Scholar]
- 44.Toth D.J., Toth J.T., Gulyas G. Acute depletion of plasma membrane phosphatidylinositol 4,5-bisphosphate impairs specific steps in endocytosis of the G-protein-coupled receptor. J Cell Sci. 2012;125:2185–2197. doi: 10.1242/jcs.097279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oakley R.H., Laporte S.A., Holt J.A., Caron M.G., Barak L.S. Differential affinities of visual arrestin, beta arrestin1, and beta arrestin2 for G protein–coupled receptors delineate two major classes of receptors. J Biol Chem. 2000;275:17201–17210. doi: 10.1074/jbc.M910348199. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.