Abstract
Background
Visual snow syndrome (VSS) is a neurological condition characterized by persistent flickering dots in the visual fields, palinopsia, enhanced entoptic phenomenon, photophobia, and nyctalopia. Neuroimaging evidence supports the role of the visual association cortex in visual snow syndrome.
Case series: We provided clinical care to three patients with visual snow syndrome, in whom [123I]-IMP single-photon emission computed tomography (SPECT) imaging was performed. Case 1 was a 21-year-old male with a past history of migraine with aura who exhibited visual snow and entoptic phenomenon. In this patient, [123I]-IMP SPECT imaging revealed right occipital and temporal hypoperfusion with a distribution matching the ventral visual stream. [123I]-IMP SPECT imaging detected only mild bilateral frontal hypoperfusion in Case 2 and no overt abnormalities in Case 3.
Conclusion
Although visual snow syndrome seems to be a heterogenous condition, our observations indicate that abnormal visual processing within the ventral visual stream may play a role in the pathogenesis of this condition.
Keywords: Visual snow, migraine, visual association area, occipital lobe, single-photon emission computed tomography, fusiform gyrus
Introduction
Visual snow was originally reported as one of the persistent positive visual phenomena experienced by patients with migraine (1). Typically, people with visual snow complain of a constant perception of black and white dots, snow, or “TV static” in the entire visual field. Although the visual snow phenomenon was initially regarded as a form of persistent migraine aura, similar symptoms have been reported in other conditions, including traumatic brain injuries and hallucinogenic drug use. Schankin et al. (2) noted that nearly all (97%) of their 78 patients with visual snow had additional symptoms, including palinopsia and blue field entoptic phenomenon. They claimed that visual snow was a unique syndrome distinct from migraine, and the term visual snow syndrome (VSS) was subsequently introduced (2). VSS seems to be a common condition, with a recent study reporting a 2% prevalence in the general population (3).
In this case series, we report a unique functional neuroimaging finding that may provide novel insights into the mechanisms underlying this condition.
Case series
Case 1
A 21-year-old male presented with continuous flickering tiny dots in the entire visual field starting at age 15. He also reported gradual development of persistent photophobia and a perception that he saw “whitish smoke” emerging on a dark background when he closed his eyes. He denied a history of palinopsia or nyctalopia, but had been diagnosed with migraine with aura at age 10. Typically, his visual auras consisted of a small scintillating scotoma that expanded for approximately 20 min with variable laterality across attacks, followed by headache, nausea, photophobia, and phonophobia. The patient had, however, been free of migraine attacks in the preceding 2 years. He denied any previous exposure to illicit recreational drugs.
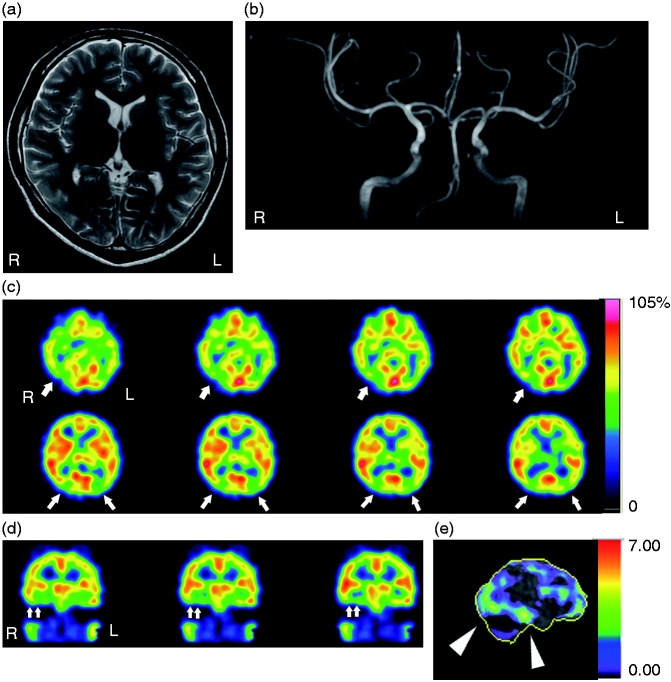
His neurological examination was unremarkable. Ophthalmological evaluation revealed a slight increase in intraocular pressure (R: 23 mm Hg, L: 24 mm Hg). His visual field and fundoscopic findings were normal. Brain magnetic resonance (MR) imaging and MR angiography were also normal (Figure 1(a),(b)).
Figure 1.
Brain magnetic resonance (MR) imaging and single-photo emission computed tomography (SPECT) findings. (a) Axial view of cranial T2-weighted MR imaging at the caudate-putamen level, revealing no structural abnormalities. (b) Coronal view of cranial MR angiography. Intracranial major arteries were normal. (c) [123I]-IMP SPECT images (consecutive axial sections), with arrows indicating areas of hypoperfusion. (d) [123I]-IMP SPECT images (consecutive coronal sections), with double arrows indicating areas of hypoperfusion. (e) Right lateral view of the Z-score image, with arrowheads indicating a hypoperfused area spanning the occipito-temporal lobes.
The patient was subsequently diagnosed with VSS. [123I]-IMP single-photon emission computed tomography (SPECT) imaging revealed decreased perfusion on bilateral sides of the occipital cortex and fusiform gyri with a preponderance on the right (Figure 1(c),(d)). This right-sided hypoperfusion was found to encompass an area extending from the occipital cortex to the inferior temporal gyrus (Figure 1(e)). The patient sought no therapy because his VSS was not severe enough to interfere with his daily activities.
Case 2
A 40-year-old female presented with recurrent episodes of throbbing headaches starting at age 39. Her headaches were always preceded by a typical scintillating scotoma lasting 10–15 min. She also complained of continuous flickering objects in the entire visual field, continuous photophobia and palinopsia, and the perception of white smoky objects appearing every time she closed her eyes. These visual symptoms had been present since her childhood. She reported no exposure to recreational drugs.
Her neurological examination and brain MRI findings were normal. Her headaches were responsive to naratriptan, and she was subsequently diagnosed with migraine with aura and VSS. Her [123I]-IMP SPECT imaging disclosed bilateral mild frontal hypoperfusion. Although lomerizine (5 mg bid) was effective at reducing her headache frequency, her VSS did not improve.
Case 3
A 19-year-old male visited our outpatient clinic due to daily headaches. He began to experience headaches at 13 years of age. Initially, his headache attacks were episodic and lasted 4–5 h with a pulsatile nature on most occasions; however, his headache frequency had increased over the 12 months prior to presentation. His headaches were worsened by physical activity and were accompanied by nausea, vomiting, and osmophobia. He also reported visual snow, palinopsia, and nyctalopia. He denied any previous exposure to recreational drugs.
There were no abnormalities identified during neurological or ophthalmic evaluations. His brain MRI, electroencephalogram (EEG), and visual evoked potential testing were also normal.
He was subsequently diagnosed with chronic migraine and VSS. His [123I]-IMP SPECT imaging disclosed a normal pattern of cerebral blood flow. He was treated with valproate (200 mg mid), which ameliorated his headache symptoms; however, his VSS-associated symptoms did not improve.
Discussion
The most remarkable finding in this case series was Case 1’s hypoperfusion of bilateral sides of the occipital cortex and fusiform gyri, with a preponderance on the right. Since we did not identify any structural alterations on brain MR imaging or angiography, this hypoperfusion likely reflected a functional abnormality. This patient did have a history of migraine with aura, and hypoperfusion of the occipital cortex in a patient with persistent aura has previously been reported (4). However, this patient had not experienced any migraine attacks for 2 years prior to presentation, and his visual snow symptoms were unlike anything he had ever experienced during a migraine aura. Therefore, the observed hypoperfusion was unlikely to be related to migraine visual aura.
Hypoperfusion involving bilateral parietal and parieto-occipital areas has previously been reported in patients with symptoms compatible with VSS; however, details about the extent and distribution of this hypoperfusion have not been provided (1). Schankin et al. (5) used [18F]-FDG positron emission tomography (PET) imaging to demonstrate hypermetabolism of the lingual gyrus in patients with VSS, noting that impaired visual sensory processing may underlie VSS (5), a theory that is also supported by a recent electrophysiological study (6). Although Case 1’s SPECT findings support this assertion, the cause for the seemingly discordant radiological findings (hypermetabolism vs. hypoperfusion) remains unclear. It has, however, been demonstrated that hemodynamic responses do not necessarily reflect neural activity in some conditions (7), and this neurovascular uncoupling may exist in the visual association areas of a subset of people with VSS.
Intriguingly, the hypoperfusion in this patient encompassed an area from the occipital cortex to the inferior temporal gyrus. It is known that there are two distinct streams originating from the primary visual cortex (V1) within the visual association area; namely, the ventral and dorsal streams (8,9). The distribution of hypoperfusion in Case 1 matched the trajectory of the ventral stream. The ventral stream is implicated in the transformation of visual information into the mental furniture that guides memory, recognition, and conscious perception, whereas the dorsal stream engages in the visual guidance of action in a real-time fashion (8). Our observations suggest that dysfunction of the ventral stream may be implicated in the development of VSS-associated symptoms.
Moreover, we also observed marked hypoperfusion in bilateral fusiform gyri of Case 1 (Figure 1(d), double arrows). The fusiform gyrus forms a functional neural network with the primary visual areas and the inferior/middle temporal gyri (10). Intriguingly, a recent study detected increased grey matter volume in the adjacent lingual gyrus-fusiform gyrus junction in VSS (11).
Nevertheless, it should be noted that these functional imaging abnormalities were detected in only one case in this series, which unambiguously indicates that VSS is a heterogenous condition with different mechanisms potentially involved in the development of visual symptoms. We were not able to identify any causes of VSS in Case 2 or Case 3. Although mild bilateral frontal hypoperfusion was detected in Case 2, it is not likely to be functionally relevant for her VSS.
The most effective therapy for patients with VSS remains to be determined. In our case series, neither lomerizine nor valproate were found to be effective at alleviating symptoms of VSS. The cellular mechanism(s) underlying the persistent visual phenomena associated with VSS, as well as the basis for the apparent neurovascular uncoupling seen in this and other cases, will need to be clarified in future studies.
Clinical implications
Visual snow syndrome seems to be a heterogenous condition with different underlying mechanisms potentially involved in the development of visual symptoms.
In some cases, visual snow syndrome may involve abnormalities in the ventral stream of the visual association area.
Elucidation of the cellular mechanisms underlying VSS and the neurovascular uncoupling seen in some cases will likely facilitate our understanding of the biological mechanisms.
Author contributions: MS saw the patients, collected clinical data, conceived the study, drafted the manuscript, and coordinated collaborations. KT, YI, MK, TN, HF, and MJ analyzed neuroimaging data. TT and JN revised the manuscript. DWD interpreted clinical and neuroimaging data and revised the manuscript.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The publication of this article was supported by JSPS KAKENHI (grant number 19K07849 to MS).
ORCID iD: Mamoru Shibata https://orcid.org/0000-0002-2416-4259
Declaration of conflicting interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: MS reports the following conflicts: Consulting fees: Amgen, Eli Lilly, Otsuka, Sanofi-Aventis. Speaking fees: Amgen, Daiichi-Sankyo, Takeda, Eisai. Reimbursement for travel: American Headache Society.
DWD reports the following conflicts: Personal fees: Amgen, AEON, Association of Translational Medicine, University Health Network, Daniel Edelman Inc., Autonomic Technologies, Axsome, Allergan, Alder BioPharmaceuticals, Biohaven, Charleston Laboratories, Clexio, Dr Reddy's Laboratories/Promius, Cerecin, Electrocore LLC, Eli Lilly, eNeura, Neurolief, Novartis, Ipsen, Impel, Satsuma, Supernus, Sun Pharma (India), Theranica, Teva, Vedanta, WL Gore, Nocira, PSL Group Services, XoC, Zosano, ZP Opco, Foresite Capital, Oppenheimer; Upjohn (Division of Pfizer), Pieris, Revance, Equinox, Salvia, Amzak Health. Speaking fees: Eli Lilly, Novartis Canada, Amgen, Lundbeck. Speakers Bureaus: None. CME fees or royalty payments: HealthLogix, Medicom Worldwide, MedLogix Communications, Mednet, Miller Medical, PeerView, WebMD Health/Medscape, Chameleon, Academy for Continued Healthcare Learning, Universal Meeting Management, Haymarket, Global Scientific Communications, Global Life Sciences, Global Access Meetings, Catamount, UpToDate (Elsevier), Oxford University Press, Cambridge University Press, Wolters Kluwer Health; Stock options: Precon Health, Aural Analytics, Healint, Theranica, Second Opinion/Mobile Health, Epien, Nocira, Matterhorn, Ontologics, King-Devick Technologies; Consulting without fee: Aural Analytics, Healint, Second Opinion/Mobile Health, Epien; Board of Directors: Paranet North America, Precon Health, Epien, Matterhorn, Ontologics, King-Devick Technologies. Patent: 17189376.1-1466:vTitle: Botulinum Toxin Dosage Regimen for Chronic Migraine Prophylaxis without fee; Research funding: American Migraine Foundation, US Department of Defense, PCORI, Henry Jackson Foundation; Professional society fees or reimbursement for travel: American Academy of Neurology, American Brain Foundation, American Headache Society, American Migraine Foundation, International Headache Society, Canadian Headache Society.
References
- 1.Liu GT, Schatz NJ, Galetta SL, et al. Persistent positive visual phenomena in migraine. Neurology 1995; 45: 664–668. [DOI] [PubMed] [Google Scholar]
- 2.Schankin CJ, Maniyar FH, Digre KB, et al. ‘ Visual snow’ – a disorder distinct from persistent migraine aura. Brain 2014; 137: 1419–1428. [DOI] [PubMed] [Google Scholar]
- 3.Kondziella D, Olsen MH, Dreier JP. Prevalence of visual snow syndrome in the UK. Eur J Neurol 2020; 27: 764–772. [DOI] [PubMed] [Google Scholar]
- 4.Relja G, Granato A, Ukmar M, et al. Persistent aura without infarction: Decription of the first case studied with both brain SPECT and perfusion MRI. Cephalalgia 2005; 25: 56–59. [DOI] [PubMed] [Google Scholar]
- 5.Schankin CJ, Maniyar FH, Sprenger T, et al. The relation between migraine, typical migraine aura and “visual snow”. Headache 2014; 54: 957–966. [DOI] [PubMed] [Google Scholar]
- 6.Eren O, Rauschel V, Ruscheweyh R, et al. Evidence of dysfunction in the visual association cortex in visual snow syndrome. Ann Neurol 2018; 84: 946–949. [DOI] [PubMed] [Google Scholar]
- 7.Logothetis NK. Neurovascular uncoupling: Much ado about nothing. Front Neuroenergetics 2010; 2: e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goodale MA. Separate visual systems for perception and action: A framework for understanding cortical visual impairment. Dev Med Child Neurol 2013; 55: 9–12. [DOI] [PubMed] [Google Scholar]
- 9.Milner AD. How do the two visual streams interact with each other? Exp Brain Res 2017; 235: 1297–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bi Y, Wang X, Caramazza A. Object domain and modality in the ventral visual pathway. Trends Cogn Sci 2016; 20: 282–290. [DOI] [PubMed] [Google Scholar]
- 11.Schankin CJ, Maniyar FH, Chou DE, et al. Structural and functional footprint of visual snow syndrome. Brain 2020; 143: 1106–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]