Abstract
Biodegradable modified natural polymers have great potential in curbing the threat of plastic pollution, but are still uncompetitive to petrochemical-based plastic. In this study, starch was hydrophobized by treating starch-dimethyl sulfoxide solutions with soybean oil at high temperature in the presence of sodium carbonate, then spray-coated on paper. The modified starch was evaluated by Fourier-transform infrared spectroscopy analysis and contact angle value measurement of coated paper. FTIR analysis confirmed the substitution of hydroxyl groups with fatty acid ester and provided an estimate of the degree of substitution. The contact angle value of starch-coated paper surfaces was 121°, and was 111° after 10 min, demonstrating the high hydrophobicity and potential of the modified starch coating as a water-resistant treatment. The high hydrophobicity of the coated paper was due to formation of a textured surface with two levels of roughness, caused by the deposition of rough hydrophobic starch particles on paper fibers.
Keywords: Materials science, Materials chemistry, Starch soybean oil catalyzed transesterification hydrophobic coating cellulose surfaces
Materials Science; Materials Chemistry; starch soybean oil catalyzed transesterification hydrophobic coating cellulose surfaces.
1. Introduction
Non-degradable petrochemical-based plastic has been found to have profoundly negative impact upon the environment, mainly from their extremely long decomposition time and creation of microplastic particles as well as endangerment of marine wildlife (Cole et al., 2011; Perkins, 2015; UNEP, 2015). As a result, petrochemical-based plastics are facing phasing out and bans in many countries around the world (Xanthos and Walker, 2017), with the most viable replacement being bioplastics. Although, bioplastic is yet to be competitive, especially in developing countries where the increased cost of bioplastic can be prohibitive (Kolybaba et al., 2003). As such, modified biomaterials derived from natural biopolymers such as cellulose, starch, and chitin are better plastic alternatives. These polymers are very abundant, driving down the cost of the final product. Yet, the hydrophilic nature and generally inferior mechanical properties of these polymers necessitate modification to grant them the advantages of plastic: imperviousness to water, durability, and malleability. Cheap and sustainable modification processes are also needed for these materials to be competitive against traditional plastic.
Starch, along with cellulose, are the most abundant biomolecules on earth as a result of botanical biosynthesis (Jane, 1995). Starch can dissolve in hot water, aiding the extraction, molding and extrusion processes. It is quickly biodegraded by microorganisms present in the environment. A drawback is the highly hydrophilic nature of native starch due to its hydroxyl-rich structure (BeMiller and Whistler, 2009) meaning starch-based materials and objects quickly lose their integrity and durability when exposed to water. Potato starch is a suitable candidate, with lower gelatinization temperature and high amylose content facilitating modification procedures (Semejin and Buwalda, 2017).
To make starch hydrophobic, the hydroxyl functional groups of starch are replaced with large, bulky groups without ability to form hydrogen bonds; reducing the starch molecule's ability to form hydrogen bonds with water (Masina et al., 2017). This is done by esterification, where the bulky functional group is a fatty acid ester from a fatty acid derivative such as anhydrides (Diop et al., 2011), chlorides (Aburto et al., 1999), imidazolides (Neumann et al., 2002) or esters (Junistia et al., 2008; Rooney, 1976). Conventionally, the chemical modification of starch is done by reacting the starch and/or the fatty acid derivative in an appropriate solvent at high temperature in the presence of a basic or acidic catalyst. Naturally, the cost, toxicity and handling of reagents are the immediate drawbacks. Firstly, with exception of certain esters and anhydrides, fatty acid derivatives are often too expensive for practical use. Secondly, complex recovery and purification schemes for the product is required, especially if the application is related to food or pharmaceuticals. Finally, strong basic or acidic catalysts can be difficult to handle in large amounts. To address these issues, investigating the use of safer, cheaper alternatives to commonly employed reagents should be conducted.
A commonly used solvent in studies regarding starch is DMSO as it is relatively safe and nonreactive, with a high boiling point. It can dissolve starch well, exposing the anhydroglucose subunits to reactants (Soyler and Meier, 2016). While more efficient esters (vinyl esters (Junistia et al., 2008), methyl esters (Muljana et al., 2010)) have been successfully used, these are often costly to purchase and tedious to produce. Triglycerides from soybean oil is a possible candidate, since it is composed of largely C18 fatty acids (Ulmasov et al., 2012) which grants better hydrophobicity than shorter fatty acids (Winkler et al., 2014) and is stable at high temperatures; as well as being widely available. Mild carbonate salts of sodium or potassium were shown to be comparable to stronger bases such as methoxides (Rooney, 1976), without their drawbacks. This paper investigates the feasibility of sustainable and green hydrophobic modification of starch using soybean oil. In this study, the effects of temperature, carbonate concentration, reaction time and starch concentration on the hydrophobicity of modified starch were examined. The degree of modification as well as its further details were evaluated by FTIR spectroscopy and staining optical microscopy while the hydrophobic properties were determined by water contact angle measurement.
2. Materials and methods
2.1. Materials
Potato starch (approximately 20% amylose and 80% amylopectin) was purchased from Sigma (Sigma Aldrich) and dried at 70 °C for 6 h to minimize its water content. Analytical grade Na2CO3, DMSO, KBr, ethanol and hexane were purchased from Sigma Aldrich and used without further purification. Pelure paper of 50 μm thickness (Taiwan) was obtained from a stationary store. Soybean oil (CALOFIC, Vietnam, purity >99%) was used in this study.
2.2. Methods
2.2.1. Starch modification
Potato starch was mixed with 10 mL of DMSO in a 20-mL glass vial and heated to 75 °C under stirring for 2 h to form a clear, homogenous solution. 9.33 g (10.74 mmol) of soybean oil was added to the starch solution and the mixture was heated to reaction temperature under vigorous stirring in an oil bath. Then the sodium carbonate was added and the reaction mixture was kept heated under stirring for a period of time. Afterwards, the mixture was left to cool to room temperature at the end of the chosen reaction time, then mixed drop-wise with stirring with 50 mL of ethanol to precipitate the crude modified starch as an oily brownish paste. This crude starch was filtered, then washed with 6 × 20 mL portions of ethanol and dried at 70 °C for 2 h. The dried modified starch was then ground and washed thoroughly with 3 × 5 mL portions of hexane, resulting in a final yellowish-brown starch powder. Finally, the modified starch powder was dried at 70 °C for 2 h, then stored in a desiccator until further tests.
2.2.2. FTIR analysis
Fourier transform infrared spectroscopy (FTIR) was used to identify the characteristic functional groups present in the modified starch as well as to provide an estimate of degree of substitution (Machell & Richards) by the ratio of the area of peaks at around 1020 cm−1 (C-O-C vibration of glucose units) and 1730-1750 cm−1 (C=O vibration of ester group) in the modified starch spectrum. 5 mg of a starch sample was mixed with 195 mg of dry KBr and pressed to form a pellet before measurement by a Fourier transform infrared spectrometer (Agilent Cary 660 FT-IR Spectrophotometer, Agilent, USA). The samples were scanned from 4000 to 800 cm−1, averaged over 64 scans with a resolution of 4 cm−1. Spectra were analyzed and processed with OriginPro version 8.5 (OriginLab, USA) and Microsoft Excel.
2.2.3. Starch coating of paper surface and contact angle measurement
A 5% (w/w) solution of modified starch in DMSO was prepared by heating 2 mL of DMSO with 0.12 g of modified starch at 75 °C for 1 h. This mixture was sprayed on dry, weighed sheets of pelure paper (2.5 × 7.5 cm) stabilized on clean glass slides at a distance of 10 cm using a pump sprayer. The coated paper sheet was dried at 70 °C for 2 h and weighed again at which point the weight of the paper sheets remain constant. The spraying was repeated until around 7 mg of starch was deposited on the paper sheet. Following that, a drop of water (20 μL) was dropped on it and the contact angle value of the water drop is then determined every minute for 10 min. The contact angle value of a particular treatment was the averaged value of three spots on the coated paper sheet.
2.2.4. Microscopic examination of modified starch and coated paper
The starch product and coated paper surfaces are examined with an optical microscope (Olympus) under 40× and 100× magnification (Olympus objectives) and compared to unmodified samples of starch and paper. Samples were dyed with Lugol's solution (5% (w/v) iodine, 10% (w/v) potassium iodide) to differentiate the structural features of starch grains and paper surfaces. The texture analysis was then performed using the Matlab (R2013A, The MathWorks Inc.) based texture analysis package.
3. Results and discussion
3.1. Exploratory experiments
This modification procedure is adapted from the two-step reaction procedure employed by several authors (Junistia et al., 2008; Mormann and Mohamed, 2004; Winkler et al., 2013), which first involves the dissolution of starch in DMSO to enable homogenous reaction conditions, which facilitates reactant contact with anhydroglucose units (AGUs), and subsequent reaction with the acylating agent, initiated by the addition of sodium carbonate. The exact reaction mechanism was not studied in depth, but likely to be similar to the transesterification of alcohols with triglycerides to form fatty acid esters, in which the alcohol is converted to an alkoxide by the basic catalyst and this alkoxide attacks the carbonyl group of the triglyceride, resulting in an alkyl ester and an anion of the diglyceride. The anion reacts with the protonated catalyst, regenerating it for further reactions (Schuchardt et al., 1998).
It was found that (data not shown) within 10 min upon the addition of sodium carbonate, the mixture quickly turned dark brown and remained so until the end of the reaction; perhaps due to the degradation of starch, accelerated by the basicity and surface area the sodium carbonate provided. In control trials without sodium carbonate, the starch solution only turned light yellow due to dextrinization at high temperatures (Guaras et al., 2017). Some of this coloration could also be contributed by the modified starch, as modified starch remains yellow-brown after washing with ethanol and hexane. Sodium carbonate was not recoverable during purification, possibly consumed by secondary reactions with triglycerides or with degraded starch products. The modified starch was not soluble in THF and chloroform, only in DMSO, unlike reported works (Soyler and Meier, 2016; Junistia et al., 2008) suggesting a lower degree of substitution.
A fixed, excess amount of oil (10.74 mmol, approximately 9.33 g) in relation to starch was provided for all trials, since previous studies suggested an excess of acylating agent to enhance reactivity (Junistia et al., 2008; Mormann and Mohamed, 2004). The molecular weight of the soybean oil was estimated using the fatty acid composition of soybean oil (Ulmasov et al., 2012) to be 868.81 g/mol. The tested reaction parameters were: reaction time, catalyst concentration, reaction temperature and initial starch concentration, since these were the most influential factors to starch substitution reactions (Ackar et al., 2015). These were investigated sequentially to find the optimal reaction conditions. The effectiveness of the reactions was determined by FTIR analysis and contact angle measurement of paper surfaces coated with modified starch. All trials were triplicated.
3.2. Characterization of modified starch
3.2.1. FTIR analysis
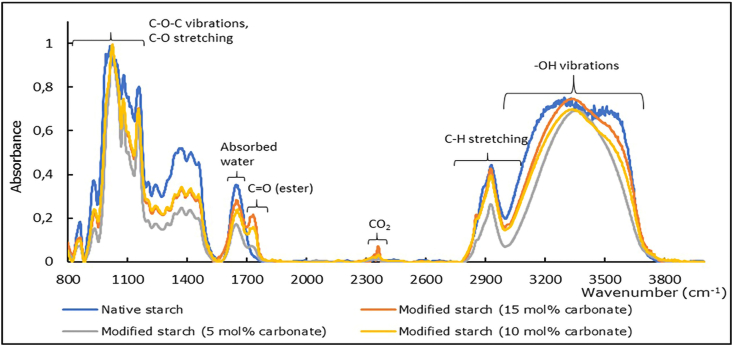
Most characteristics peaks of native starch were retained: at the 900-1200 cm−1 region, notably a strong peak at 1020 cm−1 (C-O-C vibrations, C-O stretching (Park et al., 2000; Winkler et al., 2013); at 1650 cm−1 (O-H bending of absorbed water (Park et al., 2000); at 3000-3700 cm−1 (hydroxyl vibrations (Mano et al., 2003). The peak at 1730-1750 cm−1 (C=O vibrations) (Junistia et al., 2008; Xu et al., 2004), and sharper peaks at 2920 cm−1 and 2850 cm−1 (C-H stretching) verified that long fatty acid ester groups are present in the modified starches. The reduced noise of the 3000-3700 cm−1 region alludes to the replacement of hydroxyl groups as well as the reduced level of absorbed water (Figure 1).
Figure 1.
FTIR spectra of native starch and modified starches.
The DS of the modified starches was estimated using the method of calculating peak ratio based on the work of Winkler and coworkers (Winkler et al., 2013) was used, where the area of the 1730-1750 cm−1 region (C=O vibrations of ester groups) was divided to the area of the 1020 cm−1 peak (C-O-C vibrations of AGUs). The more ester groups are present in the starch, the larger the ester peak area becomes and a higher peak area ratio is obtained. To compare among different samples, the spectra of all modified starches are normalized to a scale of 0–1 before calculating the ratio.
3.2.2. Contact angle measurement of paper coated with modified starch
Previous studies evalulated the hydrophobicity of modified starch by preparing films of modified starch for contact angle measurement. However, preliminary tests had revealed that the modified starches prepared in this study were too brittle to form a useable film. Instead, modified starch was coated on a paper surface similar to a process outlined by Ogihara and colleagues (Ogihara et al., 2012). Very thin paper sheets were used as coating substrate due to their low content of fillers, sizers, dyes; and as a result, is a close approximate to a surface of pure cellulose fibers and allow enough light to pass through for microscopic examination.
As a control, uncoated paper, paper coated with native starch and soybean oil were tested. The modified starch in this test was made at 150 °C for 6 h, with a carbonate content of 15 mol% and starch concentration of 5%.
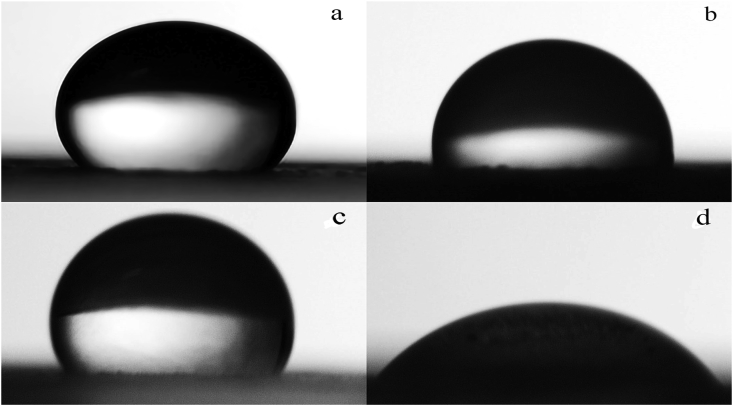
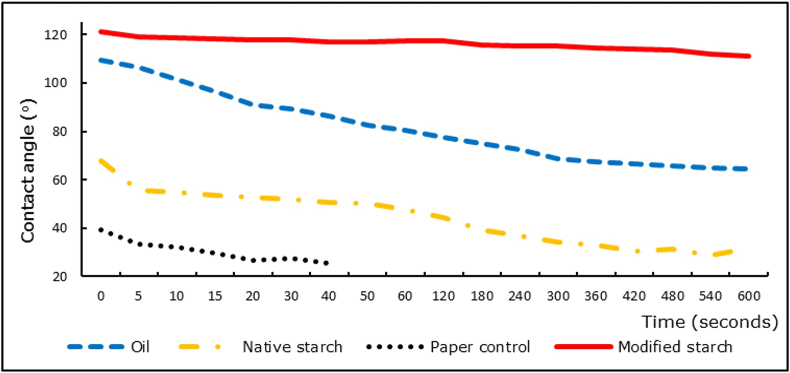
Modified starch treatment was able to make paper much more hydrophobic and less water permeable than control treatments (Figures 2 and 3). While water absorption was rapid for these control samples, the modified starch coating was able to raise the contact angle value of the paper surface to a maximum of 121°. The substantial increase in hydrophobicity is likely due to a combination of less permeable modified starch grains covering more hydrophilic cellulose fibers; as well as the rougher surface of the starch grains and paper fibers trapping air, which acts as a water resisting barrier.
Figure 2.
(a), (b) Contact angle images of modified starch (15% carbonate, 150 °C, 5% starch conc., 6 h) and native starch coated paper, respectively. (c), (d) Contact angle images of soybean oil coated paper and uncoated paper, respectively.
Figure 3.
Contact angle of paper coated with soybean oil, native starch and modified starch over 10 min. Uncoated paper served as control.
3.3. Effect of reaction parameters on characteristics of modified starch
3.3.1. Sodium carbonate catalyst concentration
Generally, reactivity increases as more sodium carbonate is introduced to the reaction mixture. This is likely caused by the secondary reactions of sodium carbonate with soybean oil, free fatty acids as well as starch and compounds created by the degradation of starch. More sodium carbonate may also provide more surface area for reactions to occur on, leading to increased DS. However, a sharp rise in DS can be observed at 15 mol%. Further increasing carbonate level to 20 mol%, interestingly, led to a decrease in DS. It can be assumed that carbonate level at or around 15 mol% is high enough to overcome consumption from secondary reactions, allowing it to approximate the performance of a catalyst.
The decreased DS at 20 mol% carbonate could be due to reaction conditions leading to starch degradation. Specifically, this involves the depolymerization of starch into oligosaccharides or monosaccharides, which can undergo further reactions to form ethanol- or fat-soluble byproducts, leading to lower starch yield after ethanol precipitation and washing. The harsh reaction conditions applied are likely responsible: high temperatures (Han and Lim, 2004; Wurzburg, 1986) leading to the dextrinization of starch; and higher concentration of the carbonate catalyst (Machell and Richards, 1958). These conditions lead to the formation of branched dextrin out of both amylose and amylopectin (Guaras et al., 2017), converting amylose to the less reactive dextrin, and thus reduces the DS of the modified starch. The dextrin structure also lacks the strong hydrogen bonding ability of amylose and amylopectin, weakening intermolecular association of the modified starch chains and eases solvation by water.
The contribution of temperature and carbonate level to starch degradation can be estimated by weighing the modified starch and comparing it against the starting amount of starch (0.5800 g) (Table 1).
Table 1.
Peak area ratio (ester/C-O-C), contact angle and yield of modified starches made with various sodium carbonate levels.
| Sodium carbonate content (mol% of starch) | Peak area ratio (ester/C-O-C) | Maximum contact angle (degrees) | Contact angle after 10 min (degrees) | % of starting material (0.5800 g) |
|---|---|---|---|---|
| 0% | - | 95.69 (±3.96) | 64.46 (±6.78) | 63.22% (±3.57%) |
| 5% | 0.05 (±0.02) | 112.63 (±0.64) | 93.95 (±6.18) | 57.78% (±4.09%) |
| 10% | 0.07 (±0.01) | 115.66 (±1.16) | 98.12 (±2.75) | 46.79% (±5.49%) |
| 15% | 0.26 (±0.06) | 121.07 (±1.07) | 110.92 (±0.98) | 41.12% (±8.76%) |
| 20% | 0.20 (±0.01) | 114.63 (±4.76) | 99.02 (±11.87) | 36.16% (±8.97%) |
Thermal damage is represented by the loss exhibited in the control sample, which was not introduced to sodium carbonate. The percent of loss shows thermal damage is likely to be the most damaging factor, while the effect of sodium carbonate is comparatively lesser. The combination of high temperature and large amount of carbonate appears to be especially damaging to the starch molecular structure. This loss might be the reason for the inferior hydrophobicity of the 20 mol% carbonate treatment, as the damage might had spread to the modified regions as well, reducing the DS of the modified starch.
The contact angle and water resistance of the coated paper are generally correlated with the FTIR ester/C-O-C peak area ratio, as progressive substitution of hydroxyl groups with fatty ester groups reduces the starch's ability to create hydrogen bonds with water and lowers hydrophilicity. Starch modified with 15 mol% carbonate was the most hydrophobic with a contact angle maximum of 121.07°. Interestingly, all starches exhibited a sharp drop in contact angle during the first minute, possibly due to water rapidly infiltrating more hydrophilic regions of the coated surface before stabilizing afterwards, slowing down the absorption rate. The lower maximum contact angle of the 20 mol% treatment could be the result of more severe starch degradation mentioned above, which reduces the fraction of fatty acid starch esters in the modified starch and make water absorption easier.
The contact angle measured on coated paper was substantially higher than previously reported on starch films (Muljana et al., 2010; Winkler et al., 2014), which is around 90–110° depending on the DS of the modified starch, despite lower estimated DS. It is reasonable to assume that the difference in hydrophobicity is more significantly contributed by changes in surface morphology, rather than the hydrophobic modified starch; not unlike how textured surfaces made from not highly hydrophobic materials can obtain superhydrophobicity (Li et al., 2007). This is likely caused by the formation of starch particles, and to a certain extent, filling of fiber surface defect and void among fibers, as the solvent evaporates. The increased surface roughness provided by their presence can trap air, which acts as a water barrier.
The trend of increasing contact angle with increasing DS could be due to two effects. Firstly, the reduced hydrogen bonding ability of the modified starch as hydroxyl groups are replaced with fatty acyl groups. Secondly, the more amorphous nature of modified starch caused by disruption of inter- and intramolecular hydrogen bonding network responsible for maintaining a crystalline structure in native starch, permitting a more textured surface trapping air that shields more hydrophilic regions of starch particles from water penetration. These effects are stronger as more substitution occurs, making high-DS modified starch more hydrophobic than its lower-DS starch counterpart.
3.3.2. Reaction temperature
Unlike the effect of carbonate concentration, the DS of modified starch seems to respond linearly to reaction temperature (Table 2). Increases in temperature steadily increases DS as the peak area ratio raises by roughly 0.04 every 10 °C. The linear response may be a result of escalating unraveling of the starch molecular structure at progressively higher temperatures, as either inter- and intramolecular hydrogen bond disintegration or dextrinization, exposing more AGUs to reactants. High temperatures also cause the soybean oil to be more fluidic and soluble in DMSO, increasing its availability in the DMSO phase and improve reactivity. Higher reaction temperature will also improve the collision rate of the reaction species which is linearly correlated with the faster mass transfer rate of the reactant molecules; and ultimately allow the reaction to overcome its activation energy barrier.
Table 2.
Peak area ratio (ester/C-O-C), contact angle and yield of modified starches made at various reaction temperatures.
| Reaction temperature (°C) | Peak area ratio (ester/C-O-C) | Maximum contact angle (degrees) | Contact angle after 10 min (degrees) | % of starting material (0.5800 g) |
|---|---|---|---|---|
| 110 | 0.01 (±0.001) | 105.31 (±1.31) | 77.53 (±0.24) | 73.19% (±1.58%) |
| 120 | 0.10 (±0.01) | 112.67 (±3.26) | 99.54 (±3.79) | 55.07% (±2.35%) |
| 130 | 0.15 (±0.04) | 115.24 (±1.34) | 104.60 (±2.82) | 51.48% (±4.02%) |
| 140 | 0.23 (±0.07) | 116.52 (±0.23) | 106.38 (±0.67) | 46.05% (±2.66%) |
| 150 | 0.26 (±0.06) | 121.07 (±1.07) | 110.92 (±0.98) | 41.12% (±8.76%) |
Overall, the contact angle value of modified starch increases with reaction temperature as suggested by its correlation with the ester/C-O-C peak area ratio. In contrast, starch degradation is consistently more severe with increasing temperature. Thus, improvements in hydrophobicity might be more limited at higher temperatures.
3.3.3. Reaction time
Increasing reaction time lead to a larger DS in the modified starch and greater ester/C-O-C peak area ratio (Table 3). The gradual increase in DS during the first 4 h may be caused by progressive changes in modified starch structure as well as carbonate content during the process. The addition of large, bulky fatty acid groups changes the conformation of modified starch chains, easing access to AGUs. The disintegration of the starch chain similarly accelerates the reaction by reducing steric hindrance. After 6 h, the numerous fatty ester groups now block unsubstituted glucose residues from interacting with triglycerides. The depletion of the sodium carbonate by secondary reactions and prolonged exposure to high temperature could be another factor as detailed above, causing a lower yield and lower DS by damaging the starch chains.
Table 3.
Peak area ratio (ester/C-O-C), contact angle and yield of modified starches made at various reaction times.
| Reaction time (hours) | Peak area ratio (ester/C-O-C) | Maximum contact angle (degrees) | Contact angle after 10 min (degrees) | % of starting material (0.5800 g) |
|---|---|---|---|---|
| 2 | 0.11 (±0.02) | 114.10 (±1.35) | 105.07 (±2.13) | 52.66% (±7.27%) |
| 4 | 0.12 (±0.01) | 115.49 (±1.48) | 106.19 (±1.52) | 48.86% (±3.60%) |
| 6 | 0.26 (±0.06) | 121.07 (±1.07) | 110.92 (±0.98) | 41.12% (±8.76%) |
| 8 | 0.23 (±0.04) | 117.49 (±2.54) | 107.48 (±2.61) | 34.24% (±4.38%) |
Shorter exposure to high temperature could have led to a more intact starch structure that still support a significant inter- and intramolecular hydrogen bond network. Upon precipitation, the hydrophilic regions may form a semi-crystalline structure, orienting the hydrophobic substituted residues outwards. This would leave the surface less irregular than more substituted and amorphous modified starches, thus lowers its maximum contact angle value, but the hydrophobic surface as well as high crystallinity would slow water absorption better than more the substituted starches do.
3.3.4. Initial starch concentration
The change in ester/C-O-C peak area ratio (Table 4) reveals a sharp decrease in reactivity as the starch concentration increases, peaking at 2.5% starch concentration and rapidly decreases with higher starch concentrations. This is in agreement with the results Mormann and coworkers (Mormann and Mohamed, 2004) obtained when starch was reacted with vinyl acetate in DMSO. The underlying cause is that soybean oil is only partially miscible with DMSO (Homrich et al., 2017). Furthermore, at lower starting starch concentrations, the anhydroglucose: triglycerides molar ratio increases; leading to higher reaction rates.
Table 4.
Peak area ratio (ester/C-O-C), contact angle and yield of modified starches made with different starting starch concentrations.
| Starting starch concentration (w/w) | Peak area ratio (ester/C-O-C) | Maximum contact angle (degrees) | Contact angle after 10 min (degrees) | % of starting material |
|---|---|---|---|---|
| 2.5% | 0.48 (±0.03) | 114.64 (±1.70) | 103.56 (±2.98) | 27.20% (±28.68%) (0.2820 g) |
| 5% | 0.29 (±0.02) | 121.07 (±1.07) | 110.92 (±0.98) | 41.12% (±8.76%) (0.5800 g) |
| 7.5% | 0.09 (±0.01) | 113.64 (±1.54) | 102.31 (±2.20) | 34.65% (±5.86%) (0.8920 g) |
| 10% | 0.08 (±0.02) | 112.12 (±1.09) | 90.72 (±1.10) | 44.02% (±1.04%) (1.2230 g) |
Evidently, stronger starch degradation is present, possibly due to the lower starch concentration allowing the starch chains greater degrees of freedom, which might result in increased chain strain; especially in heavily branched amylopectin molecules. Such strain could lead to increased chain breaking. These conditions also aid the substitution reaction by permitting easier access to glucose residues and sodium carbonate, resulting in elevated substitution and peak area ratio.
3.4. Morphology of modified starch, modified starch coated paper and its effect on hydrophobicity and water absorption of modified starch coated paper
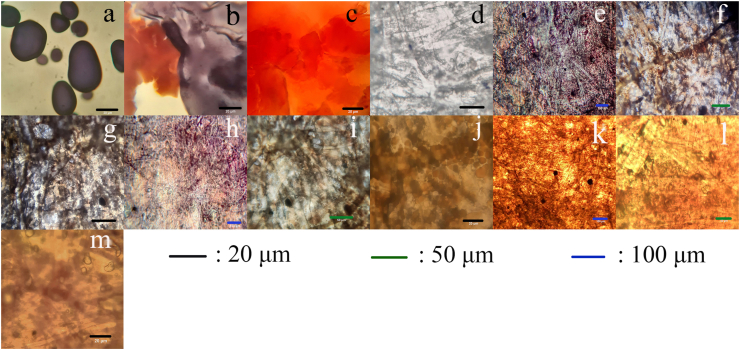
Modified starch is more amorphous in appearance than native starch, with average particle size around 20 μm (Figure 4c), forms large aggregates and retains its original orange-brown color. The lack of staining of modified starch could be caused by changes to starch structure by the modification process, with slower iodine uptake due to increased hydrophobicity. Starch heated to 150 °C without sodium carbonate (control starch) similarly form large amorphous aggregates, with separate blue/orange regions (Figure 4b). This is likely due to the separation of amylose and amylopectin during starch dissolution (Guaras et al., 2017). The different rates of precipitation of these polymers resulted in the formation of aggregates rich in either polymers.
Figure 4.
Optical microscope images of native starch (a), starch heated to 150 °C without carbonate (control starch) (b), modified starch (c) at 100× magnification. (d): Uncoated paper at 100×. (e, (f), (g): Paper coated with native starch at 10×, 40×, 100×, respectively. (h), (i), (j): Paper coated with control starch at 10×, 40×, 100×, respectively. (k), (l), (m): Paper coated with modified starch at 10×, 40× and 100×, respectively.
As a result of rapid evaporation of dilute solutions during coating, coated starch particles became smaller in size (Figure 4, d-m). Generally, coated modified starch particles remains similar to the raw modified starch (Figure 4, k-m). In contrast, coating with native starch and control starch resulted in larger particles, around 15 μm or more in size (Figure 4, e-j). The coated paper also stained dark purple (Figure 4 (e), (h)), chiefly in paper fibers, suggesting absorption of amylose into fibers. Ovular, somewhat smooth and orange-red particles, likely amylopectin-rich were present as well. This separation appeared to be caused by differences in solubility of the polymers. The more soluble amylose fraction remained in the solution and was taken up by paper fibers; while the less soluble amylopectin precipitated first, and thus was located on the surface of fibers. In all cases, starch particles were deposited on the paper fibers, giving it a coarse texture with little uniformity. It is possible that the defects present on the fibers provided nucleation sites for the starch particles to grow upon, partly influencing the particles’ distribution and the roughness of the coated paper.
The increased hydrophobicity of modified starch-coated paper is due to surface featuring two levels of roughness: the roughness of starch particle surface and the roughness caused by random distribution of starch particles. This structure provides low surface free energy and high surface roughness, which are essential for superhydrophobicity (Guo et al., 2011). The most commonly accepted models for understanding how water interacts with rough surfaces are: the Wenzel state (Wenzel, 1936); and the Cassie-Baxter (CB) state (Cassie and Baxter, 1944). These states can coexist and transition between each other, though only the CB to Wenzel transition is well-documented (Murakami et al., 2014; Parvate et al., 2020). When water first come into contact with the surface, the CB state could be dominant because of the air pockets trapped between water and the surface features, causing the high initial contact angle value. However, due to higher interfacial energy (solid/liquid + liquid/vapor), this state is less stable than the Wenzel state (solid/liquid) (Murakami et al., 2014). Thus, in combination with the large space among particles and external factors, such as evaporation and sagging due to the drop's weight, as well as the large space among asperities on this surface from random distribution of starch particles of various size and shapes, the transition from CB to Wenzel occurs very rapidly, resulting in a sharp decrease in contact angle after the first 60 s. Indeed, the texture analysis re-affirmed the highest level of roughness when the paper surface was coated with the modified starch. The entropy of the images e, h and k were calculated to be 1.63, 1.66 and 2.19 for the paper surfaces coated with native starch, control starch (non-catalyzed) and modified starch, respectively. It is worth mentioning that the entropy of the image is typically directly proportional to the roughness (number of features and chromatic depth) of the texture.
4. Conclusion
In this study, starch was hydrophobized by reacting starch with soybean oil in DMSO in the presence of sodium carbonate. FTIR analysis and contact angle measurement of paper coated with modified starch was used to investigate optimal parameters for this process. The optimal conditions for this process was found to be: a temperature of 150 °C, 6 h of reaction time, 15 mol% sodium carbonate and 5% (w/w) starch concentration. The hydrophobicity of modified starch was found to be increasing with the degree of substitution as well as changes in starch grain morphology. The presence of the fatty acid chains disrupted the hydrogen bonding among starch chains, increasing surface roughness of starch particles, as well as reduced water bonding. When coated on paper, the modified starch particles induced a textured surface with two-level roughness, leading to high hydrophobicity and prolonged water resistance, with a contact angle as high as 121°, versus a contact angle of 39° for uncoated paper and remains hydrophobic with a contact angle of 111° after 10 min of water exposure. These results demonstrated the potential of this modified starch as a sustainable hydrophobic coating for green and biodegradable materials. Further studies on the improvement of the coating and catalyzed modification procedures will lead to a wider range of practical applications for cellulose based materials.
Declarations
Author contribution statement
Phat Thinh Le: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Khoi Tan Nguyen: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by Vietnam National Foundation for Science and Technology Development (NAFOSTED) (106.02.2018.315).
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Aburto J., Alric I., Thiebaud S., Borredon E., Bikiaris D., Prinos J., Panayiotou C. Synthesis, characterization, and biodegradability of FattyAcid esters of amylose and starch. J. Appl. Polym. Sci. 1999;74:1440–1451. [Google Scholar]
- Ackar D., Babic J., Jozinovic A., Milivecic B., Jokic S. Starch modification by organic acids and their derivatives: a review. Molecules. 2015;20:19554–19570. doi: 10.3390/molecules201019554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BeMiller J., Whistler R. 2009. Starch: Chemistry and Technology. [Google Scholar]
- Cassie A.B.D., Baxter S. Wettability of porous surfaces. Trans. Faraday Soc. 1944;40:546–551. [Google Scholar]
- Cole M., Lindeque P., Halsband C., Galloway T.S. Microplastics as contaminants in the marine environment: a review. Mar. Pollut. Bull. 2011;62(12):2588–2597. doi: 10.1016/j.marpolbul.2011.09.025. [DOI] [PubMed] [Google Scholar]
- Diop C.I.K., Li H.L., Xie B.J., Shi J. Effects of acetic acid/acetic anhydride ratios on the properties of corn starch acetates. Food Chem. 2011;126:1662–1669. doi: 10.1016/j.foodchem.2010.12.050. [DOI] [PubMed] [Google Scholar]
- Guaras M.P., Luduena L.N., Alvarez V.A. Starch-Based Materials in Food Packaging. 2017. Development of biodegradable products from modified starches; pp. 77–124. [Google Scholar]
- Guo Z., Liu W., Su L. Superhydrophobic surfaces: from natural to biomimetic to functional. J. Colloid Interface Sci. 2011;353:335–355. doi: 10.1016/j.jcis.2010.08.047. [DOI] [PubMed] [Google Scholar]
- Han J.A., Lim S.T. Structural changes of corn starch by heating and stirring in DMSo measured by SEC-MALL-RI system. Carbohydr. Polym. 2004;55 [Google Scholar]
- Homrich P.O.B., Mariutti L.R.B., Bragagnolo N., Ceriani R. Solubility behavior of mixtures containing refined soybean oil and low-toxic solvents at different temperatures. Fluid Phase Equil. 2017;442:87–95. [Google Scholar]
- Jane J. Starch properties, modifications, and applications. J. Macromol. Sci. Pure Appl. Chem. 1995;32(4):751–757. [Google Scholar]
- Junistia L., Sugih A.K., Manurung R., Picchioni F., Janssen L.P.B., Heeres H.J. Synthesis of higher fatty acid starch esters using vinyl laurate and stearate as reactants. Starch Staerke. 2008;60:667–675. [Google Scholar]
- Kolybaba M., Tabil L.G., Pahigrani S., Crera W.J., Powell T., Wang B. 2003 CSAE/ASAE Annual Intersectional Meeting. 2003. Biodegradable polymers: past, present, and future. [Google Scholar]
- Li X., Reinhoudt D., Crego-Calama M. What do we need for a superhydrophobic surface? A review on the recent progress in the preparation of superhydrophobic surfaces. Chem. Soc. Rev. 2007;36:1350–1368. doi: 10.1039/b602486f. [DOI] [PubMed] [Google Scholar]
- Machell G., Richards G.N. The alkaline degradation of polysaccharides. Part III. Action of sodium hydroxide on amylose. J. Chem. Soc. 1958 [Google Scholar]
- Mano J.F., Koniarova D., Reis R.L. Thermal properties of thermoplastic starch/synthetic polymer blends with potential biomedical applicability. J. Mater. Sci. Mater. Med. 2003;14 doi: 10.1023/a:1022015712170. [DOI] [PubMed] [Google Scholar]
- Masina Y., Choonara Y.E., Kumar P., Du Toit L.C., Govender M., Indermun S., Pillay V. A review of the chemical modification techniques of starch. Carbohydr. Polym. 2017;157:1226–1236. doi: 10.1016/j.carbpol.2016.09.094. [DOI] [PubMed] [Google Scholar]
- Mormann W., Mohamed A. Acylation of starch with vinyl acetate in water. Starch Staerke. 2004;56:118–121. [Google Scholar]
- Muljana H., van der Knoop S., Keijzer D., Picchioni F., Janssen L.P.B.M., Heeres H.J. Synthesis of fatty acid starch esters in supercritical carbon dioxide. Carbohydr. Polym. 2010;82:346–354. [Google Scholar]
- Murakami D., Jinnai H., Takahara A. Wetting transition from the Cassie−Baxter state to the Wenzel state on textured polymer surfaces. Langmuir. 2014;30:2061–2067. doi: 10.1021/la4049067. [DOI] [PubMed] [Google Scholar]
- Neumann U., Wiege B., Warwel S. Synthesis of hydrophobic starch esters by reaction of starch with various carboxylic acid imidazolides. Starch Staerke. 2002;54:449–453. [Google Scholar]
- Ogihara H., Xie J., Okagaki J., Saji T. Simple method for preparing superhydrophobic paper: spray-deposited hydrophobic silica nanoparticle coatings exhibit high water-repellency and transparency. Langmuir. 2012;28:4605–4608. doi: 10.1021/la204492q. [DOI] [PubMed] [Google Scholar]
- Park J.W., Im S.S., Kim S.H., Kim Y.H. Biodegradable polymer blends of poly( L-lactic acid) and gelatinized starch. Polym. Eng. Sci. 2000;40(12) [Google Scholar]
- Parvate S., Dixit P., Chattopadhyay S. Superhydrophobic surfaces: insights from theory and experiment. J. Phys. Chem. B. 2020;124:1323–1360. doi: 10.1021/acs.jpcb.9b08567. [DOI] [PubMed] [Google Scholar]
- Perkins S. 2015. Nearly Every Seabird May Be Eating Plastic by 2050. [Google Scholar]
- Rooney M.L. Interesterification of starch with methyl palmitate. Polymer. 1976;17:555–558. [Google Scholar]
- Schuchardt U., Sercheli R., Vargas R.M. Transesterification of vegetable oils: a review. J. Braz. Chem. Soc. 1998;9(3):199–210. [Google Scholar]
- Semejin C., Buwalda P.L. Potato starch. In: Sjoo M., Nilsson L., editors. Starch in Food: Structure, Function and Applications. 2 ed. Woodhead Publishing, Elsevier; 2017. [Google Scholar]
- Soyler Z., Meier M.A.R. Catalytic transesterification of starch with plant oils: a sustainable and efficient route to fatty acid starch esters. ChemSusChem. 2016;9:1–8. doi: 10.1002/cssc.201601215. [DOI] [PubMed] [Google Scholar]
- Ulmasov T., Voelker T., Wilkes R., Cornelius J. Designing Soybeans for 21st Century Markets. 2012. High-oleic, low-saturate soybeans offer a sustainable and nutritionally enhanced solution for food applications requiring high oil stability; pp. 277–295. [Google Scholar]
- UNEP . 2015. Plastic in Cosmetics: Are We Polluting the Environment through Out Personal Care? Plastic Ingredients that Contribute to marine Microplastic Litter. [Google Scholar]
- Wenzel R. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936;28(8):988–994. [Google Scholar]
- Winkler H., Vorwerg W., Rihm R. Synthesis and properties of fatty acid starch esters. Carbohydr. Polym. 2013;98:208–216. doi: 10.1016/j.carbpol.2013.05.086. [DOI] [PubMed] [Google Scholar]
- Winkler H., Vorwerg W., Rihm R. Thermal and mechanical properties of fatty acid starch esters. Carbohydr. Polym. 2014;102:941–949. doi: 10.1016/j.carbpol.2013.10.040. [DOI] [PubMed] [Google Scholar]
- Wurzburg O.B. CRC Press; Boca Raton: 1986. Modified Starches: Properties and Uses. [Google Scholar]
- Xanthos D., Walker T.R. International policies to reduce plastic marine pollution from single-use plastics (plastic bags and microbeads): a review. Mar. Pollut. Bull. 2017 doi: 10.1016/j.marpolbul.2017.02.048. [DOI] [PubMed] [Google Scholar]
- Xu Y., Miladinov V., Hanna M.A. Synthesis and characterization of starch acetates with high substitution. Cereal Chem. 2004;81(6) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.