Abstract
Nanofibers can mimic natural tissue structure by creating a more suitable environment for cells to grow, prompting a wide application of nanofiber materials. In this review, we include relevant studies and characterize the effect of nanofibers on mesenchymal stem cells, as well as factors that affect cell adhesion and osteogenic differentiation. We hypothesize that the process of bone regeneration in vitro is similar to bone formation and healing in vivo, and the closer nanofibers or nanofibrous scaffolds are to natural bone tissue, the better the bone regeneration process will be. In general, cells cultured on nanofibers have a similar gene expression pattern and osteogenic behavior as cells induced by osteogenic supplements in vitro. Genes involved in cell adhesion (focal adhesion kinase (FAK)), cytoskeletal organization, and osteogenic pathways (transforming growth factor-β (TGF-β)/bone morphogenic protein (BMP), mitogen-activated protein kinase (MAPK), and Wnt) are upregulated successively. Cell adhesion and osteogenesis may be influenced by several factors. Nanofibers possess certain physical properties including favorable hydrophilicity, porosity, and swelling properties that promote cell adhesion and growth. Moreover, nanofiber stiffness plays a vital role in cell fate, as cell recruitment for osteogenesis tends to be better on stiffer scaffolds, with associated signaling pathways of integrin and Yes-associated protein (YAP)/transcriptional co-activator with PDZ-binding motif (TAZ). Also, hierarchically aligned nanofibers, as well as their combination with functional additives (growth factors, HA particles, etc.), contribute to osteogenesis and bone regeneration. In summary, previous studies have indicated that upon sensing the stiffness of the nanofibrous environment as well as its other characteristics, stem cells change their shape and tension accordingly, regulating downstream pathways followed by adhesion to nanofibers to contribute to osteogenesis. However, additional experiments are needed to identify major signaling pathways in the bone regeneration process, and also to fully investigate its supportive role in fabricating or designing the optimum tissue-mimicking nanofibrous scaffolds.
Keywords: Nanofiber, Stem cell, Mimicking natural tissue, Morphology, Signaling pathway
1. Introduction
Bioscaffolds in tissue engineering that provide a three-dimensional (3D) space for cells to grow and differentiate are considered superior to plain materials. Several biomaterials have been investigated and applied in various forms for bone engineering purposes. Hydrogels, including gelatin-chitosan hybrid hydrogels (Re et al., 2019), alginate hydrogels (Liu M et al., 2017), fibrin hydrogels (Chaires-Rosas et al., 2019), and a hydrogel/fiber composite scaffold (Khorshidi and Karkhaneh, 2018), are a promising branch of materials for this application. The nanocomposite scaffold, which is made of several materials such as gelatin and alginate, also has great potential (Purohit et al., 2019). A further type of bioscaffold is demineralized and decellularized bone (Abedin et al., 2018).
In addition to the applications mentioned above, nanofibrous scaffolds are widely used for tissue regeneration, including bone (Rezvani et al., 2016; Moradi et al., 2018), osteochondral tissue, musculoskeletal tissue (Carbone et al., 2014; Sankar et al., 2018), vascular tissue (Namdari et al., 2017), nerve (Shah et al., 2016; Wu et al., 2017), dental pulp (Kuang et al., 2016), and other tissues (Ribba et al., 2014). As one of the biomaterials used in tissue engineering, nanofibers have the great advantage in mimicing tissue structures (Ortega et al., 2018). This is because tissue fibers can be nano-or micro-scale, and have a highly porous structure and a high aspect ratio, which further contributes to cell adhesion, proliferation, differentiation, and tissue regeneration. Nanofibrous scaffolds can be manufactured using various biopolymers including proteins, polysaccharides, and polyhydroxyalkanoates (Ortega et al., 2018).
However, nanofibers are generally produced as two-dimensional (2D) dense mats through electrospinning, not as 3D scaffolds (Bhardwaj and Kundu, 2010). In order to obtain scaffolds with 3D pore structure, however, technological conditions should be manipulated precisely, with the help of templates when needed (Chen et al., 2017; Asencio et al., 2018). Certain specifications such as the pore size of the nanofibrous scaffold, as well as the fiber diameter, are difficult to control accurately in this process (Chen et al., 2017).
Many studies have found that, when cultured on specially designed nanofibrous scaffolds, several types of mesenchymal stem cells have the tendency to adhere to nanofibers and undergo subsequent osteogenic differentiation (Moradi et al., 2018). However, the mechanisms underlying these processes remain undiscovered. This review focuses on the effect of nanofibers on mesenchymal stem cells, as well as factors that affect cell adhesion and osteogenic differentiation, with the aim to reveal the mechanisms behind these processes.
2. Structure of bone extracellular matrix
Tissues and organs in the human body have different structures, as well as physical properties and chemical composition. It is the characteristic environment that decides the growth and differentiation of stem cells to form various tissues (Engler et al., 2006; Guilak et al., 2009; Dufort et al., 2011). Therefore, it is important that the environment for culturing bone marrow stem cells and inducing osteogenesis in vitro, as well as for improving bone regeneration in vivo afterwards, resembles that of natural tissue.
Bone is composed of organic (mainly collagen fiber I) and inorganic components. Based on the arrangement of collagen fibers, two kinds of bone structure exist: compact bone and spongy bone (Fratzl and Weinkamer, 2007). In compact bone, parallelly arranged collagen fibers are reinforced with inorganic particles (especially hydroxyapatite) to form osteons, with an average of 100 μm in diameter, which further consist of internal and external circumferential lamellae and haversian canals in between (Liu et al., 2016). The latter include osteocytes between each osteon and blood vessels supplying nutrition into the bone tissue. Spongy bone is more polyporous, consisting of bone trabeculae and bone marrow. Osteoblasts and osteoclasts, cells working on bone formation and bone healing, adhere to the inner side of the internal circumferential lamellae. In addition to the major structure made up by collagen fibers, there are nonfibrous components in bone extracellular matrix (ECM) in certain quantities such as osteocalcin (OCN) and bone morphogenetic protein (BMP), acting as regulatory molecules (Allori et al., 2008).
The direction of cell differentiation is determined by the physical properties of the environment, especially elasticity. Mesenchymal stem cells have a tendency to undergo osteogenic differentiation in an 11–40-kPa matrix (Engler et al., 2006; Huebsch et al., 2010). Besides, various growth factors (BMP, transforming growth factor (TGF), etc.) in the ECM induce specific signal pathways and help bone formation (Chen et al., 2012).
Therefore, the following requirements must be reached for bone tissue engineering, involving osteogenesis from bone marrow mesenchymal stem cells (BMSCs) on nanofibers: appropriate nanofiber structure and mechanical properties, which are close to natural bone; functional molecules to aid bone formation.
3. Nanofiber characteristics
3.1. Mimicking natural tissue structure
The major advantage of nanofibers is their ability to mimic natural tissue structure (Canha-Gouveia et al., 2015). As discussed before, the basic unit of bone is the osteon consisting of an assembly of collagen molecules with an average diameter of 100 μm. Using electrospinning, nanofibers can be efficiently produced from various polymers in specific and consistent dimensions. Fibers with diameters on the nanometer scale closely mimic fibers in the osteon. Certain kinds of nanofibrous scaffolds can be combined with nanoparticles such as hydroxyapatite (HA) and β-tricalcium phosphate (TCP) to mimic the natural bone ECM even better, thus enhancing osteogenesis without adding any osteogenic supplements (Polini et al., 2011).
3.2. Physical and structural supports
Nanofibers can be designed as mats or scaffolds providing structural support for cell adhesion and growth. Certain modifications to improve cell adhesion onto nanofibers have also been described, including the rose stem-like structure (Nasajpour et al., 2017) and nanofiber sleeve (Hwang et al., 2015). The “fiber-on-fiber” matrix using electrospun gelatin nanofibers crosslinked on the microfiber layer aims to support human pluripotent stem cell proliferation, and enhances their pluripotency by mimicking the natural ECM (Liu L et al., 2017).
3.3. Combination with other components
It is more feasible to add extra components to nanoparticles for the improvement of properties during production. For example, the fabrication of graphene oxide on nanofibrous scaffolds has been shown to promote cell adhesion, osteogenic differentiation, and further bone regeneration (Luo et al., 2015; Shao et al., 2016; Liu XY et al., 2017; Mahmoudi and Simchi, 2017; Xie et al., 2017; Marrella et al., 2018). Bioactive glass nanoparticles incorporated into 3D porous scaffolds also enhance cell adhesion and osteogenesis (Kim et al., 2017). Nano-HA improves the osteogenesis of osteoblastic cells on polycaprolactone (PCL) nanofibers as well, which may be due to its similarity to bone tissue (Zhang et al., 2018). In addition, bioactive molecules including BMP-2 (Perikamana et al., 2015), polydopamine (Yang et al., 2017), and peptides (Mobasseri et al., 2018) can also be used in nanofibrous scaffolds.
4. Effect of nanofibrous scaffolds on mesenchymal stem cells
Cells can sense the microenvironment and change their microstructure accordingly—organellar and nuclear morphology changes with the matrix provided by nanofibers (Tutak et al., 2017). The nucleus appears to occupy more space in the cell, while organelles become more tridimensional on nanofibers than on flat films. Furthermore, bioassay measurements have indicated that organelle functions may be enhanced on nanofibers. Changes in focal adhesion and cytoskeletal arrangements further lead to distinct differentiation pathways (Kennedy et al., 2017).
Here, we hypothesize that (1) the process of bone regeneration in vitro is similar to bone formation and healing in vivo; (2) the closer nanofibers or nanofibrous scaffolds are to natural bone tissue, the more efficient osteogenesis will be. By reviewing literature herein, we conclude the effect of nanofibrous scaffolds on mesenchymal stem cells, as well as factors that affect cell behavior.
4.1. Overall pattern of cell behavioral mechanisms on nanofiber
Studies have indicated that behavioral patterns of cells growing on nanofibers are similar to those in osteogenic medium and those in vivo. The osteogenic differentiation of cells is usually induced and observed via adding osteogenic supplements to the culture medium. Using ontology analysis, Liu WT et al. (2013) reported that human mesenchymal stem cells cultured on random nanofibers had a similar pattern of gene expression and osteogenic behavior, yet to a lower extent, when compared with cells induced by osteogenic supplements. According to a study by Baker et al. (2014), nanofibers and osteogenic supplements regulated similar pathways of human mesenchymal stem cells, indicating the common nature of the molecular mechanism for cell differentiation, although nanofibers appeared to influence cell adhesion/ECM-receptor pathways more strongly. In addition, nanofiber structure (3D scaffold or 2D film) seemed to matter more than nanofiber material.
In the study of Baker et al. (2014), it was also reported that TGF-β and the cell-adhesion/ECM-receptor pathways play important roles in this process, consistently with the study by Liu WT et al. (2013), who further characterized the behavior of gene expression. Liu WT et al. (2013) concluded that genes involved in cell adhesion, ECM organization, and integrin-mediated signaling pathways, such as focal adhesion kinase (FAK), were first upregulated followed by an increased expression of genes associated with cytoskeletal organization on Day 7. Genes involved in osteogenic pathways (TGF-β/BMP, mitogen-activated protein kinase (MAPK), and Wnt) and genes associated with mineralization were upregulated later at Week 2 or 3.
4.2. Factors that improve cell adhesion and osteogenesis
The physical properties of biomaterials have a key significance in cell behavior including adhesion, migration, proliferation, and differentiation (di Cio and Gautrot, 2016; Brusatin et al., 2018). Many studies have proved that biomaterial stiffness plays a remarkable role in cell fate in a manner similar to cell differentiation in the native tissue environment (Lv et al., 2017; Xing et al., 2019). Dynamic changes in scaffolds, such as the time-dependent stress relaxation of hydrogel, also regulate cell activity and differentiation (Chaudhuri et al., 2016; Lee and Kim, 2018). Accordingly, cell behavior on nanofibrous scaffolds is controlled and regulated mainly by physical properties.
4.2.1 Stiff nanofibers promoting cell adhesion and osteogenesis through altering cell tension
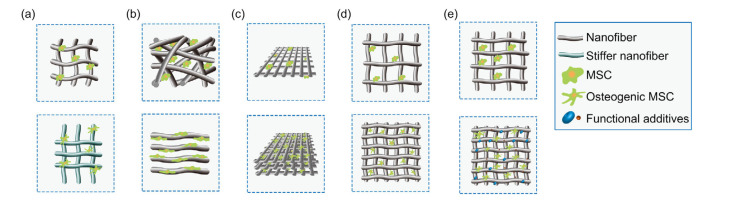
It has been reported that cells on nanofibrous scaffolds have the tendency to osteogenic differentiation upon sensing the stiffness of the matrix (Kennedy et al., 2017; Jahanmard et al., 2020) (Fig. 1a). Pathways related to cell adhesion and the cytoskeleton play key roles in this process (Discher et al., 2005). Certain specific signaling pathways are involved in the sensing of nanofibers. The cell membrane protein porin 1 (POR1) functions as a curvature sensor affecting the activity of Ras-related C3 botulinum toxin substrate 1 (Rac1) on nanofibers with various diameters to influence downstream pathways, including the expression of alkaline phosphatase (ALP) (Higgins et al., 2015). The silencing integrin subunit β1 also induces cells to lose this function of sensing their substrate (Olivares-Navarrete et al., 2017).
Fig. 1.
Factors affecting stem cell behavior on nanofibers
Cells with osteogenic tendency are more spindle-shaped. (a) The stiffness of environment affects cell fate. A stiff matrix (below) leads to more osteogenesis compared with a soft matrix (up). (b) The alignment of nanofibers affects cell fate. Stem cells cultured on aligned nanofibers are consistent with the direction of the fibers and have better osteogenesis (below) compared with random nanofibers (up). (c) The hierarchical structure of nanofibrous scaffolds affects cell adhesion and differentiation. Compared with simple scaffolds (up), scaffolds with nanofibers aligned differently on each layer mimic natural tissue better, leading to improved osteogenesis by cells (below). (d) Pore volume in nanofibrous scaffolds affects cell adhesion and growth. Compared with cells growing in overlarge pores (up), cells have better adhesion and interconnectivity when the pore size fits the cell size (below). (e) Functional additives aid cell growth. Scaffolds combined with various nanoparticles and growth factors provide better cell osteogenesis (below) compared with pure scaffolds (up). MSC: mesenchymal stem cell
Nanofibers can regulate cell behavior by using a similar mechanism, although studies attempting to reveal this mechanism came to different conclusions. Generally, cells tend to undergo osteogenic differentiation more on stiffer nanofibers, probably because their resemblance of a stiff bone tissue is better. As previously mentioned, an 11–40-kPa matrix is likely to make bone mesenchymal stem cells undergo osteogenesis (Engler et al., 2006; Huebsch et al., 2010), with associated signaling pathways of integrin and Yes-associated protein (YAP)/transcriptional co-activator with PDZ-binding motif (TAZ).
Integrin receptors on the cell membrane determine the areas of cell adhesion and the structure of focal adhesions of cells (Dalby et al., 2014), which may change cell shape, thereby altering cell phenotypes through Ras homolog gene family member A (RhoA) activity (McBeath et al., 2004). The increased stiffness of the ECM results in higher cell tension, causing the translocation of YAP/TAZ to the nucleus (Cui et al., 2003; Piccolo et al., 2014; Das et al., 2016; Sun et al., 2016; Panciera et al., 2017). Nuclear YAP/TAZ further induces downstream signaling pathways and leads to osteogenesis in turn (Dupont et al., 2011; Pan HH et al., 2017; Panciera et al., 2017; Kegelman et al., 2018; Pan JX et al., 2018).
Chang et al. (2018) designed a nanofibrous scaffold to culture single BMSCs without cell–cell interactions. The cultured cells had a smaller adhesion area, less cell tension, and higher ALP activity than BMSCs cultured on a flat film island. It was also established that the downregulated FAK/RhoA/YAP1 pathway was involved in this difference, consistent with the smaller adhesion areas and fewer stress fibers.
In contrast, Ozdemir et al. (2013) reported that cells grown on nanofibers had higher cellular stiffness, i.e., cytoskeletal integrity. The change in cell tension that led to early osteogenesis was due to activation of the RhoA/Rho-associated coiled-coil-containing protein kinase II (ROCKII) pathway and myosin IIa involvement. Stiffer nanofibers enhanced osteogenesis in a similar manner in a different study by Nam et al. (2011).
The roles of YAP and TAZ, which are transcriptional co-factors in osteogenesis, are complex. The increase in nuclear YAP suppresses osteogenesis through the repression of Runt-related transcription factor 2 (RUNX2) (Zaidi et al., 2004; Dupont et al., 2011). However, in contrast to YAP, TAZ binds to RUNX2 and promotes osteogenesis (Cui et al., 2003; Hong et al., 2005; Qian et al., 2017). Different cell types and cells in different stages of osteogenesis vary in their YAP-nucleus translocation rate (Tatapudy et al., 2017; Xiong et al., 2018). Furthermore, cell–cell contact influences cell fate decisions (Mao et al., 2016; Ye et al., 2016), which may explain the disagreement between the results of Chang et al. (2018) and Ozdemir et al. (2013).
4.2.2 Aligned nanofibers enhancing osteogenic gene expression
Based on the organization of nanofibrous scaffolds, cells undergo morphological adaptation and migration differently. Therefore, cell fate can be potentially affected by nanofiber alignment. Compared with random nanofibers, aligned fibers induce the differential expression of transcription factors, which in turn trigger various biochemical pathways resulting in differentiation (Cheng et al., 2019). This could be due to the fact that aligned nanofibers are more likely to resemble natural osteons. Certain key molecules such as integrins, FAK, ROCK, and the BMP pathways, have been investigated in this respect.
The morphology of stem cells cultured on aligned nanofibers was consistent with fiber direction with higher migration rates and integrin expression levels, thus better osteogenesis than that of random nanofibers both in vitro and in vivo (Chen et al., 2013; Lee et al., 2014) (Fig. 1b). Other molecules are also important for cell adhesion, including FAK and ROCK, which appear to be upregulated in cells on aligned nanofibers (Andalib et al., 2013, 2016). Moreover, there is a connection between nanotopographical cues and microRNA (miR) levels in cells. As reported by Izadpanahi et al. (2018), aligned nanofibers modulated long non-coding RNAs (lncRNAs), miR-125b, and their downstream molecule maternally expressed 3 (MEG3). They also regulated the H19 and BMP pathways, resulting in improved osteogenesis. Their findings show that the non-coding RNA network is involved in the regulation of aligned nanofibers during osteogenesis.
However, certain other studies have contradictory results. For example, Pandey et al. (2018) found that aligned nanofibers induced higher proliferation but lower osteogenic differentiation of canine adipose-derived mesenchymal stem cells than random nanofibers; this was confirmed by the quantitative reverse transcription polymerase chain reaction (qRT-PCR) of osteogenic markers (collagen type I α 1 (COL1A1) and osterix). Lü et al. (2012) cultured cells for 7 d on various materials. Cells grown on random nanofibers had even higher viability than those on aligned nanofibers, even though the latter were better than microfibers and flat films.
4.2.3 Hierarchical structure of nanofibrous scaffolds improving cell adhesion and osteogenesis
Beyond nanofiber alignment, cell differentiation is also affected by the hierarchical structure of scaffolds. As mentioned previously (Liu et al., 2016), natural bone is composed of multilayered compact bone and spongy bone, where osteoblasts adhere to the inner surface of compact bone. Various structures have been designed to aid cell growth. The better these structures mimic natural bone tissue, the more cell adhesion and osteogenesis resemble bone regeneration in vivo (Fig. 1c).
Scaffolds with nanofibers aligned differently on each layer have been designed, which promote the osteogenic differentiation of BMSCs (Yahia et al., 2019; Li et al., 2020). Similar results were found for cells cultured on lattice-like scaffolds, with the increased expression of integrins, RhoA, and extracellular signal-regulated kinase (ERK), compared with cells cultured on nonwoven nanofibrous scaffolds (Zhu et al., 2013). Xue et al. (2017) validated that the Wnt/β-catenin pathway, a signaling pathway confirmed to lead to osteogenesis and suppress peroxisome proliferator-activated receptor-γ (PPARγ)-mediated adipogenesis (Takada et al., 2009), contributed to the osteogenesis of BMSCs cultured on nanofibrous scaffolds.
4.2.4 Other physical and chemical properties of nanofibers
Apart from stiffness, alignment, and structure, further physical properties of nanofibers such as porosity and swelling properties, as well as chemical properties like hydrophilicity, affect the adhesion and differentiation of cells (Arslan et al., 2017).
Porosity and swelling properties determine the space for cells to grow, thus influencing cell migration, morphology, and differentiation (Kennedy et al., 2017). Sufficient pore size and interconnectivity are required for cells to enter into the inside of scaffolds and start angiogenesis (Gupte et al., 2018; Sankar et al., 2018) (Fig. 1d). The suitable pore diameter for bone formation is considered to be 100–300 μm, while that for cartilage formation is 400 μm (Zhang et al., 2010). This corresponds to the high porosity of spongy bone.
Stem cells adhere better to hydrophilic surfaces by altering the expression of relevant biological signals including the increased expression of c-fos and reduced expression of c-myc and p53 (Kim et al., 2007). Several methods (such as fabricating molecules (Luo et al., 2015), coatings (Barros et al., 2017), and the use of hydrophilic precursors as raw materials) can help improve the hydrophilicity of nanofibrous scaffolds to obtain better cell adhesion.
4.2.5 Functional additives in nanofibrous scaffolds
Since there are numerous bioactive molecules in natural bone tissue, the incorporation of similar functional additives into nanofibers or nanofibrous scaffolds helps osteogenesis (Fig. 1e). The most commonly used additives are nanoparticles, growth factors, and ECM-like molecules (Motamedian et al., 2015).
Adding nanoparticles to nanofibers such as calcium phosphate ceramics (CPCs), which resemble the inorganic components in bone tissue, enhances osteogenic processes. In a biomimetic nanocomposite nanofibrous scaffold of HA/chitosan developed by Liu HH et al. (2013), cells on nanofibrous scaffolds with HA maintained a spindle-like morphology, and showed nuclear localization of small mothers against decapentaplegic 1/5/8 (Smad1/5/8), thereby improving osteogenesis compared with those on simple nanofibrous chitosan and membranous HA/chitosan. The combination of HA crystals and chitin nanofibers also contributes to bone formation, as demonstrated by in vivo experiment in rabbits (Duan et al., 2017). β-TCP nanoparticles, a different type of CPCs, were added to nanofibers by Zhang et al. (2015). As a result, the expression of the calcium-sensing receptor was upregulated, which may be another mechanism involving the enhancement of osteogenesis by CPCs. Furthermore, bioactive glasses could enhance cell migration on nanofibrous scaffolds (Shalumon et al., 2013; Kim et al., 2017). Additives promoting the formation of human inorganic HA have similar effects (Sun et al., 2017; Wang et al., 2019).
Growth factors, such as the functional sequence of the fibronectin type III domain from native tenascin-C on self-assembled peptide nanofibers (Sever et al., 2014) and the osteoinductive collagen I-derived peptide sequence Asp-Gly-Glu-Ala (Ceylan et al., 2014), have also proved to be effective. Binding the bone marrow homing peptide 1 motif to a nanofibrous scaffold strongly activated the BMP pathway and induced osteogenesis, as reported by Tavakol et al. (2019). In an experiment by Hosseini et al. (2019), inorganic polyphosphate, an activator of the Wnt/β-catenin signaling pathway, was combined with nanofibers resulting in enhanced osteogenic differentiation, which indicated the importance of the Wnt/β-catenin pathway.
Among ECM-like molecules, collagen and fibrin are widely integrated to nanofibrous scaffolds. Collagen coating on nanofibers facilitates cell spreading and the expression of osteogenic-related genes (Yang et al., 2018; Qian et al., 2019). Fibrin has good biocompatibility and controllable biodegradability, which prompts its extensive use for modifications to nanofibers (Noori et al., 2017).
Furthermore, nanofibrous scaffolds can function as gene carriers. Scaffolds containing the BMP-2 gene could efficiently transduce cells and promote bone formation (Zhu et al., 2017; Doosti-Telgerd et al., 2020).
5. Conclusions and future perspectives
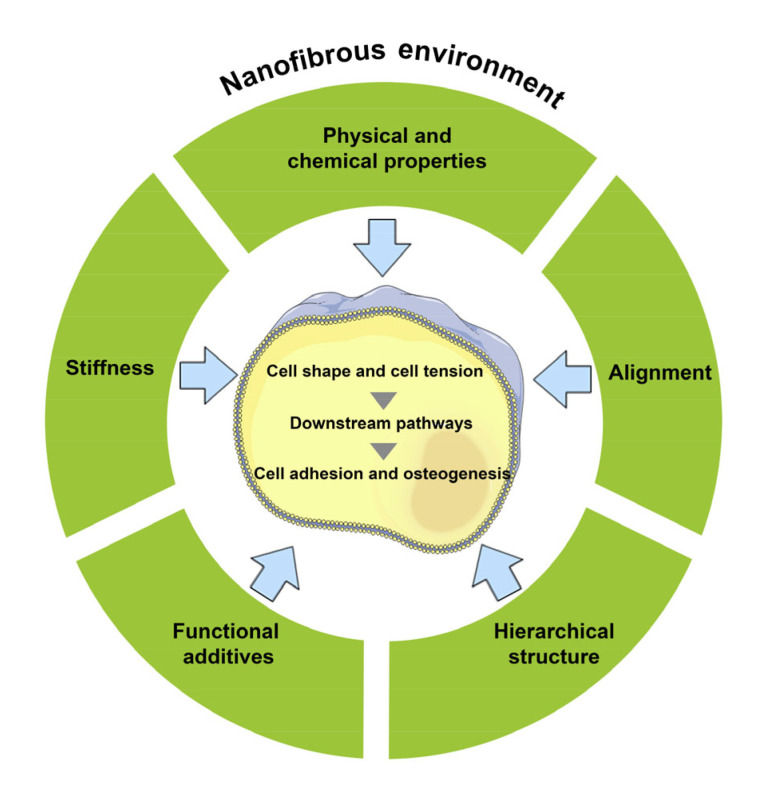
The question of what environment best promotes stem cell proliferation and osteogenesis in vitro, remains unanswered. Recently, the trend has been to develop an environment that can mimic the natural ECM the most, from physical support to biological molecules. This principle can also be applied to nanofibers and BMSCs. Owing to their great advantage in mimicking natural bone tissue, nanofibers have found a range of applications in bone regeneration. As demonstrated by past research, the more similarity there is between the nanofibrous scaffold and natural bone tissue, the better cells grow and differentiate during osteogenesis. Upon sensing the stiffness and other characteristics of nanofibers, stem cells change their shape and cell tension accordingly, and regulate downstream pathways (Fig. 2). Scientists have designed some highly structured nanofibrous scaffolds by combining multiple nanofibrous layers and various inorganic molecules, growth factors, and cells (Gao et al., 2015; Sun et al., 2017; Zhang et al., 2017).
Fig. 2.
Model of the effect of nanofibers on stem cells
Stem cells sense the stiffness and other characteristics of nanofibers, change their shape and cell tension accordingly, and regulate downstream pathways resulting in osteogenesis and bone regeneration
However, to our best knowledge, despite increasing knowledge, previous studies have not reached a standard conclusion regarding the optimal environment for osteogenesis. Certain studies describing various types and degrees of differentiation of cells, as well as using assorted materials and culture environments (for example, cells cultured singly or together), have contradictory results. Besides, there is not enough research aimed at revealing the signaling pathways (Table 1). Therefore, we were not able to describe the detailed mechanism of cell adhesion and osteogenic differentiation on nanofibrous scaffolds in the present review. Additional experiments are needed to identify the essential signaling pathways in the bone regeneration process, for which gene ontology could be a useful method.
Table 1.
Summary of research on signaling pathways and relative factors that affect cell adhesion and osteogenesis
| Reference | Nanofiber | Cell type | Relative pathway | Gene function or main discovery | Relative factor |
| Liu WT et al., 2013 | PLLA nanofibers: random and aligned | Human BMSCs | Focal adhesion kinase, TGF-β, Wnt, and MAPK pathways | A similar though weaker rhythm of dynamic cellular behavior was induced on random nanofibers when compared with that on osteogenic supplements, and mechanotransduction could trigger nonspecific and multilevel responses in human BMSCs | |
| Baker et al., 2014 | PCL-NF and PDLLA-NF scaffolds | Human BMSCs | TGF-β and cell-adhesion/ECM-receptor pathways | Nanofibers and osteogenic supplements regulated similar pathways; both amplified TGF-β and cell-adhesion/ECM-receptor pathways | |
| Higgins et al., 2015 | PLLA nanofibers in diameters of 0.1, 0.3, and 1.0 μm | MC3T3-E1 S4 cells (passage 35) | POR1, Rac1, and Artf1 | Geometry sensing | Physical and chemical properties of nanofibers |
| Chang et al., 2018 | The fabrication of an NF-MP matrix that controls one single stem cell in a nanofibrous microisland | Rat BMSCs | FAK/RhoA/YAP1 pathway | Cell adhesion | Physical and chemical properties of nanofibers |
| Ozdemir et al., 2013 | Poly(methyl methacrylate) fibers | MC3T3-E1 osteoprogenitor cells | Integrin receptors, focal adhesion proteins, actin stress fibers and Myosin IIa, RhoA/ROCKII | Cytoskeletal organization and cell morphology | Nanofiber stiffness |
| Andalib et al., 2013 | Unidirectionally aligned and randomly distributed nanofibers, both with an average diameter of approximately | MSCs, C3H10T1/2 | ROCK | Cytoskeletal organization and cell morphology | Alignment of nanofibers |
| 130 nm, fabricated with PLLA | |||||
| Andalib et al., 2016 | Aligned and randomly distributed nanofibers from PLLA to have the same diameters (about 130 nm) | C3H10T1/2 murine MSCs | FAK | Cytoskeletal organization and cell morphology | Alignment of nanofibers |
| Izadpanahi et al., 2018 | Aligned and randomly oriented PLLA scaffolds | hASCs | LncRNAs and miR-125b-MEG3, H19 modulator BMP signaling pathway | Osteogenesis | Alignment of nanofibers |
| Zhu et al., 2013 | Two kinds of electrospun nanofibrous meshes with different fiber arrangements (totally non-woven and lattice-like) | Rat BMSCs | Integrin subunits α5 and β1, RhoA, and ERK | Cell adhesion | Hierarchical structure of nanofibrous scaffolds |
| Xue et al., 2017 | Polycaprolactone nanofiber scaffold | Human UC-, BM-, and AD-derived MSCs | Wnt/β-catenin and Smad3 | Osteogenesis | Hierarchical structure of nanofibrous scaffolds |
| Liu HH et al., 2013a | Biomimetic nanocomposite nanofibrous scaffold of hydroxyapatite/chitosan | Rat BMSCs | Smad1, BMP-2/4, Runx2, ALP, collagen I, integrin subunits together with myosins; the critical proteins pSmad1/5/8 in the BMP pathway | Osteogenesis | Functional additives |
| Zhang et al., 2015 | Gelatin/β-tricalcium phosphate composite nanofibers | Rat BMSCs | Calcium-sensing receptor | Environmental sensing | Functional additives |
PLLA: poly(l)-lactic acid; PCL-NF: poly(ε-caprolactone) nanofiber; PDLLA-NF: poly(d,l-lactic acid) nanofiber; NF-MP: nanofibrous micropatterned; BMSC: bone marrow mesenchymal stem cell; MSC: mesenchymal stem cell; hASC: human adipose-derived stem cell; UC: umbilical cord; BM: bone marrow; AD: adipose tissue; TGF-β: transforming growth factor-β; MAPK: mitogen-activated protein kinase; ECM: extracellular matrix; POR1: porin 1; Rac1: Ras-related C3 botulinum toxin substrate 1; Artf1: adenosine diphosphate (ADP) ribosylation factor 1; FAK: focal adhesion kinase; RhoA: Ras homolog gene family member A; YAP1: Yes-associated protein 1; ROCK: Rho-associated coiled-coil-containing protein kinase; lncRNA: long non-coding RNA; MEG3: maternally expressed gene 3; BMP: bone morphogenic protein; ERK: extracellular signal-regulated kinase; Smad: small mothers against decapentaplegic; Runx2: Runt-related transcription factor 2; ALP: alkaline phosphatase; pSmad: phosphorylated Smad
To date, nanofibrous scaffolds have been designed using various methods to promote bone regeneration. However, no single design is in use that is widely accepted, which limits the general application of such scaffolds. Additional experiments are necessary to understand the principles of osteogenesis on classical designs of tissue-mimicking nanofibrous scaffold to further guide future research and development in this field.
Acknowledgments
We express our sincere appreciation to the First Affiliated Hospital and Department of Stomatology, Zhejiang University School of Medicine, Hangzhou, China for providing the platform for tissue engineering.
Footnotes
Project supported by the National Natural Science Foundation of China (No. 31570989), the Young Talents Project of Zhejiang Provincial Health Department (No. 2019RC151), and the Zhejiang Provincial Welfare Technology Research Project (No. LGF20H140007), China
Contributors: Dan YU was responsible for concept design, literature research, and manuscript writing. Jin WANG performed the literature research, and wrote and edited the manuscript. Ke-jia QIAN and Jing YU participated in the analysis of literature, and writing and editing of the manuscript. Hui-yong ZHU was responsible for review and quality control of the manuscript. All authors have read and approved the final manuscript and, therefore, have full access to all the data in the study and take responsibility for the integrity and security of the data.
Compliance with ethics guidelines: Dan YU, Jin WANG, Ke-jia QIAN, Jing YU, and Hui-yong ZHU declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Abedin E, Lari R, Mahdavi Shahri N, et al. Development of a demineralized and decellularized human epiphyseal bone scaffold for tissue engineering: a histological study. Tissue Cell. 2018;55:46–52. doi: 10.1016/j.tice.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Allori AC, Sailon AM, Warren SM. Biological basis of bone formation, remodeling, and repair–part II: extracellular matrix. Tissue Eng Part B: Rev. 2008;14(3):275–283. doi: 10.1089/ten.teb.2008.0083. [DOI] [PubMed] [Google Scholar]
- 3.Andalib MN, Lee JS, Ha L, et al. The role of RhoA kinase (ROCK) in cell alignment on nanofibers. Acta Biomater. 2013;9(8):7737–7745. doi: 10.1016/j.actbio.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 4.Andalib MN, Lee JS, Ha L, et al. Focal adhesion kinase regulation in stem cell alignment and spreading on nanofibers. Biochem Biophys Res Commun. 2016;473(4):920–925. doi: 10.1016/j.bbrc.2016.03.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arslan E, Hatip Koc M, Uysal O, et al. Supramolecular peptide nanofiber morphology affects mechanotransduction of stem cells. Biomacromolecules. 2017;18(10):3114–3130. doi: 10.1021/acs.biomac.7b00773. [DOI] [PubMed] [Google Scholar]
- 6.Asencio IO, Mittar S, Sherborne C, et al. A methodology for the production of microfabricated electrospun membranes for the creation of new skin regeneration models. J Tissue Eng. 2018;9:1–8. doi: 10.1177/2041731418799851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baker BA, Pine PS, Chatterjee K, et al. Ontology analysis of global gene expression differences of human bone marrow stromal cells cultured on 3D scaffolds or 2D films. Biomaterials. 2014;35(25):6716–6726. doi: 10.1016/j.biomaterials.2014.04.075. [DOI] [PubMed] [Google Scholar]
- 8.Barros RC, Gelens E, Bulten E, et al. Self-assembled nanofiber coatings for controlling cell responses. J Biomed Mater Res A. 2017;105(8):2252–2265. doi: 10.1002/jbm.a.36092. [DOI] [PubMed] [Google Scholar]
- 9.Bhardwaj N, Kundu SC. Electrospinning: a fascinating fiber fabrication technique. Biotechnol Adv. 2010;28(3):325–347. doi: 10.1016/j.biotechadv.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Brusatin G, Panciera T, Gandin A, et al. Biomaterials and engineered microenvironments to control YAP/TAZ-dependent cell behaviour. Nat Mater. 2018;17(12):1063–1075. doi: 10.1038/s41563-018-0180-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Canha-Gouveia A, Rita Costa-Pinto A, Martins AM, et al. Hierarchical scaffolds enhance osteogenic differentiation of human Wharton’s jelly derived stem cells. Biofabrication. 2015;7(3):035009. doi: 10.1088/1758-5090/7/3/035009. [DOI] [PubMed] [Google Scholar]
- 12.Carbone EJ, Jiang T, Nelson C, et al. Small molecule delivery through nanofibrous scaffolds for musculoskeletal regenerative engineering. Nanomedicine: NBM. 2014;10(8):1691–1699. doi: 10.1016/j.nano.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ceylan H, Kocabey S, Gulsuner HU, et al. Bone-like mineral nucleating peptide nanofibers induce differentiation of human mesenchymal stem cells into mature osteoblasts. Biomacromolecules. 2014;15(7):2407–2418. doi: 10.1021/bm500248r. [DOI] [PubMed] [Google Scholar]
- 14.Chaires-Rosas CP, Ambriz X, Montesinos JJ, et al. Differential adhesion and fibrinolytic activity of mesenchymal stem cells from human bone marrow, placenta, and Wharton’s jelly cultured in a fibrin hydrogel. J Tissue Eng. 2019;10:1–17. doi: 10.1177/2041731419840622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang B, Ma C, Liu XH. Nanofibers regulate single bone marrow stem cell osteogenesis via FAK/RhoA/YAP1 pathway. ACS Appl Mater Interfaces. 2018;10(39):33022–33031. doi: 10.1021/acsami.8b11449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chaudhuri O, Gu L, Klumpers D, et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat Mater. 2016;15(3):326–334. doi: 10.1038/nmat4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen GQ, Deng CX, Li YP. TGF-β and BMP signaling in osteoblast differentiation and bone formation. Int J Biol Sci. 2012;8(2):272–288. doi: 10.7150/ijbs.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen HL, Malheiro ADBF, van Blitterswijk C, et al. Direct writing electrospinning of scaffolds with multidimensional fiber architecture for hierarchical tissue engineering. ACS Appl Mater Interfaces. 2017;9(44):38187–38200. doi: 10.1021/acsami.7b07151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen XN, Fu XL, Shi JG, et al. Regulation of the osteogenesis of pre-osteoblasts by spatial arrangement of electrospun nanofibers in two- and three-dimensional environments. Nanomedicine: NBM. 2013;9(8):1283–1292. doi: 10.1016/j.nano.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 20.Cheng Z, Ye Z, Natan A, et al. Bone-inspired mineralization with highly aligned cellulose nanofibers as template. ACS Appl Mater Interfaces. 2019;11(45):42486–42495. doi: 10.1021/acsami.9b15234. [DOI] [PubMed] [Google Scholar]
- 21.Cui CB, Cooper LF, Yang XL, et al. Transcriptional coactivation of bone-specific transcription factor Cbfa1 by TAZ. Mol Cell Biol. 2003;23(3):1004–1013. doi: 10.1128/mcb.23.3.1004-1013.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dalby MJ, Gadegaard N, Oreffo ROC. Harnessing nanotopography and integrin–matrix interactions to influence stem cell fate. Nat Mater. 2014;13(6):558–569. doi: 10.1038/nmat3980. [DOI] [PubMed] [Google Scholar]
- 23.Das A, Fischer RS, Pan DJ, et al. YAP nuclear localization in the absence of cell–cell contact is mediated by a filamentous actin-dependent, myosin II- and phosphor-YAP-independent pathway during extracellular matrix mechanosensing. J Biol Chem. 2016;291(12):6096–6110. doi: 10.1074/jbc.M115.708313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.di Cio S, Gautrot JE. Cell sensing of physical properties at the nanoscale: mechanisms and control of cell adhesion and phenotype. Acta Biomater. 2016;30:26–48. doi: 10.1016/j.actbio.2015.11.027. [DOI] [PubMed] [Google Scholar]
- 25.Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310(5751):1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 26.Doosti-Telgerd M, Mahdavi FS, Moradikhah F, et al. Nanofibrous scaffolds containing hydroxyapatite and microfluidic-prepared polyamidoamin/BMP-2 plasmid dendriplexes for bone tissue engineering applications. Int J Nanomed. 2020;15:2633–2646. doi: 10.2147/IJN.S244416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duan B, Shou KQ, Su XJ, et al. Hierarchical microspheres constructed from chitin nanofibers penetrated hydroxyapatite crystals for bone regeneration. Biomacromolecules. 2017;18(7):2080–2089. doi: 10.1021/acs.biomac.7b00408. [DOI] [PubMed] [Google Scholar]
- 28.Dufort CC, Paszek MJ, Weaver VM. Balancing forces: architectural control of mechanotransduction. Nat Rev Mol Cell Biol. 2011;12(5):308–319. doi: 10.1038/nrm3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dupont S, Morsut L, Aragona M, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474(7350):179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 30.Engler AJ, Sen S, Sweeney HL, et al. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 31.Fratzl P, Weinkamer R. Hierarchical structure and repair of bone: deformation, remodelling, healing. In: van der Zwaag S , editor. Self Healing Materials. Springer Series in Materials Science, Vol. 100. Springer, Dordrecht; 2007. pp. 323–335. [DOI] [Google Scholar]
- 32.Gao X, Zhang XH, Song JL, et al. Osteoinductive peptide-functionalized nanofibers with highly ordered structure as biomimetic scaffolds for bone tissue engineering. Int J Nanomed. 2015;10(1):7109–7128. doi: 10.2147/IJN.S94045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guilak F, Cohen DM, Estes BT, et al. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell. 2009;5(1):17–26. doi: 10.1016/j.stem.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gupte MJ, Swanson WB, Hu J, et al. Pore size directs bone marrow stromal cell fate and tissue regeneration in nanofibrous macroporous scaffolds by mediating vascularization. Acta Biomater. 2018;82:1–11. doi: 10.1016/j.actbio.2018.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Higgins AM, Banik BL, Brown JL. Geometry sensing through POR1 regulates Rac1 activity controlling early osteoblast differentiation in response to nanofiber diameter. Integr Biol. 2015;7(2):229–236. doi: 10.1039/c4ib00225c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hong JH, Hwang ES, McManus MT, et al. TAZ, a transcriptional modulator of mesenchymal stem cell differentiation. Science. 2005;309(5737):1074–1078. doi: 10.1126/science.1110955. [DOI] [PubMed] [Google Scholar]
- 37.Hosseini FS, Soleimanifar F, Khojasteh A, et al. Promoting osteogenic differentiation of human-induced pluripotent stem cells by releasing WNT/β-catenin signaling activator from the nanofibers. J Cell Biochem. 2019;120(4):6339–6346. doi: 10.1002/jcb.27921. [DOI] [PubMed] [Google Scholar]
- 38.Huebsch N, Arany PR, Mao AS, et al. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat Mater. 2010;9(6):518–526. doi: 10.1038/nmat2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hwang CW, Johnston PV, Gerstenblith G, et al. Stem cell impregnated nanofiber stent sleeve for on-stent production and intravascular delivery of paracrine factors. Biomaterials. 2015;52:318–326. doi: 10.1016/j.biomaterials.2015.02.047. [DOI] [PubMed] [Google Scholar]
- 40.Izadpanahi M, Seyedjafari E, Arefian E, et al. Nanotopographical cues of electrospun PLLA efficiently modulate non-coding RNA network to osteogenic differentiation of mesenchymal stem cells during BMP signaling pathway. Mater Sci Eng C. 2018;93:686–703. doi: 10.1016/j.msec.2018.08.023. [DOI] [PubMed] [Google Scholar]
- 41.Jahanmard F, Eslaminejad MB, Amani-Tehran M, et al. Incorporation of F-MWCNTs into electrospun nanofibers regulates osteogenesis through stiffness and nanotopography. Mater Sci Eng C, 106:110163. 2020 doi: 10.1016/j.msec.2019.110163. [DOI] [PubMed] [Google Scholar]
- 42.Kegelman CD, Mason DE, Dawahare JH, et al. Skeletal cell YAP and TAZ combinatorially promote bone development. FASEB J. 2018;32(5):2706–2721. doi: 10.1096/fj.201700872R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kennedy KM, Bhaw-Luximon A, Jhurry D. Cell–matrix mechanical interaction in electrospun polymeric scaffolds for tissue engineering: implications for scaffold design and performance. Acta Biomater. 2017;50:41–55. doi: 10.1016/j.actbio.2016.12.034. [DOI] [PubMed] [Google Scholar]
- 44.Khorshidi S, Karkhaneh A. Hydrogel/fiber conductive scaffold for bone tissue engineering. J Biomed Mater Res Part A. 2018;106(3):718–724. doi: 10.1002/jbm.a.36282. [DOI] [PubMed] [Google Scholar]
- 45.Kim JJ, El-Fiqi A, Kim HW. Synergetic cues of bioactive nanoparticles and nanofibrous structure in bone scaffolds to stimulate osteogenesis and angiogenesis. ACS Appl Mater Interfaces. 2017;9(3):2059–2073. doi: 10.1021/acsami.6b12089. [DOI] [PubMed] [Google Scholar]
- 46.Kim MS, Shin YN, Cho MH, et al. Adhesion behavior of human bone marrow stromal cells on differentially wettable polymer surfaces. Tissue Eng. 2007;13(8):2095–2103. doi: 10.1089/ten.2006.0062. [DOI] [PubMed] [Google Scholar]
- 47.Kuang R, Zhang ZP, Jin XB, et al. Nanofibrous spongy microspheres for the delivery of hypoxia-primed human dental pulp stem cells to regenerate vascularized dental pulp. Acta Biomater. 2016;33:225–234. doi: 10.1016/j.actbio.2016.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee JH, Kim HW. Emerging properties of hydrogels in tissue engineering. J Tissue Eng, 9:2041731418768285. 2018 doi: 10.1177/2041731418768285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee JH, Lee YJ, Cho HJ, et al. Guidance of in vitro migration of human mesenchymal stem cells and in vivo guided bone regeneration using aligned electrospun fibers. Tissue Eng Part A. 2014;20(15-16):2031–2042. doi: 10.1089/ten.tea.2013.0282. [DOI] [PubMed] [Google Scholar]
- 50.Li HX, Wu T, Xue JJ, et al. Transforming nanofiber mats into hierarchical scaffolds with graded changes in porosity and/or nanofiber alignment. Macromol Rapid Commun. 2020;41(3):1900579. doi: 10.1002/marc.201900579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu HH, Peng HJ, Wu Y, et al. The promotion of bone regeneration by nanofibrous hydroxyapatite/chitosan scaffolds by effects on integrin-BMP/Smad signaling pathway in BMSCs. Biomaterials. 2013;34(18):4404–4417. doi: 10.1016/j.biomaterials.2013.02.048. [DOI] [PubMed] [Google Scholar]
- 52.Liu L, Kamei KI, Yoshioka M, et al. Nano-on-micro fibrous extracellular matrices for scalable expansion of human ES/iPS cells. Biomaterials. 2017;124:47–54. doi: 10.1016/j.biomaterials.2017.01.039. [DOI] [PubMed] [Google Scholar]
- 53.Liu M, Zeng X, Ma C, et al. Injectable hydrogels for cartilage and bone tissue engineering. Bone Res, 5:17014. 2017 doi: 10.1038/boneres.2017.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu WT, Wei Y, Zhang XH, et al. Lower extent but similar rhythm of osteogenic behavior in hBMSCs cultured on nanofibrous scaffolds versus induced with osteogenic supplement. ACS Nano. 2013;7(8):6928–6938. doi: 10.1021/nn402118s. [DOI] [PubMed] [Google Scholar]
- 55.Liu XY, Shen H, Song SJ, et al. Accelerated biomineralization of graphene oxide-incorporated cellulose acetate nanofibrous scaffolds for mesenchymal stem cell osteogenesis. Colloids Surf B Biointerfaces. 2017;159:251–258. doi: 10.1016/j.colsurfb.2017.07.078. [DOI] [PubMed] [Google Scholar]
- 56.Liu Y, Luo D, Wang T. Hierarchical structures of bone and bioinspired bone tissue engineering. Small. 2016;12(34):4611–4632. doi: 10.1002/smll.201600626. [DOI] [PubMed] [Google Scholar]
- 57.Lü LX, Wang YY, Mao X, et al. The effects of PHBV electrospun fibers with different diameters and orientations on growth behavior of bone-marrow-derived mesenchymal stem cells. Biomed Mater, 7:015002. 2012 doi: 10.1088/1748-6041/7/1/015002. [DOI] [PubMed] [Google Scholar]
- 58.Luo Y, Shen H, Fang YX, et al. Enhanced proliferation and osteogenic differentiation of mesenchymal stem cells on graphene oxide-incorporated electrospun poly(lactic-co-glycolic acid) nanofibrous mats. ACS Appl Mater Interfaces. 2015;7(11):6331–6339. doi: 10.1021/acsami.5b00862. [DOI] [PubMed] [Google Scholar]
- 59.Lv HW, Wang HP, Zhang ZJ, et al. Biomaterial stiffness determines stem cell fate. Life Sci. 2017;178:42–48. doi: 10.1016/j.lfs.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 60.Mahmoudi N, Simchi A. On the biological performance of graphene oxide-modified chitosan/polyvinyl pyrrolidone nanocomposite membranes: in vitro and in vivo effects of graphene oxide. Mater Sci Eng C. 2017;70:121–131. doi: 10.1016/j.msec.2016.08.063. [DOI] [PubMed] [Google Scholar]
- 61.Mao AS, Shin JW, Mooney DJ. Effects of substrate stiffness and cell–cell contact on mesenchymal stem cell differentiation. Biomaterials. 2016;98:184–191. doi: 10.1016/j.biomaterials.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marrella A, Tedeschi G, Giannoni P, et al. “Green-reduced” graphene oxide induces in vitro an enhanced biomimetic mineralization of polycaprolactone electrospun meshes. Mater Sci Eng C. 2018;93:1044–1053. doi: 10.1016/j.msec.2018.08.052. [DOI] [PubMed] [Google Scholar]
- 63.McBeath R, Pirone DM, Nelson CM, et al. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6(4):483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 64.Mobasseri R, Tian LL, Soleimani M, et al. Peptide modified nanofibrous scaffold promotes human mesenchymal stem cell proliferation and long-term passaging. Mater Sci Eng C. 2018;84:80–89. doi: 10.1016/j.msec.2017.11.017. [DOI] [PubMed] [Google Scholar]
- 65.Moradi SL, Golchin A, Hajishafieeha Z, et al. Bone tissue engineering: adult stem cells in combination with electrospun nanofibrous scaffolds. J Cell Physiol. 2018;233(10):6509–6522. doi: 10.1002/jcp.26606. [DOI] [PubMed] [Google Scholar]
- 66.Motamedian SR, Hosseinpour S, Ahsaie MG, et al. Smart scaffolds in bone tissue engineering: a systematic review of literature. World J Stem Cells. 2015;7(3):657–668. doi: 10.4252/wjsc.v7.i3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nam J, Johnson J, Lannutti JJ, et al. Modulation of embryonic mesenchymal progenitor cell differentiation via control over pure mechanical modulus in electrospun nanofibers. Acta Biomater. 2011;7(4):1516–1524. doi: 10.1016/j.actbio.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Namdari M, Negahdari B, Eatemadi A. Paediatric nanofibrous bioprosthetic heart valve. IET Nanobiotechnol. 2017;11(5):493–500. doi: 10.1049/iet-nbt.2016.0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nasajpour A, Mandla S, Shree S, et al. Nanostructured fibrous membranes with rose spike-like architecture. Nano Lett. 2017;17(10):6235–6240. doi: 10.1021/acs.nanolett.7b02929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Noori A, Ashrafi SJ, Vaez-Ghaemi R, et al. A review of fibrin and fibrin composites for bone tissue engineering. Int J Nanomed. 2017;12:4937–4961. doi: 10.2147/IJN.S124671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Olivares-Navarrete R, Lee EM, Smith K, et al. Substrate stiffness controls osteoblastic and chondrocytic differentiation of mesenchymal stem cells without exogenous stimuli. PLoS ONE. 2017;12(1):e0170312. doi: 10.1371/journal.pone.0170312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ortega Z, Alemán ME, Donate R. Nanofibers and microfibers for osteochondral tissue engineering. In: Oliveira JM Pina S, Reis RL et al., editors. Osteochondral Tissue Engineering. Advances in Experimental Medicine and Biology, Vol. 1058. Springer, Cham; 2018. pp. 97–123. [DOI] [PubMed] [Google Scholar]
- 73.Ozdemir T, Xu LC, Siedlecki C, et al. Substrate curvature sensing through myosin IIA upregulates early osteogenesis. Integr Biol. 2013;5(11):1407–1416. doi: 10.1039/c3ib40068a. [DOI] [PubMed] [Google Scholar]
- 74.Pan HH, Xie YT, Zhang ZQ, et al. YAP-mediated mechanotransduction regulates osteogenic and adipogenic differentiation of BMSCs on hierarchical structure. Colloids Surf B Biointerfaces. 2017;152:344–353. doi: 10.1016/j.colsurfb.2017.01.039. [DOI] [PubMed] [Google Scholar]
- 75.Pan JX, Xiong L, Zhao K, et al. YAP promotes osteogenesis and suppresses adipogenic differentiation by regulating β-catenin signaling. Bone Res, 6:18. 2018 doi: 10.1038/s41413-018-0018-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Panciera T, Azzolin L, Cordenonsi M, et al. Mechanobiology of YAP and TAZ in physiology and disease. Nat Rev Mol Cell Biol. 2017;18(12):758–770. doi: 10.1038/nrm.2017.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pandey S, Rathore K, Johnson J, et al. Aligned nanofiber material supports cell growth and increases osteogenesis in canine adipose-derived mesenchymal stem cells in vitro. J Biomed Mater Res Part A. 2018;106(7):1780–1788. doi: 10.1002/jbm.a.36381. [DOI] [PubMed] [Google Scholar]
- 78.Perikamana SKM, Lee J, Ahmad T, et al. Effects of immobilized BMP-2 and nanofiber morphology on in vitro osteogenic differentiation of hMSCs and in vivo collagen assembly of regenerated bone. ACS Appl Mater Interfaces. 2015;7(16):8798–8808. doi: 10.1021/acsami.5b01340. [DOI] [PubMed] [Google Scholar]
- 79.Piccolo S, Dupont S, Cordenonsi M. The biology of YAP/TAZ: hippo signaling and beyond. Physiol Rev. 2014;94(4):1287–1312. doi: 10.1152/physrev.00005.2014. [DOI] [PubMed] [Google Scholar]
- 80.Polini A, Pisignano D, Parodi M, et al. Osteoinduction of human mesenchymal stem cells by bioactive composite scaffolds without supplemental osteogenic growth factors. PLoS ONE. 2011;6(10):e26211. doi: 10.1371/journal.pone.0026211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Purohit SD, Bhaskar R, Singh H, et al. Development of a nanocomposite scaffold of gelatin-alginate-graphene oxide for bone tissue engineering. Int J Biol Macromol. 2019;133:592–602. doi: 10.1016/j.ijbiomac.2019.04.113. [DOI] [PubMed] [Google Scholar]
- 82.Qian WY, Gong LQ, Cui X, et al. Nanotopographic regulation of human mesenchymal stem cell osteogenesis. ACS Appl Mater Interfaces. 2017;9(48):41794–41806. doi: 10.1021/acsami.7b16314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Qian YZ, Zhou XF, Zhang FM, et al. Triple PLGA/PCL scaffold modification including silver impregnation, collagen coating, and electrospinning significantly improve biocompatibility, antimicrobial, and osteogenic properties for orofacial tissue regeneration. ACS Appl Mater Interfaces. 2019;11(41):37381–37396. doi: 10.1021/acsami.9b07053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Re F, Sartore L, Moulisova V, et al. 3D gelatin-chitosan hybrid hydrogels combined with human platelet lysate highly support human mesenchymal stem cell proliferation and osteogenic differentiation. J Tissue Eng. 2019;10:1–16. doi: 10.1177/2041731419845852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rezvani Z, Venugopal JR, Urbanska AM, et al. A bird’s eye view on the use of electrospun nanofibrous scaffolds for bone tissue engineering: current state-of-the-art, emerging directions and future trends. Nanomedicine: NBM. 2016;12(7):2181–2200. doi: 10.1016/j.nano.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 86.Ribba L, Parisi M, D'Accorso NB, et al. Electrospun nanofibrous mats: from vascular repair to osteointegration. J Biomed Nanotechnol. 2014;10(12):3508–3535. doi: 10.1166/jbn.2014.2046. [DOI] [PubMed] [Google Scholar]
- 87.Sankar S, Sharma CS, Rath SN, et al. Electrospun nanofibres to mimic natural hierarchical structure of tissues: application in musculoskeletal regeneration. J Tissue Eng Regen Med. 2018;12(1):e604–e619. doi: 10.1002/term.2335. [DOI] [PubMed] [Google Scholar]
- 88.Sever M, Mammadov B, Guler MO, et al. Tenascin-C mimetic peptide nanofibers direct stem cell differentiation to osteogenic lineage. Biomacromolecules. 2014;15(12):4480–4487. doi: 10.1021/bm501271x. [DOI] [PubMed] [Google Scholar]
- 89.Shah S, Solanki A, Lee KB. Nanotechnology-based approaches for guiding neural regeneration. Acc Chem Res. 2016;49(1):17–26. doi: 10.1021/acs.accounts.5b00345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shalumon KT, Sowmya S, Sathish D, et al. Effect of incorporation of nanoscale bioactive glass and hydroxyapatite in PCL/chitosan nanofibers for bone and periodontal tissue engineering. J Biomed Nanotechnol. 2013;9(3):430–440. doi: 10.1166/jbn.2013.1559. [DOI] [PubMed] [Google Scholar]
- 91.Shao WL, He JX, Sang F, et al. Enhanced bone formation in electrospun poly(L-lactic-co-glycolic acid)-tussah silk fibroin ultrafine nanofiber scaffolds incorporated with graphene oxide. Mater Sci Eng C. 2016;62:823–834. doi: 10.1016/j.msec.2016.01.078. [DOI] [PubMed] [Google Scholar]
- 92.Sun M, Spill F, Zaman MH. A computational model of YAP/TAZ mechanosensing. Biophys J. 2016;110(11):2540–2550. doi: 10.1016/j.bpj.2016.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sun TW, Yu WL, Zhu YJ, et al. Hydroxyapatite nanowire @magnesium silicate core-shell hierarchical nanocomposite: synthesis and application in bone regeneration. ACS Appl Mater Interfaces. 2017;9(19):16435–16447. doi: 10.1021/acsami.7b03532. [DOI] [PubMed] [Google Scholar]
- 94.Takada I, Kouzmenko AP, Kato S. Wnt and PPARγ signaling in osteoblastogenesis and adipogenesis. Nat Rev Rheumatol. 2009;5(8):442–447. doi: 10.1038/nrrheum.2009.137. [DOI] [PubMed] [Google Scholar]
- 95.Tatapudy S, Aloisio F, Barber D, et al. Cell fate decisions: emerging roles for metabolic signals and cell morphology. EMBO Rep. 2017;18(12):2105–2118. doi: 10.15252/embr.201744816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tavakol S, Rasoulian B, Ramezani F, et al. Core and biological motif of self-assembling peptide nanofiber induce a stronger electrostatic interaction than BMP2 with BMP2 receptor 1A. Mater Sci Eng C. 2019;101:148–158. doi: 10.1016/j.msec.2019.03.097. [DOI] [PubMed] [Google Scholar]
- 97.Tutak W, Jyotsnendu G, Bajcsy P, et al. Nanofiber scaffolds influence organelle structure and function in bone marrow stromal cells. J Biomed Mater Res B Appl Biomater. 2017;105(5):989–1001. doi: 10.1002/jbm.b.33624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang D, Jang J, Kim K, et al. “Tree to bone”: lignin/polycaprolactone nanofibers for hydroxyapatite biomineralization. Biomacromolecules. 2019;20(7):2684–2693. doi: 10.1021/acs.biomac.9b00451. [DOI] [PubMed] [Google Scholar]
- 99.Wu J, Xie LL, Lin WZY, et al. Biomimetic nanofibrous scaffolds for neural tissue engineering and drug development. Drug Discov Today. 2017;22(9):1375–1384. doi: 10.1016/j.drudis.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 100.Xie CM, Sun HL, Wang KF, et al. Graphene oxide nanolayers as nanoparticle anchors on biomaterial surfaces with nanostructures and charge balance for bone regeneration. J Biomed Mater Res Part A. 2017;105(5):1311–1323. doi: 10.1002/jbm.a.36010. [DOI] [PubMed] [Google Scholar]
- 101.Xing F, Li L, Zhou CC, et al. Regulation and directing stem cell fate by tissue engineering functional microenvironments: scaffold physical and chemical cues. Stem Cells Int, 2019:2180925. 2019 doi: 10.1155/2019/2180925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xiong JH, Almeida M, O'Brien CA. The YAP/TAZ transcriptional co-activators have opposing effects at different stages of osteoblast differentiation. Bone. 2018;112:1–9. doi: 10.1016/j.bone.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Xue RY, Qian YN, Li LH, et al. Polycaprolactone nanofiber scaffold enhances the osteogenic differentiation potency of various human tissue-derived mesenchymal stem cells. Stem Cell Res Ther, 8:148. 2017 doi: 10.1186/s13287-017-0588-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yahia S, Khalil IA, El-Sherbiny IM. Sandwich-like nanofibrous scaffolds for bone tissue regeneration. ACS Appl Mater Interfaces. 2019;11(32):28610–28620. doi: 10.1021/acsami.9b06359. [DOI] [PubMed] [Google Scholar]
- 105.Yang X, Li YY, He W, et al. Hydroxyapatite/collagen coating on PLGA electrospun fibers for osteogenic differentiation of bone marrow mesenchymal stem cells. J Biomed Mater Res A. 2018;106(11):2863–2870. doi: 10.1002/jbm.a.36475. [DOI] [PubMed] [Google Scholar]
- 106.Yang ZQ, Si JH, Cui ZX, et al. Biomimetic composite scaffolds based on surface modification of polydopamine on electrospun poly(lactic acid)/cellulose nanofibrils. Carbohydr Polym. 2017;174:750–759. doi: 10.1016/j.carbpol.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 107.Ye K, Cao LP, Li SY, et al. Interplay of matrix stiffness and cell–cell contact in regulating differentiation of stem cells. ACS Appl Mater Interfaces. 2016;8(34):21903–21913. doi: 10.1021/acsami.5b09746. [DOI] [PubMed] [Google Scholar]
- 108.Zaidi SK, Sullivan AJ, Medina R, et al. Tyrosine phosphorylation controls Runx2-mediated subnuclear targeting of YAP to repress transcription. EMBO J. 2004;23(4):790–799. doi: 10.1038/sj.emboj.7600073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang K, Wang Y, Sun T, et al. Bioinspired surface functionalization for improving osteogenesis of electrospun polycaprolactone nanofibers. Langmuir. 2018;34(50):15544–15550. doi: 10.1021/acs.langmuir.8b03357. [DOI] [PubMed] [Google Scholar]
- 110.Zhang S, Jiang GJ, Prabhakaran MP, et al. Evaluation of electrospun biomimetic substrate surface-decorated with nanohydroxyapatite precipitation for osteoblasts behavior. Mater Sci Eng C. 2017;79:687–696. doi: 10.1016/j.msec.2017.05.113. [DOI] [PubMed] [Google Scholar]
- 111.Zhang XH, Meng S, Huang Y, et al. Electrospun gelatin/β-TCP composite nanofibers enhance osteogenic differentiation of BMSCs and in vivo bone formation by activating Ca2+-sensing receptor signaling. Stem Cells Int, 2015: 507154. 2015 doi: 10.1155/2015/507154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang YF, Fan W, Ma ZC, et al. The effects of pore architecture in silk fibroin scaffolds on the growth and differentiation of mesenchymal stem cells expressing BMP7. Acta Biomater. 2010;6(8):3021–3028. doi: 10.1016/j.actbio.2010.02.030. [DOI] [PubMed] [Google Scholar]
- 113.Zhu JX, Cai Q, Zhang X, et al. Biological characteristics of mesenchymal stem cells grown on different topographical nanofibrous poly-L-lactide meshes. J Biomed Nanotechnol. 2013;9(10):1757–1767. doi: 10.1166/jbn.2013.1661. [DOI] [PubMed] [Google Scholar]
- 114.Zhu Y, Li DW, Zhang K, et al. Novel synthesized nanofibrous scaffold efficiently delivered hBMP-2 encoded in adenoviral vector to promote bone regeneration. J Biomed Nanotechnol. 2017;13(4):437–446. doi: 10.1166/jbn.2017.2361. [DOI] [PubMed] [Google Scholar]