Abstract
Aim
More than half of microRNAs are located in genes. LncRNAs are host genes of intronic microRNAs that regulate intracellular splicing to form pre-miRNAs that are processed to mature miRNAs. MicroRNAs work as partners or antagonists of their host lncRNAs by fine-tuning their target genes. However, whether lncRNA-MIR503HG (miR-503 host gene) is co-transcribed with miR-503 and affects miR-503 splicing, thereby affecting its target gene Bcl-2 expression and cell mitochondrial apoptotic pathway in diabetic nephropathy (DN) is currently unknown.
Methods
Human proximal tubular (HK-2) cells cultured in high glucose were transfected with lncRNA MIR503HG overexpression/inhibition plasmid and miR-503 mimics/inhibitor. Real-time quantitative PCR was used to measure the expression levels of lncRNA MIR503HG, pre-miR-503, miR-503 and Bcl-2. Western blot was used to measure the protein expressions of Bcl-2, Bax, Cytc and cleaved-caspase 9/3. Annexin V/PI flow cytometry was used to measure apoptosis.
Results
Host lncRNA MIR503HG was co-transcribed with miR-503. MIR503HG regulated the expression of miR-503 by affecting miR-503 splicing synthesis. In the presence of high glucose, the expression levels of lncRNA MIR503HG and miR-503 were up-regulated in HK-2 cells cultured in high glucose. Bcl-2 expression was inhibited and levels of apoptosis-related proteins Cytc and Bax were increased in HK-2 cells cultured in high glucose, all of which promoted the caspase cascade reaction, leading to increased caspase-9 and caspase-3 shear fragments inducing apoptosis of the mitochondrial pathway. Inhibition of MIR503HG led to a reduction in miR-503 expression, up-regulated its target gene Bcl-2, inhibited the expression levels of Bax and other apoptosis-related proteins and attenuated HK-2 cell apoptosis induced by high glucose. Co-transfection of miRNA-503 partially offset the effect of MIR503HG-siRNA.
Conclusion
MIR503HG indirectly regulates Bcl-2 by promoting the co-transcription of miRNA-503 to participate high-glucose-induced proximal tubular cell apoptosis, providing a new target for diabetic nephropathy treatment.
Keywords: diabetic nephropathy, lncRNA MIR503HG, miR-503, host gene, apoptosis
Introduction
According to the International Diabetes Federation (IDF) Diabetes Atlas ninth edition 2019 (www.diabetesatlas.org), approximately 463 million adults (20–79 years) were living with diabetes; by 2045 this will rise to 700 million. About 40% of diabetic patients eventually develop diabetic nephropathy.1 It is thought that glomerular damage is the main cause of kidney damage in diabetic nephropathy; however, several lines of evidence suggest that renal tubular damage is closely related to the progression of DN and plays a vital role in kidney damage, especially type 2 diabetes;2,3 nevertheless, the underlying molecular mechanism remains unclear.
Non-coding RNAs are divided into two categories: long-chain non-coding RNAs (lncRNAs) and short-chain non-coding RNAs. In the past, several studies reported that lncRNAs and microRNAs were involved in the occurrence and development of a variety of diseases. Recently, the interaction of miRNAs and lncRNAs was found to play vital physiological and pathophysiological roles in several diseases, especially lncRNAs as the host genes of miRNAs. More than half of miRNAs are located in protein-coding or non-coding genes. As a host gene of intronic microRNA, lncRNA regulates intracellular splicing to form pre-miRNA which is processed to mature miRNA. MiRNAs work as partners or antagonists of their host lncRNAs by fine-tuning their target genes.4
LncRNA MIR503HG is located in the 134543377-134546630 region of the X chromosome in the human genome and is the host gene of miR-503. MIR503HG, also known as H19X is an intergenic lncRNA and its gene locus encodes two microRNAs (miR-503, miR-424). And miR-503 is embedded in the first intron;5 therefore miR-503 is an intronic microRNA. In hepatocellular carcinoma (HCC) cell lines, the expression levels of miR-335 were significantly correlated with those of its protein-coding host gene, MEST, supporting the notion that the intronic miR-335 was co-expressed with its host gene under the control of host gene promoter.6 LncRNA MIR503HG exerted a carcinogenic effect in ALK-negative undifferentiated large cell lymphoma by inducing miR-503 expression.7 Our previous study showed that the expression of miRNA-503 was apparently higher in the glomeruli of the KKAy-untreated mice, and was inhibited by losartan treatment.8 Pescador et al reported that the combination of miR-503 and miR-138 could distinguish diabetic from obese diabetic patients.9 Sheikhbahaei et al reported that miR-503 was stable enough in the blood to be a potential diagnostic marker for ischemic stroke in diabetic patients.10 The miR-503 in the placenta tissues of patients with gestational diabetes mellitus was significantly up-regulated, and the miR-503 in peripheral blood samples increased, and it was positively correlated with the blood glucose concentration.11 Yan et al reported that the expression levels of miR-503-5p were significantly associated with diabetes and arterial stenosis. Overexpression of miR-503-5p significantly inhibited the proliferation of vascular smooth muscle cells and improved carotid artery stenosis.12 In HG-induced microvascular cells injury, miR-503/Apelin-12 enhanced inflammation and oxidative stress by regulating JNK and p38MAPK pathway, suggesting a potential therapeutic target for diabetic angiopathy (DA).13 Zha et al reported for the first time that miR-503 was highly expressed in podocytes treated with high glucose in vitro and diabetic rat kidney tissues, inhibiting E2F transcription factor 3 (E2F3), promoting podocyte apoptosis, and participating in the pathological mechanism of DN.14
Targetscan software predicted that Bcl-2 was the direct target of miR-503. Qu et al found that anti-apoptotic factor Bcl-2 was a direct target of miRNA-503 using luciferase reporter gene assay.15 Whether lncRNA MIR503HG regulates the expression of mature miR-503 through co-transcription with miR-503 remains unclear.
In the present study, we transfected lncRNA MIR503HG overexpression/inhibitor plasmid as well as miR-503 mimics/inhibitor into human renal tubular epithelial cells cultured in high glucose, and measured the expression level changes of precursor and mature miR-503, exploring whether lncRNA MIR503HG affected the splicing of miR-503 through co-transcription with miR-503, thereby indirectly affecting the expression of its target gene Bcl-2 and mitochondrial apoptosis pathway, and participating in renal tubular epithelial cell apoptosis induced by high glucose.
Materials and Methods
Cell Culture
The human proximal tubular epithelial cell line (HK-2, ATCC®CRL-2190™) was cultured in low glucose DMEM (Hyclone, Logan, UT, USA) medium containing 10% fetal calf serum (Gemini, West Sacramento, CA, USA) in a 37 °C, 5% CO2 incubator. When HK-2 cells grew to 70% to 80% confluence, they were trypsinized and passaged.
Oligonucleotides, Plasmids and Transfection
The MIR503HG small interfering (si) RNA, and inhibitor, mimics and negative control (NC) RNAs of miR-503-5p were synthesized and purified by JTS Scientific (Wuhan, China). The RNA sequences that mentioned above were as follows: MIR503HG-siRNA1: sense, CCACCCAAGUGUCCCAAAUTT, antisense, AUUUGGGACACUUGGGUGGTT; MIR503HG-siRNA2: sense, CCACACUUCUUUGUUCCAATT, antisense, UUGGAACAAAGAAGUGUGGTT; MIR503HG-siRNA3: sense, GACAAGAACUAAAGUGGAATT, antisense, UUCCACUUUAGUUCUUGUCTT; miR-503-5p mimics: sense, UAGCAGCGGGAACAGUUCUGCAG, antisense, GCAGAACUGUUCCCGCUGCUAUU; miR-503-5p inhibitor: CUGCAGAACUGUUCCCGCUGCUA; an siRNA which was not related to any human sequence was used as a negative control (NC): sense, UUCUCCGAACGUGUCACGUTT, antisense, ACGUGACACGUUCGGAGAATT; miR-503-5p inhibitor negative control: UUGUACUACACAAAAGUACUG. The MIR503HG overexpression plasmid (pcDNA 3.1-MIR503HG) was constructed using GenScript. According to the manufacturer’s instructions, oligonucleotides and plasmids mentioned above were transfected into cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA).
Real-Time qPCR Analysis
Total RNA was extracted with TRIzol (Invitrogen, Carlsbad, CA, USA). GoScript™ Reverse Transcription System (Promega, Madison, WI, USA) was used to reverse transcribe 1–2 μg RNA into template cDNA. We used the GoTaq®qPCR and RTqPCR system (Promega, Madison, WI, USA) on the CFX Connect™ optical module (Bio-Rad, Hercules, CA, USA) to perform fluorescent quantitative PCR in triplicate according to the manufacturer’s instructions. β-actin was used as an endogenous control. Levels of miR-503-5p were measured using miRNA qPCR measure kit (BioTeke, Beijing, China). U6 was used as an endogenous control. The primer sequences are shown in Table 1. The 2−ΔΔCT method was used to analyze the data, and each experiment had been run in triplicates.
Table 1.
Primers Used for Quantitative RT-PCR
| Gene | Primer Sequence5ʹ-3’ |
|---|---|
| miR-503-5p | F:TGTACCACCTTGTCGG R:TGCTGTTGCCATGAGAT |
| pre-miR-503 | F: CCCTAGCAGCGGGAACAGT R: CCTGGCAGCGGAAACAAT |
| Bcl-2 | F:CCACCAAGAAAGCAGGAAACC R:GCAGGATAGCAGCACAGGATT |
| β-actin | F:GGCACCCAGCACAATGAA R:TAGAAGCATTTGCGGTGG |
| lncRNA MIR503HG | F:ATAACCAGGGTCACAGAAA R:AAGCAGCATACAGTCATTCA |
| U6 | F:GCTTCGGCAGCACATATACT R:GTGCAGGGTCCGAGGTATTC |
Western Blotting
Cells were digested with pre-cooled RIPA lysate and protease inhibitors (Roche, Mannheim, BW, Germany) to extract total proteins and measure concentrations using the BCA method. SDS-PAGE was used to separate lanes containing 20 μg of proteins. Proteins were transferred to polyvinyl fluoride membranes (Millipore, Billerica, MA, USA) and then incubated in blocking buffer (5% skimmed milk) for 1 h. Membranes were then probed with anti-Bcl-2 (ProteinTech, 1:500), anti-Bax (ProteinTech, 1:500), anti-Cytc (Abcam, 1:500), anti-cleaved-caspase 9 (Abcam, 1:500) and anti-cleaved-caspase 3 at 4 °C overnight. The membranes were washed three times, each time for 10 minutes and then they were incubated in anti-rabbit IgG (Abcam, 1:5000) at room temperature for 45 minutes. The specific bands were revealed using the EC3 Imaging System (Analytik Jena US LLC, USA). Gel-Pro-Analyzer software was used to measure each band’s optical density.
Cell Apoptosis Analysis
We selected cells in logarithmic phase, prepared cell suspensions, and placed them in a 5% CO2, 37 °C incubator for 48 h. Cells were trypsinized and centrifuged. Cell suspensions were incubated with annexin V fluorescein isothiocyanate (FITC) and propidium iodide (PI) apoptosis kit (DOJINDO, Japan). After incubation, 400 μL 1× annexin V binding buffer was added into the cell suspensions and then analyzed using a flow cytometer (BD Bioscience, USA). The excitation wavelength was 488 nm and the emission wavelength was 530 nm. Approximately 10,000 cells were counted for each measurement.
Statistical Analysis
These experiments were repeated three times. SPSS 22.0 software was used for analysis. Measurement data were expressed as
Results
High Glucose Increased lncRNA MIR503HG Expression of HK-2 Cells
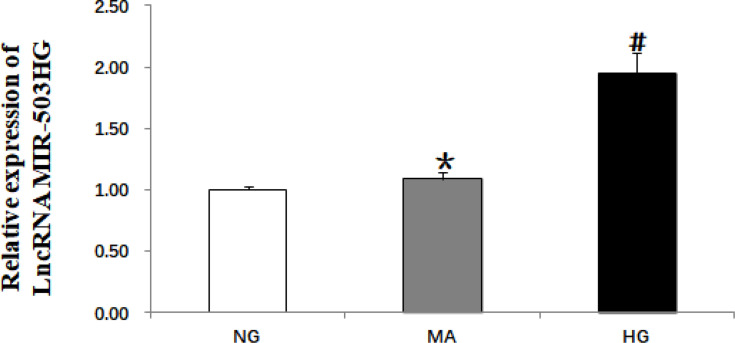
The cells were divided into normal glucose group (5.5 mmol/L glucose), hypertonic control group (5.5 mmol/L glucose + 24.5 mmol/L mannitol), and high-glucose group (30 mmol/L glucose). After culturing for 48 h, the total RNA of each group was extracted. Real-time quantitative PCR showed that, compared with the control group, the expression levels of lncRNA MIR503HG were significantly greater in the high-glucose group; the hypertonic control group showed no significant difference compared with the control group (Figure 1).
Figure 1.
High glucose induced increased expression of lncRNA MIR503HG in HK-2 cells (real-time quantitative PCR). Data from at least three separate experiments are shown. *P>0.05 vs NG, #P<0.05 vs NG.
Abbreviations: NG, normal glucose; MA, mannitol control; HG, high glucose.
LncRNA MIR503HG Was Co-Expressed with miR-503
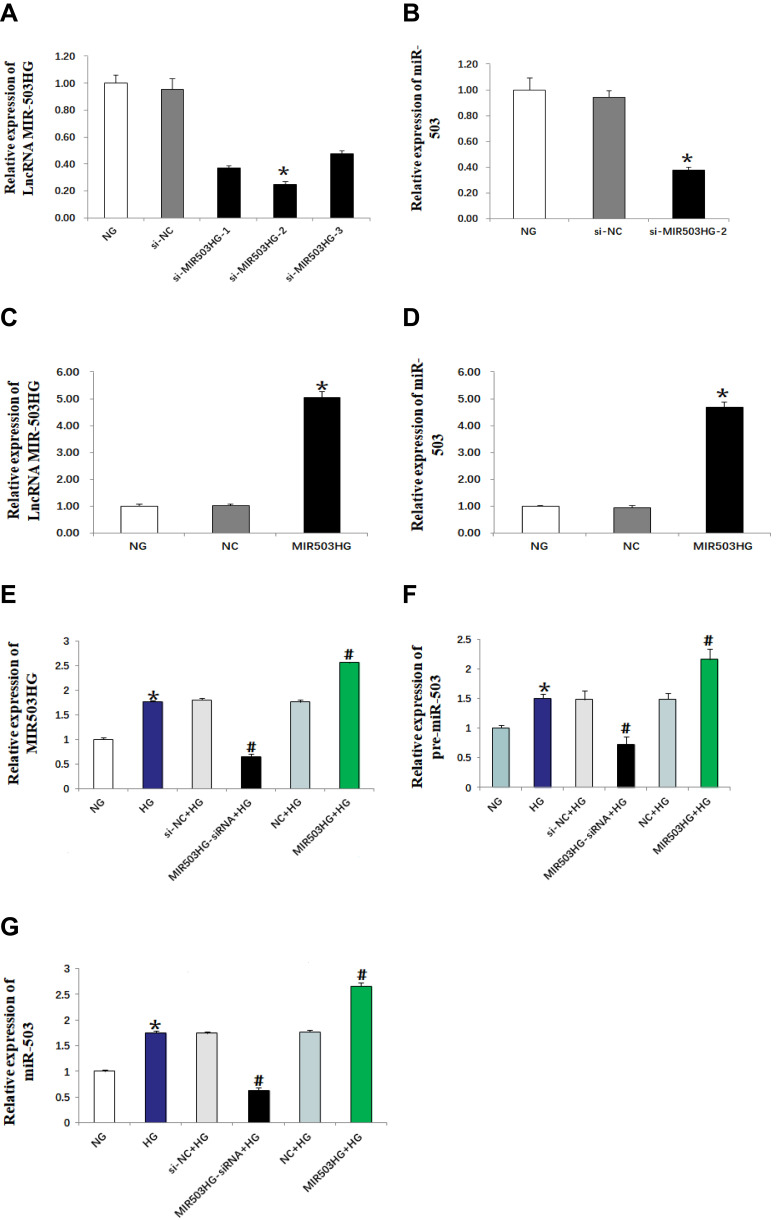
We constructed three lncRNA MIR503HG siRNAs, unrelated sequence, empty vector and overexpression vector. The siRNAs were transfected into HK-2 cells cultured in normal glucose for 48 h. The expression levels of lncRNA MIR503HG and miR-503 were measured using real-time quantitative PCR. We selected the si-MIR503HG-2 which had the best interference effect for subsequent experiments (Figure 2A). The results showed that, by inhibiting MIR503HG, the expression levels of MIR503HG and miR-503 were significantly reduced (Figure 2A and B), while overexpression of MIR503HG, MIR503HG and miR-503 were significantly increased (Figure 2C and D). HK-2 cells cultured in high glucose were transfected with unrelated sequence (siRNA control), lncRNA MIR503HG-siRNA, empty vector (NC) and lncRNA MIR503HG overexpression vector for 48 h. The results showed that the expression levels of lncRNA MIR503HG, pre-miR-503 and miR-503 in HK-2 cells cultured in high glucose were significantly greater than those of the normal glucose group. Inhibition of lncRNA MIR503HG significantly reduced expression levels of pre-miR-503 and miR-503, while overexpression of lncRNA MIR503HG significantly increased the expression levels of pre-miR-503 and miR-503 (Figure 2E–G). These results indicated that miR-503 and lncRNA MIR503HG were co-expressed. LncRNA MIR503HG regulated the expression of miR-503 by affecting the splicing synthesis of miR-503.
Figure 2.
MIR503HG and miR-503 were co-expressed (real-time quantitative PCR). Data from at least three separate experiments are shown. *P<0.05 vs NG, #P<0.05 vs HG.
Abbreviations: (A–D) NG, normal glucose; si-NC, unrelated sequence transfection; si-MIR503HG- (1–3), MIR503HG suppression plasmid transfection; NC, empty vector transfection; MIR503HG, MIR503HG overexpression vector transfection. (E–G) NG, normal glucose; HG, high-glucose; si-NC+HG, unrelated sequence transfection+high glucose; MIR503HG-siRNA+HG, MIR503HG suppression plasmid transfection+high glucose; NC+HG, empty vector transfection+high glucose; MIR503HG+HG, MIR503HG overexpression vector transfection+high glucose.
Inhibition of miR-503 Expression Up-Regulated the Expression of Its Target Gene Bcl-2
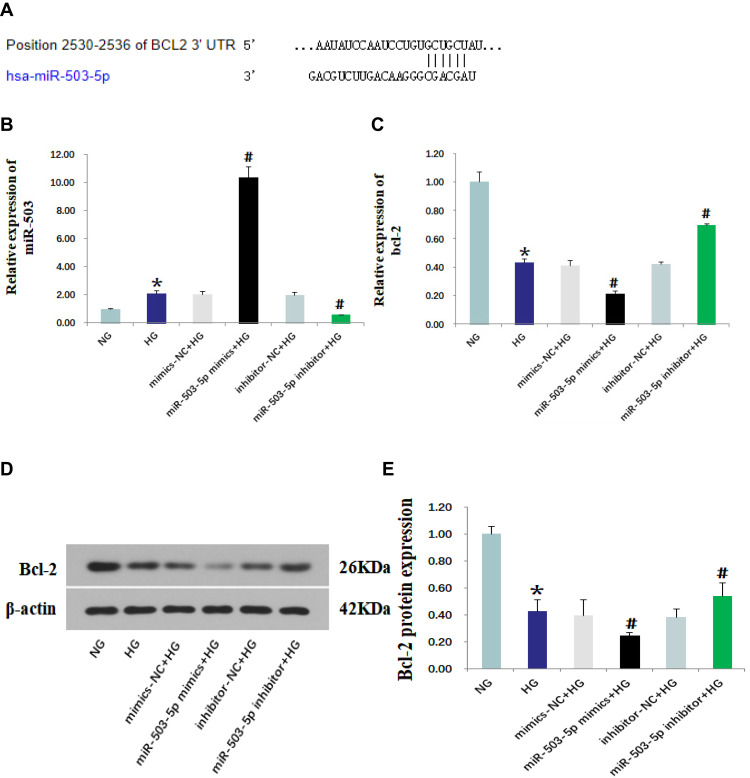
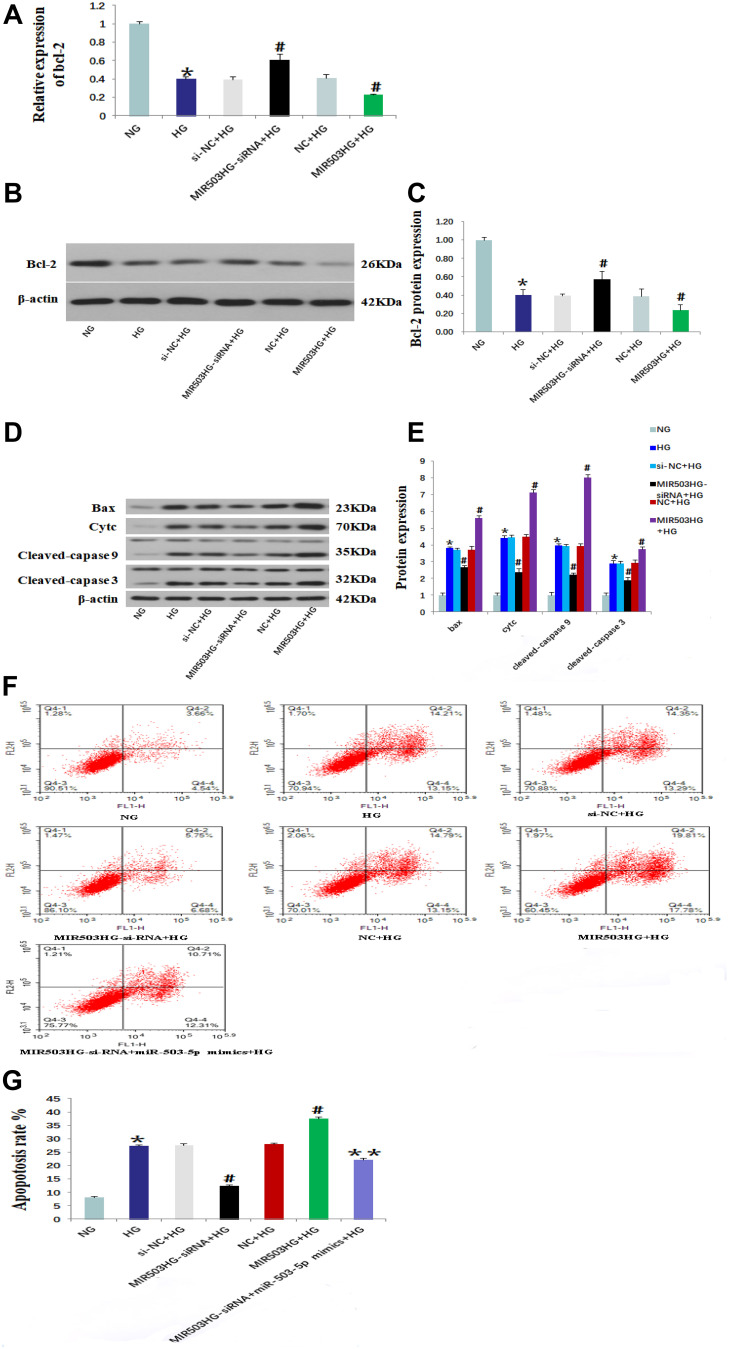
TargetScan (http://www.targetscan.org) predicted that Bcl-2 was the target gene of miR-503 (Figure 3A). The HK-2 cells cultured in high glucose were transfected with miR-503-5p mimics, mimics-NC, miR-503-5p inhibitor and inhibitor-NC. Expression levels of miR-503 and its target gene Bcl-2 were measured using RT-qPCR and Western blot. The results showed that expression levels of miR-503 in HK-2 cells cultured in high glucose were significantly higher than those cultured in normal glucose. Compared with the untransfected high-glucose group, expression levels of miR-503 in the miR-503-5p mimics transfected HK-2 cells were significantly higher, and expression levels of miR-503 in the miR-503-5p inhibitor transfected HK-2 cells were significantly lower (Figure 3B). Expression levels of Bcl-2 in the high-glucose group were significantly reduced. Overexpression of miR-503 significantly inhibited the expression of its target protein Bcl-2 (Figure 3C–E) and increased expression levels of apoptosis-related proteins (Bax, Cytc, and cleaved-caspases 9 and 3) (Figure 4A and B), while inhibition of miR-503 expression significantly up-regulated expression of Bcl-2 in HK-2 cells (Figure 3C–E).
Figure 3.
MiR-503 inhibitor up-regulated the expression of its target protein Bcl-2. (A) The binding site of miR-503 and Bcl-2 predicted by TargetScan. Levels of miR-503 (B) and Bcl-2 (C) that were measured using real-time quantitative PCR. (D) Protein expression levels of Bcl-2 in each group were measured using Western blot. (E) The intensities of the bands for Bcl-2 protein in (D) were quantified. Data from at least three separate experiments are shown. *P<0.05 vs NG, #P<0.05 vs HG.
Abbreviations: NG, normal glucose; HG, high glucose; mimics-NC+HG, mimics negative control+high glucose; miR-503-5p mimics+HG,miR-503-5p mimics+high glucose; inhibitor-NC+HG, inhibitor negative control+high glucose; miR-503-5p inhibitor+HG,miR-503-5p inhibitor+high glucose.
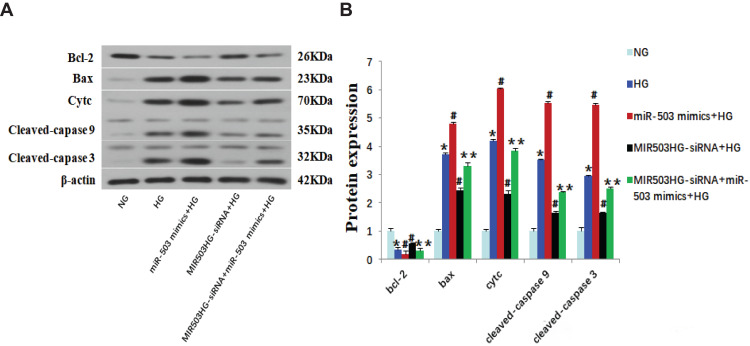
Figure 4.
MiR-503 mimics partially restored the effect of MIR503HG-siRNA. (A) Western blot of Bcl-2, Bax, Cytc and cleaved-caspase 9/3. (B) The intensities of the bands in (A) were quantified. Data from at least three separate experiments are shown. *P<0.05 vs NG, #P<0.05 vs HG, **P<0.05 vs MIR503HG-siRNA+HG.
Abbreviations: NG, normal glucose; HG, high glucose; miR-503 mimics+HG,miR-503-5p mimics+high glucose; MIR503HG-siRNA+HG, MIR503HG suppression plasmid transfection +high glucose; MIR503HG-siRNA+miR-503 mimics+HG, MIR503HG suppression plasmid and miR-503-5p mimics co-transfection+high glucose.
LncRNA MIR503HG Regulated Mitochondrial Apoptosis Pathway of HK-2 Cells Induced by High Glucose
HK-2 cells cultured in high glucose were transfected with unrelated sequence (siRNA control), lncRNA MIR503HG-siRNA, empty vector (NC), and lncRNA MIR503HG overexpression vector. RT-qPCR and Western blot were used to measure expression levels of Bcl-2 and other apoptosis-related proteins, and annexin V-FITC was used to measure apoptosis in each group. We found that expression levels of Bcl-2 in the HG group were significantly lower than those of the NG group (Figure 5A–C), and the expression levels of apoptosis-related proteins (Bax, Cytc, and cleaved-caspases 9 and 3) were significantly increased (Figure 5D and E). Compared to the NG group, the HK-2 cell apoptosis rate of HG group was greater (Figure 5F and G). Compared with the untransfected high glucose cultured HK-2 cells, inhibiting the expression of MIR503HG significantly up-regulated expression levels of anti-apoptotic factor Bcl-2 (Figure 5A–C) and reduced expression levels of apoptosis-related proteins (Bax, Cytc, and cleaved-caspases 9, and 3) (Figure 5D and E) and the apoptosis rate (Figure 5F and G). However, overexpression of MIR503HG significantly inhibited Bcl-2 expression (Figure 5A–C), and increased the expression levels of apoptosis-related proteins (Bax, Cytc, and cleaved-caspases 9 and 3) (Figure 5D and E) and apoptosis rate (Figure 5F and G).
Figure 5.
Inhibition of lncRNA MIR503HG alleviated apoptosis of HK-2 cells induced by high glucose. Levels of Bcl-2 in each group that were measured using real-time quantitative PCR (A). Western blot was used to measure the expression levels of Bcl-2 (B) and Bax, Cytc and cleaved-caspase 9/3 (D). (C) The intensities of the bands for Bcl-2 protein in (B) were quantified. (E) The intensities of the bands in (D) were quantified. (F) Annexin V-FITC was used to measure cell apoptosis in each group. (G) The apoptosis rate of (F). Data from at least three separate experiments are shown. *P<0.05 vs NG, #P<0.05 vs HG, **P<0.05 vs MIR503HG-siRNA+HG.
Abbreviations: NG, normal glucose; HG, high glucose; si-NC+HG, unrelated sequence transfection+high glucose; MIR503HG-siRNA+HG, MIR503HG suppression plasmid transfection +high glucose; NC+HG, empty vector transfection+high glucose; MIR503HG+HG, MIR503HG overexpression vector transfection +high glucose; MIR503HG-siRNA+miR-503-5p mimics+HG, MIR503HG suppression plasmid and miR-503-5p mimics co-transfection+high glucose.
MiR-503 Mimics Partially Restored the Inhibition of MIR503HG-siRNA on HK-2 Cell Apoptosis Induced by High Glucose
MIR503HG-siRNA and miR-503-5p mimics were co-transfected into HK-2 cells cultured in high glucose. Compared with the MIR503HG-siRNA transfected HK-2 cells, the expression levels of Bcl-2 were significantly lower, the expression levels of Bax, Cytc, cleaved-caspases 9 and 3 were significantly higher (Figure 4A and B), and the rate of apoptosis was increased in the MIR503HG-siRNA with miR-503 mimics co-transfected HK-2 cells (Figure 5F and G). These results suggested that miR-503 mimics partially restored the inhibitory effect of MIR503HG-siRNA on apoptosis. Under high-glucose conditions, MIR503HG regulated the expression of mature miR-503 through co-transcription with miR-503, inhibiting the expression of its target protein Bcl-2, triggering the mitochondrial apoptosis pathway, promoting apoptosis, and participating in diabetic kidney injury.
Discussion
The pathophysiology of diabetic nephropathy involves multifactorial interactions between metabolic and hemodynamic factors. A heterogeneous set of pathological mechanisms drives the development and progression of diabetic kidney disease and increases the difficulty of treatment. Apoptosis of intrinsic kidney cells plays an important role in the development of diabetic nephropathy (DN).16,17 Renal tubule damage caused by long-term persistent hyperglycemia is one of the main causes of diabetic nephropathy. The increased apoptosis of renal tubular epithelial cells leads to the loss of renal function in patients, thereby affecting outcomes.18
Many studies have shown that non-coding RNAs (lncRNAs and microRNAs) play vital roles in epigenetic regulation and are involved in many diseases including diabetic nephropathy.19,20 The interaction between lncRNAs and microRNAs has become a research hotspot, especially host lncRNAs. By analogy to mammalian snoRNAs, intronic miRNAs may be cotranscribed with the host genes by inclusion in introns of their pre-mRNAs and excision from debranched introns by an exonuclease.21 Several investigators have shown co-expression of intronic miRNAs and their host genes. LncRNA PVT1 introns have been reported to produce a cluster of six miRNAs; miR-1207-5p is among them and can be transcribed together with its host gene PVT1. Yan et al showed that miR-1207-5p and its host gene PVT1 were positively correlated in breast cancer cells and tissues.22
The RNA-seq-derived lincRNA MIR503HG is located at chromosome X, and harbors a coding sequence of miR-503 that is embedded in the first intron.5,23 MiR-503 is overexpressed in various types of diabetes, especially in complications related to diabetes. MiR-503 was involved in the protective effect of Phase II enzyme inducer (CPDT) in diabetic cardiomyopathy via Nrf2/ARE signaling pathway.24 The expression of miR-503 was significantly increased in the ischemic muscles of diabetic mice and diabetic patients whose limbs were amputated due to severe ischemia. Inhibition of miR-503 normalized blood flow in diabetic mice after ischemia, and promoted recovery through angiogenesis.25 Regulating the expression of p75NTR in endothelial cells under high-glucose conditions activated miR-503 transcription, reducing the coverage of capillaries by pericytes, increasing permeability, and damaging angiogenesis in limb muscles after ischemia.26 Hou et al found that, compared with the normal glucose group, miR-503 was overexpressed in human umbilical vein endothelial cells in high-glucose group, inhibiting the expression of IGF-1R, inhibiting cell migration and proliferation, and promoting apoptosis.27 Our previous study showed that expression of miR-503 was significantly up-regulated in the glomeruli of KKAy mice with type 2 diabetes, and losartan inhibited its high expression level, suggesting that miRNA-503 played an important role in DN.8 In addition, a study had reported that miR-503 was highly expressed in podocytes treated with high glucose in vitro and diabetic rat kidney tissues, inhibiting E2F transcription factor 3 (E2F3), promoting podocyte apoptosis, and participating in the pathological mechanism of DN.14 Nevertheless, the mechanism of miR-503 and host gene MIR503HG has not been reported to date. Qu et al reported that anti‐apoptotic factor Bcl‐2 was a direct target of miRNA‐503 using a luciferase reporter gene assay15 and Bcl-2 was an anti-apoptotic protein that inhibited the activation of Bax, thereby inhibiting the depolarization of mitochondrial inner membrane pores, the caspase cascade, and ultimately inhibiting apoptosis. A study in human pulmonary microvascular endothelial cells showed that miR-503-5p induced apoptosis in these cells by up-regulating the expression of apoptosis-related protein Bcl-2. While knocking down miR-503-5p, Bcl-2 expression increased, and apoptosis decreased.28 However, it remains unclear as to whether lncRNA MIR503HG regulates the expression of mature miR-503 through co-transcription with miR-503, thereby affecting the expression of its target gene Bcl-2 and the pathway of mitochondrial apoptosis, thereby participating in diabetic kidney damage.
Huang et al showed that MIR503HG induced the miR-503 expression in lymphoma. Overexpression of MIR503HG in ALK-positive (SR-786, KARPAS) ALCL cell lines exhibited a significant increase in miR-503 expression level. MiR-503HG-depleted MAC-1 and FePD cells (ALK-negative cell lines) exhibited significant decreases in the levels of miR-503.7 In another study, both miR-503 and MIR503HG were downregulated in hepatocellular carcinoma. However enhanced or decreased expression of MIR503HG in hepatocellular carcinoma (HCC) cell lines had no effect on the levels of miR-503, and vice versa. These results suggested that the function of MIR503HG was miR-503 independent in HCC cells.29 In the present study, by inhibiting and overexpressing lncRNA MIR503HG in human proximal tubular epithelial cells cultured in high glucose, we found that lncRNA-MIR503HG was co-expressed with miR-503, affecting miR-503 splicing, thereby affecting the expression of its target protein Bcl-2, ultimately inducing the apoptosis of proximal tubular epithelial cells. A change in the epigenetics may explain these differences in MIR503HG and miR-503 in different cells, which suggests their different roles in different cell types and diseases. The relationship between miRNAs and host genes is complicated, and at this point, additional research is required.
Conclusion
High glucose up-regulated the expression of lncRNA MIR503HG in human proximal tubular epithelial (HK-2) cells. The host lncRNA MIR503HG was co-transcribed with miR-503 and regulated miR-503 expression by affecting the splicing generation of miR-503. Expression of Bcl-2 was inhibited, and expression levels of apoptosis-related proteins Cytc and Bax were greater in HK-2 cells cultured in high glucose, promoting a caspase cascade reaction that led to increased levels of cleaved fragments of caspase-9 and caspase-3 and induced mitochondrial pathway-mediated HK-2 cell apoptosis. Inhibiting MIR503HG reduced miR-503 expression, up-regulating its target gene Bcl-2, inhibiting the expression levels of apoptosis-related proteins such as Bax, and reducing high-glucose-induced HK-2 cell apoptosis. Co-transfection of miRNA-503 partially offset the effect of MIR503HG-siRNA, suggesting that MIR503HG played a pathogenic effect by promoting the co-transcription of miRNA-503 which regulated Bcl-2. The interaction of miR-503 and its host lncRNA MiR503HG in the pathogenesis of diabetic nephropathy has not been reported to date, and MIR503HG is expected to become a new target for DN treatment.
Disclosure
The authors report no conflicts of interest associated with this work.
References
- 1.Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol. 2017;12(12):2032–2045. doi: 10.2215/CJN.11491116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang H, Zhang H, Chen X, et al. The decreased expression of electron transfer flavoprotein β is associated with tubular cell apoptosis in diabetic nephropathy. Int J Mol Med. 2016;37(5):1290–1298. doi: 10.3892/ijmm.2016.2533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Magri CJ, Fava S. The role of tubular injury in diabetic nephropathy. Eur J Intern Med. 2009;20(6):551–555. doi: 10.1016/j.ejim.2008.12.012 [DOI] [PubMed] [Google Scholar]
- 4.Liu B, Shyr Y, Cai J, Liu Q. Interplay between miRNAs and host genes and their role in cancer. Brief Funct Genomics. 2019;18(4):255–266. doi: 10.1093/bfgp/elz002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pachera E, Assassi S, Salazar GA, et al. Long noncoding RNA H19X is a key mediator of TGF-β–driven fibrosis. J Clin Invest. 2020;130(9):4888–4905. doi: 10.1172/JCI135439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dohi O, Yasui K, Gen Y, et al. Epigenetic silencing of miR-335 and its host gene MEST in hepatocellular carcinoma. Int J Oncol. 2013;42(2):411–418. doi: 10.3892/ijo.2012.1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang PS, Chung IH, Lin YH, Lin TK, Chen WJ, Lin KH. The long non-coding RNA MIR503HG enhances proliferation of human ALK-negative anaplastic large-cell lymphoma. Int J Mol Sci. 2018;19(5):1463. doi: 10.3390/ijms19051463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu X, Zhang C, Fan Q, et al. Inhibiting microRNA-503 and microRNA-181d with losartan ameliorates diabetic nephropathy in KKAy mice. Med Sci Monit. 2016;22:3902–3909. doi: 10.12659/MSM.900938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pescador N, Pérez-Barba M, Ibarra JM, et al. Serum circulating microRNA profiling for identification of potential type 2 diabetes and obesity biomarkers. PLoS One. 2013;8(10):e77251. doi: 10.1371/journal.pone.0077251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sheikhbahaei S, Manizheh D, Mohammad S, et al. Can MiR-503 be used as a marker in diabetic patients with ischemic stroke? BMC Endocr Disord. 2019;19(1):42. doi: 10.1186/s12902-019-0371-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu K, Bian D, Hao L, et al. microRNA-503 contribute to pancreatic beta cell dysfunction by targeting the mTOR pathway in gestational diabetes mellitus. EXCLI J. 2017;16:1177–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan Z, Wang H, Liang J, Li Y, Li X. MicroRNA-503-5p improves carotid artery stenosis by inhibiting the proliferation of vascular smooth muscle cells. Exp Ther Med. 2020;20(5):85. doi: 10.3892/etm.2020.9213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen K, Zhao XL, Li LB, et al. miR-503/Apelin-12 mediates high glucose-induced microvascular endothelial cells injury via JNK and p38MAPK signaling pathway. Regen Ther. 2020;14:111–118. doi: 10.1016/j.reth.2019.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zha F, Bai L, Tang B, et al. MicroRNA-503 contributes to podocyte injury via targeting E2F3 in diabetic nephropathy. J Cell Biochem. 2019;120(8):12574–12581. doi: 10.1002/jcb.28524 [DOI] [PubMed] [Google Scholar]
- 15.Qu R, Hu C, Tang Y, Yu Q, Shi G. Long non-coding RNA BLACAT1 induces tamoxifen resistance in human breast cancer by regulating miR-503/Bcl-2 axis. Cancer Manage Res. 2020;12:1771–1777. doi: 10.2147/CMAR.S239981 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Wagener FA, Dekker D, Berden JH, Scharstuhl A, van der Vlag J. The role of reactive oxygen species in apoptosis of the diabetic kidney. Apoptosis. 2009;14(12):1451–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tong Y, Chuan J, Bai L, et al. The protective effect of shikonin on renal tubular epithelial cell injury induced by high glucose. Biomed Pharmacother. 2018;98:701–708. doi: 10.1016/j.biopha.2017.12.112 [DOI] [PubMed] [Google Scholar]
- 18.Ju Y, Su Y, Chen Q, et al. Protective effects of astragaloside IV on endoplasmic reticulum stress-induced renal tubular epithelial cells apoptosis in type 2 diabetic nephropathy rats. Biomed Pharmacother. 2019;109:84–92. doi: 10.1016/j.biopha.2018.10.041 [DOI] [PubMed] [Google Scholar]
- 19.Cech TR, Steitz JA. The noncoding RNA revolution-trashing old rules to forge new ones. Cell. 2014;157(1):77–94. doi: 10.1016/j.cell.2014.03.008 [DOI] [PubMed] [Google Scholar]
- 20.Adams BD, Parsons C, Walker L, Zhang WC, Slack FJ. Targeting noncoding RNAs in disease. J Clin Invest. 2017;127(3):761–771. doi: 10.1172/JCI84424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodriguez A, Griffiths-Jones S, Ashurst JL, et al. Identification of mammalian microRNA host genes and transcription units. Genome Res. 2004;14(10A):1902–1910. doi: 10.1101/gr.2722704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yan C, Chen Y, Kong W, et al. PVT1-derived miR-1207-5p promotes breast cancer cell growth by targeting STAT6. Cancer Sci. 2017;108(5):868–876. doi: 10.1111/cas.13212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fiedler J, Breckwoldt K, Remmele CW, et al. Development of long noncoding RNA-based strategies to modulate tissue vascularization. J Am Coll Cardiol. 2015;66(18):2005–2015. doi: 10.1016/j.jacc.2015.07.081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miao Y, Wan Q, Liu X, Wang Y, Luo Y, Liu D. miR-503 is involved in the protective effect of phase II enzyme inducer (CPDT) in diabetic cardiomyopathy via Nrf2/ARE signaling pathway. Biomed Res Int. 2017;2017:9167450. doi: 10.1155/2017/9167450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caporali A, Meloni M, Vollenkle C, et al. Deregulation of microRNA-503 contributes to diabetes mellitus-induced impairment of endothelial function and reparative angiogenesis after limb ischemia. Circulation. 2011;123(3):282–291. doi: 10.1161/CIRCULATIONAHA.110.952325 [DOI] [PubMed] [Google Scholar]
- 26.Caporali A, Meloni M, Nailor A, et al. p75(NTR)-dependent activation of NF-kappaB regulates microRNA-503 transcription and pericyte-endothelial crosstalk in diabetes after limb ischaemia. Nat Commun. 2015;6:8024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hou LJ, Han JJ, Liu Y. Up-regulation of microRNA-503 by high glucose reduces the migration and proliferation but promotes the apoptosis of human umbilical vein endothelial cells by inhibiting the expression of insulin-like growth factor-1 receptor. Eur Rev Med Pharmacol Sci. 2018;22(11):3515–3523. [DOI] [PubMed] [Google Scholar]
- 28.Tang NP, Hui TT, Ma J, Mei QB. Effects of miR-503-5p on apoptosis of human pulmonary microvascular endothelial cells in simulated microgravity. J Cell Biochem. 2019;120(1):727–737. doi: 10.1002/jcb.27430 [DOI] [PubMed] [Google Scholar]
- 29.Wang H, Liang L, Dong Q, et al. Long noncoding RNA miR503HG, a prognostic indicator, inhibits tumor metastasis by regulating the HNRNPA2B1/NF-κB pathway in hepatocellular carcinoma. Theranostics. 2018;8(10):2814–2829. doi: 10.7150/thno.23012 [DOI] [PMC free article] [PubMed] [Google Scholar]