Abstract
Agriculture is the foundation of social development. Under the pressure of population growth, natural disasters, environmental pollution, climate change, and food safety, the interdisciplinary “new agriculture” is becoming an important trend of modern agriculture. In fact, new agriculture is not only the foundation of great health and new energy sources, but is also the cornerstone of national food security, energy security, and biosafety. Hydrogen agronomy focuses mainly on the mechanism of hydrogen gas (H2) biology effects in agriculture, and provides a theoretical foundation for the practice of hydrogen agriculture, a component of the new agriculture. Previous research on the biological effects of H2 focused chiefly on medicine. The mechanism of selective antioxidant is the main theoretical basis of hydrogen medicine. Subsequent experiments have demonstrated that H2 can regulate the growth and development of plant crops, edible fungus, and livestock, and enhance the tolerance of these agriculturally important organisms against abiotic and biotic stresses. Even more importantly, H2 can regulate the growth and development of crops by changing the soil microbial community composition and structure. Use of H2 can also improve the nutritional value and postharvest quality of agricultural products. Researchers have also shown that the biological functions of molecular hydrogen are mediated by modulating reactive oxygen species (ROS), nitric oxide (NO), and carbon monoxide (CO) signaling cascades in plants and microbes. This review summarizes and clarifies the history of hydrogen agronomy and describes recent progress in the field. We also argue that emerging hydrogen agriculture will be an important direction in the new agriculture. Further, we discuss several scientific problems in hydrogen agronomy, and suggest that the future of hydrogen agronomy depends on contributions by multiple disciplines. Important future research directions of hydrogen agronomy include hydrogen agriculture in special environments, such as islands, reefs, aircraft, and outer space.
Keywords: Hydrogen gas (H2), Hydrogen agronomy, New agriculture
1. Introduction
Many years ago, the significance of hydrogen gas (H2) to human life became apparent. As early as in 1937, Paneth (1937) reported that the tropospheric mixing ratio of H2 in the atmosphere was about 0.5 ppmv (parts per million by volume), equivalent to 34.5 ppbw (parts per billion by weight). Although the content of hydrogen is very low, it plays an important role in maintaining oxidation state of earth atmosphere (Constant et al., 2009). H2 affects almost every aspect of our lives. For example, due to its high energy density and ability to store and retain energy after combustion, H2 has been widely used, especially in the energy industry (Árnason and Sigfússon, 2000) for applications such as hydrogen-powered fuel cell vehicles (Dresselhaus and Thomas, 2001).
The sources of atmospheric H2 are diverse. According to previous work (Novelli et al., 1999; Constant et al., 2009), photochemical oxidation reactions of methane (CH4) and non-methane hydrocarbons (NMHC) are the largest sources of H2 in atmosphere (about 19 Tg/year (1 Tg=1×1012 g)). In addition to photochemical oxidation reactions, biomass combustion (about 16 Tg/year) and the fossil fuel industry (about 15 Tg/year) are close secondary and tertiary sources of atmospheric H2 (Novelli et al., 1999). H2 is also generated from nitrogen-fixing legumes, as a type of byproduct during nitrogen (N2) fixation (Novelli et al., 1999). The proportion of electrons utilized to generate H2 in the root nodule nitrogenases is about 40%–60% (Schubert and Evans, 1976). H2 circulates all across the globe. The whereabouts of H2 entering the earth atmosphere are also diverse. Previous research found that H2 was consumed mainly by soil microbes, and oxidized by hydroxyl radicals (Ichimasa et al., 1989; Derwent et al., 2001; Constant et al., 2009). About 80% of atmospheric H2 (about 56 Tg/year) is absorbed by soil, through a microbial-mediated process (Constant et al., 2009). Because H2 is consumed by soil microorganisms adjacent to the nodules, relatively little soil H2 gets into the atmosphere (about 3 Tg/year) (Novelli et al., 1999; Constant et al., 2009). The 56 Tg/year of soil H2 represents significant energy, about 8 EJ/year. Because this energy source is taken up by soil microbial populations, it is not surprising that H2 has biological effects on agricultural systems.
Although the production and release of H2 from algae, plants, microorganisms, and animals have been reported, the biological functions of H2 were long unknown (Gaffron, 1939; Gest and Kamen, 1949; Renwick et al., 1964; Czerkawski, 1972). Dole et al. (1975) reported positive effects of high-pressure H2 on skin cancer in mice, which is the earliest report of medical hydrogen research recognized by the scientific community. Later, Ohsawa et al. (2007) found that inhaling 2% to 4% (volume/volume ratio, v/v) of H2 can protect against ischemic brain damage in rats by selectively reducing toxic reactive oxygen species (ROS), including hydroxyl radicals and peroxynitrite anions. These findings sparked a new wave of research on H2.
By 2019, 42 scientific research institutions in China had participated in research on hydrogen biology, and a landmark event is the establishment of the Center of Hydrogen Science at Shanghai Jiao Tong University (Shanghai, China). The new center is headed by Prof. Wen-jiang DING, Academician of Chinese Academy of Engineering. In order to expand commercial applications of H2, some companies have also begun to focus their attention on hydrogen medicine and hydrogen agriculture.
In the history of hydrogen biology, hydrogen medicine was ahead of hydrogen agronomy. The effects of H2 as an anti-oxidant (Ohsawa et al., 2007), anti-inflammatory (Zhao et al., 2013), and anti-apoptotic agent (Liu et al., 2015) have been elucidated by several animal models or small-scale clinical trials (Ohta, 2014; Lu and Sun, 2018). Moreover, H2 can not only inhibit tumors (Dole et al., 1975; Saitoh et al., 2008), but it also has positive effects on oxidative stress-related diseases, such as ischemia reperfusion injury (Ohsawa et al., 2007), acute ischemic disease (Du et al., 2014), Parkinson’s disease (Fu et al., 2009), Alzheimer’s disease (Hou et al., 2018), and atherosclerosis (Song et al., 2012). Although the hypothesis of H2 as a selective antioxidant is widely accepted, in some cases, it also still questioned by academia, and the molecular mechanisms underlying anti-oxidation, anti-inflammatory, and anti-apoptosis effects of H2 in medicine need to be further elucidated (Ge et al., 2017; Shen and Sun, 2019). More importantly, the potentially important development of hydrogen agronomy cannot be ignored.
Several research groups have found that H2 has important biological effects on crops. Dong et al. (2003) put forward the concept of “H2 fertilization,” which was a milestone in the advancement of hydrogen agronomy. H2 can change the structure of microbial communities and ultimately promotes soil fertility by promoting community growth of beneficial microorganisms. Thus, the essence of H2 effects in agronomy is as a type of special biofertilizer. In this role, H2 produced from symbiotic Rhizobium leguminosarum had been shown to promote crop rotation (Golding and Dong, 2010). Furthermore, some studies suggested that H2 might enhance plant tolerance towards abiotic and biotic stresses (Xie et al., 2012, 2014; Jin et al., 2013; Zeng et al., 2013; Su et al., 2018), and improve the quality of vegetables and fruits (Hu et al., 2014, 2018; Su et al., 2014).
With support from the Ministry of Science and Technology of the People’s Republic of China, hydrogen agronomy is developing quickly. By 2019, 85 projects on hydrogen biology have been supported by the National Natural Science Foundation of China (Shen and Sun, 2019), of which nine were related to hydrogen agronomy. From the perspective of species, these hydrogen agronomy projects focused on alfalfa (Medicago sativa) (Cui et al., 2013, 2014; Chen et al., 2014; Su et al., 2018) and several horticultural plants, such as tomatoes (Solanum lycopersicum) (Zhang et al., 2019) and marigolds (Tagetes erecta) (Zhu and Liao, 2017). Hydrogen agronomy is related to a diverse range of physiological functions, including plant responses related to abiotic stress (Xie et al., 2012, 2014, 2015; Cui et al., 2013, 2014, 2020; Jin et al., 2013, 2016; Xu et al., 2013, 2017; Zeng et al., 2013; Chen et al., 2014, 2017; Wu et al., 2015; Dai et al., 2017; Su et al., 2018), root development (Lin et al., 2014; Zhu et al., 2016; Cao et al., 2017; Zhu and Liao, 2017), and quality of agricultural products (Hu et al., 2014, 2018; Su et al., 2014; Zhang XY et al., 2018; Su et al., 2019; Zhang YH et al., 2019). A long list of obvious and significant questions in the field of hydrogen agronomy awaits deeper scientific investigation.
2. Hydrogen agronomy: laboratory-based research
2.1. Agricultural status in China
Food is the fuel of life. As important sources of food, agriculture is one of the foundations of social development, and occupies an important position in people’s lives. According to data from the National Bureau of Statistics of the People’s Republic of China, the agricultural output and its gross output value keep sustainable increase (Table 1). However, pressures from increasing population, reduction in arable land, extreme weather, and increasing demand for cereals like maize (Zea mays), for both fodder and fuel, are important factors restricting the development of agriculture in China (Pimentel, 1996; Lewandrowski et al., 1997; Lesk et al., 2016; Nicolopoulou-Stamati et al., 2016).
Table 1.
Gross output value of agriculture and agricultural product output in China
| Year | Gross agricultural output value (billion CNY) | Grain output (1×104 t) | Fruit output (1×104 t) | Tea output (1×104 t) | Meat output (1×104 t) |
| 2013 | 4894.39 | 63 048.20 | 22 748.10 | 188.72 | 8632.77 |
| 2014 | 5185.11 | 63 964.83 | 23 302.63 | 204.93 | 8817.90 |
| 2015 | 5420.53 | 66 060.27 | 24 524.62 | 227.66 | 8749.52 |
| 2016 | 5565.99 | 66 043.51 | 24 405.24 | 231.33 | 8628.33 |
| 2017 | 5805.98 | 66 160.72 | 25 241.90 | 246.04 | 8654.43 |
| 2018 | 6145.26 | 65 789.22 | 25 688.35 | 261.04 | 8624.63 |
Data source: the National Bureau of Statistics of the People’s Republic of China (https://data.stats.gov.cn/easyquery.htm?cn=C01)
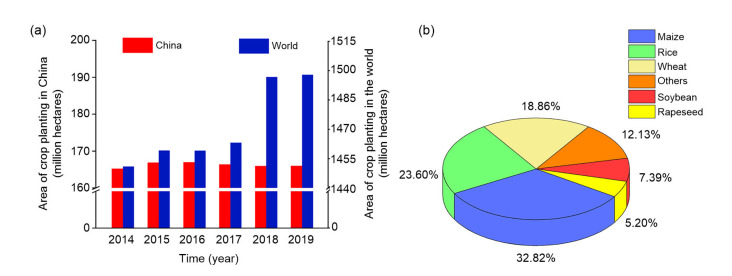
Soil plays a key role in agricultural development and biodiversity of agricultural production systems (Maeder et al., 2002; Young and Crawford, 2004; Mijangos et al., 2006; Melman et al., 2019; Qaswar et al., 2020). Soil degradation is a serious problem not only in China but also throughout the world. Crop acreage during last 6 years in China shows a volatile trend, and maize and rice are the first and second major crops (Fig. 1), based on data released by the Ministry of Natural Resources of the People’s Republic of China and the United States Department of Agriculture. Although the main reasons for reduction in crop acreage include ecological land transfer and agricultural structure adjustment, according to official findings, the potential contradiction between growing population and cropland cannot be easily ignored. As we all know, modern agriculture is closely integrated not only with traditional planting and breeding industries, but also with secondary industries, including manufacturing and food processing, and with tertiary industries, such as transportation, technology, and information services (Macrae et al., 1993; Li et al., 2018; Lytos et al., 2020). Hydrogen agronomy has also no exception.
Fig. 1.
Changes in crop area under cultivation in China and the world between 2014 and 2019 (a) and proportion of area devoted to cultivation of various crops in China in 2019 (b)
Data sources: the National Bureau of Statistics of the People’s Republic of China (https://data.stats.gov.cn) and the United States Department of Agriculture (https://apps.fas.usda.gov/psdonline/app/index.html#/app/downloads)
2.2. Hydrogen agronomy needs to be developed
Although the core of both hydrogen agronomy and hydrogen medicine is human health, the practices of these two programs are different, including targets, methods of H2 supply, and degree of difficulty (Table 2). The ultimate practical goal of hydrogen medicine is human health (Ohta, 2011, 2014). Unlike hydrogen medicine, hydrogen agronomy is not only a laboratory-based research study, but also has potential applications for agricultural production, dubbed hydrogen agriculture, with the goal of growing food in an affordable, healthy, and sustainable manner for human beings (Shen and Sun, 2019).
Table 2.
Differences in research methods between hydrogen medicine and hydrogen agronomy
| Program | Research model | Method of H2 supply | Degree of difficulty | Reference |
| Hydrogen medicine | Rat | Inhalation of H2 | Difficult | Ohsawa et al., 2007 |
| Rat | Injection of hydrogen-rich saline | Difficult | Zhao et al., 2013 | |
| Rat | Injection of hydrogen-rich saline | Difficult | Wang et al., 2011 | |
| Rat | Drinking HRW | Easy | Fu et al., 2009 | |
| Mice | Feeding magnesium hydride | Easy | Kamimura et al., 2016 | |
| Human | Drinking HRW | Easy | Kang et al., 2011 | |
| Hydrogen agronomy | Hypsizygus marmoreus | Watering HRW | Easy | Zhang et al., 2017 |
| Rice | Soak in HRW | Easy | Xu et al., 2013 | |
| Kiwifruit | Soak in HRW | Easy | Hu et al., 2014 | |
| Tomato | Soak in HRW | Easy | Lu et al., 2017 | |
| Lisianthus | Watering HRW | Easy | Su et al., 2019 |
HRW: hydrogen-rich water
Traditional agriculture consumes a large volume of agrochemicals, especially fertilizers and pesticides (Lowry et al., 2019). As a gaseous signaling molecule, H2 has been studied extensively in recent years. Hydrogen agronomy focuses mainly on the molecular mechanisms underlying hydrogen-rich water (HRW)-or H2-increased yield and/or H2-improved quality of agricultural products (Wang and Wei, 2016; Ren PJ et al., 2017; Shen and Sun, 2019).
Additionally, we should be aware of the problems probably caused by the application of H2 on human society. In particular, H2 is used for clean energy in industry, and future development of hydrogen economy in agriculture could also result in more anthropogenic emissions of H2. In developing hydrogen agriculture, the influence of increasing global H2 concentration in atmosphere cannot be easily ignored. Therefore, the impact of hydrogen economy on human beings and the planet should be carefully evaluated in the near future.
3. Hydrogen agronomy: past and present
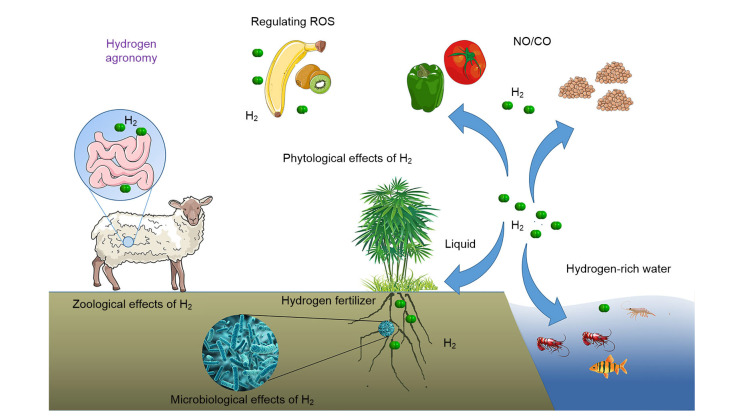
Generally speaking, both hydrogen medicine and hydrogen agriculture address the biological effects of H2 and its corresponding applications. Discoveries related to the research targets and mechanisms in hydrogen agronomy are summarized below (Fig. 2).
Fig. 2.
Hydrogen agronomy: targets and mechanisms
The targets of hydrogen agronomy mainly include plants, animals, and microbes. Hydrogen gas (H2) can influence reactive oxygen species (ROS), nitric oxide (NO), and carbon monoxide (CO) signaling in organisms. In addition, the soil microbial community composition and structure can be changed by soil hydrogen treatment, and the hydrogen-treated soil can improve plant growth
3.1. H2 biosynthesis
H2 biosynthesis occurs mainly in plants and microbes. Gas chromatography (GC), hydrogen-specific sensors, and spectrophotometric methods are normally used to determine H2 in biological contexts (Shen and Sun, 2019). H2 production was first observed in bacteria (Stephenson and Stickland, 1931). Subsequently, release of H2 was also found in green algae (Gaffron, 1939). In algae and microbes, H2 production depends mainly on hydrogenase (typically [Ni–Fe] and [Fe–Fe] hydrogenases) and nitrogenase (Das et al., 2008). [Ni–Fe] hydrogenase is mainly present in some sulfate-reducing bacteria (Yagi and Higuchi, 2013), and [Fe–Fe] hydrogenase is found in anaerobic prokaryotes such as clostridia and sulfate reducers; among eukaryotes, [Fe–Fe] hydrogenase has been found in some anaerobic eukaryotes (including fungi, cilates, and trichomonads) and some green algae, including Chlamydomonas reinhardtii (Lubitz et al., 2014). In addition, a third type of hydrogenase, [Fe] hydrogenase, has been found in methanogenic archaea (Vogt et al., 2008). For nitrogenase, H2 is a byproduct of nitrogen fixation in rhizobia (Golding and Dong, 2010).
Although metabolic synthesis of H2 has been demonstrated in microbes, algae, and higher plants, the mechanisms of H2 generation still need further investigation. Su et al. (2019) discovered that the photosynthetic electron transport chain was related to H2 production. The generation of H2 was reduced by suppressing above process using pharmacological approach.
Some researchers are currently focusing attention on the relationship among environmental stress, phytohormones, and hydrogen production. Many studies have found that the production of H2 from plants can be induced by drought, osmotic stress, salt damage, and heavy metal stress (Cui et al., 2013; Zeng et al., 2013; Jin et al., 2016; Chen et al., 2017). Phytohormones are also associated with hydrogen production. H2 production from plants can be induced or influenced by auxin, abscisic acid, and ethylene (Zeng et al., 2013; Xie et al., 2014; Cao et al., 2017). Since H2 production is quickly induced in response to abscisic acid signaling, it cannot be easily ruled out that hydrogen production may be a non-enzymatic process. We also speculate that H2 production and emission might be associated with energy metabolism in chloroplasts and mitochondria. The involvement of H2 in the signaling pathways of various phytohormones (Shen and Sun, 2019) suggests the possibility that H2 may be a “universal signaling molecule.”
3.2. Beginning of hydrogen agronomy: hydrogen fertilizer?
The essence of hydrogen agronomy in the narrow sense has been previously regarded as “hydrogen fertilizer.” Unlike traditional fertilizers that supply nutrients (N, P, and trace nutrients), “hydrogen fertilizer” exerts the roles of fertilizer in the soil through supplying H2 (Dong et al., 2003; Golding and Dong, 2010). This “hydrogen fertilizer” is not a nutrient but exerts its beneficial effects through other mechanisms. In the forms of HRW and H2 gas, H2 is the protagonist of hydrogen agronomy, and its potentials in the production, transportation, storage, and sales of agricultural products have been repeatedly demonstrated (Hu et al., 2014, 2018; Xu et al., 2017; Ji et al., 2019; Zhang et al., 2019).
Previous research has found that the rates of oxygen and carbon dioxide uptake in soils are influenced by H2 (Dong and Layzell, 2001). Further work demonstrated that soil uptake of H2, especially near nodulated legume roots, dramatically modifies microbial populations (McLearn and Dong, 2002; Dong et al., 2003; Stein et al., 2005), by selectively enhancing the populations of plant growth-promoting rhizobacteria (PGPRs). This phenomenon is used to explain the benefits of using legumes in crop rotation with wheat and other non-legumes (Kirkegaard et al., 2008). The weight of fixed nitrogen in one hectare of legume crop is about 200 kg per season; meanwhile, the volume of H2 produced during above process is about 240 000 L (Dong et al., 2003). The evolution of H2 from roots of nodulated grain legumes fluctuates between 0.06 and 0.51 mmol/(h·g nodule dry weight) (Angus et al., 2015). Therefore, it is not difficult to understand that H2 can exert tremendous biological effects in agriculture.
The growth performance of barley, soybean, canola, and spring wheat was improved in H2-treated soils compared with growth in untreated soils, and the tiller numbers of barley and spring wheat were increased by 36% and 40%, respectively (Dong et al., 2003). H2 is an obligate byproduct of nitrogen fixation in legume rhizobia. Dong and his colleagues proposed that H2, whether produced by nitrogenase or exogenously applied, could have an important “fertilization” effect in rotations with legume crops (Dong et al., 2003; Golding and Dong, 2010). H2 may then have some functions to partly minimize the use of chemical fertilizer. The relationships between microbes in soils and plants are very close (Yang and Crowley, 2000). H2 can change the microbial community structure and thus influence plant growth and development (Dong et al., 2003).
3.3. Research methods of hydrogen agronomy
Hydrogen agronomy is an interdisciplinary science that combines physiological, biochemical, molecular biology, genetics, and multi-omics to study the related scientific principles of hydrogen agriculture. Each method has its own unique characteristics. Integration of these various disciplines and use of multiple methods are needed to study hydrogen agronomy from multiple angles and at multiple organizational levels.
3.3.1 Physiological and biochemical approaches
Physiology and biochemistry form the basis of hydrogen agronomy research. By measuring indicators related to physiology and biochemistry, we can intuitively understand hydrogen agronomy.
Early research on hydrogen agronomy mainly focused on easily measurable parameters, such as yield, quality, and organ development. Golding and Dong (2010) found that H2, as a “fertilizer,” promoted rotation in non-leguminous crops, including influencing yield. The role of H2 in improving drought tolerance of alfalfa (M. sativa) was confirmed via pharmacological experiments (Jin et al., 2013). In addition, lateral root and adventitious root formation, as easily observed phenotypes, have been widely used to study the influence of H2 on plant development (Zhu et al., 2016; Cao et al., 2017). Moreover, the use of phytohormones and their synthetic inhibitors, as well as other biologically active substances, are effective experimental tools for studying hydrogen agronomy; these experiments provide strong evidence that H2 may be an essential component of the complex signaling network (Zeng et al., 2013; Xie et al., 2014; Su et al., 2018). These physiological and biochemical approaches are effective and useful methods for studying hydrogen agronomy.
3.3.2 Molecular and genetic approaches
Molecular and genetic approaches are two important methods of biological research. Although the presence of hydrogenase in algae and certain bacteria has been confirmed, no homologous gene has been found in higher plants (Gaffron, 1939; Golding and Dong, 2010; Shen et al., 2018). Therefore, finding candidate genes encoding a hydrogenase-like enzyme is of great biological and historical significance.
At present, most molecular and genetic experiments in hydrogen agronomy have been carried out by using specific, signaling-impaired mutants including mutants deficient in ROS (Xie et al., 2014) and nitric oxide (NO) (Cao et al., 2017) signaling. In addition, the study of gene expression at transcriptional and translation levels is widely used in hydrogen agronomy. For example, Wu et al. (2015) found that HRW could regulate expression of ion transporter genes, thus alleviating cadmium toxicity in Chinese cabbage (Brassica campestris spp. chinensis). The expression of microRNA528 (miR528), miR160a, miR398a, and miR159a is also regulated by H2, which explains the phenotypes that alleviate aluminum stress in rice (Oryza sativa) seed germination (Xu et al., 2017). The expression levels of jasmonic acid and salicylic acid receptors are also regulated by H2, indicating H2 controls of plant resistance against disease (Zeng et al., 2013).
3.3.3 “Multi-omics” approaches
With the development of science and technology, the use of genomics, proteomics, or newly developed metabolomics technology is becoming more and more common. These approaches offer the potential of high-throughput experiment technology for hydrogen agronomy.
At present, multi-omics approaches have been used to study the mechanisms of H2 production in algae, and to characterize of some important genes, including HydA1, HydA2, Sulp, Tla1, Sta7, and PFL1 (Xu et al., 2019). Similarly, some genes related to H2 production have been discovered (Melis et al., 2000; Vignais et al., 2001; Volgusheva et al., 2013). Using RNA-sequencing, Cui et al. (2020) identified many differentially expressed genes in cadmium-stressed alfalfa (M. sativa) seedlings in the presence of HRW, including genes involved in glutathione and sulfur metabolism. More recently, Huang et al. (2020) found that metabolism-related proteins, photosynthesis-related proteins, and stress response-related proteins might play positive roles in H2-promoted cucumber adventitious rooting by proteomic analysis.
Use of multi-omics technology allows researchers to analyze the process of H2 metabolism and understand the changes of the entire metabolic network. Since metabolomics technology has a lot of scientific potential in higher plants, metabolomics technology is bound to advance hydrogen agronomy in the near future.
3.4. Mechanisms
Generally, the concentration of dissolved H2 in HRW utilized in agriculture is about 78 μmol/L (10% HRW) (Cui et al., 2014; Zhu and Liao, 2017), and the H2 content during fumigation tests is greater than 0.2 μmol/L (Hu et al., 2018). Since H2 concentration in the troposphere is about 0.5 ppmv (equivalent to 34.5 ppbw, about 22 nmol/L H2) (Paneth, 1937), and tropospheric H2 can escape to upper atmosphere (Liu and Donahue, 1974), it is reasonably deduced that the lower concentration of H2 in the atmosphere could not directly influence the performance of plants on earth. However, there is ample evidence, showing that local H2 in the soils and plants, when endogenously produced (especially in roots of legume plants, among other sources) and/or exogenously applied, could significantly alter plant responses (Shen and Sun, 2019). Here, mechanism of hydrogen agronomy is accordingly summarized.
3.4.1 H2 and ROS signaling
Studies using various animal models have confirmed the effects of H2 or hydrogen-rich saline on Alzheimer’s disease (Nishimaki et al., 2018), cell apoptosis (Guo et al., 2015), retinal light damage (Tian et al., 2013; Qi et al., 2015), and branch retinal vein occlusion (Long et al., 2019).
In animals, ROS are byproducts of oxidative phosphorylation. Excess ROS can be produced by smoking (Tanriverdi et al., 2006; Grassi et al., 2010), immobilization stress (Liu et al., 1996), and ischemia/reperfusion injury (Zhao et al., 2013). Besides their toxic effects, ROS also play an important role in cell signaling pathways, as has been shown in the previous work (Sauer et al., 2001; Kim and Byzova, 2014). In addition, the functions of H2 are related to anti-apoptosis, metabolic diseases, and neurodegenerative diseases (Table 3). The main goal of hydrogen medicine is reduction of toxic ROS, especially selective scavenging of ROS (Ohsawa et al., 2007).
Table 3.
Mechanisms of hydrogen biology in hydrogen medicine and hydrogen agronomy
| Process | Mechanism of action | Reference |
| Hydrogen medicine | ||
| Hydrogen-mediated oxidative stress | H2 can attenuate severe burn-induced early AKI by regulating the MAPKs, Akt, and NF-κB signaling pathway | Guo et al., 2015 |
| H2 can increase the antioxidant effect by activating the Nrf2 transcription system | Kawamura et al., 2013 | |
| Hydrogen-regulated metabolic diseases and neurodegenerative diseases | H2 can improve Alzheimer’s disease caused by apolipoprotein ApoE4 mutation | Nishimaki et al., 2018 |
| HRW can reduce the damage of type 2 diabetes mellitus patients to glucose tolerance to a certain extent | Kajiyama et al., 2008 | |
| Hydrogen-regulated autophagy | Hydrogen-rich saline can enhance anti-apoptosis of cells by regulating Bax/Bcl-2 ratio and ASK-1/JNK pathway | Liu et al., 2015 |
| H2 can increase the expression of phosphorylated p-AMPK, AIF, Caspase 3, etc., thereby enhancing the 5-fluorouracil-induced apoptosis of cancer cells | Runtuwene et al., 2015 | |
| Hydrogen agronomy | ||
| Hydrogen-mediated ROS signaling | H2 can reduce the accumulation of cadmium and mercury, improve the antioxidant capacity of plant seedlings, and reduce the accumulation of ROS | Cui et al., 2014; Dai et al., 2017 |
| H2 can maintain a low-ROS level, thereby extending the shelf life of kiwifruit | Hu et al., 2014 | |
| Interaction of hydrogen and NO | H2 mobilizes NO signaling to promote stomata closure, thereby improving its drought tolerance | Xie et al., 2014 |
| H2 enhances the resistance of tomato fruits to Botrytis cinerea by increasing the activity of polyphenol oxidase and NO content | Lu et al., 2017 | |
| Interaction of hydrogen and CO | H2 regulates CO, a downstream signaling molecule that depends on heme oxygenase-1, thereby improving alfalfa’s drought tolerance | Jin et al., 2013 |
| H2 modulates target gene expression related to adventitious root development and auxin signaling pathway through the CO pathway, thereby promoting the development of cucumber adventitious roots | Lin et al., 2014 |
ROS, reactive oxygen species; NO, nitric oxide; CO, carbon monoxide; AKI, acute kidney injury; MAPK, mitogen-activated protein kinase; NF-κB, nuclear factor-κB; Nrf2, nuclear factor erythroid 2-related factor 2; ApoE4, apolipoprotein E4; HRW, hydrogen-rich water; Bcl-2, B cell lymphoma-2; ASK-1, apoptosis signal-regulating kinase 1; JNK, c-Jun N-terminal kinase; p-AMPK, phospho-adenosine monophosphate (AMP)-activated protein kinase; AIF, apoptosis-inducing factor
Unlike hydrogen medicine, H2 may induce ROS signaling in plants. Xie et al. (2014) found that H2 first rapidly induced ROS signaling (as early as 10 min after treatment) and then mobilized NO signaling to promote stomatal closure in Arabidopsis. Further, by using a mutant deficient in nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, we further confirmed that NADPH oxidase-dependent ROS were downstream signaling molecules of H2 (Xie et al., 2014).
However, there is also strong evidence that H2 might improve the antioxidant capacity of plants and reduce ROS accumulation (24 h after stress), thereby alleviating the inhibitory effects of metal pollution on seedling growth (Cui et al., 2014; Dai et al., 2017). Moreover, H2 can increase superoxide dismutase (SOD) activity in kiwifruit (Actinidia chinesis), therefore maintaining ROS at a low level (Hu et al., 2014). Additionally, H2 enhances pyruvate kinase activity by reducing lipid peroxidation and intracellular ROS levels, thereby alleviating the damage of abiotic stress to Hypsizygus marmoreus (Zhang et al., 2017). Ren A et al. (2017) found that the homeostasis of ROS in Ganoderma lucidum can be regulated by H2.
Overall, ROS play important roles in H2 function in both hydrogen medicine and hydrogen agronomy. In agronomy, we propose that H2 may quickly induce ROS signaling to trigger the gene expression of antioxidant genes. Afterwards, the redox homeostasis was reestablished.
3.4.2 Crosstalk between H2 and NO
In plants, NO generation is induced by phytohormones and environmental stimuli to trigger a wide range of cellular responses. Although NO has been widely studied, the causal relationship between NO and H2 is a matter of strong research interest in the study of hydrogen agronomy.
In Arabidopsis, NO was found to be involved in H2-induced stomatal closure and drought tolerance (Xie et al., 2014). In addition, interaction between H2 and NO was shown to be a factor in plant biotic stress and other abiotic stresses. For example, NO can contribute to H2-improved osmotic tolerance in alfalfa (Su et al., 2018). Pharmacological experiments showed that H2 can alleviate the inhibition of root growth caused by aluminum stress by inhibiting synthesis of NO in alfalfa (Chen et al., 2014). H2 also enhances the resistance of tomato (S. lycopersicum) to the fungal pathogen Botrytis cinerea by increasing polyphenol oxidase activity and NO content (Lu et al., 2017).
Furthermore, interactions between H2 and NO have been found in plant organ development, especially in root development. For instance, the development of lateral roots in tomato and Arabidopsis is regulated by H2 (Cao et al., 2017). As a downstream signaling molecule, NO might participate in H2-induced cucumber adventitious roots (Zhu et al., 2016).
These research efforts have shown several apparent interactions between H2 and NO. As a downstream signaling molecule, NO participates in H2-induced stress resistance, and in plant growth and development. These data strongly suggest that the interaction between H2 and NO may be as tight as that of antigens and antibodies.
3.4.3 Crosstalk between H2 and carbon monoxide
For plant stress tolerance, carbon monoxide (CO) is also a potential downstream signaling molecule. Previous research reported that CO might be a downstream signaling molecule for H2 control of drought tolerance in alfalfa (Jin et al., 2013). Moreover, CO is involved in plant organogenesis induced by H2, especially in root development (Lin et al., 2014). The expression of target genes related to adventitious root formation and auxin signaling is mediated by H2 in CO-dependent fashion, thereby promoting the development of cucumber adventitious rooting. Additionally, CO may participate in the adventitious root development process induced by H2 under drought stress, and reduce the oxidative damage as well (Chen et al., 2017).
According to current research, both H2 and CO are thought to be involved in plant stress tolerance and adventitious root development, both of which closely interact with phytohormone activity (Shen and Sun, 2019). Thus, the combination of genetic and multi-omics approaches should be adopted to reveal corresponding mechanisms in the near future.
3.5. Nanotechnology and hydrogen agronomy
Although application of HRW is an effective and safe method to exert the biological effects of H2, the high diffusivity and the low solubility of H2 in water normally results in several difficulties when used in hydrogen agronomy and thereafter in hydrogen agriculture.
Yang et al. (2018) packed the hydrogen-producing prodrug into a spherical mesoporous structure to increase hydrogen delivery time and to achieve higher concentrations. In addition, nanocarriers can also be modified to release H2 in some specific areas or contexts, such as cancer cells (Zhao et al., 2018; Kou et al., 2019). Combining nanotechnology and H2 agronomy should have great future potential.
3.6. Hydrogen agronomy in future: a challenging
Unlike the processes used in hydrogen medicine, hydrogen agronomy has its own unique mechanisms (Table 3). In addition to ROS, the functions of H2 are closely related to those of many other gaseous signaling molecules, including NO and CO. Hydrogen agronomy is a broad field that includes beneficial roles of H2 in plants, microbes, and animals (Fig. 2).
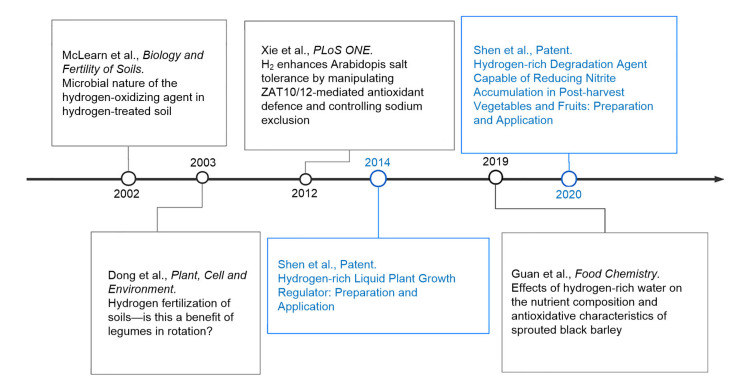
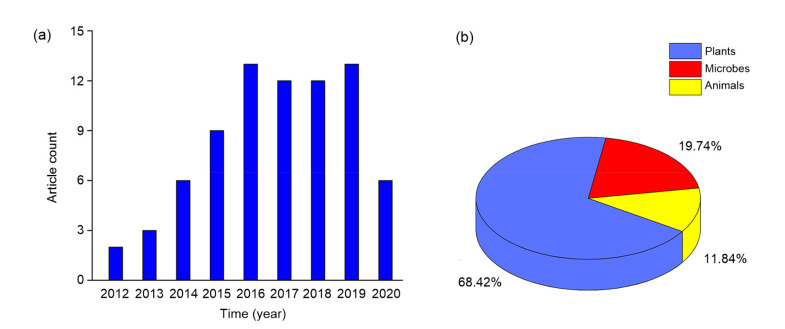
With the development of hydrogen science, some important papers and patents of hydrogen agronomy have been pointed out (Fig. 3). We searched hydrogen agronomy-related papers in the database of Web of Science, and found that the number of the papers shows an increasing tendency since 2012 (Fig. 4; Table S1). Among these papers, the plant studies ranked first, at least in terms of the number of papers. More importantly, the development of hydrogen agronomy is just beginning. Although it was recently found that H2 can alter enzyme activity (Ma et al., 2020), there is still an urgent need for basic research to fully understand the process of endogenous H2 metabolism and its consumption in agriculturally relevant plants and microbes, and also to identify the cellular targets or receptors of H2 and their functions.
Fig. 3.
Growth of research on hydrogen agronomy
Fig. 4.
Number of papers focused on hydrogen agronomy from Jan. 2012 to July 2020 (a) and the proportion of papers focused on biological effects of hydrogen on plants, microbes, and animals in the core collection section of Web of Science (b)
Overall, hydrogen agronomy has developed gradually as a multidisciplinary and integrative field, and its interconnections among agronomy, medicine, chemistry, and other disciplines are apparent. Research on aquaculture and space agriculture may be the next direction in hydrogen agronomy.
List of electronic supplementary materials
Articles about hydrogen agronomy
Footnotes
Project supported by the National Natural Science Foundation of China (No. 31972396), the Foshan Agriculture Science and Technology Project (Foshan City Budget, No. 140, 2019), and the Funding from Center of Hydrogen Science, Shanghai Jiao Tong University, China
Contributors: Wen-biao SHEN conceptualized and designed this study. Yue-qiao WANG and Wen-biao SHEN wrote this article. Yu-hao LIU and Shu WANG collected the data and drew the figures and tables. Wen-biao SHEN and Hong-mei DU checked the final version. All authors have read and approved the final manuscript and, therefore, have full access to all the data in the study and take responsibility for the integrity and security of the data.
Electronic supplementary materials: The online version of this article (https://doi.org/10.1631/jzus.B2000386) contains supplementary materials, which are available to authorized users
Compliance with ethics guidelines: Yue-qiao WANG, Yu-hao LIU, Shu WANG, Hong-mei DU, and Wen-biao SHEN declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Angus JF, Kirkegaard JA, Hunt JR, et al. Break crops and rotations for wheat. Crop Pasture Sci. 2015;66(6):523–552. doi: 10.1071/CP14252. [DOI] [Google Scholar]
- 2.Árnason B, Sigfússon TI. Iceland–a future hydrogen economy. Int J Hydrogen Energy. 2000;25(5):389–394. doi: 10.1016/S0360-3199(99)00077-4. [DOI] [Google Scholar]
- 3.Cao ZY, Duan XL, Yao P, et al. Hydrogen gas is involved in auxin-induced lateral root formation by modulating nitric oxide synthesis. Int J Mol Sci. 2017;18(10):2084. doi: 10.3390/ijms18102084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen M, Cui WT, Zhu KK, et al. Hydrogen-rich water alleviates aluminum-induced inhibition of root elongation in alfalfa via decreasing nitric oxide production. J Hazard Mater. 2014;267:40–47. doi: 10.1016/j.jhazmat.2013.12.029. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y, Wang M, Hu LL, et al. Carbon monoxide is involved in hydrogen gas-induced adventitious root development in cucumber under simulated drought stress. Front Plant Sci, 8:128. 2017 doi: 10.3389/fpls.2017.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Constant P, Poissant L, Villemur R. Tropospheric H2 budget and the response of its soil uptake under the changing environment. Sci Total Environ. 2009;407(6):1809–1823. doi: 10.1016/j.scitotenv.2008.10.064. [DOI] [PubMed] [Google Scholar]
- 7.Cui WT, Gao CY, Fang P, et al. Alleviation of cadmium toxicity in Medicago sativa by hydrogen-rich water. J Hazard Mater. 2013;260:715–724. doi: 10.1016/j.jhazmat.2013.06.032. [DOI] [PubMed] [Google Scholar]
- 8.Cui WT, Fang P, Zhu KK, et al. Hydrogen-rich water confers plant tolerance to mercury toxicity in alfalfa seedlings. Ecotoxicol Environ Saf. 2014;105:103–111. doi: 10.1016/j.ecoenv.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 9.Cui WT, Yao P, Pan JC, et al. Transcriptome analysis reveals insight into molecular hydrogen-induced cadmium tolerance in alfalfa: the prominent role of sulfur and (homo)glutathione metabolism. BMC Plant Biol, 20:58. 2020 doi: 10.1186/s12870-020-2272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Czerkawski JW. Fate of metabolic hydrogen in the rumen. Proc Nutr Soc. 1972;31(2):141–146. doi: 10.1079/pns19720028. [DOI] [PubMed] [Google Scholar]
- 11.Dai C, Cui WT, Pan JC, et al. Proteomic analysis provides insights into the molecular bases of hydrogen gas-induced cadmium resistance in Medicago sativa . J Proteomics. 2017;152:109–120. doi: 10.1016/j.jprot.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 12.Das D, Khanna N, Veziroğlu NT. Recent developments in biological hydrogen production processes. Chem Ind Chem Eng Quart. 2008;14(2):57–67. doi: 10.2298/CICEQ0802057D. [DOI] [Google Scholar]
- 13.Derwent RG, Collins WJ, Johnson CE, et al. Transient behaviour of tropospheric ozone precursors in a global 3-D CTM and their indirect greenhouse effects. Clim Change. 2001;49(4):463–487. doi: 10.1023/A:1010648913655. [DOI] [Google Scholar]
- 14.Dole M, Wilson FR, Fife WP. Hyperbaric hydrogen therapy: a possible treatment for cancer. Science. 1975;190(4210):152–154. doi: 10.1126/science.1166304. [DOI] [PubMed] [Google Scholar]
- 15.Dong Z, Layzell DB. H2 oxidation, O2 uptake and CO2 fixation in hydrogen treated soils. Plant Soil. 2001;229:1–12. doi: 10.1023/A:1004810017490. [DOI] [Google Scholar]
- 16.Dong Z, Wu L, Kettlewell B, et al. Hydrogen fertilization of soils–is this a benefit of legumes in rotation? Plant Cell Environ. 2003;26(11):1875–1879. doi: 10.1046/j.1365-3040.2003.01103.x. [DOI] [Google Scholar]
- 17.Dresselhaus MS, Thomas IL. Alternative energy technologies. Nature. 2001;414(6861):332–337. doi: 10.1038/35104599. [DOI] [PubMed] [Google Scholar]
- 18.Du ZM, Jia HP, Liu J, et al. Protective effects of hydrogen-rich saline in uncontrolled hemorrhagic shock. Exp Ther Med. 2014;7(5):1253–1258. doi: 10.3892/etm.2014.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fu Y, Ito M, Fujita Y, et al. Molecular hydrogen is protective against 6-hydroxydopamine-induced nigrostriatal degeneration in a rat model of Parkinson’s disease. Neurosci Lett. 2009;453(2):81–85. doi: 10.1016/j.neulet.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 20.Gaffron H. Reduction of carbon dioxide with molecular hydrogen in green algae. Nature. 1939;143(3614):204–205. doi: 10.1038/143204a0. [DOI] [Google Scholar]
- 21.Ge L, Yang M, Yang NN, et al. Molecular hydrogen: a preventive and therapeutic medical gas for various diseases. Oncotarget. 2017;8(60):102653–102673. doi: 10.18632/oncotarget.21130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gest H, Kamen MD. Studies on the metabolism of photosynthetic bacteria IV: photochemical production of molecular hydrogen by growing cultures of photosynthetic bacteria. J Bacteriol. 1949;58(2):239–245. [PMC free article] [PubMed] [Google Scholar]
- 23.Golding AL, Dong ZM. Hydrogen production by nitrogenase as a potential crop rotation benefit. Environ Chem Lett. 2010;8(2):101–121. doi: 10.1007/s10311-010-0278-y. [DOI] [Google Scholar]
- 24.Grassi D, Desideri G, Ferri L, et al. Oxidative stress and endothelial dysfunction: say NO to cigarette smoking! Curr Pharm Design. 2010;16(23):2539–2550. doi: 10.2174/138161210792062867. [DOI] [PubMed] [Google Scholar]
- 25.Guan Q, Ding XW, Jiang R, et al. Effects of hydrogen-rich water on the nutrient composition and antioxidative characteristics of sprouted black barley. Food Chem, 299: 125095. 2019 doi: 10.1016/j.foodchem.2019.125095. [DOI] [PubMed] [Google Scholar]
- 26.Guo SX, Fang Q, You CG, et al. Effects of hydrogen-rich saline on early acute kidney injury in severely burned rats by suppressing oxidative stress induced apoptosis and inflammation. J Transl Med, 13:183. 2015 doi: 10.1186/s12967-015-0548-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hou C, Peng YH, Qin C, et al. Hydrogen-rich water improves cognitive impairment gender-dependently in APP/PS1 mice without affecting Aβ clearance. Free Radic Res. 2018;52(11-12):1311–1322. doi: 10.1080/10715762.2018.1460749. [DOI] [PubMed] [Google Scholar]
- 28.Hu HL, Li PX, Wang YN, et al. Hydrogen-rich water delays postharvest ripening and senescence of kiwifruit. Food Chem. 2014;156:100–109. doi: 10.1016/j.foodchem.2014.01.067. [DOI] [PubMed] [Google Scholar]
- 29.Hu HL, Zhao SP, Li PX, et al. Hydrogen gas prolongs the shelf life of kiwifruit by decreasing ethylene biosynthesis. Postharvest Biol Tech. 2018;135:123–130. doi: 10.1016/j.postharvbio.2017.09.008. [DOI] [Google Scholar]
- 30.Huang DJ, Bian BT, Zhang ML, et al. The role and proteomic analysis of ethylene in hydrogen gas-induced adventitious rooting development in cucumber (Cucumis sativus L.) explants. PeerJ. 2020;8(15):e8896. doi: 10.7717/peerj.8896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ichimasa M, Ichimasa Y, Yagi Y, et al. Oxidation of atmospheric molecular tritium in plant leaves, lichens and mosses. J Radiat Res. 1989;30(4):323–329. doi: 10.1269/jrr.30.323. [DOI] [PubMed] [Google Scholar]
- 32.Ji X, Zhang Q, Zheng WJ, et al. Morphological and molecular response of small intestine to lactulose and hydrogen-rich water in female piglets fed Fusarium mycotoxins contaminated diet. J Anim Sci Biotechnol, 10:9. 2019 doi: 10.1186/s40104-019-0320-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jin QJ, Zhu KK, Cui WT, et al. Hydrogen gas acts as a novel bioactive molecule in enhancing plant tolerance to paraquat-induced oxidative stress via the modulation of heme oxygenase-1 signalling system. Plant Cell Environ. 2013;36(5):956–969. doi: 10.1111/pce.12029. [DOI] [PubMed] [Google Scholar]
- 34.Jin QJ, Cui WT, Dai C, et al. Involvement of hydrogen peroxide and heme oxygenase-1 in hydrogen gas-induced osmotic stress tolerance in alfalfa. Plant Growth Regul. 2016;80(2):215–223. doi: 10.1007/s10725-016-0159-x. [DOI] [Google Scholar]
- 35.Kajiyama S, Hasegawa G, Asano M, et al. Supplementation of hydrogen-rich water improves lipid and glucose metabolism in patients with type 2 diabetes or impaired glucose tolerance. Nutr Res. 2008;28(3):137–143. doi: 10.1016/j.nutres.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 36.Kamimura N, Ichimiya H, Iuchi K, et al. Molecular hydrogen stimulates the gene expression of transcriptional coactivator PGC-1α to enhance fatty acid metabolism. NPJ Aging Mech Dis, 2:16008. 2016 doi: 10.1038/npjamd.2016.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kang KM, Kang YN, Choi IB, et al. Effects of drinking hydrogen-rich water on the quality of life of patients treated with radiotherapy for liver tumors. Med Gas Res, 1:11. 2011 doi: 10.1186/2045-9912-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kawamura T, Wakabayashi N, Shigemura N, et al. Hydrogen gas reduces hyperoxic lung injury via the Nrf2 pathway in vivo. Am J Physiol Lung Cell Mol Physiol. 2013;304(10):L646–L656. doi: 10.1152/ajplung.00164.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim YW, Byzova TV. Oxidative stress in angiogenesis and vascular disease. Blood. 2014;123(5):625–631. doi: 10.1182/blood-2013-09-512749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kirkegaard J, Christen O, Krupinsky J, et al. Break crop benefits in temperate wheat production. Field Crops Res. 2008;107(3):185–195. doi: 10.1016/j.fcr.2008.02.010. [DOI] [Google Scholar]
- 41.Kou Z, Zhao PH, Wang ZH, et al. Acid-responsive H2-releasing Fe nanoparticles for safe and effective cancer therapy. J Mater Chem B. 2019;7(17):2759–2765. doi: 10.1039/C9TB00338J. [DOI] [PubMed] [Google Scholar]
- 42.Lesk C, Rowhani P, Ramankutty N. Influence of extreme weather disasters on global crop production. Nature. 2016;529(7584):84–87. doi: 10.1038/nature16467. [DOI] [PubMed] [Google Scholar]
- 43.Lewandrowski J, Tobey J, Cook Z. The interface between agricultural assistance and the environment: chemical fertilizer consumption and area expansion. Land Econ. 1997;73(3):404–427. doi: 10.2307/3147176. [DOI] [Google Scholar]
- 44.Li CX, Gong TY, Bian BT, et al. Roles of hydrogen gas in plants: a review. Funct Plant Biol. 2018;45(8):783–792. doi: 10.1071/FP17301. [DOI] [PubMed] [Google Scholar]
- 45.Lin YT, Zhang W, Qi F, et al. Hydrogen-rich water regulates cucumber adventitious root development in a heme oxygenase-1/carbon monoxide-dependent manner. J Plant Physiol. 2014;171(2):1–8. doi: 10.1016/j.jplph.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 46.Liu JK, Wang XY, Shigenaga MK, et al. Immobilization stress causes oxidative damage to lipid, protein, and DNA in the brain of rats. FASEB J. 1996;10(13):1532–1538. doi: 10.1096/fasebj.10.13.8940299. [DOI] [PubMed] [Google Scholar]
- 47.Liu SC, Donahue TM. The aeronomy of hydrogen in the atmosphere of the earth. J Atoms Sci. 1974;31(4):1118–1136. doi: 10.1175/1520-0469(1974)031<1118:TAOHIT>2.0.CO;2. [DOI] [Google Scholar]
- 48.Liu YQ, Liu YF, Ma XM, et al. Hydrogen-rich saline attenuates skin ischemia/reperfusion induced apoptosis via regulating Bax/Bcl-2 ratio and ASK-1/JNK pathway. J Plast Reconstr Aesthet Surg. 2015;68(7):e147–e156. doi: 10.1016/j.bjps.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 49.Long P, Yan WM, He MS, et al. Protective effects of hydrogen gas in a rat model of branch retinal vein occlusion via decreasing VEGF-α expression. BMC Ophthalmol, 19:112. 2019 doi: 10.1186/s12886-019-1105-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lowry GV, Avellan A, Gilbertson LM. Opportunities and challenges for nanotechnology in the agri-tech revolution. Nat Nanotechnol. 2019;14(6):517–522. doi: 10.1038/s41565-019-0461-7. [DOI] [PubMed] [Google Scholar]
- 51.Lu H, Wu BQ, Wang YF, et al. Effects of hydrogen-rich water treatment on defense responses of postharvest tomato fruit to Botrytis cinerea . J Henan Agric Sci. 2017;46(2):64–68. (in Chinese) [Google Scholar]
- 52.Lu HT, Sun XJ. Hydrogen medicine: research advance, controversy and challenges. Acad J Second Mil Med Univ. 2018;39(11):1181–1187. (in Chinese) [Google Scholar]
- 53.Lubitz W, Ogata H, Rüdiger O, et al. Hydrogenases. Chem Rev. 2014;114(8):4081–4148. doi: 10.1021/cr4005814. [DOI] [PubMed] [Google Scholar]
- 54.Lytos A, Lagkas T, Sarigiannidis P, et al. Towards smart farming: systems, frameworks and exploitation of multiple sources. Comput Netw, 172:107147. 2020 doi: 10.1016/j.comnet.2020.107147. [DOI] [Google Scholar]
- 55.Ma XM, Zhang X, Xie F, et al. Bio-enzyme basis of hydrogen in biological system. Curr Biotech. 2020;10(1):15–22. (in Chinese) [Google Scholar]
- 56.Macrae RJ, Henning J, Hill SB. Strategies to overcome barriers to the development of sustainable agriculture in Canada: the role of agribusiness. J Agric Environ Ethics. 1993;6(1):21–51. doi: 10.1007/BF01965613. [DOI] [Google Scholar]
- 57.Maeder P, Fliessbach A, Dubois D, et al. Soil fertility and biodiversity in organic farming. Science. 2002;296(5573):1694–1697. doi: 10.1126/science.1071148. [DOI] [PubMed] [Google Scholar]
- 58.McLearn N, Dong ZM. Microbial nature of the hydrogen-oxidizing agent in hydrogen-treated soil. Biol Fertil Soils. 2002;35(6):465–469. doi: 10.1007/s00374-002-0495-z. [DOI] [Google Scholar]
- 59.Melis A, Zhang LP, Forestier M, et al. Sustained photobiological hydrogen gas production upon reversible inactivation of oxygen evolution in the green alga Chlamydomonas reinhardtii . Plant Physiol. 2000;122(1):127–136. doi: 10.1104/pp.122.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Melman DA, Kelly C, Schneekloth J, et al. Tillage and residue management drive rapid changes in soil macrofauna communities and soil properties in a semiarid cropping system of Eastern Colorado. Appl Soil Ecol. 2019;143:98–106. doi: 10.1016/j.apsoil.2019.05.022. [DOI] [Google Scholar]
- 61.Mijangos I, Pérez R, Albizu I, et al. Effects of fertilization and tillage on soil biological parameters. Enzyme Microb Technol. 2006;40(1):100–106. doi: 10.1016/j.enzmictec.2005.10.043. [DOI] [Google Scholar]
- 62.Nicolopoulou-Stamati P, Maipas S, Kotampasi C, et al. Chemical pesticides and human health: the urgent need for a new concept in agriculture. Front Public Health, 4:148. 2016 doi: 10.3389/fpubh.2016.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nishimaki K, Asada T, Ohsawa I, et al. Effects of molecular hydrogen assessed by an animal model and a randomized clinical study on mild cognitive impairment. Curr Alzheimer Res. 2018;15(5):482–492. doi: 10.2174/1567205014666171106145017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Novelli PC, Lang PM, Masarie KA, et al. Molecular hydrogen in the troposphere: global distribution and budget. J Geophys Res Atmos. 1999;104(D23):30427–30444. doi: 10.1029/1999JD900788. [DOI] [Google Scholar]
- 65.Ohsawa I, Ishikawa M, Takahashi K, et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13(6):688–694. doi: 10.1038/nm1577. [DOI] [PubMed] [Google Scholar]
- 66.Ohta S. Recent progress toward hydrogen medicine: potential of molecular hydrogen for preventive and therapeutic applications. Curr Pharm Design. 2011;17(22):2241–2252. doi: 10.2174/138161211797052664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ohta S. Molecular hydrogen as a preventive and therapeutic medical gas: initiation, development and potential of hydrogen medicine. Pharmacol Therapeut. 2014;144(1):1–11. doi: 10.1016/j.pharmthera.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 68.Paneth FA. The chemical composition of the atmosphere. Quart J Roy Meteorol Soc. 1937;63(271):433–443. doi: 10.1002/qj.49706327114. [DOI] [Google Scholar]
- 69.Pimentel D. Green revolution agriculture and chemical hazards. Sci Total Environ. 1996;188:S86–S98. doi: 10.1016/0048-9697(96)05280-1. [DOI] [PubMed] [Google Scholar]
- 70.Qaswar M, Jing H, Ahmed W, et al. Yield sustainability, soil organic carbon sequestration and nutrients balance under long-term combined application of manure and inorganic fertilizers in acidic paddy soil. Soil Tillage Res, 198:104569. 2020 doi: 10.1016/j.still.2019.104569. [DOI] [Google Scholar]
- 71.Qi LS, Yao L, Liu W, et al. Sirtuin type 1 mediates the retinal protective effect of hydrogen-rich saline against light-induced damage in rats. Invest Ophth Vis Sci. 2015;56(13):8268–8279. doi: 10.1167/iovs.15-17034. [DOI] [PubMed] [Google Scholar]
- 72.Ren A, Liu R, Miao ZG, et al. Hydrogen-rich water regulates effects of ROS balance on morphology, growth and secondary metabolism via glutathione peroxidase in Ganoderma lucidum . Environ Microbiol. 2017;19(2):566–583. doi: 10.1111/1462-2920.13498. [DOI] [PubMed] [Google Scholar]
- 73.Ren PJ, Jin X, Liao WB, et al. Effect of hydrogen-rich water on vase life and quality in cut lily and rose flowers. Hortic Environ Biotechnol. 2017;58(6):576–584. doi: 10.1007/s13580-017-0043-2. [DOI] [Google Scholar]
- 74.Renwick GM, Giumarro C, Siegel SM. Hydrogen metabolism in higher plants. Plant Physiol. 1964;39(3):303–306. doi: 10.1104/pp.39.3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Runtuwene J, Amitani H, Amitani M, et al. Hydrogen-water enhances 5-fluorouracil-induced inhibition of colon cancer. PeerJ, 3:e859. 2015 doi: 10.7717/peerj.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Saitoh Y, Okayasu H, Xiao L, et al. Neutral pH hydrogen-enriched electrolyzed water achieves tumor-preferential clonal growth inhibition over normal cells and tumor invasion inhibition concurrently with intracellular oxidant repression. Oncol Res. 2008;17(6):247–255. doi: 10.3727/096504008786991620. [DOI] [PubMed] [Google Scholar]
- 77.Sauer H, Wartenberg M, Hescheler J. Reactive oxygen species as intracellular messengers during cell growth and differentiation. Cell Physiol Biochem. 2001;11(4):173–186. doi: 10.1159/000047804. [DOI] [PubMed] [Google Scholar]
- 78.Schubert KR, Evans HJ. Hydrogen evolution: a major factor affecting the efficiency of nitrogen fixation in nodulated symbionts. Proc Natl Acad Sci USA. 1976;73(4):1207–1211. doi: 10.1073/pnas.73.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shen WB, Sun XJ. Hydrogen biology: it is just beginning. Chin J Biochem Mol Biol. 2019;35(10):1037–1050. (in Chinese) [Google Scholar]
- 80.Shen WB, Xie YJ, Cao ZY, et al. Hydrogen-rich Liquid Plant Growth Regulator: Preparation and Application. CN201210154005.0; 2014. (in Chinese) [Google Scholar]
- 81.Shen WB, Zhang YH, Su JC, et al. Hydrogen-rich Degradation Agent Capable of Reducing Nitrite Accumulation in Post-harvest Vegetables and Fruits: Preparation and Application. CN201710124039.8; 2020. (in Chinese) [Google Scholar]
- 82.Shen WB, Su JC, Sun XJ. Research progress in the botanical effects of hydrogen gas. J Nanjing Agric Univ. 2018;41(3):392–401. doi: 10.7685/jnau.201803059. (in Chinese) [DOI] [Google Scholar]
- 83.Song GH, Tian H, Qin SC, et al. Hydrogen decreases athero-susceptibility in apolipoprotein B-containing lipoproteins and aorta of apolipoprotein E knockout mice. Atherosclerosis. 2012;221(1):55–65. doi: 10.1016/j.atherosclerosis.2011.11.043. [DOI] [PubMed] [Google Scholar]
- 84.Stein S, Selesi D, Schilling R, et al. Microbial activity and bacterial composition of H2-treated soils with net CO2 fixation. Soil Biol Biochem. 2005;37(10):1938–1945. doi: 10.1016/j.soilbio.2005.02.035. [DOI] [Google Scholar]
- 85.Stephenson M, Stickland LH. Hydrogenase: a bacterial enzyme activating molecular hydrogen: the properties of the enzyme. Biochem J. 1931;25(1):205–214. doi: 10.1042/bj0250205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Su JC, Zhang YH, Nie Y, et al. Hydrogen-induced osmotic tolerance is associated with nitric oxide-mediated proline accumulation and reestablishment of redox balance in alfalfa seedlings. Environ Exp Bot. 2018;147:249–260. doi: 10.1016/j.envexpbot.2017.12.022. [DOI] [Google Scholar]
- 87.Su JC, Nie Y, Zhao G, et al. Endogenous hydrogen gas delays petal senescence and extends the vase life of lisianthus cut flowers. Postharvest Biol Technol. 2019;147:148–155. doi: 10.1016/j.postharvbio.2018.09.018. [DOI] [Google Scholar]
- 88.Su NN, Wu Q, Liu YY, et al. Hydrogen-rich water reestablishes ROS homeostasis but exerts differential effects on anthocyanin synthesis in two varieties of radish sprouts under UV-A irradiation. J Agric Food Chem. 2014;62(27):6454–6462. doi: 10.1021/jf5019593. [DOI] [PubMed] [Google Scholar]
- 89.Tanriverdi H, Evrengul H, Kuru O, et al. Cigarette smoking induced oxidative stress may impair endothelial function and coronary blood flow in angiographically normal coronary arteries. Circ J. 2006;70(5):593–599. doi: 10.1253/circj.70.593. [DOI] [PubMed] [Google Scholar]
- 90.Tian L, Zhang L, Xia F, et al. Hydrogen-rich saline ameliorates the retina against light-induced damage in rats. Med Gas Res. 2013;3(1):19. doi: 10.1186/2045-9912-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vignais PM, Billoud B, Meyer J. Classification and phylogeny of hydrogenases. FEMS Microbiol Rev. 2001;25(4):455–501. doi: 10.1111/j.1574-6976.2001.tb00587.x. [DOI] [PubMed] [Google Scholar]
- 92.Vogt S, Lyon EJ, Shima S, et al. The exchange activities of [Fe] hydrogenase (iron–sulfur-cluster-free hydrogenase) from methanogenic archaea in comparison with the exchange activities of [FeFe] and [NiFe] hydrogenases. J Biol Inorg Chem. 2008;13(1):97–106. doi: 10.1007/s00775-007-0302-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Volgusheva A, Styring S, Mamedov F. Increased photosystem II stability promotes H2 production in sulfur-deprived Chlamydomonas reinhardtii . Proc Natl Acad Sci USA. 2013;110(18):7223–7228. doi: 10.1073/pnas.1220645110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang F, Yu G, Liu SY, et al. Hydrogen-rich saline protects against renal ischemia/reperfusion injury in rats. J Surg Res. 2011;167(2):e339–e344. doi: 10.1016/j.jss.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 95.Wang YP, Wei C. Effect of hydrogen-rich water concentration on rooting of Nepenthes . Mod Agric Sci Technol. 2016;(14):136–137. doi: 10.3969/j.issn.1007-5739.2016.14.079. (in Chinese) [DOI] [Google Scholar]
- 96.Wu Q, Su NN, Cai JT, et al. Hydrogen-rich water enhances cadmium tolerance in Chinese cabbage by reducing cadmium uptake and increasing antioxidant capacities. J Plant Physiol. 2015;175:174–182. doi: 10.1016/j.jplph.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 97.Xie YJ, Mao Y, Lai DW, et al. H2 enhances Arabidopsis salt tolerance by manipulating ZAT10/12-mediated antioxidant defence and controlling sodium exclusion. PLoS ONE. 2012;7(11):e49800. doi: 10.1371/journal.pone.0049800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xie YJ, Mao Y, Zhang W, et al. Reactive oxygen species-dependent nitric oxide production contributes to hydrogen-promoted stomatal closure in Arabidopsis. Plant Physiol. 2014;165(2):759–773. doi: 10.1104/pp.114.237925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xie YJ, Zhang W, Duan XL, et al. Hydrogen-rich water-alleviated ultraviolet-B-triggered oxidative damage is partially associated with the manipulation of the metabolism of (iso)flavonoids and antioxidant defence in Medicago sativa . Funct Plant Biol. 2015;42(12):1141–1157. doi: 10.1071/FP15204. [DOI] [PubMed] [Google Scholar]
- 100.Xu DK, Cao H, Fang W, et al. Linking hydrogen-enhanced rice aluminum tolerance with the reestablishment of GA/ABA balance and miRNA-modulated gene expression: a case study on germination. Ecotoxicol Environ Saf. 2017;145:303–312. doi: 10.1016/j.ecoenv.2017.07.055. [DOI] [PubMed] [Google Scholar]
- 101.Xu LL, Fan JH, Wang QX. Omics application of bio-hydrogen production through green alga Chlamydomonas reinhardtii . Front Bioeng Biotechnol, 7:201. 2019 doi: 10.3389/fbioe.2019.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xu S, Zhu SS, Jiang YL, et al. Hydrogen-rich water alleviates salt stress in rice during seed germination. Plant Soil. 2013;370(1-2):47–57. doi: 10.1007/s11104-013-1614-3. [DOI] [Google Scholar]
- 103.Yagi T, Higuchi Y. Studies on hydrogenase. Proc Jpn Acad Ser B Phys Biol Sci. 2013;89(1):16–33. doi: 10.2183/pjab.89.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yang CH, Crowley DE. Rhizosphere microbial community structure in relation to root location and plant iron nutritional status. Appl Environ Microbiol. 2000;66(1):345–351. doi: 10.1128/AEM.66.1.345-351.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yang T, Jin ZK, Wang ZH, et al. Intratumoral high-payload delivery and acid-responsive release of H2 for efficient cancer therapy using the ammonia borane-loaded mesoporous silica nanomedicine. Appl Mater Today. 2018;11:136–143. doi: 10.1016/j.apmt.2018.01.008. [DOI] [Google Scholar]
- 106.Young IM, Crawford JW. Interactions and self-organization in the soil-microbe complex. Science. 2004;304(5677):1634–1637. doi: 10.1126/science.1097394. [DOI] [PubMed] [Google Scholar]
- 107.Zeng JQ, Zhang MY, Sun XJ. Molecular hydrogen is involved in phytohormone signaling and stress responses in plants. PLoS ONE. 2013;8(8):e71038. doi: 10.1371/journal.pone.0071038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang JJ, Hao HB, Chen MJ, et al. Hydrogen-rich water alleviates the toxicities of different stresses to mycelial growth in Hypsizygus marmoreus . AMB Express. 2017;7(1):107. doi: 10.1186/s13568-017-0406-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang XY, Wei JY, Huang YF, et al. Increased cytosolic calcium contributes to hydrogen-rich water-promoted anthocyanin biosynthesis under UV-A irradiation in radish sprouts hypocotyls. Front Plant Sci, 9:1020. 2018 doi: 10.3389/fpls.2018.01020.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang YH, Zhao G, Cheng PF, et al. Nitrite accumulation during storage of tomato fruit as prevented by hydrogen gas. Int J Food Prop. 2019;22(1):1425–1438. doi: 10.1080/10942912.2019.1651737. [DOI] [Google Scholar]
- 111.Zhao L, Wang YB, Qin SR, et al. Protective effect of hydrogen-rich saline on ischemia/reperfusion injury in rat skin flap. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2013;14(5):382–391. doi: 10.1631/jzus.B1200317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhao PH, Jin ZK, Chen Q, et al. Local generation of hydrogen for enhanced photothermal therapy. Nat Commun. 2018;9(1):4241. doi: 10.1038/s41467-018-06630-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhu YC, Liao WB. The metabolic constituent and rooting-related enzymes responses of marigold explants to hydrogen gas during adventitious root development. Theor Exp Plant Physiol. 2017;29(2):77–85. doi: 10.1007/s40626-017-0085-y. [DOI] [Google Scholar]
- 114.Zhu YC, Liao WB, Wang M, et al. Nitric oxide is required for hydrogen gas-induced adventitious root formation in cucumber. J Plant Physiol. 2016;195:50–58. doi: 10.1016/j.jplph.2016.02.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Articles about hydrogen agronomy