Abstract
Cardiovascular and non-cardiovascular comorbidities are frequently observed in heart failure patients, complicating the therapeutic management and leading to poor prognosis. The prompt recognition of associated comorbid conditions is of great importance to optimize the clinical management, the follow-up, and the treatment of patients affected by chronic heart failure. Anaemia and iron deficiency are commonly reported in all heart failure forms, have a multifactorial aetiology and are responsible for reduced exercise tolerance, impaired quality of life, and poor long-term prognosis. Diabetes mellitus is highly prevalent in heart failure and a poor glycaemic control is associated with worst outcome. Two specific heart failure forms are usually observed in diabetic patients: an ischaemic cardiomyopathy or a typical diabetic cardiomyopathy. The implementation of use of sodium-glucose cotransporter-2 inhibitors will much improve in the near future the long-term prognosis of patients affected by heart failure and diabetes. Among cardiovascular comorbidities, atrial fibrillation is the most common arrhythmic disease of heart failure patients and it is still not clear whether its presence should be considered as a prognostic indicator or as a marker of advanced disease. The aim of the present review was to explore the clinical and prognostic impact of anaemia and iron deficiency, diabetes mellitus, and atrial fibrillation in patients affected by chronic heart failure.
Keywords: Heart failure, anaemia, iron deficiency, diabetes mellitus, atrial fibrillation
Introduction
Comorbidities are quite frequent in patients affected by heart failure and represent a major issue that frequently complicates the management of the disease.1
The presence of multiple cardiovascular and non-cardiovascular comorbidities, in fact, impacts on the diagnostic and therapeutic management of heart failure patients and may lead to poor outcome, increased rates of hospitalization and mortality.
The early recognition of those associated pathological conditions in heart failure patients is of great value, allowing a strict follow-up to avoid or delay an episode of acute decompensated heart failure, facilitating the prompt use of targeted drugs for each condition and accelerating the inclusion of multiple medical specialists in the management of the disease.
The aim of the present review was to focus the attention on two major non-cardiovascular comorbidities of heart failure, anaemia and iron deficiency, and diabetes mellitus, and on the most important cardiovascular associated condition of heart failure patients, atrial fibrillation, focusing the attention on the clinical and prognostic impact of those conditions and directing on promising therapeutic interventions.
Anaemia and iron deficiency
Anaemia and iron deficiency are frequently observed in heart failure patients, independently from disease type, aetiology or stage. As regards anaemia, the World Health Organization defines its presence as haemoglobin (Hb) level <12 g/dl in women and <13 g/dl in men. However, substantial prevalence variability in heart failure has been described, ranging from 22% to 37%, with some reports also describing much higher prevalence rates.2,3 Patients exhibiting both anaemia and heart failure have a reduced functional capacity, a worse quality of life, and increased rates of major cardiovascular events, hospitalizations and death compared with non-anaemic heart failure patients. As regards exercise limitation, Agostoni et al.,4 in a large cohort of patients with heart failure and reduced left ventricular ejection fraction (HFrEF), demonstrated that each gram of Hb accounts, on average, for a 0.97 ml/min per kg change in O2 uptake at peak exercise (peak VO2), clarifying the impact of anaemia on functional capacity in heart failure patients. In terms of prognostic relevance, the MAGGIC dataset, including 13,295 heart failure patients, recognized anaemia as an independent prognostic predictor both in HFrEF and in heart failure with preserved ejection fraction (HFpEF).5 Moreover, Hb is one of the six independent predictors of total and cardiovascular mortality of the MECKI score, together with peak VO2, ventilation versus carbon dioxide production relationship slope (VE/VCO2 slope), ejection fraction, renal function, and sodium plasma levels.6
Multiple elements contribute to the occurrence of anaemia in heart failure: impaired renal function, a state of chronic inflammation, bone-marrow dysfunction, haemodilution and, of most importance, iron deficiency (Table 1).
Table 1.
Causes of anaemia and iron deficiency in patients affected by heart failure.
| Anaemia | Iron deficiency |
|---|---|
| Impaired renal function | Impaired iron absorption |
| Chronic inflammation | Reduced iron intake (malnutrition) |
| Bone-marrow dysfunction | Gastrointestinal blood losses |
| Haemodilution | Chronic inflammation (with impaired iron transition in the circulation and its sequestration into the reticuloendothelial system) |
| Iron deficiency |
Iron deficiency is a condition characterized by insufficient iron to satisfy the metabolic needs. Two distinct forms of iron deficiency can be recognized: absolute and functional; both are accompanied or not by a status of anaemia. Absolute iron deficiency is characterized by ferritin levels <100 µg/ml; in functional iron deficiency, ferritin is in the range 100–300 μg/l with a transferrin saturation <20%. The prevalence of iron deficiency in patients with chronic heart failure ranges from 35% to 55%; in acute decompensated heart failure it can reach a prevalence up to 80% in the first days after admission.7 As already discussed for anaemia, also iron deficiency, alone or combined with an anaemic status, is responsible for reduced exercise tolerance, affects quality of life and results in higher rates of hospitalizations and death in heart failure patients.8 As regards exercise intolerance, Jankowska et al.9 reported in 443 chronic heart failure patients the independent role of iron deficiency in determining reduced exercise capacity.
Absolute iron deficiency is usually due to malabsorption, malnutrition or gastrointestinal blood losses, whereas functional iron deficiency is related to a state of chronic inflammation10 (Table 1). Chronic inflammation is characterized by cytokine production, responsible for the synthesis of hepcidin and consequent reduction of ferroportin expression, resulting in a decreased iron transition into the circulation as well as in its sequestration in the macrophages of the reticuloendothelial system.10
The presence of anaemia and/or iron deficiency might complicate the prognostic assessment of heart failure patients, since anaemic patients are often excluded or poorly represented in heart failure trials and the prognostic role of commonly used prognostic predictors in chronic heart failure patients might change in anaemic patients. In this setting, the MECKI score research group studied a population of 3913 heart failure patients grouped according to Hb values, demonstrating that cardiopulmonary exercise test (CPET) can be safely performed also in anaemic patients and that peak VO2 and ejection fraction preserve their prognostic power also in heart failure patients with Hb <11 g/dl.11 Differently, the VE/VCO2 slope loses its prognostic power in severe anaemic heart failure patients (Hb <11 g/dl), despite the highest (and thus worst) values being found in this group.11 These results suggest the use of CPET also in anaemic heart failure patients and underline that a multiparametric approach can be useful also in heart failure patients with low Hb levels.
As regards the treatment of anaemia and iron deficiency, several trials have been conducted in recent years. The RED-HF trial12 failed to demonstrate a beneficial role of darbepoetin alpha in heart failure patients, since the treatment did not improve clinical outcomes in patients with systolic heart failure and a mild-to-moderate anaemia degree. Thus, the use of erythropoietin-stimulating agents is currently not recommended in heart failure patients. As regards iron deficiency, oral iron replacement therapy has many concerns regarding its reduced tolerability and low gastrointestinal absorption, mostly in chronic disease, as heart failure, where gastrointestinal absorption is already impaired.10 All the trials assessing the effects of oral iron support on quality of life and functional capacity failed to demonstrate a clinical benefit. In particular, the recent IRONOUT-HF trial13 demonstrated that oral iron supplementation minimally increases iron stores and does not improve exercise capacity in HFrEF patients with iron deficiency. Moreover, an IRONOUT-HF sub-analysis14 failed to identify a subset of responders more likely to develop a clinical benefit from oral iron therapy, confirming that its routine use in patients with symptomatic HFrEF and iron deficiency is not recommended. Thus, in recent years, several trials concentrated on the role of intravenous ferric carboxymaltose in heart failure patients, thus avoiding the gastrointestinal tract, and increasing circulating iron levels and its availability for target organs. The FAIR-HF15 and the CONFIRM-HF16 trial were designed to assess the effects on quality of life, New York Heart Association (NYHA) class, and exercise capacity of (intravenous) ferric carboxymaltose compared to placebo in HFrEF patients with iron deficiency. The trials’ results reported an improvement of symptoms, functional capacity, and quality of life with an acceptable side-effect profile in the group treated with ferric carboxymaltose regardless of the presence of an anaemic status. Moreover, the CONFIRM-HF trial,16 as secondary endpoint, reported a significant reduction in the risk of hospitalizations for worsening heart failure, whereas the number of deaths were comparable between groups. In these trials, exercise capacity was assessed through the 6-min walking test, whereas the FERRIC-HF trial17 confirmed the effects of ferric carboxymaltose on exercise capacity also with the use of CPET, reporting a significant improvement in maximal exercise capacity as measured by peak VO2 (ml/min per kg) and a trend toward an increase in absolute peak VO2 values and exercise duration. Similarly, the EFFECT-HF trial18 confirmed a slight improvement in peak VO2 in heart failure patients with iron deficiency treated with ferric carboxymaltose. Thus, European Society of Cardiology guidelines19 recommend to consider the use of ferric carboxymaltose in symptomatic heart failure patients (if serum ferritin <100 μg/, or if ferritin between 100 and 299 μg/l and transferrin saturation <20%) in order to alleviate heart failure symptoms and improve exercise capacity and quality of life (Class of recommendations: IIa, Level of evidence A). However, no robust data are at the moment available on the effects of ferric carboxymaltose on major cardiovascular outcomes and long-term prognosis; large randomized studies are needed in this setting in order to integrate the clinical benefit of intravenous iron supplementation with a relevant prognostic role.
Diabetes
Diabetes mellitus is a common comorbidity in heart failure and has a significant negative impact on prognosis. Diabetes ranges from 10% to 30% in HFrEF and is present in about 45% of patients with HFpEF, and the prevalence of comorbid diabetes mellitus is increasing most significantly in those with new-onset heart failure.20,21 In particular, type 2 diabetes and HFpEF are frequently seen together in older, hypertensive, and female patients and often underdiagnosed, hence the importance of correct risk stratification in heart failure patients also in presence of a preserved systolic function.22 In addition, newly diagnosed type 2 diabetes and prediabetes are highly prevalent in patients hospitalized for worsening HFrEF and independently associated with an increased risk of both all-cause and cardiovascular mortality.23 On the other side, heart failure is frequently observed in diabetes mellitus patients. In the Framingham Heart Study,24 heart failure was shown to be twice as common in men and five times more common in women with diabetes mellitus between the ages of 45 and 74 years when compared with age-matched non-diabetic controls. Older age, longer duration of diabetes, use of insulin and lower body mass index were independent risk factors for the presence of heart failure.
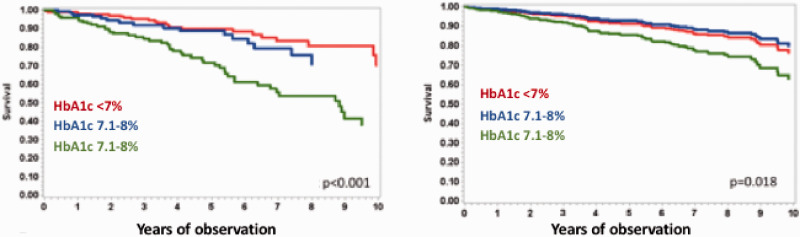
As regards prognosis, in the Swedish Heart Failure Registry,25 mortality was 37% in diabetic patients with heart failure. In older adults enrolled in the Medicare programme, those with diabetes and heart failure had a mortality of 32.7 per 1000 person-years compared with 3.7 per 1000 person-years among those without heart failure.25 In the REACH Registry,26 diabetes was associated with a 33% greater risk of hospitalization for heart failure and the presence of heart failure at baseline was independently associated with cardiovascular death and hospitalization for heart failure. However, in a recent analysis of the MECKI score database27 performed in 3927 HFrEF patients, a worse prognosis was observed in patients with poor glycaemic control (HbA1c >8%) (Figure 1), whereas the presence of a diabetic status and ongoing anti-diabetic treatment were not related to prognosis after correction for multiple confounders.8
Figure 1.
Effect of glycaemic control on long-term prognosis (composite of cardiovascular death, urgent heart transplant, or left ventricular assist device implantation) in diabetic heart failure patients at baseline (panel on the left) and after correction for multiple confounders (ejection fraction, oxygen uptake at peak exercise, haemoglobin, minute ventilation/carbon dioxide production relationship slope, renal function and sodium plasma levels) (panel on the right).
Data from the MECKI score database. Modified from Johansson et al.25
HbA1c: glycated haemoglobin.
Left ventricular (LV) dysfunction in patients with type 2 diabetes may present as HFpEF, HFrEF or heart failure with mid-range ejection fraction. LV diastolic dysfunction is frequent in both pre-diabetes and overt diabetes, and its severity correlates with insulin resistance and the degree of glucose dysregulation. The complex relationship between diabetes and heart failure can be summarized in two situations: 1) diabetes as a risk factor favouring and worsening coronary artery disease (CAD) and consequent ischaemic systolic dysfunction; 2) diabetes as a direct cause of cardiomyopathy and heart failure, both in the form of HFpEF or HFrEF, also known as diabetic cardiomyopathy.28 The main mechanisms of cardiac dysfunction are the resistance to the metabolic actions of insulin in heart tissue, compensatory hyperinsulinaemia and the progression of hyperglycaemia. Hyperglycaemia causes up-regulation of the sodium-glucose cotransporter-2 (SGLT2), leading to increased proximal renal sodium absorption, volume expansion and decreased responsiveness to diuretics.28 Patients with diabetes have increased sympathetic and renin–angiotensin system activation and alterations in sodium handling, which predisposes to congestion, cardiorenal syndrome and decreased diuretic responsiveness.28
Diabetes mellitus as risk factor of CAD and consequent heart failure
Diabetes has a different impact on prognosis according to its duration, comorbidities and organ damage. We can identify three different cardiovascular risk classes in diabetic patients:29
Very high cardiovascular risk. Diabetes and existing cardiovascular disease, or end organ damage, or ≥3 cardiovascular risk factors or diabetes duration >20 years;
High cardiovascular risk. Diabetes with duration >10 years without end organ damage, but with an additional cardiovascular risk factor;
Moderate cardiovascular risk. Young patients (type 1 diabetes <35 years; type 2 diabetes <50 years) with a diabetes duration <10 years without other cardiovascular risk factors.
The presence of type 2 diabetes does not worsen per se the rate of overall survival, but it does interact significantly with the aetiology, substantially increasing the risk of death among patients with ischaemic heart failure by 32%, irrespective of echocardiographic parameters. High levels of plasma High Sensitivity Troponin I were also found to be stronger predictors of overall mortality in heart failure patients with type 2 diabetes than in their counterparts without diabetes.30
Diabetic cardiomyopathy
Diabetic cardiomyopathy is a distinctive heart failure form observed in the diabetic population and occurs in absence of other cardiovascular disease, such as CAD, hypertension, valvular and congenital heart disease.28 The exact pathophysiology of diabetic cardiomyopathy is still under investigation; however, a main role is represented by the state of insulin resistance. Insulin resistance leads to change in substrate metabolism and cardiac lipotoxicity, advanced glycated end-products deposition, endothelial and microvascular dysfunction, inappropriate neurohormonal response, oxidative stress and subcellular component abnormalities promoting all the baseline components of cardiac dysfunction. The disease can have two distinct phenotypes: a hypertrophic-restrictive dominant pathophysiology and HFpEF or a dilatative phenotype with HFrEF. It is still under investigation whether the two forms are the evolution of the same disease or are two different diseases and this distinction is pivotal in terms of pharmacological treatment, because currently we have efficacious tools for dilated/HFrEF, but still few evidences for the treatment of restrictive/HFpEF.31 The key problem is the absence of a universally accepted definition of diabetic cardiomyopathy, which makes studies of epidemiology, pathophysiology, clinical characteristics, and prognosis challenging. Irrespective of the disease phenotype, its occurrence is responsible for an adverse prognosis. However, in the setting of diabetic cardiomyopathy, no target treatments have been tested, thus clinical trials are needed to define the role of available heart failure therapies and/or to find new therapeutic targets for this clinical condition.
Treatment of diabetic patients with heart failure
Diabetes has received increasing attention due to the results of clinical trials that have shown beneficial effects of new oral antidiabetics on heart failure outcomes. Guideline-based medical and device therapies are the same in heart failure patients with and without diabetes;19,32 some dose adjustments in diabetic patients may be necessary because of renal dysfunction.33
In the treatment of patients affected by both diabetes and cardiovascular diseases, of most importance is a continuous exercise training programme that contributes to improve many metabolic functions, such as peripheral sensitivity to insulin, impaired lipid profile, vascular reactivity and functional capacity. On this topic, the European Association of Preventive Cardiology reported in a recent position paper34 the positive effects of exercise training in diabetes, providing practical recommendations and methods to prescribe exercise training in order to reach specific targets and improve quality of life, glycaemic control, cardiovascular fitness, and prognosis.
As regards the pharmacological approach, first-line treatment of diabetes in heart failure should include metformin and SGLT2 inhibitors;29 saxagliptin, pioglitazone and rosiglitazone are not recommended for patients with diabetes and heart failure.9
Cardiovascular outcome trials with SGLT2 inhibitors (empagliflozin, canagliflozin, dapagliflozin, and ertugliflozin) in the last few years demonstrated beneficial effects in terms of heart failure hospitalization in diabetic patients with a risk reduction of about 30%, regardless of the presence of heart failure at baseline. Moreover, dapagliflozin and empagliflozin reduced the risk of worsening heart failure/heart failure hospitalizations or cardiovascular death in patients with HFrEF, regardless of the presence of diabetes at baseline.35,36 A sub-analysis of the DAPA-HF trial37 demonstrated significant and similar benefit and safety of dapagliflozin in patients taking or not sacubitril/valsartan, thus dispelling the doubt that the association between the two drugs caused excessive diuresis and hypotension. Furthermore, the results of this recent sub-analysis37 indicated that the use of both agents together could lower morbidity and mortality in diabetic patients with HFrEF, suggesting that the mechanisms of action of these drugs are likely to be distinct and potentially complementary. The US Food and Drug Administration recently approved the use of dapagliflozin for HFrEF patients regardless of the presence of diabetes at baseline and the drug is under evaluation by the European Medical Agency. Thus, in the near future, gliflozins will be introduced as heart failure specific drugs, newly changing the therapeutic and prognostic pathway of such a complex disease.
Atrial fibrillation
Atrial fibrillation is common in patients affected by chronic heart failure and its prevalence is growing in the more advanced heart failure stages with increased morbidity and mortality.38 Data from the EuroHeart Failure Survey reported that about 20% of patients with heart failure exhibit atrial fibrillation and that its prevalence reaches 40% in patients with advanced disease. There is a complex interaction between these two conditions since heart failure predisposes to atrial fibrillation and occurrence of atrial fibrillation in patients with heart failure worsens symptoms and complicates therapeutic management. This is due to several detrimental effects, including heart rate increase, reduced left ventricular loading, irregular periods of ventricular filling and decreased cardiac output and a range of pathophysiologic mechanisms, including rapid ventricular rates, irregularity and loss of atrial systole. In turn, heart failure can lead to atrial fibrillation through elevated atrial pressure and activation of the sympathetic and renin–angiotensin systems.
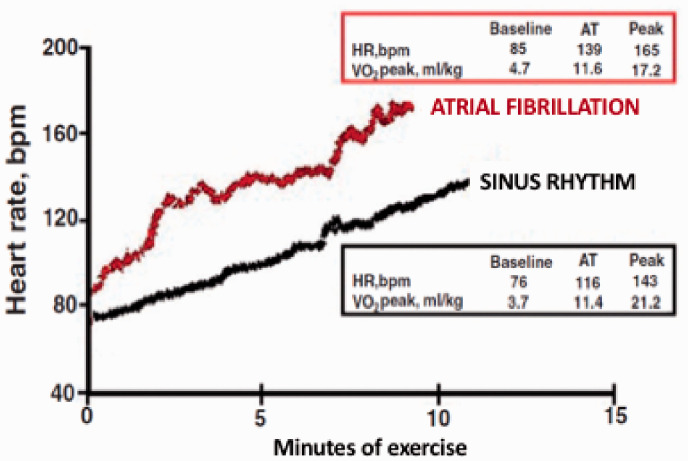
The impact of atrial fibrillation on heart failure patients during exercise is complex. CPET with gas exchange measurements is nowadays a cornerstone in the clinical management of patients with heart failure. Accordingly, besides classic risk factors – including age, NYHA class and LV ejection fraction – both peak VO2 and oxygen uptake at the anaerobic threshold (VO2AT) have proven to be strong independent predictors of outcome in heart failure patients. The analysis of patients that are part of the MECKI score database underlined the importance of CPET also in patients with atrial fibrillation. In particular it allowed to observe in HFrEF patients affected by atrial fibrillation a peculiar response to exercise. First, permanent atrial fibrillation is associated with more compromised exercise performance at CPET, expressed by lower values of peak VO2, lower value of O2 pulse and lower workload achieved at peak exercise of about 20% when compared with HFrEF patients in sinus rhythm, but higher values of VO2AT.39 The postponed anaerobic threshold is likely due to different heart rate kinetics during exercise so that the increase at the regimen of exercise is higher in atrial fibrillation patients; consequently cardiac output (CO) increase is anticipated and the anaerobic threshold, which is CO dependent, postponed40 (Figure 2). Anaerobic threshold is used to confirm the clinical value of CPET information obtained at peak exercise and it has been proposed as a strong alternative to peak VO2, being independent of exercise protocol and exercise duration. It is important to note that VO2AT has a prognostic value in patients with atrial fibrillation as in those with sinus rhythm, but VO2AT in atrial fibrillation patients should be analysed differently from that of sinus rhythm patients with a different cut-off for poor prognosis. As a matter of fact, the prognostic negative VO2AT cut-off value is <11.7 ml/kg per min in sinus rhythm heart failure patients and <12.8 ml/kg per min in atrial fibrillation heart failure patients.41
Figure 2.
Explicative example of different kinetics of the exercise-induced heart rate (HR) response between a heart failure patient (male, 55 years old) on sinus rhythm (black line) and another patient (male, 51 years old) with atrial fibrillation (red line). In spite of a significantly lower peak oxygen uptake (VO2 peak), the atrial fibrillation patient shows an oxygen uptake at anaerobic threshold (AT) similar to the sinus rhythm patient. Modified from Magri et al.40
Other important information about the impact of atrial fibrillation in heart failure patients is derived from the analysis of a population of patients who were part of the MECKI score database.42 First, the analysis of 3447 heart failure patients (85% males) with mean age of 61.5±11.8 years and median ejection fraction of 34.9% followed for a median period of 3.15 years documented that atrial fibrillation in HFrEF is a marker of disease severity but not an independent prognostic indicator. In particular, applying a multivariable model based on all variables significant at univariable analysis (ejection fraction, peak VO2, VE/VCO2 slope, sodium, kidney function, Hb, beta-blockers and digoxin), atrial fibrillation was no longer associated with adverse outcome, either in the whole cohort or in a subgroup of patients with atrial fibrillation or sinus rhythm matched for clinical characteristics and follow-up.5 This suggests that atrial fibrillation is linked to heart failure prognosis because it more frequently presents in severe heart failure but it does not directly influence heart failure prognosis, that is, atrial fibrillation should be seen a red flag for poor prognosis. However, this undeniable datum has nothing to do with cardioversion need or utility.
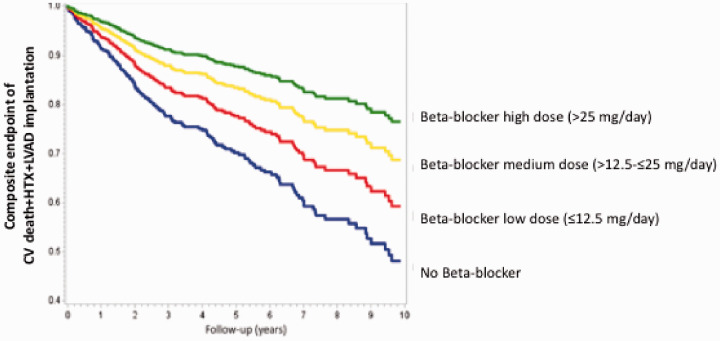
Another debated question was about the efficacy of β-blockers in patients with heart failure and concomitant atrial fibrillation. The analysis of data derived from 958 heart failure patients with atrial fibrillation as part of the MECKI score database allowed to define that, at 10-year follow-up, patients treated with β-blockers had a better outcome with no effects as regards β1-selective drugs (53%) versus β1-β2 blockers (47%) and that survival improved in parallel with β-blocker dose increase (Figure 3).43
Figure 3.
Kaplan–Meier analysis of study endpoint (composite of cardiovascular (CV) death, urgent heart transplant (HTX) or left ventricular assist device (LVAD) implantation) according to β-blocker equivalent dose at a 10-year follow-up (p < 0.0001). Blue line = no β-blocker, red line = low dose (≤12.5 mg/day), yellow line = medium dose (>12.5–≤25 mg/day), green line = high dose (25 mg/day). Modified from Campodonico et al.42
New studies are still needed regarding the dangerous liaison between atrial fibrillation and heart failure: first, on the effects of sinus rhythm restoration on exercise performance, drugs, treatment and prognosis; similarly, different rate control strategies in atrial fibrillation heart failure patients.
Footnotes
Author contribution: SP, ABS and JC contributed to the conception or design of the work, drafted the manuscript and critically revised the manuscript. All gave final approval and agree to be accountable for all aspects of work ensuring integrity and accuracy.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Correale M, Paolillo S, Mercurio V, et al. Comorbidities in chronic heart failure: An update from Italian Society of Cardiology (SIC) Working Group on Heart Failure. Eur J Intern Med 2020; 71: 23–31. [DOI] [PubMed] [Google Scholar]
- 2.Groenveld HF, Januzzi JL, Damman K, et al. Anemia and mortality in heart failure patients a systematic review and meta-analysis. J Am Coll Cardiol 2008; 52: 818–827. [DOI] [PubMed] [Google Scholar]
- 3.Tang YD, Katz SD. Anemia in chronic heart failure: Prevalence, etiology, clinical correlates, and treatment options. Circulation 2006; 113: 2454–2461. [DOI] [PubMed] [Google Scholar]
- 4.Agostoni P, Salvioni E, Debenedetti C, et al. Relationship of resting hemoglobin concentration to peak oxygen uptake in heart failure patients. Am J Hematol 2010; 85: 414–417. [DOI] [PubMed] [Google Scholar]
- 5.Berry C, Poppe KK, Gamble GD, et al. Prognostic significance of anaemia in patients with heart failure with preserved and reduced ejection fraction: Results from the MAGGIC individual patient data meta-analysis. QJM 2016; 109: 377–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agostoni P, Corra U, Cattadori G, et al. Metabolic exercise test data combined with cardiac and kidney indexes, the MECKI score: A multiparametric approach to heart failure prognosis. Int J Cardiol 2013; 167: 2710–2718. [DOI] [PubMed] [Google Scholar]
- 7.Von Haehling S, Jankowska EA, van Veldhuisen DJ, et al. Iron deficiency and cardiovascular disease. Nat Rev Cardiol 2015; 12: 659–669. [DOI] [PubMed] [Google Scholar]
- 8.Dinatolo E, Dasseni N, Metra M, Lombardi C, et al. Iron deficiency in heart failure. J Cardiovasc Med (Hagerstown) 2018; 19: 706–716. [DOI] [PubMed] [Google Scholar]
- 9.Jankowska EA, Rozentryt P, Witkowska A, et al. Iron deficiency predicts impaired exercise capacity in patients with systolic chronic heart failure. J Card Fail 2011; 17: 899–906. [DOI] [PubMed] [Google Scholar]
- 10.Magri D, de Martino F, Moscucci F, et al. Anemia and iron deficiency in heart failure: Clinical and prognostic role. Heart Fail Clin 2019; 15: 359–369. [DOI] [PubMed] [Google Scholar]
- 11.Cattadori G, Agostoni P, Corra U, et al. Heart failure and anemia: Effects on prognostic variables. Eur J Intern Med 2017; 37: 56–63. [DOI] [PubMed] [Google Scholar]
- 12.Swedberg K, Young JB, Anand IS, et al. Treatment of anemia with darbepoetin alfa in systolic heart failure. N Engl J Med 2013; 368: 1210–1219. [DOI] [PubMed] [Google Scholar]
- 13.Lewis GD, Malhotra R, Hernandez AF, et al. Effect of oral iron repletion on exercise capacity in patients with heart failure with reduced ejection fraction and iron deficiency: The IRONOUT HF randomized clinical trial. JAMA 2017; 317: 1958–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ambrosy AP, Lewis GD, Malhotra R, et al. Identifying responders to oral iron supplementation in heart failure with a reduced ejection fraction: A post-hoc analysis of the IRONOUT-HF trial. J Cardiovasc Med (Hagerstown) 2019; 20: 223–225. [DOI] [PubMed] [Google Scholar]
- 15.Anker SD, Comin Colet J, Filippatos G, et al. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med 2009; 361: 2436–2448. [DOI] [PubMed] [Google Scholar]
- 16.Ponikowski P, van Veldhuisen DJ, Comin-Colet J, et al. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency. Eur Heart J 2015; 36: 657–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okonko DO, Grzeslo A, Witkowski T, et al. Effect of intravenous iron sucrose on exercise tolerance in anemic and nonanemic patients with symptomatic chronic heart failure and iron deficiency FERRIC-HF: A randomized, controlled, observer-blinded trial. J Am Coll Cardiol 2008; 51: 103–112. [DOI] [PubMed] [Google Scholar]
- 18.Van Veldhuisen DJ, Ponikowski P, van der Meer P, et al. Effect of ferric carboxymaltose on exercise capacity in patients with chronic heart failure and iron deficiency. Circulation 2017; 136: 1374–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 20.McHugh K, DeVore AD, Wu J, et al. Heart failure with preserved ejection fraction and diabetes: JACC State-of-the-Art Review. J Am Coll Cardiol 2019; 73: 602–611. [DOI] [PubMed] [Google Scholar]
- 21.Solang L, Malmberg K, Ryden L. Diabetes mellitus and congestive heart failure. Further knowledge needed. Eur Heart J 1999; 20: 789–795. [DOI] [PubMed] [Google Scholar]
- 22.Gohar A, Kievit RF, Valstar GB, et al. Opportunistic screening models for high-risk men and women to detect diastolic dysfunction and heart failure with preserved ejection fraction in the community. Eur J Prev Cardiol 2019; 26: 613–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pavlovic A, Polovina M, Ristic A, et al. Long-term mortality is increased in patients with undetected prediabetes and type-2 diabetes hospitalized for worsening heart failure and reduced ejection fraction. Eur J Prev Cardiol 2019; 26: 72–82. [DOI] [PubMed] [Google Scholar]
- 24.Kannel WB, McGee DL. Diabetes and cardiovascular disease. The Framingham study. JAMA 1979; 241: 2035–2038. [DOI] [PubMed] [Google Scholar]
- 25.Johansson I, Edner M, Dahlstrom U, et al. Is the prognosis in patients with diabetes and heart failure a matter of unsatisfactory management? An observational study from the Swedish Heart Failure Registry. Eur J Heart Fail 2014; 16: 409–418. [DOI] [PubMed] [Google Scholar]
- 26.Cavender MA, Steg PG, Smith SC, Jr, et al. Impact of diabetes mellitus on hospitalization for heart failure, cardiovascular events, and death: Outcomes at 4 years from the Reduction of Atherothrombosis for Continued Health (REACH) Registry. Circulation 2015; 132: 923–931. [DOI] [PubMed] [Google Scholar]
- 27.Paolillo S, Salvioni E, Perrone Filardi P, et al. Long-term prognostic role of diabetes mellitus and glycemic control in heart failure patients with reduced ejection fraction: Insights from the MECKI Score database. Int J Cardiol 2020; 317: 103–110. [DOI] [PubMed] [Google Scholar]
- 28.Paolillo S, Marsico F, Prastaro M, et al. Diabetic cardiomyopathy: Definition, Diagnosis, and therapeutic implications. Heart Fail Clin 2019; 15: 341–347. [DOI] [PubMed] [Google Scholar]
- 29.Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J 2020; 41: 255–323. [DOI] [PubMed] [Google Scholar]
- 30.Rorth R, Jhund PS, Kristensen SL, et al. The prognostic value of troponin T and N-terminal pro B-type natriuretic peptide, alone and in combination, in heart failure patients with and without diabetes. Eur J Heart Fail 2019; 21: 40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seferovic PM, Petrie MC, Filippatos GS, et al. Type 2 diabetes mellitus and heart failure: A position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2018; 20: 853–872. [DOI] [PubMed] [Google Scholar]
- 32.Scalvini S, Grossetti F, Paganoni AM, et al. Impact of in-hospital cardiac rehabilitation on mortality and readmissions in heart failure: A population study in Lombardy, Italy, from 2005 to 2012. Eur J Prev Cardiol 2019; 26: 808–817. [DOI] [PubMed] [Google Scholar]
- 33.Cunha FM, Pereira J, Marques P, et al. Diabetic patients need higher furosemide doses: A report on acute and chronic heart failure patients. J Cardiovasc Med (Hagerstown) 2020; 21: 21–26. [DOI] [PubMed] [Google Scholar]
- 34.Kemps H, Krankel N, Dorr M, et al. Exercise training for patients with type 2 diabetes and cardiovascular disease: What to pursue and how to do it. A Position Paper of the European Association of Preventive Cardiology (EAPC). Eur J Prev Cardiol 2019; 26: 709–727. [DOI] [PubMed] [Google Scholar]
- 35.McMurray JJV, DeMets DL, Inzucchi SE, et al. A trial to evaluate the effect of the sodium-glucose co-transporter 2 inhibitor dapagliflozin on morbidity and mortality in patients with heart failure and reduced left ventricular ejection fraction (DAPA-HF). Eur J Heart Fail 2019; 21: 665–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Packer M, Anker SD, Butler J, et al.; on behalf of the EMPEROR-Reduced Trial Investigators. Cardiovascular and Renal Outcomes With Empagliflozin in Heart Failure. N Engl J Med. Epub ahead of print 29 August 2020. DOI: 10.1056/NEJMoa2022190.
- 37.Solomon SD, Jhund PS, Claggett BL, et al. Effect of dapagliflozin in patients with HFrEF treated with sacubitril/valsartan: The DAPA-HF trial. JACC Heart Fail. Epub ahead of print 8 July 2020. DOI: 10.1016/j.jchf.2020.04.008. [DOI] [PubMed] [Google Scholar]
- 38.Maisel WH, Stevenson LW. Atrial fibrillation in heart failure: Epidemiology, pathophysiology, and rationale for therapy. Am J Cardiol 2003; 91: 2D–8D. [DOI] [PubMed] [Google Scholar]
- 39.Agostoni P, Emdin M, Corra U, et al. Permanent atrial fibrillation affects exercise capacity in chronic heart failure patients. Eur Heart J 2008; 29: 2367–2372. [DOI] [PubMed] [Google Scholar]
- 40.Palermo P, Magri D, Sciomer S, et al. Delayed anaerobic threshold in heart failure patients with atrial fibrillation. J Cardiopulm Rehabil Prev 2016; 36: 174–179. [DOI] [PubMed] [Google Scholar]
- 41.Magri D, Agostoni P, Corra U, et al. Deceptive meaning of oxygen uptake measured at the anaerobic threshold in patients with systolic heart failure and atrial fibrillation. Eur J Prev Cardiol 2015; 22: 1046–1055. [DOI] [PubMed] [Google Scholar]
- 42.Paolillo S, Agostoni P, Masarone D, et al. Prognostic role of atrial fibrillation in patients affected by chronic heart failure. Data from the MECKI score research group. Eur J Intern Med 2015; 26: 515–520. [DOI] [PubMed] [Google Scholar]
- 43.Campodonico J, Piepoli M, Clemenza F, et al. Dose-dependent efficacy of beta-blocker in patients with chronic heart failure and atrial fibrillation. Int J Cardiol 2018; 273:141–146. [DOI] [PubMed] [Google Scholar]