Abstract
Objective
To examine the learning curves of atrial fibrillation (AF) ablation comparing the cryoballoon (CB) and radiofrequency (RF) catheters.
Methods
We performed a retrospective data analysis from the initiation of AF ablation program in our center. For CB ablation, a second generation 28 mm balloon was utilized and for RF ablation.
Results
A total of 100 consecutive patients (50 in each group) have been enrolled in the study (male 74%, mean age 58.9 ± 10 years, paroxysmal AF 85%). The mean procedure time was shorter for CB (116.6 ± 39.8 min) than RF group (191.8 ± 101.1 min) (p < 0.001). There was no difference in the mean fluoroscopy time, 24.2 ± 10.6 min in RF and 22.4 ± 11.7 min in CB group, (p = 0.422). Seven major complications occurred during the study; 5 in RF group (10%) and 2 in CB group (4%) (p = 0.436). After the mean follow up of 14.5 ± 2.4 months, 15 patients in RF group (30%) and 11 in CB group (26%) experienced AF recurrences (P = 0.300).
Conclusion
When starting a new AF ablation program, our results suggest that CB significantly shortens procedure while fluoroscopy time and clinical outcomes are comparable to RF ablation.
Keywords: Atrial fibrillation, Ablation, Second generation cryoballoon, Contact force sensing radiofrequency catheters
1. Introduction
The prevalence of atrial fibrillation (AF) is on the rise and the current estimates suggest that up to 3% of the adult population suffers from the disease [1]. This also translates to 120 000–215 000 newly diagnosed patients per year in the European Union [2]. Pulmonary vein isolation (PVI) has become the standard of care for patients with AF [3] and there is an ever growing demand for the procedure. Point-by-point catheter ablation using radiofrequency (RF) energy is still the most prevalent technique [4], but recently, “single shot” devices have been developed with the aim of reducing the complexity of the procedure. The cryoballoon (CB) technology has become an attractive alternative to point-by-point RF ablation with similar treatment outcomes [5]. The number of electrophysiology (EP) labs that are performing AF ablation is increasing and there is a need for simple, safe and reproducible PVI technique [6]. Modern advancements in the area of RF technology with contact force sensing catheters alongside electroanatomic mapping (EAM) systems and the second generation CB represent important achievements towards these targets. Therefore, the introduction of AF ablation procedures into the new EP centers might be less challenging than in the past.
2. Methods
2.1. Aim of the study
The aim of this study was to examine the introduction of AF ablation procedure to a new EP center comparing two different modern approaches. We included the first 100 consecutive AF procedures that were performed in our center in the analysis. Two approaches consisted of the second generation CB technology and advanced EAM system with contact force sensing RF catheters (CSRF). The focus of the study was on safety, efficacy and procedural characteristics. We retrospectively analyzed the data that are standardly recorded for all ablation procedures. We studied the learning curves in adopting AF ablation procedures for three senior operators in a single center that started the AF ablation program. All three operators were experienced in basic EP procedures, familiar with the Carto 3 EAM system and had limited training in transseptal puncture. The procedure time was measured from the application of local anesthesia until the sheath removal in the case of CB ablation, or, until the sheath exchange in the case of RF ablation.
2.2. Study population
Data was retrospectively collected from the beginning of the AF ablation program in our center. The first pulmonary vein isolation procedure was performed in February 2015, and the last patient included in the study was in October 2016. We included a total of 100 consecutive patients for symptomatic paroxysmal or early persistent AF. Half of patients were treated by CB and the other half by RF catheters with EAM technology. Our lab adopted both technologies simultaneously. Exclusion criteria were: continuous AF for >3 months, intracardiac thrombi, uncontrolled heart failure (NYHA III-IV), significant valvular disease and a left atrial (LA) diameter >55 mm. All patients provided written informed consent for the ablation procedure. All research was conducted in accordance with the principles of the Declaration of Helsinki.
2.3. Pre-procedural management
A transthoracic echocardiogram (TTE) was performed within 3 months prior to ablation enabling assessment of the left ventricular ejection fraction and to rule out any significant structural and/or valvular disease. To exclude the presence of thrombi, trans-esophageal echocardiography (TEE) was performed before the procedure. For patients taking novel anticoagulant agents our practice was to stop anticoagulation for 24 h before the procedure (last doses of dabigatran, rivaroxaban and apixaban were given in the morning on the day before the procedure). An uninterrupted vitamin K antagonist (VKA) strategy was used and the ablation was performed when the INR was between 2 and 3, measured on the day of the procedure [7].
2.4. Cryoballoon ablation procedure
All procedures were performed under conscious sedation combining fentanyl and diazepam. Two left and one right femoral venous access points were obtained. The right puncture was used for the CB catheter and left punctures for a decapolar electrophysiology (EP) catheter and intracardiac echo (ICE) catheter (AcuNav Acuson, Siemens) positioned in the right atrium (RA). Access to LA was achieved under fluoroscopic and ICE guidance using a trans-septal sheath (SL1, St Jude Medical) which was exchanged with a steerable 15 Fr sheath (FlexCath Advance, Medtronic). The Flex sheath was placed in mid LA and the decapolar catheter was positioned in the right ventricular (RV) apex for pacing. Rotational angiography was performed in all patients (Siemens Axiom Artis, Siemens) to evaluate LA anatomy and possible pulmonary vein (PV) variations. From acquired images, 3D volume of LA was created to guide the ablation procedure (Fig. 1). After the rotational angiography, mapping catheter (Achieve, Medtronic) was advanced in each PV ostium to obtain baseline electrical information. A 28 mm CB (Arctic Front Advance, Medtronic) was positioned at each PV antrum/ostium to achieve the optimal occlusion, always having in mind not to put the CB too distally, in the vein. Optimal vessel occlusion was considered to have been achieved when selective contrast injection showed total contrast retention (Fig. 2). All the procedures were performed with a single 3-min application for each PV. In order to avoid right phrenic nerve palsy (PNP), diaphragmatic stimulation was performed by pacing the ipsilateral nerve with the decapolar catheter via the subclavian vein with a 1500 ms cycle and a 20 mA output. Phrenic capture was monitored by tactile feedback placing the operator’s hand on the patient’s abdomen and by measuring the central venous pressure increase in relation to the diaphragmatic movement [8,9]. PVI was confirmed by using the Achieve catheter (Fig. 3). If real time recordings were not visible during the freeze application, isolation was verified after the freeze-thaw cycle. A second 3 min freeze was delivered in the case of no isolation after the first cycle or early spontaneous PV reconnection. At the end of procedure, all PVs were revisited to confirm the isolation. During the whole procedure, activated clotting time (ACT) was maintained over 300 s by supplementing heparin infusion as required. At the end of procedure we routinely performed heparin reversal with protamine for the sheath removal.
Fig. 1.
Image of the left atrium generated from 3D rotational angiography (posterior-anterior view)
LSPV – left superior pulmonary vein, LIPV – left inferior pulmonary vein, RSPV – right superior pulmonary vein, RIPV – right inferior pulmonary vein.
Fig. 2.
Real time pulmonary vein isolation visualized by the Achieve catheter. Please note the typical progressive delay of pulmonary vein potentials (arrows) and final disappearance when achieving the entrance block.
Fig. 3.
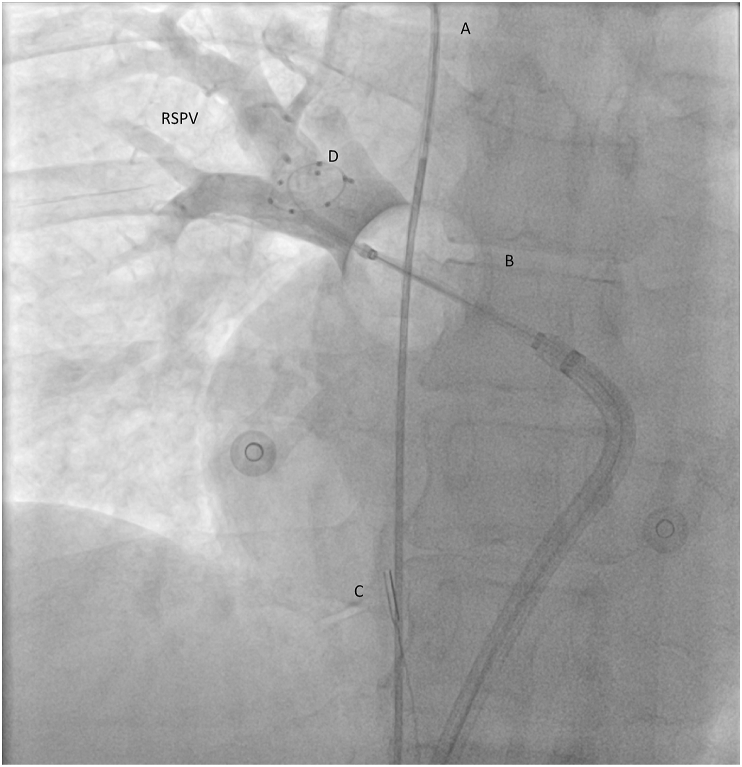
Cryoballoon ablation, anterior-posterior view. The CB is positioned at the RSPV antrum/ostium. The optimal pulmonary vein occlusion is documented by contrast injection from the distal tip of the catheter. A) A decapolar catheter in superior vena cava/right v. subclavia junction for the phrenic nerve pacing B) Cryoballoon C) ICE catheter in the right atrium D) Achieve circular catheter in the RSPV
CB – Cryoballoon, ICE – intracardiac echo catheter, RSPV – right superior pulmonary vein.
2.5. Radiofrequency ablation procedure
The same strategy of conscious sedation combining fentanyl and diazepam was used as in CB group. Two femoral venous access points were obtained in both groins. Left sided access was used for ICE catheter and decapolar catheter as previously described. Right sided access was used for double transseptal puncture which was performed under ICE and fluoroscopy guidance. After the first transseptal puncture, standard transseptal SL1 sheath was exchanged for deflectable sheath (Agilis, St Jude Medical) and the second transseptal puncture with SL1 sheath was performed. The Agilis sheath was used for rotational angiography as previously described. A 3D image was created and imported to the CARTO 3 system (Biosense-Webster). After the image acquisition, deflectable sheath was used for 3.5 mm contact sensing, open-irrigated tip ablation catheter (Thermocool, SmartTouch TM, Biosense-Webster) and SL1 sheet was used for deflectable duodecapolar circular catheter (Lasso Nav, Biosense-Webster). Quick anatomical map of LA was created with a circular catheter and baseline electrical information from all veins was acquired. The ablation strategy consisted of the encirclement of ipsilateral PVs by creating contiguous lesions at a distance of more than 5 mm from the ostia to achieve wide antral circumferential ablation (WACA). A power controlled mode with a power limit of 30 W and at a maximum temperature of 43 C with 20 mL/min of irrigation was used. The Visitag software (Biosense-Webster) was used to guide the ablation with the force time integral (FTI) goal of 400 g s in posterior wall and 550 g s in anterior wall [10]. If the veins were not isolated after the initial ablation, segmental ablation was performed guided by the earliest signals on the circular catheter. Entry and exit block was confirmed in all veins (Fig. 4). At the end of procedure, all veins were revisited to confirm PVI. During the whole procedure, ACT was maintained over 300 s by supplementing heparin infusion as required. At the end of procedure, right sided sheets were exchanged for the short ones and the sheath removal was performed at the hospital ward when ACT fell under 200 s.
Fig. 4.
Radiofrequency ablation. A) PV potentials before ablation can be seen on circular catheter (20A) and ablation catheter (MAP) located in LSPV (thin arrow). B) After the PV isolation only far field electrograms could be detected on circular catheter (thick arrow).
LSPV – left superior pulmonary vein, PV pulmonary vein.
2.6. Post-ablation management
Patients were discharged the day following ablation if the clinical status was stable. After the intervention, patients were continuously monitored with ECG telemetry until discharge. Before hospital discharge, all patients underwent TTE in order to evaluate pericardium for potential effusion. Oral anticoagulation was continued the same evening after ablation and prescribed for at least 3 months. Previously ineffective antiarrhythmic drugs (AADs) were continued for 3 months and after that their discontinuation was recommended if the patient was free from AF.
2.7. Follow-up
After hospital discharge, patients were scheduled for follow-up visits at 3, 6 and 12 months. Twenty-four hour Holter recordings were obtained at each follow-up visit. Furthermore, telephone calls to patients were made during the follow-up. All documented AF episodes of >30 s after the index procedure were considered as a recurrence. A blanking period of 3 months was applied. All re-do ablations were performed with the RF catheters and 3D mapping.
2.8. Statistical analysis
Categorical variables are expressed as absolute and relative frequencies. Continuous variables are expressed as mean + SD or median and range as appropriate. Comparisons of continuous variables were done with a Student’s t-test and binomial variables with χ2 or Fisher’s exact test as appropriate. Event-free survival was estimated by Kaplan–Meier method and was compared by log-rank test. A two-tailed probability value of <0.05 was deemed significant. Statistical analyses were conducted using the SPSS software (SPSS v22).
3. Results
3.1. Study population
A total of 100 patients (male 74%, mean age 58.9 ± 10 years) with drug-resistant AF were included in the study. Paroxysmal AF was present in 85 patients and the rest presented with early persistent AF (non-interrupted duration of AF <3 months). Mean CHA2DS2-VASc score was 1.45 ± 1.2 and the mean LA diameter was 41.4 ± 5.5 mm.
At a pre-procedural rotational angiography, a distinct 4 PV pattern was present in 84% of patients. A discrete left common ostium was observed in 13%, and right middle PV was found in 3% of patients. There were no significant differences in baseline characteristics between the two studied groups. Table 1 shows the baseline clinical and anatomical characteristics of the study population.
Table 1.
Baseline characteristics.
| RF N = 50 |
CB N = 50 |
P | |
|---|---|---|---|
| Demographic variables | |||
| Male gender, n (%) | 34 (68) | 40 (80) | 0.254 |
| Age at the time of procedure, years (mean ± SD) | 60.4 ± 9.5 | 57.5 ± 10.5 | 0.124 |
| Medical history | |||
| Paroxysmal AF, n (%) | 45 (90) | 40 (80) | 0.262 |
| Early-persistent AF, n (%) | 5 (10) | 10 (20) | 0.262 |
| Hypertension, n (%) | 33 (66) | 28 (56) | 0.406 |
| Diabetes, n (%) | 1 (2) | 3 (6) | 0.617 |
| Ischemic heart disease, n (%) | 4 (8) | 3 (6) | 1 |
| OSAS, n (%) | 1 (2) | 1 (2) | 1 |
| Heart failure, n (%) | 1 (2) | 3 (6) | 0.617 |
| Dyslipidemia, n (%) | 33 (66) | 27 (54) | 0.307 |
| Previous TIA/stroke, n (%) | 1(2) | 2 (4) | 1 |
| CHA2DS2VASc score (mean ± SD) | 1.6 ± 1.1 | 1.3 ± 1.3 | 0.2159 |
| Echocardiography | |||
| LA diameter, mm (mean ± SD) | 41.5 ± 6.5 | 41.4 ± 4.6 | 0.929 |
| LVEF, % (mean ± SD) | 61.8 ± 5.3 | 61.3 ± 6.6 | 0.677 |
| PV anatomy | |||
| Left common PV, n (%) | 5 (10) | 8 (16) | 0.553 |
| Right middle PV, n (%) | 1 (2) | 2 (4) | 1 |
| Medical treatment before procedure | |||
| Class I AAD, n (%) | 22 | 20 | 0.839 |
| Class II AAD, n (%) | 35 | 24 | 0.041 |
| Class III AAD, n (%) | 18 | 22 | 0.540 |
| Class IV AAD, n (%) | 0 | 0 | 1 |
AAD – anti-arrhythmic drug, AF – atrial fibrillation, CB – cryoballoon, LA – left atrium, LVEF – left ventricular ejection fraction, OSAS – obstructive sleep apnea syndrome, PV – pulmonary vein, RF – radiofrequency, TIA – transient ischemic attack.
3.2. Procedural characteristics and outcomes
In both groups, acute procedural success was achieved, all veins were isolated at the end of the procedure. In the CB group, no additional focal catheter applications were needed. Procedure time, which included 3D rotational angiography, was significantly longer in the RF group (191.8 ± 101.1 min) in relation to the CB group (116.6 ± 39.8 min) (p < 0.001). There was no difference in fluoroscopy times, 24.2 ± 10.6 min and 22.4 ± 11.7 min for RF and CB group, respectively (p = 0.422). However, the RF group received lower radiation doses (measured as dose area product) than CB group, 2976 ± 1812 μGy/m2 and 9585 ± 5610 μGy/m2 respectively (p < 0.001). These figures include the dose for 3D rotational angiography. Ablation time was shorter in CB group (1227 ± 141.5 s) vs RF group (2627.3 ± 745 s) (p < 0.001) Table 2. Development of the procedural characteristics over time is depicted in Fig. 5.
Table 2.
Procedure characteristics.
| RF N = 50 |
CB N = 50 |
P | |
|---|---|---|---|
| Procedure time, min (mean ± SD) | 191.8 ± 101.1 | 116.6 ± 39.8 | <0.001 |
| Fluoroscopy time, min (mean ± SD) | 24.2 ± 10.6 | 22.4 ± 11.7 | 0.422 |
| Dose area product μGy/m2, (mean ± SD) | 2976 ± 1812 | 9585 ± 5610 | <0.001 |
| Ablation time, sec (mean ± SD) | 2627 ± 745 | 1227 ± 141 | <0.001 |
Fig. 5.
Evolution of procedural characteristics during the study period. Panel A – Radiofrequency point by point procedures. Panel B – Cryoballoon procedures. Major complications are denoted on y axis, for the given patient when the complication happened.
At a mean follow-up of 14.5 ± 2.4 months after the procedure, there were 26% patients with AF recurrences; 15 in the RF group (30%) and 11 in CB group (22%) (Fig. 6). However, 9 (60%) patients in the RF group underwent re-do ablation, and 4 in the CB group (36%) (p = 0.428). In the RF group, 26/36 veins were reconnected (72.2%) and in CB group 7/15 had reconnections (46.6%) (p = 0.111). Re-do ablations were longer in the RF group (190.5 ± 69.8 min) with more ablation time required (1904 ± 608 s) than in the CB group (122.8 ± 40.8 min and 558 ± 320 s) but this difference did not reach statistical significance.
Fig. 6.
Kaplan-Meier arrhythmia free survival curves. Blue line - CB group; green line - RF group. Lower, the table indicates the remaining patients free from the AF during the follow up.
3.3. Complications
Seven major complications occurred during the study period (7%); 5 in the RF group (10%) and 2 in the CB group (4%) (p = 0.436). Pericardial tamponade (N = 2) occurred solely in RF group and in both cases successful pericardiocentesis was performed and no surgical intervention was needed. One persistent PNP occurred in a CB group which fully recovered at 6 months follow up, confirmed by normal chest X-ray. Furthermore, one right sided femoral arterio-venous fistula occurred in the CB group which required surgical closure and prolonged hospitalization. In the RF group, other than pericardial complications, two femoral vein thrombosis and one arterial pseudoaneurysm occurred. These puncture site complications resolved by conservative treatment. There were no atrio-esophageal fistulae, no symptomatic PV stenosis and no deaths related to the procedure.
4. Discussion
The main findings of our study are: (i) in unexperienced hands in the new EP lab, second generation CB AF ablation could be performed faster than standard point by point ablation, (ii) fluoroscopy times were similar, but the radiation doses were higher when using CB, and finally (iii) complication rates might hypothetically be lower when using CB.
Despite multiple different catheter ablation strategies to treat both paroxysmal and persistent AF, PVI is still the cornerstone of the therapy and the only intervention with excellent supporting evidence [11]. There is plenty of published data comparing the two most popular techniques to achieve PVI, but these reports are coming usually from the most experienced electrophysiology labs in the world. For instance, probably the most important study about that topic (the Fire and Ice Trial), came from sixteen high volume centers in eight western countries and showed that CB is non inferior to old golden standard, in terms of success and complications rates [5]. The publications that followed reported a possible superiority of CB in terms of repeat ablations, direct-current cardioversions and all-cause rehospitalizations which resulted in improved health care economics [12,13]. However, PVI procedure is being increasingly performed in the newly opened EP labs throughout the world and most AF ablations are performed in low volume centers [14]. Only in Croatia, in the last 2 years 4 new EP labs were opened (population <5 million). Similar scenarios are seen in the rest of Central and Eastern Europe. In contrast to the abundance of data coming from the highest volume EP labs regarding the AF ablation, there is a lack of literature from new and low volume laboratories. This represents an important issue since success and complication rates in AF ablation procedures are directly related to the centers’ experience and volume [15]. Moreover, recent publications show that along with the increase in the annual number of catheter ablation procedures in the US, the rate of periprocedural complications also increased [16]. Therefore, quick adoption and safe performance of EP procedures, including AF ablation, is crucial. There are already some published data that second generation CB ablation has excellent learning curves for the new operators but the direct comparison with RF is lacking [17]. To our knowledge, this is the first study that directly compared learning curves of AF ablation using CB and RF point by point ablation.
4.1. Procedure characteristics and outcomes
In our cohort, as in most published studies comparing RF and cryo-energy, CB ablation required lower procedure times, with a mean time of less than 2 h, while point by point ablations needed more than 3 h to be completed. Recent paper by Leitz et al. that compared CB to Multielectrode Pulmonary Vein Ablation Catheter (PVAC) ablation showed very similar procedure times in the beginning of learning curves for second generation CB (122 ± 32 min) [18]. Furthermore, standard deviation of procedure time for CB ablation in our cohort was around 30 min which is consistent with the published data from the more experienced centers [5,18,19]. In our opinion, this is an important finding, since low variance in procedure time for AF ablation allows easier EP lab management and planning. It seems that even in the beginning of learning curves, CB ablation allows shorter and more predictable procedure times, that greatly eases the lab time management.
Considering the fluoroscopy time, we did not find significant differences between RF and CB group. Compared to the Fire and Ice Trial [5], our fluoroscopy time was quite similar in CB patients (22 min) but longer when compared with more recent reports in which fluoroscopy times are usually <10–15 min for CB ablation [17,20]. In the RF group, fluoroscopy times were longer than in experienced centers (17 vs 24 min), once again emphasizing the importance of learning curves [5]. More important than fluoroscopy times are radiation doses and in this regard RF was clearly superior. In the CB group, radiation dose was >30% larger than in RF patients, which is probably due to the occlusion assessment using cine-mode of fluoroscopy. Most published studies did not report radiation doses, which are more relevant than fluoroscopy times. However, our data is consistent with recent larger multicenter studies that also reported similar fluoroscopy times in RF and CB groups with higher radiation doses in CB patients [21].
In our study, one year success rates did not significantly differ in the two studied groups. Outcomes were similar to already published data from high volume centers but it is important to state that in the beginning of our AF ablation program, we have been choosing a relatively healthy population with paroxysmal and short persistent AF. The differences in outcomes could be proven in pooled data from French AF registry, but they are not that pertinent to be detected in smaller population studies. In the same registry, CB seems to be less operator-dependent and more reproducible than RF, especially in the low volume centers [22]. Furthermore, it seems that a lower number of patients required re-do ablation in the CB group, but because of the small patient sample, this finding is not statistically significant. This is in accordance with the recently published big Swedish/EHRA registry which also found that CB patients less commonly need re-do ablations [23]. Our data might suggest that when performing re-do ablation, CB patients need less ablation and shorter procedures to achieve PV re-isolation. Lower number of reconnected veins might explain shorter procedure and ablation times after CB ablation. Recent single center studies and also the Fire and Ice Trial found higher rates of reconnections following RF ablation [24,25].
4.2. Complications
In the total study population major complications occurred in 7% of patients, which is in line with published results on >9000 ablations [15]. Interestingly, cardiac tamponades exclusively occurred in the RF group. It seems that over the wire balloon technique might be safer in terms of tamponades, especially in less experienced hands. Recently published big German registry reported low rates of tamponades in CB ablation procedures, less than 0.5% both in low and high volume centers. On the contrary, point by point RF ablation resulted in significant differences of pericardial complications in high (0.9%) and low volume centers (2%) [26]. Not surprisingly, PNP was limited to CB ablation, and the rest of the complications were related to puncture sites.
5. Conclusion
When starting an AF ablation program in an inexperienced center, our results suggest that CB significantly shortens the procedure and ablation time with comparable fluoroscopy duration and clinical outcomes to RF ablation. However, RF ablation seems superior regarding the radiation doses applied, but CB could result in lower complication rates, especially when considering cardiac tamponade.
6. Limitations
This study has several limitations. It is a single center, non-randomized, retrospective study with all inherent limitations of the study design. The number of patients is not high, but it reflects initial learning curves of adopting AF ablation technologies in parallel which was the aim of the study. Important to note, due to the small number of patients, any firm conclusions could not be given but our study could be the basis for the future larger research. The decision to perform RF or CB ablation was not based on preprocedural imaging or specific characteristic of the patients. Rather, the availability of the catheters and general EP lab scheduling were more decisive in choosing the technology. Finally, using the newer RF (ablation index) and CB (4th generation) technologies might change these results.
Declarations
No funding was received.
Availability of data
No associated data.
Author’s contribution
VV was responsible for conception of the study and drafting of the manuscript. VP, IP and PM were responsible for acquisition and analysis of the data. JS, IP, DP and DM were responsible for critical revision of the manuscript and important intellectual content.
Ethics approval
Ethical approval was waived by the local Ethics Committee of University Hospital Centre Zagreb in view of the retrospective nature of the study and all the procedures being performed were part of the routine care.
Declaration of competing interest
Conflict of interest: VV received an educational grants by St Jude Medical, travel grants and lecture fees from Medtronic and Biosense-Webster. MP received educational and travel grants from Medtronic and Biosense-Webster. Conflict of interest: VV received an educational grants by St Jude Medical, travel grants and lecture fees from Medtronic and Biosense-Webster. MP received educational and travel grants from Medtronic and Biosense-Webster.
Footnotes
Peer review under responsibility of Indian Heart Rhythm Society.
References
- 1.Haim M., Hoshen M., Reges O., Rabi Y., Balicer R., Leibowitz M. Prospective national study of the prevalence, incidence, management and outcome of a large contemporary cohort of patients with incident non-valvular atrial fibrillation. J Am Heart Assoc. 2015;4 doi: 10.1161/JAHA.114.001486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krijthe B.P., Kunst A., Benjamin E.J., Lip G.Y., Franco O.H., Hofman A., Witteman J.C., Stricker B.H., Heeringa J. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J. 2013;34:2746–2751. doi: 10.1093/eurheartj/eht280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calkins H., Kuck K.H., Cappato R. HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Europace. 2012;14:528–606. doi: 10.1093/europace/eus027. 2012. [DOI] [PubMed] [Google Scholar]
- 4.Arbelo E., Brugada J., Hindricks G. Atrial fibrillation ablation pilot study investigators: ESCEURObservational research programme: the atrial fibrillation ablation pilot study, conducted by the European heart rhythm association. Europace. 2012;14:1094–1103. doi: 10.1093/europace/eus153. [DOI] [PubMed] [Google Scholar]
- 5.Kuck K.H., Brugada J., Fürnkranz A., Metzner A., Ouyang F., Chun K.R. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med. 2016;374:2235–2245. doi: 10.1056/NEJMoa1602014. [DOI] [PubMed] [Google Scholar]
- 6.G. Hindricks, J. Camm, B. Merkely, P. Raatikainen, D.O. Arnar The EHRA white book 2016
- 7.Nairooz R., Sardar P., Payne J., Aronow W.S., Paydak H. Meta-analysis ofmajor bleeding with uninterrupted warfarin compared to interrupted warfarin and heparin bridging in ablation of atrial fibrillation. Int J Cardiol. 2015;187:426–429. doi: 10.1016/j.ijcard.2015.03.376. [DOI] [PubMed] [Google Scholar]
- 8.Ghosh J., Singarayar S., Kabunga P., McGuire M.A. Subclavian vein pacing and venous pressure waveform measurement for phrenic nerve monitoring during cryoballoon ablation of atrial fibrillation. Europace. 2015;17:884–890. doi: 10.1093/europace/euu341. [DOI] [PubMed] [Google Scholar]
- 9.Ghosh J., Sepahpour A., Chan K.H., Singarayar S., McGuire M.A. Immediate balloon deflation for prevention of persistent phrenic nerve palsy during pulmonary vein isolation by balloon cryoablation. Heart Rhythm. 2013;10:646–652. doi: 10.1016/j.hrthm.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 10.Nakagawa H., Jackman W.M. The role of contact force in atrial fibrillation ablation. J Atr Fibrillation. 2014 Jun 30;7(1):1027. doi: 10.4022/jafib.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calkins H., Hindricks G., Cappato R., Kim Y.H., Saad E.B., Aguinaga L. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace. 2018 Jan 1;20(1):e1–e160. doi: 10.1093/europace/eux274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chun K.R.J., Brugada J., Elvan A., Gellér L., Busch M., Barrera A. The impact of cryoballoon versus radiofrequency ablation for paroxysmal atrial fibrillation on healthcare utilization and costs: an economic analysis from the fire and ICE trial. J Am Heart Assoc. 2017 Jul 27;(8):6. doi: 10.1161/JAHA.117.006043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuck K.H., Fürnkranz A., Chun K.R., Metzner A., Ouyang F., Schlüter M Cryoballoon or radiofrequency ablation for symptomatic paroxysmal atrial fibrillation: reintervention, rehospitalization, and quality-of-life outcomes in the FIRE AND ICE trial. Eur Heart J. 2016 Oct 7;37(38):2858–2865. doi: 10.1056/NEJMoa1602014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheung J.W., Ilhwan Y., Cheng E., James E.I., Thomas G., Liu C.F. Impact of hospital procedural volume on complications and thirty-day readmissions following catheter ablation of atrial fibrillation: nationwide readmissions database 2010 – 2014. Heart Rhythm. 2019;15(5S) S-PO01-123 (abstr) [Google Scholar]
- 15.Deshmukh A., Patel N.J., Pant S., Shah N., Chothani A., Mehta K. In-hospital complications associated with catheter ablation of atrial fibrillation in the United States between 2000 and 2010: analysis of 93801 procedures. Circulation. 2013;128:2104–2112. doi: 10.1161/CIRCULATIONAHA.113.003862. [DOI] [PubMed] [Google Scholar]
- 16.Hosseini S.M., Rozen G., Saleh A., Vaid J., Biton Y., Moazzami K. Catheter ablation for cardiac arrhythmias: utilization and in-hospital complications, 2000 to 2013. JACC Clin Electrophysiol. 2017 Nov;3(11):1240–1248. doi: 10.1016/j.jacep.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Velagić V., de Asmundis C., Mugnai G., Hünük B., Hacioğlu E., Ströker E. Learning curve using the second-generation cryoballoon ablation. J Cardiovasc Med. 2017 Jul;18(7):518–527. doi: 10.2459/JCM.0000000000000493. [DOI] [PubMed] [Google Scholar]
- 18.Leitz P., Mönnig G., Güner F., Dechering D.G., Wasmer K., Reinke F. Comparing learning curves of two established single-shot devices for ablation of atrial fibrillation. J Intervent Card Electrophysiol. 2018;53:317–322. doi: 10.1007/s10840-018-0361-z. [DOI] [PubMed] [Google Scholar]
- 19.Irfan G., de Asmundis C., Mugnai G., Poelaert J., Verborgh C. One-year follow-up after second-generation cryoballoon ablation for atrial fibrillation in a large cohort of patients: a single-centre experience. Europace. 2015 Dec 23 doi: 10.1093/europace/euv365. [DOI] [PubMed] [Google Scholar]
- 20.Salghetti F., Abugattas J.P., Regibus V., Iacopino S., Takarada K., Ströker E. Real-time recordings in cryoballoon pulmonary veins isolation: comparison between the 25mm and the 20mm achieve catheters. J Atr Fibrillation. 2018 Apr 30;10(6):1855. doi: 10.4022/jafib.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoffmann E., Straube F., Wegscheider K., Kuniss M., Andresen D., Wu L.Q. Outcomes of cryoballoon or radiofrequency ablation in symptomatic paroxysmal or persistent atrial fibrillation. Europace. 2019 Jun 14 doi: 10.1056/NEJMoa1602014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Providencia R., Defaye P., Lambiase P.D., Pavin D., Cebron J.P., Halimi F. Results from a multicentre comparison of cryoballoon vs. radiofrequency ablation for paroxysmal atrial fibrillation: is cryoablation more reproducible Europace. 2017 Jan;19(1):48–57. doi: 10.1093/europace/euw080. [DOI] [PubMed] [Google Scholar]
- 23.Mörtsell D., Arbelo E., Dagres N., Brugada J., Laroche C., Trines S.A. Cryoballoon vs. radiofrequency ablation for atrial fibrillation: a study of outcome and safety based on the ESC-EHRA atrial fibrillation ablation long-term registry and the Swedish catheter ablation registry. Europace. 2019 Apr 1;21(4) doi: 10.1093/europace/euy239. 581-58. [DOI] [PubMed] [Google Scholar]
- 24.Ciconte G., Velagić V., Mugnai G., Saitoh Y., Irfan G., Hunuk B. Electrophysiological findings following pulmonary vein isolation using radiofrequency catheter guided by contact-force and second-generation cryoballoon: lessons from repeat ablation procedures. Europace. 2016 Jan;18(1):71–77. doi: 10.1093/europace/euv224. [DOI] [PubMed] [Google Scholar]
- 25.Kuck K.H., Albenque J.P., Chun J., Fürnkranz A., Busch M., Elvan A. Repeat ablation for atrial fibrillation recurrence post cryoballoon or radiofrequency ablation in the fire and ICE trial. Circ Arrhythm Electrophysiol. 2019;12 doi: 10.1056/NEJMoa1602014. [DOI] [PubMed] [Google Scholar]
- 26.Bollmann A., Ueberham L., Schuler E., Wiedemann M., Reithmann C., Sause A. Cardiac tamponade in catheter ablation of atrial fibrillation: German-wide analysis of 21 141 procedures in the Helios atrial fibrillation ablation registry (SAFER) Europace. 2018 Dec 1;20(12):1944–1951. doi: 10.1093/europace/euy131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No associated data.