Abstract
Background
Systematic review and meta-analyses of observational studies on maternal vitamin D status and risk of respiratory allergic conditions indicated that mothers who had supplementation during pregnancy could decrease the risk of recurrent wheeze or asthma in their offspring.
Objectives
We conducted this meta-analysis of Randomized Controlled Trials with the primary intention of detecting the effect of prenatal vitamin D supplementation on the offspring's asthma. Secondary outcomes under respiratory health include eczema, lower respiratory tract infections, Immunoglobulin E positive test, upper respiratory tract infections, and allergic rhinitis.
Methods
A comprehensive search of PubMed, ScienceDirect, Google Scholar, and Cochrane Library databases was performed to retrieve randomized controlled trials. Risk Ratio with 95% confidence intervals was computed from dichotomous data using a random-effects model, with I2 >50% representing notable heterogeneity.
Results
Six clinical trials met the inclusion criteria, involving a total of 2898 subjects (1461 experimental group and 1437 control group). There was non-significant inverse relationship between vitamin D intake during pregnancy and the occurrence of asthma in offspring (pooled RR = 0.89, 95% CI = 0.69–1.15, I2 = 46% and Z-static = 0.90, P-value = 0.37). There is no significant difference in the risk of assessed childhood respiratory problems due to maternal supplementation of vitamin D during pregnancy.
Conclusion and implications
Currently, there is no fertile evidence to promote vitamin D supplementation in pregnancy for childhood respiratory health. Future clinical trials should emphasize early initiation of vitamin D supplementation, consider 6 weeks to 6 months postnatal critical window for vitamin D deficiency for offspring, lower risk dose of vitamin D, and identify different phenotypes of asthma and response to vitamin D supplementation.
Keywords: Allergy, Asthma, Childhood, Meta-analysis, Prenatal, Vitamin D
Abbreviations: IgE, Immunoglobulin E; IU, International unit; LRTI, Lower respiratory tract infection; PRISMA, preferred reporting items for systematic reviews and meta-analysis; RCT, randomized controlled trial; RR, relative risk; URTI, Upper respiratory tract infection
Introduction
About 300 million people worldwide suffer from asthma and it is the most common chronic disease in children. Asthma is among the top chronic conditions for the global ranking of disability-adjusted life years in children.1,2 Pediatric asthma is a serious global health problem; currently, the trends indicate the increasing number of hospitalizations in young children.3 The prevalence of respiratory symptoms is increasing and varies in different geographic regions; for example, the prevalence of severe asthma ranges from 0 to 20.3% and wheezing from 2.4 to 37.6% in 6–7 years old children.4
Numerous studies have suggested that vitamin D can promote a tolerogenic phenotype that protects against allergic sensitization. Vitamin D has been shown to promote immune tolerance by the generation of myeloid-derived dendritic cells, increased activity of regulatory Th cells, and suppression of immunoglobulin E (IgE) production.5 Other mechanistic studies highlighted the inhibitory effect of vitamin D on passively sensitized airway smooth muscle cell affecting growth and contractility.6,7 This inhibition prevents airway remodeling which is common in asthma. In vitro studies also indicated that vitamin D can increase glucocorticoid bioavailability on bronchial smooth muscle which further extends the benefit.8
So far evidence has been gathered to resolve the dispute about the role of vitamin D on childhood asthma. In recent systematic reviews and meta-analyses, vitamin D deficiency was associated with decreased lung function in asthmatic children;9,10 other systematic reviews also found that vitamin D supplementation reduced the rate of asthma exacerbation requiring treatment with systemic glucocorticoids and suggested high dose vitamin D may prevent asthma exacerbation.11,12
Even though, the primary intention is for bone and teeth health, vitamin D supplementation for infants have been widely recommended. The World Health Organization, after going through different literature, recommended a daily dose of 200–400 IU vitamin D supplement to maintain optimum plasma vitamin D level up to 1 year.13 Different countries and organizations have recommendations for infants. The American Academy of Pediatrics (400IU),14 Australia and New Zeeland (200–400 IU),15 and Canada (400 IU),16 and the Committee on Nutrition of the French Society of Pediatrics recommended up to 1000 IU for children less than 18 months of age.17
Besides the children's vitamin D status or supplementation, gestational vitamin D concentration was evaluated on the risk of offspring's asthma and other allergic conditions. Systematic review and meta-analyses conducted on randomized controlled trials (RCTs) and prospective cohort studies on maternal vitamin D status and risk of respiratory allergic conditions indicated that mothers who had supplementation during pregnancy could decrease the risk of recurrent wheeze or asthma.18,19
Li et al20 conducted a meta-analysis of prospective cohort and clinical trials published up to December 2017. The pooled effect of vitamin D effect during pregnancy showed an inverse relationship with the offspring's wheeze but not eczema. Since then, plenty of clinical trials have been published.21, 22, 23 We planned to conduct this meta-analysis with the primary intention of detecting the effect of prenatal vitamin D supplementation on the offspring's asthma. Secondary outcomes under respiratory health include eczema, lower respiratory tract infections (LRTIs), IgE positive test, upper respiratory tract infections (URTI), and allergic rhinitis.
Methods
Literature searches
A comprehensive search of the following databases was performed: PubMed, ScienceDirect, Google Scholar, and Cochrane Library. Reports were compiled according to the preferred reporting items for systematic reviews and meta-analysis (PRISMA) guideline.24 Unpublished articles were searched from clinical trial registration platforms. Preprint articles were also retrieved from websites. A manual search was conducted by screening the reference lists of inclusive studies. The search strategy involves pregnant mothers and intervention relevant terms (vitamin D) with both Medical Subject Headings (MeSH) and free text. The following search terms were used with Boolean search operators: “randomized controlled trial” OR “clinical trial” OR “random” OR “placebo” OR “intervention” OR “double-blind” OR “single-blind” OR “supplement∗” AND “prenatal” OR “pregnan∗” OR “antenatal” OR “maternal” OR “antepartum” AND “Vitamin D” OR “cholecalciferol” OR “calciferol” OR “vitamin D3” OR “25 hydroxy-vitamin D” OR “25 hydroxy-vitamin D3” AND “asthma” OR “wheeze” OR “wheezing” OR “respiratory tract infection” OR “eczema” OR “allerg∗”. All relevant articles published or unpublished available until May 1, 2020, were included.
Study selection and outcomes
Studies which fulfill the following criteria were included after systematically reviewing the manuscripts. The studies were considered for inclusion if they were randomized controlled trials (RCTs), performed among pregnant mothers; evaluating the effect of vitamin D supplementation; and reporting respiratory health events of intervention and control groups.
Studies lacking a control group were excluded. This meta-analysis excluded in vitro and animal studies, review articles, studies with a cohort, case-control, and cross-sectional design, and trials with combination therapy (combine vitamin D with other nutrients).
Due to feasibility, articles published in the English language/have English version were included. All the above-mentioned designed research conducted on adults and conducted as of May 1, 2020, were included. Incomplete articles, conference proceedings, and duplicates were excluded.
The primary outcome of this study is a diagnosis of asthma in offspring and secondary outcomes were eczema, LRTIs, IgE positive test, URTI, and allergic rhinitis. The two authors independently screened the titles, abstracts, and full-text of retrieved articles to identify their eligibility, and disagreement was resolved by the third author.
Data extraction and quality assessment
Data extraction conducted independently by the 2 authors using a standardized data collection form, which includes study characteristics (author, year of publication, country, study design, and sample size), intervention characteristics (dosage, duration [time of recruitment]), and outcomes (asthma, eczema, LRTIs, IgE positive tests, URTIs, and allergic rhinitis).
The risk of bias of inclusive was assessed following the Cochrane Collaboration Risk of Bias Tool.25 The risk of bias of individual study will be rated as low, moderate, or high.26
Statistical analysis
Risk Ratio (RR) with 95% confidence intervals (CIs) was computed from dichotomous data using a random-effects model, with I2 >50% representing notable heterogeneity.27 Subgroup analysis was performed based on different moderators.
To detect the robustness of the results, a sensitivity analysis was conducted by sequential elimination of each study from the pool. Potential publication bias was assessed using funnel plots. The statistical analysis was performed using the Review Manager (RevMan) [Computer program]. Version 5.3.5. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014, with P ≤ 0.05 indicating a statistically significant difference.
Results
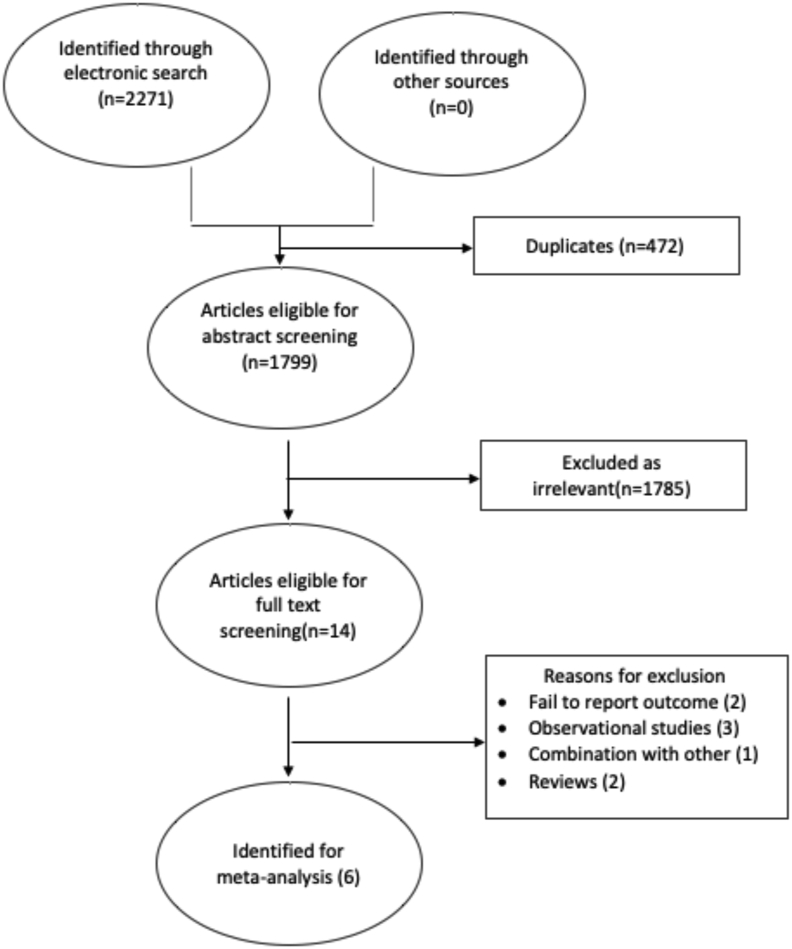
Overall 2271 articles were identified. Fourteen studies were eligible for full text screening, in which 6 were included. The studies analyzed a total of 2898 subjects (1461 experimental group and 1437 control group). The details of the exclusions are presented in Fig. 1. Six studies reported the primary outcome (Asthma), 2–5 studies reported the secondary outcomes; Eczema, LRTI, IgE, allergic rhinitis, and URTI.
Fig. 1.
Literature search results and study selection
All studies were randomized controlled trials that recruited pregnant mothers from 10 to 27 weeks of gestation and evaluated children's respiratory conditions from 1.5 to 6 years. Studies supplement 2400–8000 IU vitamin D for mothers in the experimental group. One study had supplementation for infant for a period of 6 months 800IU vs placebo in experimental and control groups, respectively. Other studies have prenatal supplementation for mothers from recruitment time to delivery or 1 week postpartum. Table 1 summarizes the characteristics of the included studies.
Table 1.
Characteristics of included studies
| Author-year | Country | Design | participants |
Recruitment time | Measurement time | Intervention | Outcomes | |
|---|---|---|---|---|---|---|---|---|
| Vit. D | Control | |||||||
| Goldring et al. 201323 | UK | RCT | 50 | 56 | 27wk | 3 years | 8000IU-ergocalciferol/800 IU ergocalciferol | Wheeze, API, Eczema, Atopy, allergic rhinitis |
| Grant et al. 201617 | Australia | RCT | 76 | 80 | 27wk | 18 months | 2000/800IU vs placebo/placebo vitamin D | Cold or influenza, Otitis media, URTI, croup, bronchiolitis, LRTI, fever, cough, number of asthma visits |
| Brustad et al. 201918 | Denmark | RCT | 274 | 268 | 24wk | 6 years | 2400IU vs 400IU Vitamin D3 |
Asthma, lung function tests, IgE test, skin prink test, allergic rhinitis |
| Litonjua et al. 201624 | USA | RCT | 405 | 401 | 10–18wks | 3 years | 4000IU vs 400 IU cholecalciferol |
Asthma, eczema, LRTI, IgE concentration, IgE tests, allergy sensitization |
| Chaws et al. 201625 | Denmark | RCT | 295 | 286 | 24wk | 3 years | 2400IU vs placebo vitamin D3 |
Wheeze, asthma, LRTI, URTI, eczema, skin prink test, IgE test |
| Litonjua et al. 202016 | USA | RCT | 361 | 346 | 10–18wks | 6 years | 4400IU vs 400 IU cholecalciferol |
Asthma, wheeze, LRTI, eczema, allergic rhinitis, allergic sensitization, IgE test |
API; Asthma predictive index, IgE; Immunoglobulin E, IU; International unit, LRTI; Lower respiratory tract infection, RCT; randomized controlled trial, UK; United Kingdom, URTI; Upper respiratory tract infection, USA; United States of America, Wk; weeks.
Primary outcome
Our analysis showed non-significant inverse relationship between vitamin D intake during pregnancy and the occurrence of asthma in offspring (pooled RR = 0.89, 95% CI = 0.69–1.15, I2 = 46% and Z-static = 0.90, P-value = 0.37), forest plot is given in Fig. 2A.
Fig. 2.
Forest plot showing: A) the pooled effect of prenatal vitamin D supplementation on offspring's risk of asthma, B) the pooled effect of prenatal vitamin D supplementation on offspring's risk of eczema, C) the pooled effect of prenatal vitamin D supplementation on offspring's LRTIs, D) the pooled effect of prenatal vitamin D supplementation on offspring's IgE; subgroup analysis performed on infant's postnatal vitamin D status, E) the pooled effect of prenatal vitamin D supplementation on offspring's URTIs, F) the pooled effect of prenatal vitamin D supplementation on the offspring's status of allergic rhinitis.
Secondary outcomes
Table 2 summarizes all secondary outcomes of respiratory health comparing prenatal intake of vitamin D and the risk of respiratory problems. We found that there was no significant difference in the risk of respiratory problems whether there was maternal supplementation of vitamin D during pregnancy or not. Because of moderate heterogeneity, we conducted a subgroup analysis for IgE positive tests based on the offspring's vitamin D supplementation status. With notable heterogeneity, the risk of IgE positive test was not different for maternal intake of vitamin D in pregnancy overall, but the risk of airway sensitization could be lower after vitamin D supplementation of infants for 6 months beyond the prenatal vitamin D daily supplement RR = 0.34, 95% CI = 0.12–1.00, Z-static = 1.96, P-value = 0.05). Fig. 2(B–F) shows the forest plot of primary and secondary outcomes of this meta-analysis.
Table 2.
Summary of the effect of prenatal vitamin D supplementation on respiratory health parameters (pooled RR)
| Outcome | Meta-analysis |
Heterogeneity |
||||
|---|---|---|---|---|---|---|
| RR | (95% CI) | P-value | I2 | P-value | ||
| 1 | Asthma | 0.89 | (0.69–1.15) | 0.37 | 46% | 0.10 |
| 2 | Eczema | 0.95 | (0.82–1.10) | 0.51 | 0% | 0.95 |
| 3 | LRTIs | 0.95 | (0.85–1.06) | 0.37 | 0% | 0.95 |
| 4 | IgEa | 0.34 | (0.12–1.00) | 0.05 | – | |
| IgEb | 1.03 | (0.79–1.33) | 0.85 | 60% | 0.06 | |
| IgE total | 0.97 | (0.73–1.29) | 0.82 | 63% | 0.03 | |
| 5 | URTIs | 0.89 | (0.73–1.07) | 0.22 | 0% | 0.58 |
| 6 | Allergic rhinitis | 1.03 | (0.69–1,54) | 0.87 | 43% | 0.17 |
IgE; Immunoglobulin E, LRTI; Lower respiratory tract infection, URTI; Upper respiratory tract infection, RR; relative risk.
Subgroup analysis of children who received vitamin D in the first 6 months.
children who didn't receive vitamin D supplementation.
Risk of bias and Publication bias: the risk of bias of original articles (individually) and quality of evidence was given in the Supplementary file. Generally included studies have low risk of bias when evaluated by Cochrane risk of bias tool. Funnel plot for each individual outcome indicated no asymmetry with visual inspection (Supplementary file).
Discussion
This meta-analysis found out that there was no significant difference in relative risk of asthma or secondary outcomes of offspring regardless of prenatal vitamin D supplementation. While the scientific world is pushing for better evidence from clinical trials, this meta-analysis of RCTs is odd from the previous meta-analysis of observational studies.18,19 Other meta-analyses of observational studies indicated a modest association of low maternal vitamin D level with increased risk of childhood eczema, but failed to find any apparent association with childhood asthma or wheeze.28
There are several concerns that need to be raised. First, what is the optimum prenatal plasma concentration of vitamin D level for normal lung function in offspring and association with baseline vitamin D? Baseline vitamin D level is an important confounder in some clinical trials included in this meta-analysis. Insufficient maternal vitamin D status appears to be associated with a significantly higher prevalence of allergen sensitization. Furthermore, high maternal vitamin D level is significantly associated with reduced risk of developing eczema and asthma.29 In addition to the daily supplementation of vitamin D dose, the baseline plasma vitamin D status is crucial to reach an optimal level.
Second, to evaluate the role of prenatal vitamin D supplementation on childhood respiratory health, we have to take into consideration the U-shaped association of maternal vitamin D status and risk of childhood asthma.30, 31, 32 Both high (>100 nmol/l) and low (<25–50 nmol/l) vitamin D levels were significantly associated with childhood asthma and increased IgE, which subsequently associated with a high risk of asthma. The lowest risk of childhood asthma was approximately 70 nmol/l of maternal vitamin D.32 The mean vitamin D level of the intervention group in the eligible clinical trials for this meta-analysis was either lower33 or higher (107.5 nmol/l,34 98.0 nmol/l35) than the supposed lowest risk belt.
Third, in the first 6–8 weeks of postnatal life, vitamin D status of infants is mainly dependent on placental transfer in utero,36 and in most infants, the acquired vitamin D stores are depleted by approximately 8 weeks of age.37 Thereafter, the infant's vitamin D supplement is derived from diet, sunlight, and supplementation. Human milk contains a very low amount which is not sufficient to maintain an optimal vitamin D level especially if exposure to sunlight is limited.38 Exclusively breastfed infants have hypovitaminosis D due to poor content of human milk.39,40 If the placental transfer of vitamin D keeps the requirement for 6–8 weeks and exclusive breastfeeding is recommended for 6 months, this period (6 weeks–6 months postnatal) is a critical window for vitamin D deficiency. Besides prenatal supplementation, infant supplementation up to 6 months might work better. In a randomized controlled trial, vitamin D supplementation during the third trimester of pregnancy and the first 6 months of infancy resulted in a decreased proportion of children sensitized to aeroallergens at 18 months.22
Fourth, deficiency of vitamin D in breastfeeding is a global problem. Dawodu et al41 found a higher magnitude of vitamin D deficiency from 17% in Cincinnati to 62% in Mexican mothers. Lower maternal vitamin D status in lactation fails to keep the infant's minimum requirement and exposes both of them to vitamin deficiency. High dose postpartum vitamin D supplementation to mothers alone was enough to optimize maternal vitamin D status, breast milk vitamin D content, and adequate infant serum vitamin D.42 Along with the prenatal supplementation continuing it to up to 6 months postpartum might be the key to lung maturation and respiratory health.
Fifth, human lung development starts at 3–4 weeks of gestation.43 Although more than 63% of vitamin D regulated genes are expressed later in gestation than earlier,44 vitamin D signaling pathway is active in early fetal life.45 It is also unclear whether early vitamin D status provides silent risk for asthma and other allergic diseases. Vitamin D supplementation usually in the third trimester (as occurs in most of the clinical trials) might be the loophole for the non-significant difference in childhood risk of respiratory problems whether there was maternal supplementation of vitamin D during pregnancy or not.
And, diagnosing asthma in childhood is very difficult. It is also possible that prenatal vitamin D supplementation affected some phenotypes and others may need interventions other than vitamin D. Still, there is no consensus on how much is a clinically significant reduction, and we can't rule out the importance of prenatal vitamin D supplementation.
There are some limitations of this meta-analysis. There are discrepancies in the included studies on the definition of asthma/recurrent wheeze; this may have affected the result. On the other hand, serum vitamin D level of pregnant mothers at the entrance to study, different gestational age, or during birth was not analyzed and related to childhood asthma because most clinical trials fail to report it.
Conclusion and implications
There was a non-significant inverse relationship between vitamin D intake during pregnancy and the occurrence of asthma in offspring. There was no statistically significant difference in the risk of respiratory problems due to maternal supplementation of vitamin D during pregnancy. Currently, there is no fertile evidence to promote vitamin D supplementation in pregnancy for childhood respiratory health. Future clinical trials should emphasize early initiation of vitamin D supplementation, consider 6 weeks to 6 months postnatal critical window for vitamin D deficiency for offspring, lower risk dose of vitamin D, and identify different phenotypes of asthma and response to vitamin D supplementation.
Funding
This research did not receive any specific grant from funding agencies.
Informed consent
Not applicable.
Ethical approval
Not applicable.
Consent for publication
All authors approved the publication of this work.
Author contributions
Amare Abera made substantial contributions to conception, design, literature search, data extraction and writing. Addis Alem performed literature searching, data extraction and contributed to the drafting of the article. Andualem Mossie and Tadesse Alemu revised the study critically, contributed substantially to the interpretation of data and drafting of the article.
Availability of data and materials
Not applicable.
Declaration of competing interest
The authors have declared that there are no competing interests.
Acknowledgements
Not applicable.
Footnotes
Full list of author information is available at the end of the article
Supplementary data to this article can be found online at https://doi.org/10.1016/j.waojou.2020.100486.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Wang H., Naghavi M., Allen C. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1459–1544. doi: 10.1016/S0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asher I., Pearce N. Global burden of asthma among children. Int J Tubercul Lung Dis. 2014;18:1269–1278. doi: 10.5588/ijtld.14.0170. [DOI] [PubMed] [Google Scholar]
- 3.Serebrisky D., Wiznia A. Pediatric asthma: a global epidemic. Annals of Global Health. 2019;85:6. doi: 10.5334/aogh.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lai C.K.W., Beasley R., Crane J., Group, the I.P.T.S Global variation in the prevalence and severity of asthma symptoms: phase three of the International Study of Asthma and Allergies in Childhood (ISAAC) Thorax. 2009;64:476–483. doi: 10.1136/thx.2008.106609. [DOI] [PubMed] [Google Scholar]
- 5.Hollams E.M. Vitamin D and atopy and asthma phenotypes in children. Curr Opin Allergy Clin Immunol. 2012;12:228–234. doi: 10.1097/ACI.0b013e3283534a32. [DOI] [PubMed] [Google Scholar]
- 6.Damera G., Fogle H.W., Lim P. Vitamin D inhibits growth of human airway smooth muscle cells through growth factor-induced phosphorylation of retinoblastoma protein and checkpoint kinase 1. Br J Pharmacol. 2009;158:1429–1441. doi: 10.1111/j.1476-5381.2009.00428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song Y., Qi H., Wu C. Effect of 1,25-(OH)2D3 (a vitamin D analogue) on passively sensitized human airway smooth muscle cells. Respirology. 2007;12:486–494. doi: 10.1111/j.1440-1843.2007.01099.x. [DOI] [PubMed] [Google Scholar]
- 8.Bossé Y., Maghni K., Hudson T.J. 1α,25-Dihydroxy-vitamin D3 stimulation of bronchial smooth muscle cells induces autocrine, contractility, and remodeling processes. Physiol Genom. 2007;29:161–168. doi: 10.1152/physiolgenomics.00134.2006. [DOI] [PubMed] [Google Scholar]
- 9.Zhang L.L., Gong J., Liu C.T. Vitamin D with asthma and COPD: not a false hope? A systematic review and meta-analysis. Genet Mol Res. 2014;13:7607–7616. doi: 10.4238/2014.february.13.10. [DOI] [PubMed] [Google Scholar]
- 10.Jat K.R., Khairwa A. Vitamin D and asthma in children: a systematic review and meta-analysis of observational studies. Lung India. 2017;34:355–363. doi: 10.4103/0970-2113.209227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pojsupap S., Iliriani K., Sampaio T.Z.A.L. Efficacy of high-dose vitamin D in pediatric asthma: a systematic review and meta-analysis. J Asthma. 2015;52:382–390. doi: 10.3109/02770903.2014.980509. [DOI] [PubMed] [Google Scholar]
- 12.Jolliffe D.A., Greenberg L., Hooper R.L. Vitamin D supplementation to prevent asthma exacerbations: a systematic review and meta-analysis of individual participant data. The Lancet Respiratory Medicine. 2017;5:881–890. doi: 10.1016/S2213-2600(17)30306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO | Vitamin D Supplementation for Infants Available online: http://www.who.int/elena/titles/bbc/vitamind_infants/en/(accessed on Oct 9, 2020).
- 14.Wagner C.L., Greer F.R. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122:1142–1152. doi: 10.1542/peds.2008-1862. [DOI] [PubMed] [Google Scholar]
- 15.Nutrient Reference Values for Australia and New Zealand Including Recommended Dietary Intakes | NHMRC Available online: https://www.nhmrc.gov.au/about-us/publications/nutrient-reference-values-australia-and-new-zealand-including-recommended-dietary-intakes (accessed on Oct 9 2020).
- 16.Godel J.C., Society, C.P. First Nations, I. and M.H.C. Vitamin D supplementation: recommendations for Canadian mothers and infants. Paediatr Child Health. 2007;12:583–589. doi: 10.1093/pch/12.7.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vidailhet M., Mallet E., Bocquet A. Vitamin D: still a topical matter in children and adolescents. A position paper by the Committee on Nutrition of the French Society of Paediatrics. Arch Pediatr. 2012;19:316–328. doi: 10.1016/j.arcped.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 18.Shi D., Wang D., Meng Y., Chen J., Mu G., Chen W. Maternal vitamin D intake during pregnancy and risk of asthma and wheeze in children: a systematic review and meta-analysis of observational studies. J Matern Fetal Neonatal Med. 2019:1–7. doi: 10.1080/14767058.2019.1611771. 0. [DOI] [PubMed] [Google Scholar]
- 19.Vahdaninia M., Mackenzie H., Helps S., Dean T. Prenatal intake of vitamins and allergic outcomes in the offspring: a systematic review and meta-analysis. J Allergy Clin Immunol: In Pract. 2017;5:771–778. doi: 10.1016/j.jaip.2016.09.024. e5. [DOI] [PubMed] [Google Scholar]
- 20.Li W., Qin Z., Gao J. Vitamin D supplementation during pregnancy and the risk of wheezing in offspring: a systematic review and dose-response meta-analysis. J Asthma. 2019;56:1266–1273. doi: 10.1080/02770903.2018.1536142. [DOI] [PubMed] [Google Scholar]
- 21.Litonjua A.A., Carey V.J., Laranjo N. Six-year follow-up of a trial of antenatal vitamin D for asthma reduction. N Engl J Med. 2020;382:525–533. doi: 10.1056/NEJMoa1906137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grant C.C., Crane J., Mitchell E.A. Vitamin D supplementation during pregnancy and infancy reduces aeroallergen sensitization: a randomized controlled trial. Allergy. 2016;71:1325–1334. doi: 10.1111/all.12909. [DOI] [PubMed] [Google Scholar]
- 23.Brustad N., Eliasen A.U., Stokholm J., Bønnelykke K., Bisgaard H., Chawes B.L. High-dose vitamin D supplementation during pregnancy and asthma in offspring at the age of 6 years. J Am Med Assoc. 2019;321:1003–1005. doi: 10.1001/jama.2019.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liberati A., Altman D.G., Tetzlaff J. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6 doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higgins J.P.T., Altman D.G., Gøtzsche P.C. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343 doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mustafa R.A., Santesso N., Brozek J. The GRADE approach is reproducible in assessing the quality of evidence of quantitative evidence syntheses. J Clin Epidemiol. 2013;66:736–742. doi: 10.1016/j.jclinepi.2013.02.004. e5. [DOI] [PubMed] [Google Scholar]
- 27.Higgins J.P.T., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei Z., Zhang J., Yu X. Maternal vitamin D status and childhood asthma, wheeze, and eczema: a systematic review and meta-analysis. Pediatr Allergy Immunol. 2016;27:612–619. doi: 10.1111/pai.12593. [DOI] [PubMed] [Google Scholar]
- 29.Chiu C.-Y., Huang S.-Y., Peng Y.-C. Maternal vitamin D levels are inversely related to allergic sensitization and atopic diseases in early childhood. Pediatr Allergy Immunol. 2015;26:337–343. doi: 10.1111/pai.12384. [DOI] [PubMed] [Google Scholar]
- 30.Rothers J., Wright A.L., Stern D.A., Halonen M., Camargo C.A. Cord blood 25-hydroxyvitamin D levels are associated with aeroallergen sensitization in children from Tucson, Arizona. J Allergy Clin Immunol. 2011;128:1093–1099. doi: 10.1016/j.jaci.2011.07.015. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maslova E., Hansen S., Thorne-Lyman A.L. Predicted vitamin D status in mid-pregnancy and child allergic disease. Pediatr Allergy Immunol. 2014;25:706–713. doi: 10.1111/pai.12295. [DOI] [PubMed] [Google Scholar]
- 32.Song H., Yang L., Jia C. Maternal vitamin D status during pregnancy and risk of childhood asthma: a meta-analysis of prospective studies. Mol Nutr Food Res. 2017;61:1600657. doi: 10.1002/mnfr.201600657. [DOI] [PubMed] [Google Scholar]
- 33.Goldring S.T., Griffiths C.J., Martineau A.R. Prenatal vitamin D supplementation and child respiratory health: a randomised controlled trial. PloS One. 2013;8 doi: 10.1371/journal.pone.0066627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chawes B.L., Bønnelykke K., Stokholm J. Effect of vitamin D3 supplementation during pregnancy on risk of persistent wheeze in the offspring: a randomized clinical trial. J Am Med Assoc. 2016;315:353–361. doi: 10.1001/jama.2015.18318. [DOI] [PubMed] [Google Scholar]
- 35.Litonjua A.A., Carey V.J., Laranjo N. Effect of prenatal supplementation with vitamin D on asthma or recurrent wheezing in offspring by age 3 Years: the VDAART randomized clinical trial. J Am Med Assoc. 2016;315:362–370. doi: 10.1001/jama.2015.18589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hillman L.S., Haddad J.G. Human perinatal vitamin D metabolism I: 25-Hydroxyvitamin D in maternal and cord blood. J Pediatr. 1974;84:742–749. doi: 10.1016/S0022-3476(74)80024-7. [DOI] [PubMed] [Google Scholar]
- 37.Ala-Houhala M. 25-Hydroxyvitamin D levels during breast-feeding with or without maternal or infantile supplementation of vitamin D. J Pediatr Gastroenterol Nutr. 1985;4:220–226. doi: 10.1097/00005176-198504000-00011. [DOI] [PubMed] [Google Scholar]
- 38.Hollis B.W., Roos B.A., Draper H.H., Lambert P.W. Vitamin D and its metabolites in human and bovine milk. J Nutr. 1981;111:1240–1248. doi: 10.1093/jn/111.7.1240. [DOI] [PubMed] [Google Scholar]
- 39.Dawodu A., Agarwal M., Hossain M., Kochiyil J., Zayed R. Hypovitaminosis D and vitamin D deficiency in exclusively breast-feeding infants and their mothers in summer: a justification for vitamin D supplementation of breast-feeding infants. J Pediatr. 2003;142:169–173. doi: 10.1067/mpd.2003.63. [DOI] [PubMed] [Google Scholar]
- 40.Balasubramanian S., Ganesh R. Vitamin D deficiency in exclusively breast-fed infants. Indian J Med Res. 2008;127:250–255. [PubMed] [Google Scholar]
- 41.Dawodu A., Davidson B., Woo J.G. Sun exposure and vitamin D supplementation in relation to vitamin D status of breastfeeding mothers and infants in the global exploration of human milk study. Nutrients. 2015;7:1081–1093. doi: 10.3390/nu7021081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dawodu A., Salameh K.M., Al-Janahi N.S., Bener A., Elkum N. The effect of high-dose postpartum maternal vitamin D supplementation alone compared with maternal plus infant vitamin D supplementation in breastfeeding infants in a high-risk population. A randomized controlled trial. Nutrients. 2019;11:1632. doi: 10.3390/nu11071632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schittny J.C. Development of the lung. Cell Tissue Res. 2017;367:427. doi: 10.1007/s00441-016-2545-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kho A.T., Sharma S., Qiu W. Vitamin D related genes in lung development and asthma pathogenesis. BMC Med Genom. 2013;6:47. doi: 10.1186/1755-8794-6-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Evans K.N., Bulmer J.N., Kilby M.D., Hewison M. Vitamin D and placental-decidual function. J Soc Gynecol Invest. 2016;11:263–271. doi: 10.1016/j.jsgi.2004.02.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.