Abstract
Amyotrophic lateral sclerosis (ALS) is the most common and severe adult-onset motoneuron disease and has currently no effective therapy. Approximately 20% of familial ALS cases are caused by dominantly-inherited mutations in the gene encoding Cu/Zn superoxide dismutase (SOD1), which represents one of the most frequent genetic cause of ALS. Despite the overwhelming majority of ALS-causing missense mutations in SOD1, a minority of premature termination codons (PTCs) have been identified. mRNA harboring PTCs are known to be rapidly degraded by nonsense-mediated mRNA decay (NMD), which limits the production of truncated proteins. The rules of NMD surveillance varying with PTC location in mRNA, we analyzed the localization of PTCs in SOD1 mRNA to evaluate whether or not those PTCs can be triggered to degradation by the NMD pathway. Our study shows that all pathogenic PTCs described in SOD1 so far can theoretically escape the NMD, resulting in the production of truncated protein. This finding supports the hypothesis that haploinsufficiency is not an underlying mechanism of SOD1 mutant-associated ALS and suggests that PTCs found in the regions that trigger NMD are not pathogenic. Such a consideration is particularly important since the availability of SOD1 antisense strategies, in view of variant treatment assignment.
Subject terms: Genetics, Medical genetics, Motor neuron disease, Amyotrophic lateral sclerosis, Molecular medicine, RNA decay
Introduction
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease characterized by the selective loss of both upper and lower motoneurons, leading to a progressive paralysis and death within 3–5 years1.
About 20% of familial ALS cases are caused by mutations in the gene encoding the detoxifying copper-zinc superoxide dismutase (SOD1)2. Currently, over 180 different mutations throughout the five exons of the SOD1 gene (MIM 147450) have been described2,3, the vast majority of which being missense point mutations resulting in a dominant mode of inheritance of ALS (with the exception of the D91A mutation) and spreading over the entire 154 amino acid sequence4,5.
It has been well-established that SOD1 mutants-mediated toxicity is caused by a gain-of-function rather than the loss of the detoxifying activity of SOD12 and that mutant SOD1 can adopt multiple misfolded conformations that mediate toxicity2. Moreover, mice with genetic ablation of Sod1 do not recapitulate disease phenotype5–8. Instead, Sod1-deficient mice show accelerated rate of muscle denervation, locomotor deficits and tremors, as well as increased vulnerability to stress. It is noteworthy that the 50% loss of Sod1 activity described in heterozygous Sod1+/- mice leads to an increased susceptibility to axonal injury, ischemia or glutamate-induced toxicity9.
Nonsense-mediated mRNA decay (NMD) is an eukaryotic quality control pathway that degrades mRNAs containing Premature termination codons (PTCs) caused by nonsense or frameshift mutations10,11. It is important to note that some PTCs can escape NMD. This capability is governed by four rules12: (1) the 50 nucleotides rule: PTCs less than 50–55 nucleotides upstream of the last exon–exon junction typically do not trigger NMD; (2) the last exon rule: PTCs in the last exon of a gene also do not trigger NMD; (3) the long exon rule: exons greater than approximately 400 nucleotides inhibit NMD; (4) the start-proximal rule: PTCs located below 150 nucleotides from the start codon typically fail to trigger NMD.
PTCs that escape NMD in SOD1 are thus expected to lead to the production of truncated SOD1 protein, which can be highly unstructured with elevated toxicity as illustrated with the non-sense mutation p.Leu127*13,14.
To evaluate the capacity of PTCs associated with ALS in SOD1 to trigger NMD, we analyzed their localization through the gene.
Results
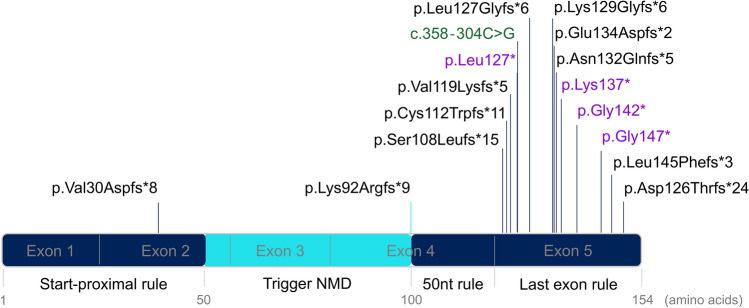
Regarding the NMD rules we estimated that the region of SOD1 obeying the NMD is located between nucleotides 151–301, which correspond to amino acids 50–100 (Fig. 1). Accordingly, all PTCs located within this region are expected to trigger the NMD, resulting in the degradation of messenger RNAs, and leading to haploinsufficiency. Conversely, all PTCs located outside this region can result in the production of truncated SOD1, prone to induce misfolded protein. We found a total of 16 disease-associated-PTCs mutations in SOD1 in the literature15,16, including 4 nonsense mutations, 11 frameshift mutations and 1 deep intronic splicing mutation (Table 1, Fig. 1).
Figure 1.
Schematic representation of the SOD1 gene showing the localization of PTCs causing ALS and NMD escaping regions. Frameshift mutations and the deep intronic splicing mutation are placed according to the localization of their resulting PTCs (and not the mutation site itself). Nonsense mutations are indicated in purple, deep intronic splicing mutation in green and frameshifts mutations in black. The region of SOD1 where PTCs trigger NMD is represented in cyan. The regions that escape NMD are represented in dark blue.
Table 1.
List of truncating mutations in SOD1 associated with ALS.
| Mutation (GRCh37)a | Protein variant b | Resulting PTC position | References |
|---|---|---|---|
| Splicing mutation | |||
| c.358-304C > G | p.Val120Glnfs*8 | 127 | 19 |
| Non-sense mutations | |||
| c.380 T > A | p.Leu127* | 127 | 16 |
| c.409A > T | p.Lys137* | 137 | 15 |
| c.424G > T | p.Gly142* | 142 | 36 |
| c.441 T > A | p.Cys147* | 147 | 20,37 |
| Frameshift mutations | |||
| c.88_89insA | p.Val30Aspfs*8 | 37 | 20,38,39 |
| c.275_276delAA | p.Lys92Argfs*9 | 100 | 40 |
| c.320dupT | p.Ser108Leufs*15 | 122 | 41 |
| c.335dupG | p.Cys112Trpfs*11 | 122 | 28,29 |
| c.355delGinsAAAAC | p.Val119Lysfs*5 | 123 | 20,42 |
| c.379_380delTT | p.Leu127Glyfs*6 | 132 | 43–45 |
| c.380_383dupTGGG | p.Lys129Glyfs*6 | 134 | 20,46,47 |
| c.401_402insTT | p.Glu134Aspfs*2 | 135 | 20,48 |
| c.383_392dupGCAAAGGTGG | p.Asn132Glnfs*5 | 136 | 49 |
| c.435delGinsCGTTTA | p.Leu145Phefs*3 | 147 | 50 |
| c.376delG | p.Asp126Thrfs*24 | 149 | 51 |
a Human genome variation society (HGVS) nomenclature V2.0 according to mRNA reference sequence GenBank: NM_000454.4. Nucleotide numbering uses + 1 as the A of the ATG translation initiation codon in the reference sequence, with the initiation codon as codon 1. b HGVS nomenclature according to protein reference sequence GenPept: NP_000445.1. Amino acid numbering uses p.1 as the Methionine corresponding to the initiation codon. This implies a 1-amino acid switch compared to former SOD1 nomenclature (eg. L127X mutation was formerly known as L126X or, in some articles, L126Z).
Among them, fourteen are predicted to escape the NMD according to the last exon rule and one (p.Val30Aspfs*8) is predicted to escape the NMD, obeying to the start proximal rule (Fig. 1). By faithfully following the 50 nucleotides rule, one frameshift mutation (p.Lys92Argfs*9) was found to introduce a PTC at the position 100, which is the last position predicted to trigger NMD. However, it is likely that at such borderline position, NMD is not completely activated and that truncated protein is at least partly produced.
To evaluate the impact of NMD on PTCs across the SOD1 gene, we have analyzed experimental data from a large-scale analysis of approximately 80,000 matched tumor exomes and transcriptomes available on the cBio Cancer Genomics Portal17,18. Among them, we identified 31 mutations in SOD1 in 40 samples. 24 were missense mutations, 2 were translation start site mutations and 5 were truncating mutations (2 splicing, 2 frameshift and 1 non-sense mutation). The heterozygous non-sense mutation (p.Glu79*, E79*) identified in one sample by whole exome sequencing (WES) was almost not detected on the RNA sequencing (RNA-Seq) data from the same sample, suggesting a massive degradation of the SOD1 mutated transcript by the NMD (Fig. 2). In contrast, heterozygous frameshift mutation (p.Lys137Aspfs*26, K137Dfs*26), located in the last exon of SOD1, was detected in both WES and RNA-Seq data, confirming NMD escape for this other PTC (Fig. 2).
Figure 2.
(A) Evaluation of NMD effect on SOD1 transcripts by comparison of matched tumor exomes and transcriptomes. Visualization with the Integrative Genome Viewer software (version 2.8.4, http://software.broadinstitute.org/software/igv/)35 of WES and RNA-Seq alignments of 3 samples from the Cancer Cell Line Encyclopedia52. The missense mutation p.Ile150Val (I150V, sample 3) is used here as a control as it has no effect on NMD activity and is thus found heterozygous in both WES and RNA-Seq alignments. The non-sense mutation p.Glu79* located in exon 3 of SOD1, (E79*, sample 1) was detected on 9% of RNA-Seq reads versus 46% of WES reads, showing the degradation of the SOD1 mutated transcript by NMD. In contrast, heterozygous frameshift mutation p.Lys137Aspfs*26 (K137Dfs*26, sample 2), located in the last exon of SOD1 was detected in 32% of RNA-Seq reads versus 56% of WES reads, highlighting NMD escape. (B) SOD1 mRNA expression correlation with SOD1 mutations in 2029 samples. This plot was generated from the cBio Cancer Genomics Portal (http://cbioportal.org)17,18. Although not statistically significant, sample 1 with the E79* mutation appears to have a lower SOD1 mRNA expression (z-score = −1.31) compared to sample 2 with the K137Dfs*26 mutation (z-score = −0.21, equivalent to the mean mRNA expression in the wild type group). Deep Deletion indicates a deep loss, possibly a homozygous deletion; Shallow Deletion indicates a shallow loss, possibly a heterozygous deletion; Gain indicates a low-level gain (a few additional copies, often broad); Amplification indicate a high-level amplification (more copies, often focal); Not profiled for CNA indicate the samples for which copy-number analysis was not performed. These levels are derived from copy-number analysis algorithms and indicate the copy-number level per gene.
Discussion
In this study, we have explored the impact of NMD on SOD1 and shown that the activity of the NMD pathway is of broad importance for ALS caused by PTC in SOD1. Through a large-scale analysis of human cancer exomes and transcriptomes we were able to confirm that SOD1 standardly obeys to the NMD pathway and its rules.
Our conclusion is supported by several arguments from the literature: (1) The presence of PTC in a region that escape NMD has been detected in mRNA extracted from immortalized lymphoblast cell lines from two patients harboring the c.358-304C > G mutation, thus confirming the impact on the protein level p.Val120Glnfs*819; (2) Conformational changes of truncated proteins have been well characterized for PTC located in the region that escape NMD20; (3) Heterozygous PTCs located in the region that trigger NMD seem to be more frequent in individuals from general population: in gnomAD database, for example, we found 6 individuals with a PTC theoretically triggering NMD (p.Glu50Glyfs*39, p.Leu68Glufs*19) versus 3 individuals aged between 40 and 65 years with a PTC theoretically escaping NMD (p.Val6Cysfs*4, p.Asp97Metfs*8).
Interestingly, the fact that all PTCs associated with ALS in SOD1 can escape the NMD comforts the hypothesis that haploinsufficiency is not an underlying mechanism of the disease. Instead, the production of a misfolded truncated SOD1 protein could cause a toxic gain-of-function. Therefore, even if we confirmed the massive degradation of mRNA harboring a PTC in the region triggering NMD, as we could detect a small remaining amount of mutated mRNA (9% of reads on RNA-Seq data, see sample 1 on Fig. 2A), we cannot exclude a very late onset form of SLA in such situation.
Dimer destabilization, oligomerization and increased aggregation are the proposed mechanisms for mutant SOD1 toxicity4. Recently it has been demonstrated that SOD1 acts as a H2O2-responsive regulatory protein in the expression of ALS-linked genes. Both sequence preference and affinity of SOD1 interactions with DNA depend on SOD1 conformation21. Thereby, PTCs that escape NMD in SOD1 are expected to cause toxic conformational changes. Indeed, some of the truncating mutations described here were proven to cause SOD1 misfolding capable to interact with Derlin-1, triggering endoplasmic reticulum stress and contributing to motoneuron death (i.e. p.Val30Aspfs*8, p.Val119Lysfs*5, p.Leu127*, p.Glu134Aspfs*2, p.Gly142*)20.
The toxic gain-of-function mechanism evidence provides a strong rationale for gene silencing as a therapy for SOD1-mediated ALS. Thus, clinically promising therapies, all aimed at enhancing specifically the degradation of the mutated SOD1 RNA, such as anti-sense oligonucleotides (ASO) and RNA interference (RNAi) are being tested in preclinical and clinical studies22–25. In the first clinical trial of ASO treatment in human beings, only ASO targeting missense mutations were developed. This trial had favorable safety outcomes, and a trial to assess the safety, tolerability and pharmacokinetics of a second generation SOD1 ASO is currently in progress (ClinicalTrials.gov, NTC02623699)26. To our knowledge, no ASO targeting a PTC has been investigated so far. This could be explained by the low proportion of patients carrying such mutations. For example, PTCs in SOD1 are absent from MinE Database which includes 4366 whole genomes from ALS patients and 1832 whole genomes from controls, from different European ancestry27.
Recently, complete loss of function of SOD1 in human has been reported in a 2 years old girl with a homozygous truncating mutation and an absence of SOD1 activity. The patient presented with axial hypotonia and loss of gross and fine motor function at 6 months of age, after which severe, progressive spastic tetraparesis developed and Babinski’s sign was present in both feet. Atrophy, fasciculations, and other signs of lower motor neuron involvement were not noted. Her parents, both heterozygous for the mutation were healthy at the time of the report while the level of SOD1 activity was half that of the normal level28. Another report of the same homozygous truncating variant c.335dupG (p.Cys112Trpfs*11) in SOD1 was identified in another patient with tetraspasticity. In contrast with Andersen et al. 2019 report, heterozygous carriers from this family had a markedly reduced enzyme activity when compared to wild-type controls but show no overt neurologic phenotype29. Thus, while caution might be exercised regarding the use of gene therapies that may markedly depresses SOD1 activity, reduction of SOD1 appears to be well tolerated, as outlined by the favorable clinical trial safety outcomes.
Animal models, particularly SOD1 rodent model30, initially developed to investigate the complex processes occurring in ALS, had played a major role in performance evaluation of these silencing approaches22. More recently, other models like zebrafish31, Drosophila32 or patient-derived induced pluripotent stem cell33 have also been designed and tested to investigate the physiopathology of ALS. These models, particularly useful since the availability of SOD1 antisense strategies, offer the possibility to study the pathogenicity of novel SOD1 variants, especially complex intronic mutations that could either lead to an amino-acid(s) insertion or deletion and/or to the creation of a PTC.
In conclusion, we highlight that all described PTCs in SOD1 causing ALS are predicted to escape the nonsense-mediated mRNA decay. More importantly, this observation suggests that truncating mutations found in the region of SOD1 that trigger NMD may have no pathogenic significance. Such a consideration is particularly important since the availability of SOD1 antisense strategies, in view of variant treatment assignment.
Methods
The PTCs of the human SOD1 gene (NM_000454.4) resulting from nonsense, frameshift and splicing mutations that are associated with ALS were obtained from the Human gene mutation database (HGMD)34, which provides systematic and in-depth qualitative and quantitative overviews of genetic research in both familial and sporadic ALS. Intronic mutations located outside the canonical sites and not confirmed by transcript analysis were excluded from this study.
The cBio Cancer Genomics Portal (http://cbioportal.org)17,18, an open platform for exploring multidimensional cancer genomics data, was used to select tumor samples with PTC in SOD1 for which whole exome sequencing (WES) and RNA-sequencing (RNA-seq) experimental data were performed. We generated plot from cBio Cancer Genomics Portal17,18 to analyze SOD1 mRNA expression correlation with SOD1 mutations.
Raw data (Bam files) from sample of interest were downloaded, when available, from NCBI Sequence Read Archive (SRA) and visualized with the Integrative Genome Viewer software (version 2.8.4, http://software.broadinstitute.org/software/igv/)35. SRA accession numbers and detailed information about the samples are available in the Supplementary Table S1.
Supplementary information
Author contributions
Conceptualization, C.G. and S.L.; methodology, C.G.; formal analysis, C.G.; investigation, C.G.; resources, S.L.; data curation, A.P., B.L., C.G., C.R., J.K., K.M., P.V. and S.L.; writing—original draft preparation, C.G.; writing and editing, C.G., C.R., K.M. and S.L.; supervision, S.L.; All authors reviewed the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-77716-5.
References
- 1.Kiernan MC, et al. Amyotrophic lateral sclerosis. Lancet (London, England) 2011;377:942–955. doi: 10.1016/S0140-6736(10)61156-7. [DOI] [PubMed] [Google Scholar]
- 2.Huai J, Zhang Z. Structural properties and interaction partners of familial ALS-associated SOD1 mutants. Front. Neurol. 2019;10:527. doi: 10.3389/fneur.2019.00527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Broom HR, Rumfeldt JAO, Meiering EM. Many roads lead to Rome? Multiple modes of Cu, Zn superoxide dismutase destabilization, misfolding and aggregation in amyotrophic lateral sclerosis. Essays Biochem. 2014;56:149–165. doi: 10.1042/bse0560149. [DOI] [PubMed] [Google Scholar]
- 4.Pansarasa O, et al. SOD1 in amyotrophic lateral sclerosis: ‘ambivalent’ behavior connected to the disease. Int. J. Mol. Sci. 2018;19:1345. doi: 10.3390/ijms19051345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sangwan S, Eisenberg DS. Perspective on SOD1 mediated toxicity in Amyotrophic Lateral Sclerosis. Postepy Biochem. 2016;62:362–369. [PubMed] [Google Scholar]
- 6.Rosen DR, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- 7.Deng HX, et al. Amyotrophic lateral sclerosis and structural defects in Cu, Zn superoxide dismutase. Science. 1993;261:1047–1051. doi: 10.1126/science.8351519. [DOI] [PubMed] [Google Scholar]
- 8.Reaume AG, et al. Motor neurons in Cu/Zn superoxide dismutase-deficient mice develop normally but exhibit enhanced cell death after axonal injury. Nat. Genet. 1996;13:43–47. doi: 10.1038/ng0596-43. [DOI] [PubMed] [Google Scholar]
- 9.Saccon RA, Bunton-Stasyshyn RKA, Fisher EMC, Fratta P. Is SOD1 loss of function involved in amyotrophic lateral sclerosis? Brain. 2013;136:2342–2358. doi: 10.1093/brain/awt097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lykke-Andersen S, Jensen TH. Nonsense-mediated mRNA decay: an intricate machinery that shapes transcriptomes. Nat. Rev. Mol. Cell Biol. 2015;16:665–677. doi: 10.1038/nrm4063. [DOI] [PubMed] [Google Scholar]
- 11.Popp MW, Maquat LE. Leveraging rules of nonsense-mediated mRNA decay for genome engineering and personalized medicine. Cell. 2016;165:1319–1322. doi: 10.1016/j.cell.2016.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindeboom RGH, Vermeulen M, Lehner B, Supek F. The impact of nonsense-mediated mRNA decay on genetic disease, gene editing and cancer immunotherapy. Nat. Genet. 2019;51:1645–1651. doi: 10.1038/s41588-019-0517-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim L, Lee X, Song J. Mechanism for transforming cytosolic SOD1 into integral membrane proteins of organelles by ALS-causing mutations. Biochim. Biophys. Acta - Biomembr. 2015;1848:1–7. doi: 10.1016/j.bbamem.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 14.Lim L, Song J. SALS-linked WT-SOD1 adopts a highly similar helical conformation as FALS-causing L126Z-SOD1 in a membrane environment. Biochim. Biophys. Acta - Biomembr. 2016;1858:2223–2230. doi: 10.1016/j.bbamem.2016.06.027. [DOI] [PubMed] [Google Scholar]
- 15.Corrado L, et al. SOD1 gene mutations in Italian patients with Sporadic Amyotrophic Lateral Sclerosis (ALS) Neuromuscul. Disord. 2006;16:800–804. doi: 10.1016/j.nmd.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 16.Siddique T, Deng HX. Genetics of amyotrophic lateral sclerosis. Hum. Mol. Genet. 1996;5:1465–1470. doi: 10.1093/hmg/5.Supplement_1.1465. [DOI] [PubMed] [Google Scholar]
- 17.Cerami E, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao J, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal complementary data sources and analysis options. Sci Signal. 2014;6:1–20. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valdmanis PN, et al. A mutation that creates a pseudoexon in SOD1 causes familial ALS. Ann. Hum. Genet. 2009;73:652–657. doi: 10.1111/j.1469-1809.2009.00546.x. [DOI] [PubMed] [Google Scholar]
- 20.Fujisawa T, et al. A novel monoclonal antibody reveals a conformational alteration shared by amyotrophic lateral sclerosis-linked SOD1 mutants. Ann. Neurol. 2012;72:739–749. doi: 10.1002/ana.23668. [DOI] [PubMed] [Google Scholar]
- 21.Li X, et al. A new function of copper zinc superoxide dismutase: as a regulatory DNA-binding protein in gene expression in response to intracellular hydrogen peroxide. Nucl. Acids Res. 2019;47:5074–5085. doi: 10.1093/nar/gkz256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bravo-Hernandez M, et al. Spinal subpial delivery of AAV9 enables widespread gene silencing and blocks motoneuron degeneration in ALS. Nat. Med. 2020;26:118–130. doi: 10.1038/s41591-019-0674-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Zundert B, Brown RH. Silencing strategies for therapy of SOD1-mediated ALS. Neurosci. Lett. 2017;636:32–39. doi: 10.1016/j.neulet.2016.07.059. [DOI] [PubMed] [Google Scholar]
- 24.Raoul C, et al. Lentiviral-mediated silencing of SOD1 through RNA interference retards disease onset and progression in a mouse model of ALS. Nat. Med. 2005;11:423–428. doi: 10.1038/nm1207. [DOI] [PubMed] [Google Scholar]
- 25.McCampbell A, et al. Antisense oligonucleotides extend survival and reverse decrement in muscle response in ALS models. J. Clin. Investig. 2018;128:3558–3567. doi: 10.1172/JCI99081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller TM, et al. An antisense oligonucleotide against SOD1 delivered intrathecally for patients with SOD1 familial amyotrophic lateral sclerosis: a phase 1, randomised, first-in-man study. Lancet Neurol. 2013;12:435–442. doi: 10.1016/S1474-4422(13)70061-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Rheenen W, et al. Project MinE: study design and pilot analyses of a large-scale whole-genome sequencing study in amyotrophic lateral sclerosis. Eur. J. Hum. Genet. 2018;26:1537–1546. doi: 10.1038/s41431-018-0177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andersen PM, et al. Phenotype in an infant with SOD1 homozygous truncating mutation. N. Engl. J. Med. 2019;381:486–488. doi: 10.1056/NEJMc1905039. [DOI] [PubMed] [Google Scholar]
- 29.Park JH, et al. SOD1 deficiency: a novel syndrome distinct from amyotrophic lateral sclerosis. Brain. 2019;142:2230–2237. doi: 10.1093/brain/awz182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gurney ME, et al. Motor neuron degeneration in mice that express a human Cu, Zn superoxide dismutase mutation. Science (80-.) 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- 31.Shaw MP, et al. Stable transgenic C9orf72 zebrafish model key aspects of the ALS/FTD phenotype and reveal novel pathological features. Acta Neuropathol. Commun. 2018;6:125. doi: 10.1186/s40478-018-0629-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walters R, Manion J, Neely GG. Dissecting motor neuron disease with Drosophila melanogaster. Front. Neurosci. 2019;13:331. doi: 10.3389/fnins.2019.00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fujimori K, et al. Modeling sporadic ALS in iPSC-derived motor neurons identifies a potential therapeutic agent. Nat. Med. 2018;24:1579–1589. doi: 10.1038/s41591-018-0140-5. [DOI] [PubMed] [Google Scholar]
- 34.Stenson PD, et al. The Human Gene Mutation Database: towards a comprehensive repository of inherited mutation data for medical research, genetic diagnosis and next-generation sequencing studies. Hum. Genet. 2017;136:665–677. doi: 10.1007/s00439-017-1779-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robinson JT, et al. Integrative genome viewer. Nat. Biotechnol. 2011;29:24–26. doi: 10.1038/nbt.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andersen PM, et al. Sixteen novel mutations in the Cu/Zn superoxide dismutase gene in amyotrophic lateral sclerosis: a decade of discoveries, defects and disputes. Amyotroph. Lateral Scler. Other Motor Neuron Disord. 2003;4:62–73. doi: 10.1080/14660820310011700. [DOI] [PubMed] [Google Scholar]
- 37.Wu J, Shen E, Shi D, Sun Z, Cai T. Identification of a novel Cys146X mutation of SOD1 in familial amyotrophic lateral sclerosis by whole-exome sequencing. Genet. Med. 2012;14:823–826. doi: 10.1038/gim.2012.50. [DOI] [PubMed] [Google Scholar]
- 38.Hu J, et al. A novel SOD1 mutation in amyotrophic lateral sclerosis with a distinct clinical phenotype. Amyotroph. Lateral Scler. 2012;13:149–154. doi: 10.3109/17482968.2011.621437. [DOI] [PubMed] [Google Scholar]
- 39.Shi S, Li L, Chen K, Liu X. Identification of the mutation of SOD1 gene in a familial amyotrophic lateral sclerosis. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2004;21:149–152. [PubMed] [Google Scholar]
- 40.Tripolszki K, et al. Genetic analysis of the SOD1 and C9ORF72 genes in Hungarian patients with amyotrophic lateral sclerosis. Neurobiol. Aging. 2017;53(195):e1–195.e5. doi: 10.1016/j.neurobiolaging.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 41.Canosa A, et al. A novel p.Ser108LeufsTer15 SOD1 mutation leading to the formation of a premature stop codon in an apparently sporadic ALS patient: insights into the underlying pathomechanisms. Neurobiol. Aging. 2018;72(189):e11–189. doi: 10.1016/j.neurobiolaging.2018.08.014. [DOI] [PubMed] [Google Scholar]
- 42.Jackson M, et al. Copper/zinc superoxide dismutase 1 and sporadic amyotrophic lateral sclerosis: analysis of 155 cases and identification of a novel insertion mutation. Ann. Neurol. 1997;42:803–807. doi: 10.1002/ana.410420518. [DOI] [PubMed] [Google Scholar]
- 43.Pramatarova A, et al. A two basepair deletion in the SOD 1 gene causes familial amyotrophic lateral sclerosis. Hum. Mol. Genet. 1994;3:2061–2062. [PubMed] [Google Scholar]
- 44.Watanabe Y, et al. Absence of the mutant SOD1 in familial amyotrophic lateral sclerosis (FALS) with two base pair deletion in the SOD1 gene. Acta Neurol. Scand. 1997;95:167–172. doi: 10.1111/j.1600-0404.1997.tb00090.x. [DOI] [PubMed] [Google Scholar]
- 45.Prudencio M, Hart PJ, Borchelt DR, Andersen PM. Variation in aggregation propensities among ALS-associated variants of SOD1: correlation to human disease. Hum. Mol. Genet. 2009;18:3217–3226. doi: 10.1093/hmg/ddp260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Andersen PM, et al. Phenotypic heterogeneity in motor neuron disease patients with CuZn-superoxide dismutase mutations in Scandinavia. Brain. 1997;120:1723–1737. doi: 10.1093/brain/120.10.1723. [DOI] [PubMed] [Google Scholar]
- 47.Jonsson PA, et al. Minute quantities of misfolded mutant superoxide dismutase-1 cause amyotrophic lateral sclerosis. Brain. 2004;127:73–88. doi: 10.1093/brain/awh005. [DOI] [PubMed] [Google Scholar]
- 48.Orrell RW, et al. Clinical and functional investigation of 10 missense mutations and a novel frameshift insertion mutation of the gene for copper-zinc superoxide dismutase in UK families with amyotrophic lateral sclerosis. Neurology. 1997;48:746–751. doi: 10.1212/WNL.48.3.746. [DOI] [PubMed] [Google Scholar]
- 49.Chen S, et al. A novel 10-base pair insertion mutation in exon 5 of the SOD1 gene in a Chinese family with amyotrophic lateral sclerosis. Neurobiol. Aging. 2016;45(212):e1–212.e4. doi: 10.1016/j.neurobiolaging.2016.04.021. [DOI] [PubMed] [Google Scholar]
- 50.Kawamata C, Morita M, Shibata N, Nakano I. Familial amyotrophic lateral sclerosis (FALS) with a novel SOD1 gene mutation: a clinicopathological study. Rinsho Shinkeigaku. 2007;47:211–216. [PubMed] [Google Scholar]
- 51.Brown JA, et al. SOD1, ANG, TARDBP and FUS mutations in amyotrophic lateral sclerosis: a United States clinical testing lab experience. Amyotroph. Lateral Scler. 2012;13:217–222. doi: 10.3109/17482968.2011.643899. [DOI] [PubMed] [Google Scholar]
- 52.Ghandi M, et al. Next-generation characterization of the cancer cell line encyclopedia. Nature. 2019;569:503–508. doi: 10.1038/s41586-019-1186-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.